Abstract
Chitin and β-glucan are major cell wall components of Aspergillus spp. We investigated the antifungal activity of chitin synthesis inhibitors nikkomycin Z, polyoxin D, flufenoxuron, lufenuron, and teflubenzuron, alone and combined with the β-glucan synthesis inhibitor caspofungin. Only nikkomycin Z and caspofungin were found to act synergistically. The nikkomycin Z-induced chitin decrease corresponded with a β-glucan increase, while with the caspofungin-induced β-glucan decrease, an increase in chitin was found. This could explain the synergistic activity of this combination of drugs.
TEXT
Chitin and β-glucan, major constituents of the fungal cell wall and not found in humans, are interesting targets for new antifungal strategies. Inhibitors of chitin synthesis have been classified as either peptidyl nucleosides or acylureas. The peptidyl nucleosides, such as nikkomycins and polyoxins, function as substrate analogues and inhibit chitin synthase at its catalytic site (9). The exact mechanism of the acylurea compounds, such as teflubenzuron, lufenuron, and flufenoxuron, is unknown (13).
β-Glucan synthesis is inhibited by the echinocandins, such as caspofungin, which are cyclic lipopeptide compounds inhibiting the enzyme 1,3-β-d-glucan synthase. Lack of chitin and β-glucan in the cell wall leads to osmotic lysis of the fungal cell (9). Therefore, combining a chitin and β-glucan inhibitor may enhance fungal killing.
This study reports the in vitro susceptibility of Aspergillus fumigatus to different classes of chitin synthesis inhibitors used alone and combined with caspofungin, a β-glucan synthesis inhibitor.
MICs of caspofungin (Merck and Co., Rahway, NJ), and for nikkomycin Z, flufenoxuron, lufenuron, teflubenzuron (all from Sigma-Aldrich, Zwijndrecht, The Netherlands), and polyoxin D (Kaken Pharmaceutical Co., Tokyo, Japan) were determined for 10 A. fumigatus strains, including A. fumigatus ATCC 204305. For caspofungin, the minimal effective concentration (MEC) of caspofungin was also determined. Nikkomycin Z and polyoxin D were dissolved in water, and all other agents were dissolved in dimethyl sulfoxide (DMSO). MICs and MECs were determined in triplicate in RPMI 1640 medium, according to the CLSI broth microdilution method (7). Caspofungin was the only agent that inhibited growth of A. fumigatus (median MICs and median MEC for 10 strains are shown in Table 1). These MICs were in agreement with previously described MICs for A. fumigatus (7). None of the chitin synthesis inhibitors inhibited growth of A. fumigatus (Table 1). Microscopic evaluation revealed some morphological alterations after exposure to nikkomycin Z at ≥8 μg/ml: some cells were rounded and swollen (Fig. 1), though most cells appeared normal.
Table 1.
Median MICs and median MEC of various cell wall synthesis inhibitors for 10 A. fumigatus strains
| Class and antifungal agent | MIC and MEC (μg/ml) (range)a | FICI in combination with caspofunginb |
|---|---|---|
| β-Glucan synthesis inhibitors | ||
| Echinocandins | ||
| Caspofungin | 128 (128 to >128) | |
| 0.25 (0.06 to 0.5)* | ||
| Chitin synthesis inhibitors | ||
| Peptidyl nucleosides | ||
| Nikkomycin Z | >64 (>64) | 0.15 |
| Polyoxin D | >64 (>64) | 2.0 |
| Acylureas | ||
| Flufenoxuron | >16 (>16) | 2.0 |
| Lufenuron | >16 (>16) | 2.0 |
| Teflubenzuron | >16 (>16) | 2.0 |
Minimal effective concentration (MEC; indicated by the asterisk) was determined for caspofungin only.
Median fractional inhibitory concentration index (FICI) of caspofungin in combination with various chitin synthesis inhibitors for 10 A. fumigatus strains.
Fig 1.
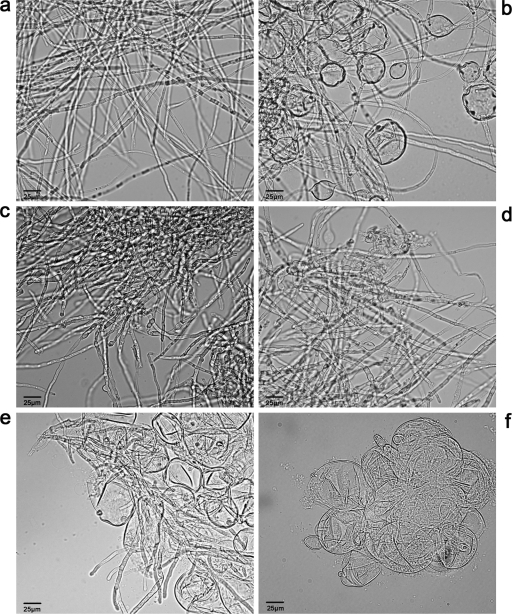
Cells of A. fumigatus (original magnification, ×400). (a) A. fumigatus not exposed to any antifungal agent. Elongated hyphae are noted; no swollen cells are present. (b) A. fumigatus exposed to 8 μg/ml nikkomycin Z. Elongated hyphae are still seen, but some of the hyphae appear to be swollen and rounded. (c) A. fumigatus exposed to 0.125 μg/ml caspofungin. Hyphae are shortened, stubby, and broad based. (d) A. fumigatus exposed to 4 μg/ml caspofungin. Hyphae are further shortened, stubby, and broad based. This morphology is more evident than with 0.125 μg/ml caspofungin. (e) A. fumigatus exposed to 0.125 μg/ml caspofungin and 8 μg/ml nikkomycin Z. Cell morphology is disrupted, and many cells are swollen and rounded, though normal hyphae are still seen. (f) A. fumigatus exposed to 4 μg/ml caspofungin and 8 μg/ml nikkomycin Z. Cell morphology is completely disturbed, and no normal hyphae are seen.
Synergy between the antifungal agents was investigated by a checkerboard antifungal susceptibility assay. The fractional inhibitory concentration index (FICI) was calculated using method 1 according to Bonapace et al. (4) with the following formula: FICI = [(MICA in combination)/MICA] + [(MICB in combination)/MICB]. Drug interactions were classified as synergistic (FICI ≤ 0.5), indifferent (0.5 < FICI < 4), or antagonistic (FICI ≥ 4). For each isolate, FICIs were determined in triplicate. Nikkomycin Z combined with caspofungin resulted in synergy (median FICI of 0.15 for 10 strains), which was not observed with other chitin synthesis inhibitors (median FICI of 2.00; Table 1). Synergy between caspofungin and nikkomycin Z is in agreement with previously published data (9).
To assess antifungal activity with respect to fungal metabolic activity, the colorimetric 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt hydroxide (XTT) assay was used (16). Exposure to none of the agents alone led to >80% decreased metabolic activity of the fungal cells, except for caspofungin at 128 μg/ml. Similar results were described by Antachopoulos et al. (1). However, combining caspofungin and nikkomycin Z mostly affected the decrease in fungal metabolic activity. For example, 0.125 μg/ml caspofungin alone resulted in an 8% decrease in metabolic activity, whereas 0.125 μg/ml caspofungin combined with 2 μg/ml nikkomycin Z resulted in a 56% decrease in metabolic activity (data not shown). The morphology of fungal cells exposed to the drugs in combination was also studied (Fig. 1). This figure shows that when A. fumigatus was exposed to 8 μg/ml nikkomycin Z, elongated hyphae were still present but some of the cells became rounded (Fig. 1b). Exposure to caspofungin caused short, stubby branched hyphae (Fig. 1c and d). When both agents were combined, the rounded cells became more prevalent (Fig. 1e). At a concentration of 4 μg/ml caspofungin combined with 8 μg/ml nikkomycin Z, the cell morphology was completely disturbed and no hyphae were identified anymore (Fig. 1f).
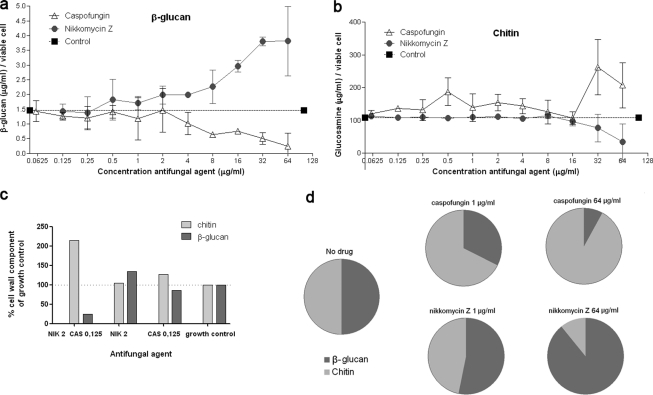
To elucidate the observed synergy between caspofungin and nikkomycin Z, 5 × 104 conidia/ml were exposed to these drugs for 48 h and the changes in cell wall components chitin and β-glucan were determined as previously published (10, 11, 14, 17). At caspofungin concentrations of 4 μg/ml and greater, a decrease in β-glucan content was observed (Fig. 2A). Additionally, with caspofungin concentrations of 32 μg/ml and greater, chitin concentrations were elevated (Fig. 2B). With nikkomycin Z, the opposite was observed: β-glucan concentrations increased when a concentration of 0.5 μg/ml or greater was used, while chitin concentrations decreased when a concentration of 16 μg/ml or greater was used. This observation is in agreement with Fortwendel et al., who reported that exposure to nikkomycin Z in concentrations up to 16 μg/ml did not influence chitin concentrations (8).
Fig 2.
Concentrations of the cell wall components β-glucan and chitin in A. fumigatus ATCC 204305 after exposure to caspofungin and nikkomycin Z at various concentrations. (A and B) Glucosamine concentrations represent chitin concentrations. Means from three independent experiments are shown. Bars represent standard deviations. β-Glucan and chitin concentrations were corrected to the number of viable cells with the XTT assay by the following formula: (amount of β-glucan or chitin measured) × (number of viable cells in the growth control/number of viable cells in the well tested). (C) Fungal response in cell wall components. Concentrations of β-glucan and chitin after exposure to 0.125 μg/ml of caspofungin combined with 2 μg/ml nikkomycin Z, each agent alone, and the growth control. This graph shows that combining caspofungin and nikkomycin Z causes a drastic decrease in β-glucan content, resulting in a compensatory increase in chitin content. (D) Shift in cell wall components β-glucan and chitin after exposure to caspofungin and nikkomycin Z at two concentrations (schematic representation). Both components are standardized to 50% in the drug-free control.
The combination of nikkomycin Z and caspofungin caused instability of the fungal cell wall. Figure 2C shows that β-glucan concentrations were strongly decreased after exposure to 0.125 μg/ml caspofungin combined with 2 μg/ml nikkomycin Z. As a consequence, chitin concentrations were increased in order to survive. This effect was also found for other combinations (data not shown).
Exposure to a single drug, either caspofungin or nikkomycin Z, causes a reshuffling of cell wall components chitin and β-glucan (schematically shown in Fig. 2D). Inhibition of chitin synthesis in A. fumigatus resulted in increased synthesis of β-glucan and vice versa. This would explain the synergy between both agents as well as the altered morphology after exposure to caspofungin and nikkomycin Z. This alteration in cell wall components is not unique for A. fumigatus. Stevens et al. showed that Candida albicans reacts to caspofungin concentrations above the MIC by compensatory increases of the chitin contents of the cell wall, resulting in survival (15). Furthermore, Walker et al. (18) reviewed several studies that investigated the enhancement of echinocandin activity when combined with nikkomycin Z in yeasts and fungi.
Nikkomycin Z was the only chitin synthesis inhibitor that inhibited A. fumigatus growth when combined with caspofungin. Since polyoxin D is also a peptidyl nucleoside, we expected to find the same synergy. However, polyoxin D, either alone or combined with caspofungin, did not inhibit growth of A. fumigatus. Polyoxin D is capable of inhibiting growth of Saccharomyces cerevisiae, (5, 6) C. albicans, and Cryptococcus neoformans (3). Archer (2) showed that polyoxin D does inhibit A. fumigatus chitin synthase. However, in the present study, the inhibition of chitin synthase by polyoxin D, even when combined with caspofungin, was not sufficient to result in fungal growth arrest.
In summary, we have shown that the inhibition of the synthesis of a single cell wall component (either β-glucan or chitin) results in a subsequent increase in synthesis of the other cell wall component. The observed synergy between caspofungin and nikkomycin Z is probably the result of an inability of the fungus to compensate for the altered ratio of chitin to β-glucan. Indeed, Luque et al. showed that the β-glucan synthesis inhibitor micafungin combined with the chitin synthase inhibitor nikkomycin Z improved survival in mice with systemic aspergillosis (12). Additional studies are needed to investigate the in vivo potential of nikkomycin Z and caspofungin as a new antifungal combination for the treatment of aspergillosis.
ACKNOWLEDGMENT
We thank Kaken Pharmaceutical Co., Ltd., for kindly providing the polyoxin D.
Footnotes
Published ahead of print 27 December 2011
REFERENCES
- 1. Antachopoulos C, et al. 2007. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob. Agents Chemother. 51:881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Archer DB. 1977. Chitin biosynthesis in protoplasts and subcellular fractions of Aspergillus fumigatus. Biochem. J. 164:3–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Becker JM, et al. 1983. Polyoxin D inhibits growth of zoopathogenic fungi. Antimicrob. Agents Chemother. 23:926–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonapace CR, et al. 2002. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 44:363–366 [DOI] [PubMed] [Google Scholar]
- 5. Bowers B, Levin G, Cabib E. 1974. Effect of polyoxin D on chitin synthesis and septum formation in Saccharomyces cerevisiae. J. Bacteriol. 119:564–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabib E. 1991. Differential inhibition of chitin synthetases 1 and 2 from Saccharomyces cerevisiae by polyoxin D and nikkomycins. Antimicrob. Agents Chemother. 35:170–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard M38-A2, second editionvol 28 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Fortwendel JR, et al. 2009. Differential effects of inhibiting chitin and 1,3-β-d-glucan synthesis in Ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 53:476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganesan LT, et al. 2004. In-vitro activity of nikkomycin Z alone and in combination with polyenes, triazoles or echinocandins against Aspergillus fumigatus. Clin. Microbiol. Infect. 10:961–966 [DOI] [PubMed] [Google Scholar]
- 10. Hector RF, Davidson AP, Johnson SM. 2005. Comparison of susceptibility of fungal isolates to lufenuron and nikkomycin Z alone or in combination with itraconazole. Am. J. Vet. Res. 66:1090–1093 [DOI] [PubMed] [Google Scholar]
- 11. Lehmann PF, White LO. 1975. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect. Immun. 12:987–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luque JC, Clemons KV, Stevens DA. 2003. Efficacy of micafungin alone or in combination against systemic murine aspergillosis. Antimicrob. Agents Chemother. 47:1452–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merzendorfer H. 2006. Insect chitin synthases: a review. J. Comp. Physiol. B 176:1–15 [DOI] [PubMed] [Google Scholar]
- 14. Shedletzky E, Unger C, Delmer DP. 1997. A microtiter-based fluorescence assay for (1,3)-beta-glucan synthases. Anal. Biochem. 249:88–93 [DOI] [PubMed] [Google Scholar]
- 15. Stevens DA, et al. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:60–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van de Sande WWJ, et al. 2005. Testing of the in vitro susceptibilities of Madurella mycetomatis to six antifungal agents by using the Sensititre system in comparison with a viability-based 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5- [(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) assay and a modified NCCLS method. Antimicrob. Agents Chemother. 49:64–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van de Sande WWJ, et al. 2010. Madurella mycetomatis is not susceptible to the echinocandin class of antifungal agents. Antimicrob. Agents Chemother. 54:2738–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker LA, Gow NA, Munro CA. 2010. Fungal echinocandin resistance. Fungal Genet. Biol. 47:117–126 [DOI] [PMC free article] [PubMed] [Google Scholar]