Abstract
TD-1792 is a novel glycopeptide-cephalosporin heterodimer investigational antibiotic that displays potent bactericidal effects against clinically relevant Gram-positive organisms in vitro. The present studies evaluated the in vivo pharmacokinetics (PK) and pharmacodynamics (PD) of TD-1792 in the neutropenic murine thigh infection animal model. TD-1792, dosed subcutaneously (SC), produced dose-dependent reduction in the thigh bacterial burden of several organisms, including methicillin-susceptible and -resistant strains of Staphylococcus aureus and Staphylococcus epidermidis (MSSA, MRSA, MSSE, MRSE, respectively), penicillin-susceptible strains of Streptococcus pneumoniae (PSSP), Streptococcus pyogenes, and vancomycin-intermediate-susceptible Staphylococcus aureus (VISA). In single-dose efficacy studies, the 1-log10 CFU kill effective dose (ED1-log kill) estimates for TD-1792 ranged from 0.049 to 2.55 mg/kg of body weight administered SC, and the bacterial burden was reduced by up to 3 log10 CFU/g from pretreatment values. Against S. aureus ATCC 33591 (MRSA), the total 24-h log10 stasis dose (EDstasis) and ED1-logkill doses for TD-1792 were 0.53 and 1.11 mg/kg/24 h, respectively, compared to 23.4 and 54.6 mg/kg/24 h for vancomycin, indicating that TD-1762 is 44- to 49-fold more potent than vancomycin. PK-PD analysis of data from single-dose and dose-fractionation studies for MRSA (ATCC 33591) demonstrated that the total-drug 24-h area under the concentration-time curve-to-MIC ratio (AUC/MIC ratio) was the best predictor of efficacy (r2 = 0.826) compared to total-drug maximum plasma concentration of drug-to-MIC ratio (Cmax/MIC ratio; r2 = 0.715) and percent time that the total-drug plasma drug concentration remains above the MIC (%Time>MIC; r2 = 0.749). The magnitudes of the total-drug AUC/MIC ratios associated with net bacterial stasis, a 1-log10 CFU reduction from baseline and near maximal effect, were 21.1, 37.2, and 51.8, respectively. PK-PD targets based on such data represent useful inputs for analyses to support dose selection decisions for clinical studies of patients.
INTRODUCTION
Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), are a major cause of serious nosocomial infections worldwide (18). The glycopeptide vancomycin still serves as the cornerstone in the treatment of drug-resistant Gram-positive infections, but its microbiological limitations are a growing concern (9). The introduction of newer agents, including linezolid, daptomycin, tigecycline, telavancin, and ceftaroline, has offered additional therapeutic options to the clinician (16). Nevertheless, given the recognized therapeutic limitations of existing drugs and the threat of emerging resistance, the need for new antibiotics that operate through novel mechanisms remains high.
TD-1792 (Fig. 1) is a bactericidal multivalent glycopeptide-cephalosporin antibiotic that simultaneously targets the D-Ala-D-Ala-containing peptidoglycan precursors and the active site of penicillin-binding protein, resulting in potent activity against Gram-positive bacteria, including multiresistant strains (10, 13, 14, 15). The objective of the present studies was to characterize the pharmacodynamics of TD-1792 against a collection of Gram-positive pathogens in the neutropenic murine thigh model of soft tissue infection. In addition, pharmacokinetic-pharmacodynamic (PK-PD) studies were also conducted to elucidate the PK-PD index associated with TD-1792 efficacy and the magnitude of such an index required to achieve various levels of reduction in bacterial burden.
Fig 1.
Chemical structure of TD-1792.
MATERIALS AND METHODS
Antimicrobial agents.
TD-1792 was synthesized at Theravance, Inc., South San Francisco, CA. Vancomycin was obtained from Sigma-Aldrich (St. Louis, MO). Both drugs were dissolved in 5% dextrose in water for dosing.
Bacterial strains.
Eight strains were evaluated in this study. These strains tested included an MRSA strain (ATCC 33591), a methicillin-susceptible S. aureus (MSSA) strain (ATCC 13709), a methicillin-resistant Staphylococcus epidermidis (MRSE) strain (MED 820), a methicillin-susceptible S. epidermidis (MSSE) strain (SU03), two penicillin-susceptible Streptococcus pneumoniae (PSSP) strains (MED 35 and MED 1119), a Streptococcus pyogenes strain (MED 2040), and a vancomycin-intermediate-susceptible S. aureus (VISA) strain (HIP5836). The MRSA and MSSA strains (ATCC 33591 and 13709, respectively) were obtained from the American Type Culture Collection (Manassas, VA). All other strains were obtained from U.S. hospitals or academic institutions.
In vitro susceptibility testing.
MICs were determined using the CLSI broth microdilution method (6). Studies to investigate the effect of serum on in vitro activity were carried out by performing susceptibility testing against MRSA (ATCC 33591) in the presence of 50% (vol/vol) mouse serum.
Protein binding determination.
The in vitro binding of TD-1792 to mouse and human plasma proteins for concentrations ranging from 0.1 to 10 μg/ml was evaluated by ultrafiltration using a Centrifree micropartition device. Samples (1 ml) of spiked plasma containing 14C-TD-1792 and unlabeled TD-1792 were loaded into the Centrifree micropartition devices with a 30,000-molecular-weight-cutoff regenerated cellulose membrane and then centrifuged at 1,000 × g for 20 min at 37°C. The fortified plasma and the resulting protein-free ultrafiltrate were transferred into a scintillation cocktail and counted with a beta counter.
Efficacy studies in a murine neutropenic thigh infection model.
All animal studies were approved by the Institutional Animal Care and Use Committee at Theravance, Inc., and conducted in the Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility at Theravance, Inc.
The experimental methodology and design were similar to that described previously (12). Animals (female NSA mice, 18 to 30 g) were acquired from Charles Rivers Laboratories (Gilroy, CA). Mice were housed 5 per cage and allowed at least 3 days after their arrival date to acclimate before the study was initiated. Throughout the study, animals had access to food and water ad libitum. Neutropenia (approximately 100 cells/mm3) was induced by administering 200 mg of cyclophosphamide per kg of body weight intraperitoneally 4 and 2 days prior to inoculation of bacteria.
Animals were lightly anesthetized with isoflurane (2.5% for induction followed by 1% for maintenance), and 50 μl of the bacterial inoculum was injected into the posterior thigh. The bacterial inoculum administered ranged from 106 to 107 CFU/ml. One hour after inoculation, animals were dosed subcutaneously (SC) with vehicle or various dose regimens of the test drug. At 0 h and 24 h, cohorts of animals were euthanized (CO2 asphyxiation) and the thigh was aseptically removed. The thigh was weighed and placed into 10 ml sterile saline and homogenized. For the majority of strains, dilutions of the homogenate were plated on tryptic soy agar plates (Teknova, Hollister, CA) which were incubated overnight. In the case of the PSSP strains, dilutions of the homogenate were plated on tryptic soy blood agar plates, which were incubated overnight in a CO2 incubator. In all cases, the bacterial burden was expressed as log10 CFU/g of thigh weight.
Pharmacokinetic studies.
A separate single-dose pharmacokinetic study of TD-1792 was performed with neutropenic NSA mice infected in the thigh with MRSA 33591. Infected animals were dosed, at 1 h postinoculation, with 3 mg/kg of TD-1792 SC. Groups of three mice were sacrificed at predose and at 0.033, 0.083, 0.25, 0.5, 1, 2, 4, 8, and 24 h postdose. Animals were euthanized with CO2, and blood samples were collected by cardiac puncture. Drug concentrations were assayed by liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). Samples (100 μl) were extracted using solid-phase extraction and were injected on a Discovery HS C18, 5-μm high-performance liquid chromatography (HPLC) column (2.1 × 50 mm) with a flow rate of 0.6 ml/min. Mobile phase A consisted of 0.1% formic acid in water, and mobile phase B consisted of 0.1% formic acid in acetonitrile. The gradient elution was started at 5% B and ramped to 16% B and 95% B at 2.9 and 3.1 min, respectively, and held for 0.4 min. The HPLC column was reequilibrated for 1.3 min at 5% B. The mass spectrometer (Sciex API4000; Applied Biosystems, Foster City, CA) was operated in positive-ion multiple-reaction monitoring mode. TD-1792 was monitored using mass transitions of 992.4/952.8. D5-TD-1792 was used as the internal standard and monitored using mass transitions of 997.1/955.5. The dynamic range of the assay was 10.0 to 10,000 ng/ml. The percent accuracy of the standard curve ranged from 96.0 to 104%. The percent coefficient of variation (%CV) ranged from 1.0 to 9.2%.
Experimental treatments.
The murine experiments consisted of a single-dose study and multiple dose-fractionation studies. In the single-dose study, TD-1792 was administered to infected mice over a dose range of 0.03 to 10 mg/kg SC. In dose-fractionation studies, the total 24-h dose of TD-1792 of 0.1, 0.3, or 1 mg/kg, SC, was administered either as a single dose (every 24 h) or as two (every 12 h), three (every 8 h), or four (every 6 h) divided doses. As a comparator, vancomycin was tested for efficacy in MRSA 33591-infected mice. Vancomycin was administered every 12 h, and total daily doses in the range of 1 to 300 mg/kg, SC, were tested.
Data analysis. (i) Pharmacodynamic analysis.
Dose-response curves were fit with a four-parameter logistic equation using GraphPad Prism, version 3.00 for Microsoft Windows (GraphPad Software, San Diego, CA). The equation used was: Y = Min + (Max − Min)/(1 + 10 [log ED50 − X] × Hillslope), where X is the logarithm of dose, Y is the response (log10 CFU/g), with Y starting at a minimum (Min) (fixed to the 24-h vehicle control response) and asymptotically approaching a maximum (Max) with a sigmoidal shape, and ED50 is defined as the dose required to produce 50% of the maximum response. ED50 estimates were expressed as means with 95% confidence intervals (CI). Two-tailed Student's t test was used to compare ED50 estimates between treatments. A P value of <0.05 was considered to be statistically significant. The log10 stasis dose (EDstasis) was defined as the dose producing no net change in the thigh bacterial burden compared to the pretreatment bacterial burden. A 1-log10 kill dose (ED1-log kill) was defined as the dose required to produce a decrease in bacterial burden of 1 log CFU/g from pretreatment controls. Similarly, 2- and 3-log10 kill doses were defined as the doses required to produce a decrease in bacterial burden of 2 and 3 log10 CFU/g, respectively, from pretreatment controls.
(ii) Pharmacokinetic analysis.
Pharmacokinetic models were fit to pooled plasma exposure data using weighted nonlinear regression in S-ADAPT 1.53 software (4). Model discrimination was accomplished according to the rule of parsimony based on the Akaike information criterion (1). Weighting of the plasma data was based on the reciprocal of the estimated observation variance, which was predicted as a function of the fitted concentration, using a constant (additive) plus proportional residual error model. Parameter estimates from the final pharmacokinetic model were used to simulate concentration-time profiles for various dosing regimens for mice studied in the single dose and dose-fractionation pharmacodynamic (PD) studies. Measures of exposures were generated from these simulated profiles.
(iii) Pharmacokinetic-pharmacodynamic analysis.
Data obtained from mice infected with MRSA ATCC 33591 were used for the PK-PD analysis. The PK-PD measures chosen for evaluation were the percentage of the 24-h period of study for which the total-drug plasma concentration remained above the MIC (%Time>MIC), the ratio of the 24-h total-drug area under the concentration-time profile to the MIC (AUC/MIC ratio), and the ratio of the maximum total-drug plasma concentration (Cmax) to the MIC (Cmax/MIC ratio).
The %Time>MIC was obtained by calculating the percentage of time the total-drug plasma concentration was above the nominal MIC (0.031 μg/ml) in a 24-h period. Given the short half-life of TD-1792 in mice, the AUC from 0 h to infinity (AUC0-inf) was assumed to be equal to the AUC from 0 to 24 h (AUC0-24). AUC0-inf was calculated using the equation AUC0-inf = D/CLt, where D is the dose in mg/kg and CLt is the clearance of TD-1792. Cmax for each dosing regimen was calculated from the simulated concentration-time data. AUC0-inf and Cmax were divided by the MIC value to obtain the AUC/MIC and Cmax/MIC ratios, respectively.
Using S-ADAPT 1.53 software, data were modeled using a Hill-type function, with nonlinear least-squares regression, which described the relationships between the change in log10 CFU/g from baseline and the PK-PD measure of interest (AUC/MIC ratio, Cmax/MIC ratio, and %Time>MIC). The PK-PD targets based on such relationships which were associated with no change in log10 CFU/g (net bacterial stasis) or a 1 log10 CFU/g reduction from baseline (1 log kill) were also calculated.
RESULTS
In vitro activity of TD-1792.
As shown in Table 1, the MIC values for TD-1792 against the collection of the staphylococcus and streptococcus isolates ranged from 0.0005 to 0.12 μg/ml. The MIC range for vancomycin against these strains was 0.25 to 8 μg/ml. Against MRSA ATCC 33591, the MIC for TD-1792 (MIC = 0.03 μg/ml) was 32-fold lower than that for vancomycin (MIC = 1 μg/ml). In the presence of mouse serum/albumin, the MIC of TD-1792 increased from 0.015 to 0.06 μg/ml.
Table 1.
Point estimates of the TD-1792 dose required to achieve various PD endpoints for each Gram-positive bacterial strain studied in the neutropenic murine thigh model
| Strain | MICa (μg/ml) | Doses (mg/kg, SC) of TD-1792 required to achieve various PD endpointsb |
||||
|---|---|---|---|---|---|---|
| EDstasis | Emax | ED1-logkill | ED2-logkill | ED3-logkill | ||
| MSSA 29213 | 0.015 (1) | 0.44 | 2.38 | 0.79 | 1.48 | ND |
| MRSA (ATCC 33591) | 0.03 (1) | 0.53 | 1.67 | 1.11 | 10 | ND |
| VISA (HIP5836) | 0.12 (4) | 1.34 | 3.17 | 2.55 | 5.33 | 10 |
| MSSE (SU03) | 0.008 (2) | 0.069 | 3.79 | 0.17 | 0.40 | 1.07 |
| MRSE (MED 820) | 0.03 (2) | 0.03 | 2.15 | 0.13 | 0.60 | ND |
| PSSP (MED 35) | 0.004 (0.5) | 0.23 | 4.66 | 0.43 | 0.71 | 1.38 |
| PSSP (MED 1119) | 0.002 (0.5) | 0.031 | 4.60 | 0.049 | 0.07 | 0.122 |
| S. pyogenes (MED 2040) | 0.0005 (0.25) | 0.72 | 3.79 | 0.98 | 1.56 | 2.47 |
Values in parentheses denote the corresponding MICs for vancomycin.
Refer to Materials and Methods for the definition of PD endpoints. ND, not determined; endpoint was not achieved at the doses tested.
Protein binding.
TD-1792 exhibited concentration-independent protein binding in mouse and human plasma that ranged from 48% to 54% and 44% to 49%, respectively.
Single-dose pharmacokinetics of TD-1792 in infected neutropenic mice.
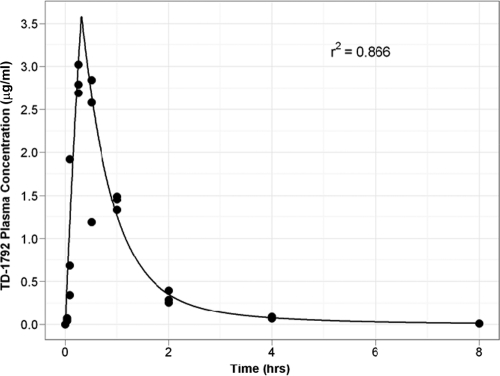
TD-1792 mouse plasma concentration-time data following a single SC dose of 3 mg/kg was fitted using a two-compartment model (one central and one peripheral) with zero-order absorption (approximating the subcutaneous injection) and first-order elimination. The model-fitted plasma concentration-time profile overlaid with the observed data is shown in Fig. 2. The correlation coefficient (r2) for the relationship between the observed and fitted concentrations was 0.866, indicating that the data were well described by the model.
Fig 2.
Observed and fitted single-dose (3 mg/kg, SC) concentration-versus-time pharmacokinetic profile for TD-1792 in neutropenic mice infected in the thigh with MRSA ATCC 33591. Abscissa shows the time (hours), and ordinate shows the plasma drug concentration (μg/ml) (n = 3 per group).
The pharmacokinetic parameter estimates for the clearance out of the central compartment, the volume of the central compartment, the distributional clearance between the central compartment and the peripheral compartment, and the volume of the peripheral compartment were 0.907 liters/kg/h, 0.659 liters/kg, 0.144 liters/kg/h, and 0.255 liters/kg, respectively. The alpha half-life estimate in mice was 0.41 h, and the terminal half-life was 1.7 h.
Pharmacodynamic effects of TD-1792 against multiple Gram-positive organisms in the neutropenic murine thigh model.
TD-1792, dosed SC, produced a dose-dependent reduction of thigh bacterial burden when tested against multiple Gram-positive pathogens in the neutropenic murine thigh model. A summary of the point estimates of the TD-1792 dose required to achieve various PD endpoints for each strain studied is provided in Table 1. The magnitude of kill was strain dependent. TD-1792 produced a maximal of approximately 1- to 2-log10 kill against MRSA ATCC 33591, MSSA ATCC 29213, and MRSE MED 820 and ≥3-log10 kill against VISA HIP 5836, MSSE SU03, PSSP MED35, PSSP MED1119, and S. pyogenes MED 2040 strains. The potency of TD-1792, assessed by the ED1-log kill, ranged from 0.049 to 2.55 mg/kg, SC.
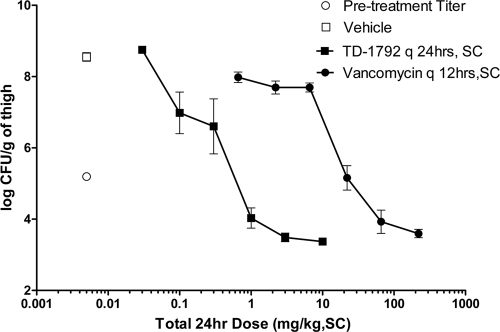
The efficacy of TD-1792 was compared to that of vancomycin against MRSA ATCC 33591 in the neutropenic thigh model infection. The dose-response curves of the two drugs are shown in Fig. 3. Against this organism, vancomycin produced a dose-dependent reduction in the thigh bacterial burden with EDstasis and ED1-logkill values of 23.4 and 54.6 mg/kg, respectively, which were 44- and 49-fold higher than those for TD-1792. The maximum kill (Emax) was 1.67 (0.71 to 2.01) log10 CFU/g for TD-1792 and 1.46 (1.16 to 1.79) log10 CFU/g for vancomycin, and these values were not significantly different from each other.
Fig 3.
Efficacy of TD-1792 and vancomycin against MRSA ATCC 33591 in the neutropenic murine thigh model. The abscissa shows the total 24-h dosage in mg/kg, SC, and the ordinate shows the thigh bacterial burden in log10 CFU/g. Vehicle and TD-1792 were dosed every 24 h, whereas vancomycin was dosed every 12 h. Data are expressed as means ± standard deviations (SD) (n = 5 per group).
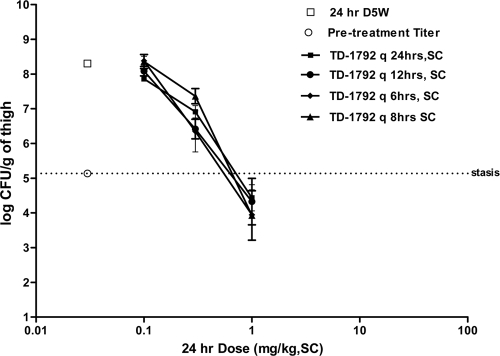
Effects of dose fractionation on the efficacy of TD-1792 in the murine neutropenic thigh model.
Figure 4 shows the effects of TD-1792 against MRSA ATCC 33591 on the bacterial burden in mice dosed with total 24-h doses of TD-1792 ranging from 0.03 to 1 mg/kg, SC, administered in one, two, or four divided fractions. The pretreatment bacterial burden was 5.13 ± 0.06 log10 CFU/g (n = 10 mice). In vehicle control-treated animals, the bacterial burden was 8.30 ± 0.01 log10 CFU/g (n = 10). TD-1792 produced a dose-dependent reduction of the thigh bacterial burden in all dose-fractionation groups (Fig. 4). At any given dose, there was no significant difference (P > 0.05) in efficacy among the different dose-fractionation groups. The EDstasis of TD-1792 was 0.71, 0.61, 0.64, and 0.54 mg/kg in groups treated every 24, 12, 8, and 6 h, respectively.
Fig 4.
Effect of TD-1792 against MRSA ATCC 33591 on thigh bacterial burden in mice dosed with various total 24-h doses of TD-1792 (0.03 to 1 mg/kg, SC) administered in one, two, or four divided fractions. The abscissa shows the total 24-h dosage in mg/kg, SC, and the ordinate shows the thigh bacterial burden in log10 CFU/g. Data are expressed as means ± SD (n = 5 to 10).
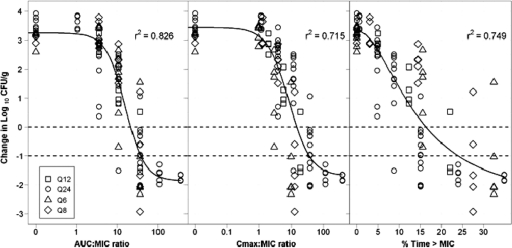
PK-PD analysis.
Relationships between each of the three PK-PD indices and change in log10 CFU/g thigh bacterial burden from baseline were evaluated using a Hill-type function (Fig. 5). The AUC/MIC ratio was most predictive of efficacy (r2 = 0.826) compared to the Cmax/MIC ratio (r2 = 0.715) or %Time>MIC (r2 = 0.749). The equation for the function describing the relationship between efficacy and the AUC/MIC ratio is as follows: change in log10 CFU/g = 3.25 − (5.12 × AUC/MIC1.83)/(15.61.83 + AUC/MIC1.83).
Fig 5.
Relationship between AUC/MIC ratio (left), Cmax/MIC ratio (middle), %Time>MIC (right), and the change in log10 CFU/g from pretreatment values. Each point represents data from one mouse. The fitted line represents the Hill-type function.
The PK-PD model parameter estimates and the 95% CI for these estimates are shown in Table 2.
Table 2.
Parameter estimates of the model describing the relationship between the AUC/MIC ratio and the efficacy for TD-1792 against MRSA ATCC 33591 in the neutropenic murine thigh model of infectiona
| Parameter | Estimate | 95% CI |
|---|---|---|
| Econ | 3.25 | 2.87–3.63 |
| Emax | 5.12 | 4.23–6.00 |
| Hill | 1.83 | 1.12–2.54 |
| EC50 | 15.6 | 11.3–19.9 |
Econ is the change in log10 CFU/g from the baseline after 24 h when no drug is administered; Emax is the maximum change in log10 CFU/g from the baseline at 24 h after administering TD-1792; the AUC/MIC ratio is the PK-PD index of interest; EC50 is the value for the AUC/MIC ratio at which there is half-maximal change in log10 CFU/g; and Hill is the Hill's coefficient of sigmoidicity.
The magnitudes of the AUC/MIC ratios associated with net bacterial stasis, a 1 log10 CFU/g reduction from baseline, and near maximal effect (EC90) were estimated and are summarized in Table 3.
Table 3.
TD-1792 AUC/MIC ratios associated with net bacterial stasis, 1 log10 CFU/g reduction from baseline, and EC90 against MRSA ATCC 33591 in the neutropenic murine thigh animal model
| Endpoint | Estimate | 95% CI |
|---|---|---|
| Net bacterial stasis | 21.1 | 17.0–25.3 |
| 1 log10 CFU/g reduction from baseline | 37.2 | 26.9–47.5 |
| EC90a | 51.8 | 29.9–73.8 |
EC90, value of the AUC/MIC ratio at which there is 90% of maximal effect.
DISCUSSION
TD-1792 is a novel antibiotic that is chemically a covalently linked heterodimer of a glycopeptide (vancomycin) and a cephalosporin moiety (14). Its two component moieties simultaneously target D-Ala-D-Ala-containing peptidoglycan precursors and the active site of penicillin-binding protein (15). We have hypothesized that simultaneous and/or sequential binding of the vancomycin component to the nascent peptidoglycan substrate presents a high effective concentration of the cephalosporin component at the active site of both PBP2 and PBP2a, thereby conferring high potency to the molecule. TD-1792 is a bactericidal drug that exerts potent antibacterial activity against clinically relevant Gram-positive pathogens, including multidrug-resistant organisms such as MRSA and VISA, and its activity cannot be reproduced by the individual monomer tested either alone or in combination (10, 13). In the present study, we evaluated the PK and PD properties of TD-1792 in the neutropenic murine thigh model to assess its in vivo potency and to determine the PK-PD index and the magnitude of this index associated with efficacy.
In vitro susceptibility studies demonstrated that TD-1792 was active and potent against all Gram-positive strains that were tested, including those which are highly resistant to oxacillin (MRSA and MRSE strains) and demonstrate intermediate susceptibility to vancomycin (VISA HIP 5836). The MIC of TD-1792 against MRSA ATCC 33591 was shifted 4-fold in the presence of mouse serum, from 0.015 to 0.06 μg/ml. This observed shift was slightly but not meaningfully greater than the 2-fold shift predicted from the magnitude of protein binding (∼50%) of the drug.
TD-1792 displayed potent antibacterial efficacy in vivo against all the organisms tested. At doses of 10 mg/kg and lower, TD-1792 produced ≥1-log kill against all organisms, consistent with its potent in vitro bactericidal activity. The in vivo rank order of potency of TD-1792 versus the different strains was somewhat consistent with the in vitro potencies. For example, TD-1792's MIC against VISA HIP 5836, which was 4-fold-higher than that against MRSA ATCC 33591, also translated to higher EDstasis and ED1-3 log kill doses in vivo. Furthermore, TD-1792's 32-fold-greater in vitro potency over vancomycin against the MRSA ATCC 33591 was reflected in the >40-fold improvement in vivo potency based on the data from the neutropenic murine thigh model.
It is now well recognized that achieving optimal efficacy for any given antibacterial agent requires a careful understanding of its PK-PD properties (3, 7, 8, 11). Dose-fractionation efficacy studies provide a useful way to elucidate PK-PD relationships for antibacterials (8). Using this approach, we demonstrated that the antibacterial potency of TD-1792 in the neutropenic thigh model was unaffected by changing the dosing interval, consistent with total exposure serving as the principal determinant of efficacy. Additionally, PK-PD analysis of the data demonstrated that the AUC/MIC ratio was the PK-PD measure most predictive of reduction in bacterial burden. Although the PD effects were also reasonably well correlated with %Time>MIC and Cmax/MIC, visual examination of the plots revealed a larger degree of scatter of observed data around fitted functions for these PK-PD indices. If one examines the plot for %Time>MIC in Fig. 5, one can see that the regimens, differentiated by interval, stack on top of one another. In contrast, for the AUC/MIC ratio plot, the symbols for the regimen are dispersed and not layered as they appear to be for %Time>MIC.
Given the similar magnitudes of protein binding in mouse and human plasma, total-drug AUC/MIC ratios associated with various levels of bacterial reduction from baseline for TD-1792 are reported herein. AUC/MIC ratios of 21.1 and 37.2 were associated with net bacterial stasis and a 1 log10 CFU/g reduction from baseline, respectively. Additionally, since previous PK-PD analyses using data from both patients with complicated skin and skin structure infections (cSSSI) and neutropenic murine infection models have demonstrated the magnitude of the PK-PD measure required for efficacy in infected patients to be similar to that required for net bacterial stasis or a 1-log10 CFU/g reduction from baseline in the neutropenic murine thigh infection model (2). Monte Carlo simulations using such PK-PD targets served to provide a reasonable minimum exposure threshold upon which to base early dose selection decisions for patients with cSSSI (5). Although plasma concentrations were well described by the murine PK model, the single-dose nature of the PK study may have resulted in some degree of underestimation of the above-described AUC/MIC ratio targets. The assumption of linearity in clearance across all doses evaluated in the PK-PD study should, therefore, be confirmed in a future study.
Overall, the data generated in the current studies using a neutropenic murine thigh infection model demonstrated that TD-1792 is a potent and efficacious antibiotic in vivo against a collection of susceptible and resistant Gram-positive organisms. PK-PD evaluations for MRSA demonstrated that the AUC/MIC ratio is the best predictor of efficacy for this drug. The integration of PK-PD targets for efficacy, based on PK-PD relationships derived using data from such in vivo infection models, together with data from phase 1 pharmacokinetic studies, provides informative dose selection support for phase 2 studies (5). The results of PK-PD target attainment analyses for TD-1792, which were based on the AUC/MIC ratio targets described herein, were used to support dose selection for a phase 2 study of patients with cSSSI. Data from this study subsequently showed that a 2-mg/kg intravenous dose of TD-1792 administered once daily was well-tolerated and noninferior to vancomycin in the treatment of cSSSI caused by Gram-positive bacteria (17). TD-1792 has the potential to be a valuable addition to the therapeutic armamentarium for the treatment of Gram-positive infections.
Footnotes
Published ahead of print 12 December 2011
REFERENCES
- 1. Akaike H. 1979. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 66:237–242 [Google Scholar]
- 2. Ambrose PG, et al. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 3. Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261–268 [DOI] [PubMed] [Google Scholar]
- 4. Bauer RJ. 2006. S-ADAPT/MCPEM user's guide: software for pharmacokinetic, pharmacodynamic and population data analysis. Biomedical Simulations Resource, Los Angeles, CA: http://bmsr.usc.edu/Software/ADAPT/SADAPTsoftware.html [Google Scholar]
- 5. Bhavnani, et al. 2011. Abstr. 51th Intersci. Conf. Antimicrob. Agents Chemother., abstr A2-044 Chicago, IL, 16 to 20 September 2011 http://www.icaac.org/ [Google Scholar]
- 6. CLSI 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—eighth edition. CLSI document M7-A8. CLSI, Wayne, PA [Google Scholar]
- 7. Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501 [DOI] [PubMed] [Google Scholar]
- 8. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–12 [DOI] [PubMed] [Google Scholar]
- 9. Deresinski S. 2007. Vancomycin: does it still have a role as an antistaphylococcal agent? Expert Rev. Anti Infect. Ther. 5:393–401 [DOI] [PubMed] [Google Scholar]
- 10. Difuntorum S, et al. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., 17 to 20 September 2007, abstr F1-2110 American Society for Microbiology, Washington, DC [Google Scholar]
- 11. Drusano GL. 1988. Role of pharmacokinetics in the outcome of infections. Antimicrob. Agents Chemother. 32:289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegde SS, et al. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krause K, et al. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., 17 to 20 September 2007, abstr E-1626 American Society for Microbiology, Washington, DC [Google Scholar]
- 14. Long DD, et al. 2009. Exploring the positional attachment of glycopeptide/beta-lactam heterodimers. J. Antibiot. (Tokyo) 61:603–614 [DOI] [PubMed] [Google Scholar]
- 15. Lunde C, et al. 2009. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., abstr C1-1344 American Society for Microbiology, Washington, DC [Google Scholar]
- 16. Nailor MD, Sobel JD. 2009. Antibiotics for gram-positive bacterial infections: vancomycin, teicoplanin, quinupristin/dalfopristin, oxazolidinones, daptomycin, dalbavancin, and telavancin. Infect. Dis. Clin. North Am. 23:965–982 [DOI] [PubMed] [Google Scholar]
- 17. Stryjewski, et al. 2007. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., 17 to 20 September 2007, abstr L-1147a American Society for Microbiology, Washington, DC [Google Scholar]
- 18. Woodford N, Livermore DM. 2009. Infections caused by Gram-positive bacteria: a review of the global challenge. J. Infect. 59(Suppl. 1):S4–S16 [DOI] [PubMed] [Google Scholar]