Abstract
TMC207 is a first-in-class diarylquinoline with a new mode of action against mycobacteria targeting the ATP synthase. It is metabolized to an active derivative, N-desmethyl TMC207, and both compounds are eliminated with long terminal half-lives (50 to 60 h in mice) reflecting slow release from tissues such as lung and spleen. In vitro, TMC207 is 5-fold more potent against Mycobacterium tuberculosis than N-desmethyl TMC207, and the effects of the two compounds are additive. The pharmacokinetic and pharmacodynamic (PK-PD) response was investigated in the murine model of tuberculosis (TB) infection following oral administration of different doses of TMC207 or N-desmethyl TMC207 at 5 days per week for 4 weeks starting the day after intravenous infection with M. tuberculosis and following administration of different doses of TMC207 at various dosing frequencies for 6 weeks starting 2 weeks after infection. Upon administration of N-desmethyl TMC207, maximum plasma concentration (Cmax), area under the plasma concentration-time curve from time zero to 168 h postdose (AUC168h), and minimum plasma concentration (Cmin) were approximately dose proportional between 8 and 64 mg/kg, and the lung CFU counts were strongly correlated with these pharmacokinetic parameters using an inhibitory sigmoid maximum effect (Emax) model. Administration of the highest dose (64 mg/kg) produced a 4.0-log10 reduction of the bacillary load at an average exposure (average concentration [Cavg] or AUC168h divided by 168) of 2.7 μg/ml. Upon administration of the highest dose of TMC207 (50 mg/kg) 5 days per week for 4 weeks, the total reduction of the bacillary load was 4.7 log10. TMC207 was estimated to contribute to a 1.8-log10 reduction and its corresponding exposure (Cavg) was 0.5 μg/ml. Optimal bactericidal activity with N-desmethyl TMC207 was reached at a high exposure compared to that achieved in humans, suggesting a minor contribution of the metabolite to the overall bactericidal activity in TB-infected patients treated with TMC207. Following administration of TMC207 at a total weekly dose of 15, 30, or 60 mg/kg fractionated for either 5 days per week, twice weekly, or once weekly, the bactericidal activity was correlated to the total weekly dose and was not influenced by the frequency of administration. Exposures (AUC168h) to TMC207 and N-desmethyl TMC207 mirrored this dose response, indicating that the bactericidal activity of TMC207 is concentration dependent and that AUC is the main PK-PD driver on which dose optimization should be based for dosing frequencies up to once weekly. The PK-PD profile supports intermittent administration of TMC207, in agreement with its slow release from tissues.
INTRODUCTION
Tuberculosis (TB) is one of the most lethal infectious diseases in the world, killing about 1.3 million people annually (19). Current treatments are inadequate, taking 6 to 9 months to treat drug-susceptible TB and as much as 30 months to treat multidrug-resistant (MDR) TB. New anti-TB agents that shorten the duration of TB treatment, simplify concomitant medications, provide more effective therapy for drug-resistant TB, and/or improve the treatment of latent TB infection are urgently needed. Currently, five new chemical classes are in various stages of clinical development for tuberculosis (21). However, optimization of dosing strategies for investigational and companion drugs is a critical obstacle. Pharmacokinetic and pharmacodynamic (PK-PD) analyses should play a vital role in the rational development of dosing regimens for new anti-TB drugs and combination regimens (3, 15). Dose fractionation studies have been performed in the murine model of tuberculosis in order to identify the PK-PD index that correlates with bactericidal activity (1, 8, 9, 17). Rifampin (8), isoniazid (9), and fluoroquinolones (17) have been reported to display concentration-dependent bactericidal activity, with the area under the plasma concentration-time curve (AUC)/MIC being the most predictive PK-PD index of the bactericidal effect, whereas PA-824 (1) has been shown to have time-dependent activity, with time of free PA-824 concentration above MIC (fT>MIC) being the most predictive index.
TMC207 is a first-in-class diarylquinoline with a new mode of action against mycobacteria targeting ATP synthase (2, 6, 10, 11). In vitro, TMC207 has been shown to be active against sensitive and resistant strains of Mycobacterium tuberculosis (2, 6). In the murine model of tuberculosis, daily administration of TMC207 monotherapy during 4 weeks exceeded the bactericidal activity of the standard WHO regimen, which combines rifampin, isoniazid, and pyrazinamide (2). The efficacy of once weekly administration of TMC207 was similar to that of daily (5 days per week) administration (18). TMC207 synergized with pyrazinamide (7), and addition of TMC207 to first- or second-line drug regimens led to accelerated clearance of bacilli (2, 12). A phase II efficacy study has been conducted in patients with MDR pulmonary TB who took TMC207 in addition to a standard background regimen (5). TMC207 was safe and well tolerated and showed significant bactericidal efficacy.
No TMC207 PK-PD analysis has been reported so far. The PK-PD properties of TMC207 and its major metabolite, N-desmethyl TMC207, were explored in the murine model of tuberculosis, and the results obtained are described in this paper.
MATERIALS AND METHODS
Bacteria.
The H37Rv strain of M. tuberculosis was grown on 7H11 medium supplemented with 5% bovine serum albumin (BSA). Colonies were subcultured in 7H9 broth supplemented with 10% oleic acid, albumin, dextrose, and catalase (OADC; Difco, le Pont de Claix, France) for 7 days at 37°C. The turbidity of the resulting suspension was adjusted with phosphate-buffered saline (PBS) to match that of a MacFarland 2 suspension with 108 CFU/ml (CFU/ml) of microorganisms. A 10-fold dilution in PBS was used for mouse inoculation.
Determination of MICs, MBCs, and FBC index.
MICs and minimal bactericidal concentrations ([MBCs] >99.9% killing) were determined for TMC207 and N-desmethyl TMC207 against the H37Rv strain using 7H9 broth and 7H11 agar medium. Fractional bactericidal concentrations (FBC) were determined for combination of the parent compound, TMC207, and its N-desmethyl metabolite by using a method previously described (20). Briefly, TMC207 and N-desmethyl TMC207 were added to 7H9 broth in different combinations so that each drug represented 0, 0.2, 0.4, 0.6, 0.8, or 1.0 times its MBC. The tubes were inoculated with approximately 105 CFU/ml of the H37Rv strain of M. tuberculosis and were subcultured on 7H11 agar to determine survival after incubation for 14 days at 37°C. The FBC was calculated as the MBC of the test compound in combination divided by the MBC of the compound alone, and the FBC index was determined as the sum of the FBC of each test compound in the combination. For the purpose of this study, synergy was defined as an FBC index of <0.5, an additive effect was defined as an FBC index of 0.5 to 1, and antagonism was defined as an FBC index of >1.
Animals, infection, and compound administration.
Male and female 4- to 5-week-old Swiss mice were purchased from the Janvier Breeding Center (Le Genest Saint-Isle, France). All animals were housed under controlled conditions (specific pathogen free, 23°C, 60% humidity, and normal light-dark cycle) and had access to food and water ad libitum. Noninfected male mice were used for the single-dose pharmacokinetic study and noninfected female mice were used for the PK-PD studies. The female mice were inoculated in the tail vein with 0.2 ml of a bacterial suspension containing approximately 107 CFU of M. tuberculosis H37Rv. TMC207 and N-desmethyl TMC207 were administered by gavage as an aqueous 20% 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) solution. The local Johnson & Johnson Ethical Committee approved all experimental protocols.
Single-dose pharmacokinetic experiment.
Male Swiss mice were given a single oral dose of 30 mg/kg of TMC207. Blood samples were taken from three mice per time point at 1, 3, 8, 24, 48, 72, 96, and 168 h postdose. Blood samples were collected in K3-EDTA-coated tubes and centrifuged at 1,500 × g for 10 min, and plasma was separated and stored at −70°C before bioanalysis.
Dose-response study (dosing frequency of 5 days/week).
Following infection, female Swiss mice were randomly distributed to one control group of six nontreated mice and eight test groups receiving either TMC207 (6 mice per group) or N-desmethyl TMC207 (10 mice per group). Treatment was initiated the day after infection and continued for 4 weeks. TMC207 was given at 6.25, 12.5, 25, and 50 mg/kg of body weight 5 days per week. N-Desmethyl TMC207 was given at 8, 16, 32, and 64 mg/kg 5 days per week. At the end of the treatment, mice were sacrificed, and the lungs were removed for measurement of the CFU counts. Control mice were sacrificed the day after infection for measurement of the CFU counts. Noninfected female Swiss mice were used for pharmacokinetic evaluation. These mice were divided in groups receiving the same treatment as the infected mice. Blood samples were taken from three mice per time point at 2, 8, 24, and 72 h after the last administration. Blood samples were centrifuged at 1,500 × g for 10 min, and plasma was separated and stored at −70°C before bioanalysis. Lungs were collected in special tissue containers at the same time points as blood. These were placed on melting ice and then stored at −70°C before bioanalysis.
Dose fractionation study (dosing frequencies of 5 days/week, 2 days/week, 1 day/week, 1 day/2 weeks).
Following infection, female Swiss mice were randomly distributed to one control group of eight nontreated mice and 11 test groups of eight mice each. In this study, treatment was initiated 2 weeks after infection. TMC207 was given for 6 weeks at 3, 6, 12 mg/kg of body weight 5 days per week; at 7.5, 15, and 30 mg/kg twice per week; at 15, 30, and 60 mg/kg once per week; and at 30 and 60 mg/kg once per 2 weeks. At the end of the treatment period, mice were sacrificed by CO2 asphyxiation, and the lungs were removed for measurement of the CFU counts. Control mice were sacrificed 2 weeks after infection for measurement of the CFU counts. Noninfected mice were used for pharmacokinetic evaluation. They were divided in groups receiving 3, 6, and 12 mg/kg 5 days per week or 15, 30, and 60 mg/kg once per week. Blood samples were taken at 0.5, 1, 2, 4, 8, 24, and 96 h after the last administration in groups treated five times per week and at 24, 48, 72, 96, 120, and 144 h after the last administration in groups treated once weekly. Blood samples were centrifuged at 1,500 × g for 10 min, and plasma was separated and stored at −70°C before bioanalysis.
Determination of the CFU counts in the lungs.
Lungs were homogenized in 2.5 ml of sterile phosphate-buffered saline (PBS), and CFU counts in the lungs were determined by plating six serial 10-fold dilutions of homogenized suspensions onto 7H11–5% BSA plates. Results of the cultures were recorded after incubation at 37°C for 4 weeks.
Determination of TMC207 and N-desmethyl TMC207 in plasma and lung.
Plasma and lung concentrations of TMC207 and N-desmethyl TMC207 were determined using a qualified research liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Lung samples were homogenized in buffer (1/10, wt/wt, or in a minimal volume of 500 μl). After solubilization and protein precipitation with methanol, plasma and lung samples were quantified on a reversed-phase LC column (Polaris C18; 5-μm particle size; 30 by 4.6 mm; Varian). Mobile phases consisted of 10 mM ammonium formate (solvent A) and methanol (solvent B). Chromatographic separation was obtained as follows: 25% solvent A, 75% solvent B starting conditions for 0.5 min and a step increase to 2% solvent A, and 98% solvent B in 0.1 min followed by an isocratic hold for 3 min at a flow rate of 1.5 ml/min. LC-MS/MS analysis was carried out on an API-4000 MS/MS (Applied Biosystems, Toronto, Canada), which was coupled to a high-performance liquid chromatography (HPLC) system (Agilent, Palo Alto, CA). The MS/MS operated in the positive ion mode using a TurboIonSpray interface (electrospray ionization) and was optimized for the quantification of both compounds and their internal standards (IS) (multiple-reaction monitoring [MRM] transition for TMC207, 555.2 to 58 (m/z); stable isotope-labeled IS for TMC207, 561.2 to 64; MRM transition for N-desmethyl TMC207, 541.1 to 480; stable isotope-labeled IS for N-desmethyl TMC207, 545.2 to 480). The limit of quantification was 5 ng/ml for plasma samples and 50 ng/g for lung samples for both analytes. The accuracy (intrabatch accuracy from independent quality control samples) was between 85% and 115% of the nominal value over the entire range for plasma and between 80% and 120% of the nominal value over the entire range for lung samples.
Data analysis.
The following pharmacokinetic parameters were calculated from the mean plasma concentrations of TMC207 and N-desmethyl TMC207 determined after single administration using a noncompartmental analysis (WinNonlin, version 5.2.1; Pharsight, Mountain View, CA): the maximum plasma concentration (Cmax), the terminal elimination half-life (t1/2), and the area under the plasma concentration-time curve from time zero to 168 h postdose (AUC168h) using the linear up/log down trapezoidal rule. Following administration 5 days per week, the following pharmacokinetic parameters were determined for the two compounds: Cmax, the trough plasma concentration determined at 72 h after the last administration (Cmin), and AUC168h that was estimated using the linear up/log down trapezoidal rule and AUC summation. The values of AUC168h were also determined from the mean lung concentrations. Following once weekly administration, plasma concentrations at 168 h after the last dose for intermittent dosing were calculated by extrapolation of the terminal curve, and AUC168h over the last week of treatment was estimated for N-desmethyl TMC207 by taking concentration at zero hour (C0h) equal to C24h considering its flat plasma profile. The average concentration (Cavg) was calculated as AUC168h divided by 168.
Steady-state plasma profiles of TMC207 and N-desmethyl TMC207 were simulated using WinNonlin from the mean plasma concentrations determined after single TMC207 administration of 30 mg/kg, assuming linear kinetics upon repeated administration. They were simulated by nonparametric superposition for a TMC207 total weekly dose of 30 mg/kg with administration 5 days per week (6 mg/kg per administration) and once weekly (30 mg/kg per administration).
Inhibitory sigmoid maximum effect (Emax) models were used to describe the relationship between the CFU counts in the lungs and TMC207 dose or N-desmethyl TMC207 Cmax or AUC168h or Cmin at the end of the treatment. WinNonlin was used to fit the data. Two models with the following equations were assessed. In the first, E = Emax × [1 − (Xy/(Xy + EX50y)] where complete inhibition is achieved with increasing dose (WinNonlin model 107). In the second, E = Emax − (Emax − E0) × [Xy/(Xy + EX50y)], where E0 is the lowest achievable number of CFU in the lungs with increasing dose (WinNonlin model 108). E is the number of CFU determined in the lungs (expressed as log10 CFU count), Emax is the number of CFU in the untreated mice before treatment with TMC207 or N-desmethyl TMC207, X is the pharmacokinetic parameter or the dose, y is the Hill coefficient, and EX50 is the pharmacokinetic parameter or the dose required to achieve 50% reduction of Emax. In order to evaluate the contribution of the two compounds to the overall activity, the mycobacterial counts measured at the end of the treatment with TMC207 were plotted versus the values of AUC168h determined for N-desmethyl TMC207. At the high TMC207 dose, TMC207 contribution to the overall activity was estimated by subtracting the activity of N-desmethyl TMC207 determined at the same value of AUC168h when N-desmethyl TMC207 was given alone. CFU counts were analyzed by means of Student's t test. Differences were considered significant at the 95% level of confidence.
RESULTS
MIC, MBC, and FBC index of TMC207 and N-desmethyl TMC207.
The MICs of TMC207 and N-desmethyl TMC207 were 0.06 and 0.3 μg/ml, respectively, and MBCs were 0.4 and 2.0 μg/ml, respectively. Combinations of TMC207 and N-desmethyl TMC207 resulted in an FBC index of 1.04 ± 0.52, suggesting additive effects.
Single-dose pharmacokinetic study.
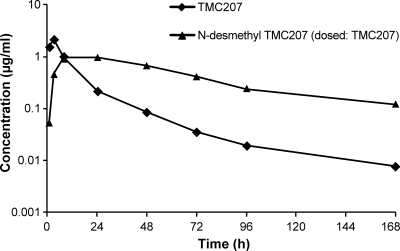
After single oral administration of TMC207 to male Swiss mice at 30 mg/kg, maximum TMC207 plasma concentrations of about 2 μg/ml were reached around 3 h (Table 1). Then, plasma concentrations decreased rapidly up to 24 h and more slowly thereafter (Fig. 1). N-Desmethyl TMC207 concentrations were on a plateau at around 1 μg/ml between 8 and 24 h and then decreased slowly. The terminal elimination half-life was 50 to 60 h for both compounds. The N-desmethyl TMC207/TMC207 ratio was about 0.5 for Cmax and 3 for AUC168h. Male mice were used in this PK study, whereas female mice were used in the PK-PD studies. No significant gender differences in the pharmacokinetic profiles of TMC207 and N-desmethyl TMC207 were observed (unpublished data).
Table 1.
TMC207 and N-desmethyl TMC207 pharmacokinetic parameters obtained after single administration of 30 mg/kg TMC207 to Swiss mice
| Parametera | Value for the parameter |
|
|---|---|---|
| TMC207 | N-Desmethyl TMC207 | |
| Cmax (μg/ml) | 2.14 | 0.97 |
| Tmax (h) | 3 | 24 |
| t1/2, 72–168h (h)b | 53 | 56 |
| AUC168h (μg · h/ml) | 26.3 | 71.5 |
Cmax, maximum plasma concentration; Tmax, time to Cmax; AUC168h, area under the plasma concentration-time curve over 168 h after administration; t1/2, 72–168h, terminal elimination half-life determined between 72 and 168 h after administration.
Approximate values were used because the terminal curve was not well defined.
Fig 1.
Mean (n = 3) plasma concentrations of TMC207 and N-desmethyl TMC207 in Swiss mice following single oral administration (30 mg/kg) of TMC207.
Dose-response study (dosing frequency of 5 days/week).
Mice were treated with either TMC207 or N-desmethyl TMC207 at different doses 5 days per week for 1 month. The mean CFU counts in the lungs of untreated animals before starting treatments (1 day after infection) were 5.39 ± 0.46 log10.
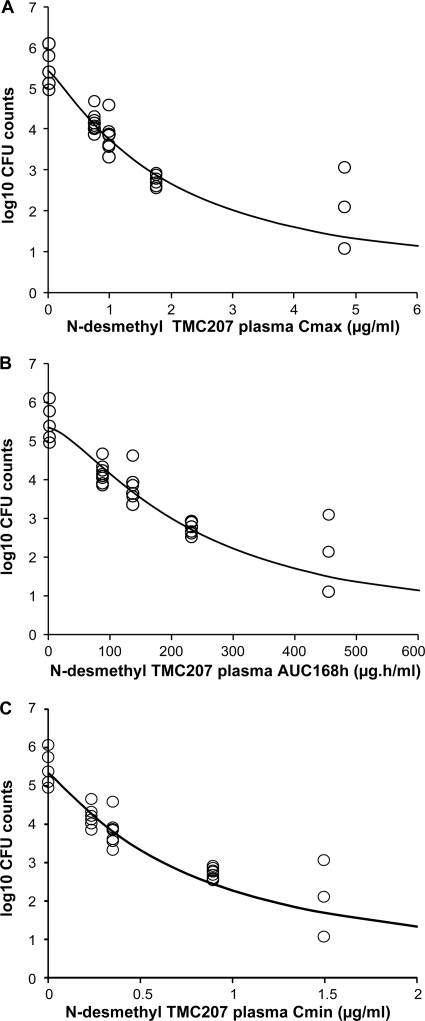
After administration of N-desmethyl TMC207 at 8, 16, 32, and 64 mg/kg for 4 weeks, the mean CFU counts were lower than the pretreatment values at all doses (P < 0.05) (Table 2). Reduction of the bacillary load was dose dependent, with about a 1-log10 reduction at the low dose (8 mg/kg) and a 4-log10 reduction at the high dose (64 mg/kg), compared to the counts at the beginning of the treatment. Cmax, AUC168h, and Cmin at the end of the treatment were approximately dose proportional between 8 and 64 mg/kg. Distribution to the lung was extensive. Lung AUC168h values increased dose-proportionally and were between 100- and 200-fold higher than plasma AUC168h values. The relationship between the CFU counts and the values of N-desmethyl TMC207 plasma Cmax, AUC168h, and Cmin was well described by inhibitory sigmoid Emax models, with zero or nonzero minimum (E0) for the CFU counts in the lungs, where Emax was the number of CFU in the untreated mice before starting treatment and E0 was the lowest achievable number of CFU in the lungs. The values estimated for E0 were low, especially for AUC168h data fitting (E0 = 0.88 log10 CFU for Cmax data fitting and 0.0002 log10 CFU for AUC168h data fitting). Therefore, the model with zero minimum for the CFU counts was selected. A strong correlation was obtained for the three parameters in agreement with the approximately dose-proportional increase in exposure. The coefficient of correlation was about 0.95, and the coefficient of variation on the parameter estimates was lower than 10% for data fitting of the three parameters. The values of N-desmethyl TMC207 Cmax, AUC168h, and Cmin required to achieve 50% reduction of Emax were 1.93 μg/ml, 237 μg · h/ml, and 0.77 μg/ml, respectively (Fig. 2). N-Desmethyl TMC207 trough plasma concentrations at the end of the treatments at 72 h after administration of the last dose (Cmin) were close to the N-desmethyl TMC207 MIC (0.3 μg/ml) at the lowest dose (8 mg/kg) and above MIC at the other doses. The average plasma concentration (Cavg or AUC168h divided by 168) was 4-fold lower than MBC (2 μg/ml) at the low dose and 1.4-fold higher at the high dose.
Table 2.
Bacterial count (mean ± standard deviation) in the lungs of mice after 4 weeks of treatment with TMC207 or N-desmethyl TMC207 given 5 days per week
| TMC207 treatment and response |
N-Desmethyl TMC207 treatment and response |
||
|---|---|---|---|
| Dose (mg/kg of body weight) | log10 CFU ± SD (n = 6)a | Dose (mg/kg of body weight) | log10 CFU ± SD (n = 10)b |
| Untreated (day 1) | 5.39 ± 0.46 | Untreated (day 1) | 5.39 ± 0.46 |
| 6.25 | 4.19 ± 0.48 | 8 | 4.16 ± 0.24 |
| 12.5 | 3.51 ± 0.32 | 16 | 3.81 ± 0.37 |
| 25 | 2.41 ± 0.73 | 32 | 2.74 ± 0.14 |
| 50 | 0.66 ± 0.85c | 64 | 1.43 ± 0.71 |
At a dose of 12.5 mg/kg, n = 4.
For untreated animals, n = 6; at a dose of 50 mg/kg, n = 9.
Includes three mice with negative culture.
Fig 2.
Relationship between the mycobacterial counts in the lungs (expressed as log10 CFU) versus N-desmethyl TMC207 plasma pharmacokinetic parameters Cmax (A), AUC168h (B), and Cmin (C) in mice after oral administration of N-desmethyl TMC207 for 5 days per week at 8, 16, 32, and 64 mg/kg for 28 days. The open circles represent the individual experimental values, and the solid lines represent the model-derived relationship as determined using an inhibitory sigmoid Emax model.
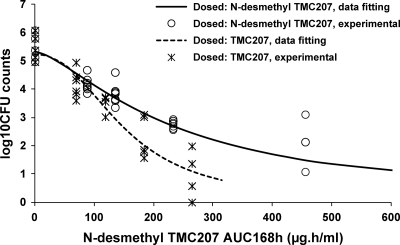
After administration of TMC207 at 6.25, 12.5, 25, and 50 mg/kg at 5 days per week for 4 weeks, N-desmethyl TMC207 was the major circulating compound. AUC168h of N-desmethyl TMC207 was 2.6-fold higher than the AUC168h of TMC207 at the low dose and 3.3-fold higher at the high dose. Distribution to the lung was extensive for both compounds. AUC168h lung/plasma ratios amounted to about 20 for TMC207 and 100 to 200 for N-desmethyl TMC207. Lung concentrations (AUC168h) of the metabolite were between 15- and 30-fold higher than those of TMC207. The reduction of the bacillary load showed a trend to be higher after administration of TMC207 than after administration of N-desmethyl TMC207, even though 28% higher doses of the latter were used (Table 2). The mean CFU counts at the high dose of TMC207 (50 mg/kg) was 0.66 ± 0.85 log10, with three mice out of six being culture negative, versus 1.43 ± 0.71 log10 at the high dose of N-desmethyl TMC207 (P = 0.23) (64 mg/kg). In order to evaluate the contribution of the two compounds to the overall activity, the mycobacterial counts measured at the end of the treatments with TMC207 or N-desmethyl TMC207 were plotted versus the values of AUC168h determined for N-desmethyl TMC207 (Fig. 3). Data point fitting using an inhibitory sigmoid Emax model showed that the response was clearly different after administration of TMC207 from that after administration of N-desmethyl TMC207. This indicates that the contribution of TMC207 to the overall activity was significant despite its 3-fold lower systemic exposure. At the high dose of TMC207 (50 mg/kg), N-desmethyl TMC207 plasma AUC168h was 264 μg · h/ml, which corresponds to a 2.9-log10 reduction of the bacillary load on the N-desmethyl TMC207 curve of the inhibitory sigmoid Emax model for N-desmethyl TMC207 alone. Therefore, since the total reduction of the bacillary load was 4.7 log10, TMC207 contribution was estimated to be 1.8 log10, or about 40% of the total effect, assuming the bactericidal effects of the two compounds were additive, as observed in vitro. The corresponding plasma average concentrations (Cavg) were 0.5 μg/ml for TMC207 and 1.6 μg/ml for N-desmethyl TMC207.
Fig 3.
Plot of mycobacterial counts in the lungs (expressed as log10 CFU) versus N-desmethyl TMC207 plasma AUC168h in mice after oral administration of TMC207 for 5 days per week at 6.25, 12.5, 25, and 50 mg/kg or N-desmethyl TMC207 for 5 days per week at 8, 16, 32, and 64 mg/kg for 28 days. The solid line represents the N-desmethyl TMC207 model-derived relationship determined using an inhibitory sigmoid Emax model following N-desmethyl TMC207 administration. The dotted line represents the fitting of the mycobacterial counts in the lungs and the desmethyl TMC207 plasma AUC168h to an inhibitory sigmoid Emax model.
Dose fractionation study (dosing frequencies of 5 days/week, 2 days/week, 1 day/week, and 1 day/2 weeks).
Mice were treated with different TMC207 weekly doses at different dosing frequencies for 6 weeks. The mean CFU counts in the lungs of untreated animals before starting treatments (2 weeks after infection) were 6.65 ± 0.46 log10 CFU.
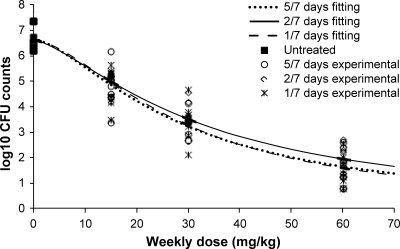
The mean CFU counts determined after 6 weeks of treatment with TMC207 with total weekly doses of 15, 30, and 60 mg/kg, fractionated either 5 days per week, twice weekly, once weekly, or once every 2 weeks, are summarized in Table 3. The mean reduction of the bacillary load increased with increasing weekly doses, with about a 5-log10 reduction at the highest weekly dose. For every total weekly dose that was assessed, the bactericidal efficacy was independent of the frequency of administration if given 5 days per week, twice weekly, or once weekly (P > 0.05). The activity was slightly lower when TMC207 was given once every 2 weeks (P < 0.05). As shown in Fig. 4, the data (mycobacterial counts versus weekly dose) were well described by an inhibitory sigmoid Emax model with no significant difference between the dosing frequencies, i.e., 5 days per week, twice weekly, or once weekly (coefficients of correlation between 0.95 and 0.97).
Table 3.
Bacterial count (mean ± standard deviation) in the lungs of mice after 6 weeks of treatment with TMC207 given at different dosing schedules
| TMC207 weekly dose (mg/kg) | log10 CFU ± SD (n = 8) by dosing schedulea |
|||
|---|---|---|---|---|
| 5 days/week | 2 days/week | 1 day/week | 1 day/2 weeks | |
| 15 | 4.83 ± 0.80 | 4.87 ± 0.49 | 4.86 ± 0.75 | 5.56 ± 0.53 |
| 30 | 3.27 ± 0.49 | 3.72 ± 0.48b | 3.47 ± 0.74 | 4.28 ± 0.36 |
| 60 | 1.60 ± 0.68 | 1.88 ± 0.52 | 1.46 ± 0.61 | |
The bacterial count before starting treatment (2 weeks after infection) was 6.65 log10 CFU ± 0.46.
n = 7.
Fig 4.
Relationship between the mycobacterial counts in the lungs (expressed as log10 CFU) versus a TMC207 weekly dose in mice after oral administration of TMC207 given 5 days per week (5/7), twice weekly (2/7), or once weekly (1/7) for 6 weeks. The lines represent the fitting of the data to an inhibitory sigmoid Emax model.
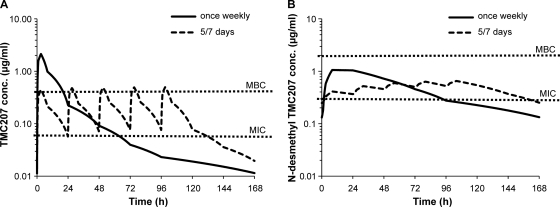
Limited blood sampling was performed in the fractionation study. Estimation of N-desmethyl TMC207 pharmacokinetic parameters was nevertheless possible given its flat plasma profile. Simulated steady-state plasma profiles for repeated administration of a TMC207 total weekly dose of 30 mg/kg assuming linear kinetics showed that TMC207 and N-desmethyl TMC207 Cmax values are expected to be lower with dosing 5 days per week than with dosing once weekly (Fig. 5). Conversely, trough C168h concentrations or time above MIC or MBC are expected to be higher with dosing 5 days per week. Such differences between dosing frequencies were observed with the Cmaxs estimated for N-desmethyl TMC207 and the trough concentrations estimated for both compounds (Tables 4 and 5). Therefore, Cmax and time above MIC or MBC cannot correlate with the bactericidal activity since the activity is the same irrespective of the frequency of administration. AUC168h values estimated for N-desmethyl TMC207 increased approximately dose proportionally with increases in the total weekly dose between 15 and 60 mg/kg and were similar with dosing 5 days per week and once weekly (Table 4). Exposure to TMC207 increased less than dose proportionally with increasing total weekly doses, but the concentration ratios of N-desmethyl TMC207/TMC207 at 24, 96, and 168 h were similar with dosing at 5 days per week or once weekly, suggesting that AUC values of TMC207 were also similar. Therefore, the dose response obtained for efficacy can be explained by similar exposures (AUC) to N-desmethyl TMC207 and TMC207, irrespective of the frequency of administration.
Fig 5.
Simulated steady-state 168-hour plasma profiles of TMC207 (A) and N-desmethyl TMC207 (B) assuming linear kinetics upon repeated administration of a total TMC207 weekly dose of 30 mg/kg given once weekly (30 mg/kg per administration) or 5 days per week (6 mg/kg per administration). conc, concentration.
Table 4.
Values of Cmax and AUC168h of N-desmethyl TMC207 estimated at the end of 6-week treatment with TMC207 given 5 days per week or once weekly
| TMC207 weekly dose (mg/kg) | Value for the parameter by dosing schedulea |
|||
|---|---|---|---|---|
|
Cmax (μg/ml) |
AUC168h (μg · h/ml) |
|||
| 5 days/week | 1 day/week | 5 days/week | 1 day/week | |
| 15 | 0.27 | 0.42 | 30.4 | 30.7 |
| 30 | 0.38 | 0.70 | 54.9 | 69.1 |
| 60 | 1.11 | 1.73 | 126 | 135 |
See Table 1 for abbreviations.
Table 5.
Values of the plasma trough 168-hour concentrations estimated at the end of a 6-week treatment with TMC207 given 5 days per week or once weekly
| Weekly dose (mg/kg) | Plasma concn at 168 h (μg/ml) by dosing and compound |
|||
|---|---|---|---|---|
| 5 days/week |
1 day/week |
|||
| TMC207 | N-Desmethyl TMC207 | TMC207 | N-Desmethyl TMC207 | |
| 15 | 0.039 | 0.098 | 0.023 | 0.051 |
| 30 | 0.081 | 0.26 | 0.031 | 0.10 |
| 60 | 0.081 | 0.40 | 0.052 | 0.23 |
DISCUSSION
TMC207 is rapidly absorbed and slowly metabolized in mice after oral administration. N-Desmethyl TMC207 is the major circulating metabolite. Upon repeated administration, the maximum plasma concentrations of N-desmethyl TMC207 are slightly lower than those of TMC207, and the overall exposure to N-desmethyl TMC207 in terms of AUC is about 3-fold higher. Both compounds distribute extensively to tissues and are slowly eliminated with terminal half-lives of 50 to 60 h. In humans, N-desmethyl TMC207 is also the major circulating metabolite, but its formation is less extensive than in mice. The exposure to N-desmethyl TMC207 in humans is about 4 to 5-fold lower than that of TMC207. In vitro, N-desmethyl TMC207 is less potent than TMC207 (the MBC against M. tuberculosis is 2.0 μg/ml for N-desmethyl TMC207 and 0.4 μg/ml for TMC207). It may contribute to the bactericidal activity to a higher extent in mice than in humans due to its higher relative exposure in mice. In order to evaluate its in vivo activity and its contribution in the murine model of tuberculosis after TMC207 administration, N-desmethyl TMC207 was directly administered to mice infected with M. tuberculosis.
Two different pharmacokinetic-pharmacodynamic studies were conducted: a dose-response study with 4-week administration of different doses of TMC207 or N-desmethyl TMC207 at a single dosing frequency (5 days per week) and a fractionation study with 6-week administration of various weekly doses of TMC207 using different dosing frequencies. Treatment started the day after intravenous infection with M. tuberculosis (nonestablished infection) in the first study and 2 weeks after infection (established infection) in the fractionation study. With the latter mode of infection, the TB infection is already established at the start of treatment, and the bacterial load in spleen and lungs is generally similar to that observed in human pulmonary TB. Following administration of N-desmethyl TMC207, the bactericidal activity was dose dependent, with up to a 4-log10 reduction of the bacillary load for a dose of 64 mg/kg given 5 days per week for 4 weeks, compared to the bacillary load at the beginning of the treatment. For this frequency of administration, the reduction in bacillary load was strongly correlated with N-desmethyl TMC207 Cmax, AUC168h, and Cmin using an inhibitory sigmoid Emax model, in agreement with a dose-proportional increase in exposure between 8 and 64 mg/kg. This indicates that N-desmethyl TMC207 plasma concentrations are a good indicator of bactericidal activity in the dose range investigated, despite extensive distribution of N-desmethyl TMC207 to sites of activity such as lung and spleen. Following administration of TMC207 at 50 mg/kg 5 days per week for 1 month, the contribution of TMC207 to the overall bactericidal activity was estimated to be 40%, as determined from the relative activities obtained upon administration of N-desmethyl TMC207 or TMC207, assuming TMC207 and its metabolite have additive effects as observed in vitro. This was consistent with the relative systemic exposures to TMC207 and N-desmethyl TMC207 since the AUC168h ratio of N-desmethyl TMC207 to TMC207 was of the same order of magnitude as the inverse of the MBC ratio. In contrast, the lung exposure ratio of N-desmethyl TMC207 to TMC207 (lung AUC168h ratio) was 3- to 6-fold higher than the inverse of the MBC ratio. This lung exposure ratio suggests that the contribution of TMC207 should be markedly lower than 40%, considering that the intracellular bactericidal activity of TMC207 was shown to be greater than its extracellular bactericidal activity (4). This indicates that the high lung concentrations of N-desmethyl TMC207, 15- to 30-fold higher than those of TMC207, are not representative for the intracellular activity. Both TMC207 and N-desmethyl TMC207 are cationic amphiphilic compounds (CAD), and both have been shown to induce phospholipidosis in vitro (13). N-Desmethyl TMC207 distributes more extensively to tissues than TMC207, in agreement with its higher potential to induce phospholipidosis (13), and a large proportion of N-desmethyl TMC207 distributed to the lung may not be available for activity as it becomes bound to membrane phospholipids and/or trapped in acidic cellular compartments of cells such as lysosomes, as described for compounds inducing phospholipidosis (16). TMC207 and N-desmethyl TMC207 undergo extensive distribution throughout the body despite a very high plasma protein binding, most probably due to a higher affinity to tissues than to plasma proteins, and in agreement with their CAD properties.
This is consistent with previous observations that tissue concentrations as determined from tissue homogenates are often not representative of a drug's efficacy (14). Therefore, only plasma concentrations were taken into account in the PK-PD analysis. Total drug concentrations and MICs or MBCs, not corrected for functional protein binding, were used as they were assumed to correlate with the free-drug concentrations (free fraction of TMC207 of ≤0.1% in plasma; MIC and MBC increase 10-fold in the presence of 5% BSA [unpublished data]). The highest values of TMC207 Cavg and Cmin in the fractionation study were 0.50 μg/ml and 0.08 μg/ml, respectively. The corresponding estimated free-drug concentrations (0.0005 and 0.00008 μg/ml) are 100- to 1,000-fold lower than the MIC of TMC207 (0.06 μg/ml). Plasma concentrations of unbound N-desmethyl TMC207 are also much lower than its MIC. Therefore, the very low free-drug concentrations do not explain the dose responses observed in these studies.
The average plasma concentration (Cavg or AUC168h divided by 168) of TMC207 after administration of the highest dose tested, 50 mg/kg given 5 days per week, was 0.5 μg/ml. The corresponding reduction of the bacillary load resulting from the combined effects of TMC207 and its metabolite was 4.7 log10. By subtracting the effect of the metabolite from the efficacy of the combination (TMC207 and the metabolite), one can estimate that at a Cavg of 0.5 μg/ml, TMC207 contributed an average 1.8 log10 to the total reduction of the bacillary load. This estimate cannot be validated in the mouse model by an experiment in which only TMC207 contributes to the efficacy. In patients with MDR TB treated with 400 mg of TMC207 once daily for 2 weeks followed by 200 mg of TMC207 three times per week for 6 weeks in combination with a standard five-drug, second-line antituberculosis regimen, a significant bactericidal efficacy was obtained at average TMC207 concentrations of 1.8 μg/ml at week 2 and 0.9 μg/ml at week 8 (5). The corresponding N-desmethyl TMC207 average concentrations (0.39 and 0.25 μg/ml, respectively) were clearly lower than the concentration of 2.7 μg/ml which was needed in the murine model to achieve optimal bactericidal activity. This suggests that N-desmethyl TMC207 has a minor contribution to the overall bactericidal activity in TB-infected patients following administration of TMC207.
In the dose fractionation study, the bactericidal activity increased when the total weekly dose of TMC207 increased from 15 to 60 mg/kg and was not influenced by the dosing frequency. PK-PD analysis of the data obtained in this study was complicated by the high systemic exposure to the active metabolite. However, the dose response did not appear to be related to Cmax or time above MIC or MBC but to the overall exposure (AUC168h) to TMC207 and N-desmethyl TMC207. AUC is the main PK-PD index for dosing frequencies up to once weekly, and TMC207 displays concentration-dependent bactericidal activity in this model, as previously described for rifampin (8), isoniazid (9), and fluoroquinolones (17). In contrast, time above MIC for free drug (fT>MIC) was found to be the most predictive index for PA-824 and activity was time dependent (1). However, free-drug AUC/MIC was also found to be well correlated with the bactericidal activity of this new anti-TB agent when the data set corresponding to the higher dosing intervals (every 72 h or every 144 h) was removed from the analysis. Thus, the bactericidal activity appears to be AUC related, irrespective of the frequency of administration for dosing intervals up to once daily for isoniazid (terminal t1/2, 0.4 to 1.6 h), up to every 2 days for PA-824 (terminal t1/2, 4 to 6 h), and up to once weekly for TMC207 (terminal t1/2, 50 to 60 h). The long terminal half-life of TMC207 corresponding to slow release from tissues and the slow replication of M. tuberculosis probably both contribute to the ability to dose TMC207 as infrequently as once weekly in mice. In humans, release of TMC207 and N-desmethyl TMC207 from tissues is even slower than in mice, as suggested by the longer terminal half-lives for both compounds, further supporting the strong potential of TMC207 for the development of an intermittent dosing regimen.
In conclusion, despite extensive biotransformation to the active metabolite N-desmethyl TMC207 in the murine model of tuberculosis, TMC207 itself significantly contributes to the bactericidal activity as a consequence of its higher in vitro potency against M. tuberculosis, which compensates for the relatively low exposure. Optimal bactericidal activity of the N-desmethyl TMC207 metabolite in mice (about a 4-log10 reduction of the bacillary load) requires a higher exposure than that achieved in humans, indicating that in TB-infected patients the majority of the bactericidal efficacy is achieved by unchanged TMC207 and not by the metabolite. An average TMC207 plasma concentration of 0.5 μg/ml was shown to result in a 1.8-log10 reduction of the bacillary load. The two compounds display concentration-dependent bactericidal activity. The AUC is the main PK-PD index on which dose optimization should be based for dosing frequencies up to once weekly, supporting development of intermittent dosing regimens of TMC207.
Footnotes
Published ahead of print 12 December 2011
REFERENCES
- 1. Ahmad Z, et al. 2011. PA-824 exhibits time-dependent activity in a murine 1 model of tuberculosis. Antimicrob. Agents Chemother. 55:239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andries K, et al. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227 [DOI] [PubMed] [Google Scholar]
- 3. Davies GR, Nuermberger EL. 2008. Pharmacokinetics and pharmacodynamics in the development of anti-tuberculosis drugs. Tuberculosis. 88:S65–S74 [DOI] [PubMed] [Google Scholar]
- 4. Dhillon J, Andries K, Phillips PPJ, Mitchison DA. 2010. Bactericidal activity of the diarylquinoline TMC207 against Mycobacterium tuberculosis outside and within cells. Tuberculosis 90:301–305 [DOI] [PubMed] [Google Scholar]
- 5. Diacon AH, et al. 2009. The diarylquinoline TMC207 in multidrug-resistant tuberculosis. N. Engl. J. Med. 360:2397–2405 [DOI] [PubMed] [Google Scholar]
- 6. Huitric E, Verhasselt P, Andries K, Hoffner S. 2007. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob. Agents Chemother. 51:4202–4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ibrahim M, et al. 2007. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob. Agents Chemother. 51:1011–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jayaram R, et al. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayaram R, et al. 2004. Isoniazid Pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 48:2951–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koul A, et al. 2007. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 3:323–324 [DOI] [PubMed] [Google Scholar]
- 11. Lounis N, et al. 2010. R207910 (TMC207): a new antibiotic for the treatment of tuberculosis. Med. Mal. Infect. 40:383–390 [DOI] [PubMed] [Google Scholar]
- 12. Lounis N, et al. 2006. Combinations of R207910 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration. Antimicrob. Agents Chemother. 50:3543–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mesens N, et al. 2010. Screening for phospholipidosis induced by central nervous drugs: comparing the predictivity of an in vitro assay to high throughput in silico assays. Toxicol. in vitro 24:1417–1425 [DOI] [PubMed] [Google Scholar]
- 14. Mouton JW, et al. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235–237 [DOI] [PubMed] [Google Scholar]
- 15. Pasipanodya J, Gumbo T. 2011. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob. Agents Chemother. 55:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reasor MJ, Kacew S. 2001. Drug-induced phospholipidosis: are there functional consequences? Exp. Biol. Med. (Maywood) 226:825–830 [DOI] [PubMed] [Google Scholar]
- 17. Shandil RK, et al. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob. Agents Chemother. 51:576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Veziris N, et al. 2009. A once-weekly R207910-containing regimen exceeds activity of the standard daily regimen in murine tuberculosis. Am. J. Respir. Crit. Care Med. 179:75–79 [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization 2009. Global tuberculosis control 2009: epidemiology, strategy, financing. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/2009/pdf/full_report.pdf [Google Scholar]
- 20. Yajko DM, Kirihara J, Sanders C, Nassos P, Hadley WK. 1988. Antimicrobial synergism against Mycobacterium avium complex strains isolated from patients with acquired immune deficiency syndrome. Antimicrob. Agents Chemother. 32:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yew WW, Cynamon M, Zhang Y. 2011. Emerging drugs for the treatment of tuberculosis. Expert Opin. Emerg. Drugs 16:1–21 [DOI] [PubMed] [Google Scholar]