Abstract
A possible link between a deficient neurogenesis at the dentate gyrus, and the loss of memory found in Alzheimer’s disease patients is discussed. First is indicated that Alzheimer disease is a memory disorder. Since adult neurogenesis at the dentate gyrus plays a role in declarative memory, a brief comment on the structure and function of dentate gyrus has been included. At molecular level, we have discussed the function of the enzyme GSK3 on the development of neurogenesis at the subgranular zone of the dentate gyrus. Finally, we have indicated that in Alzheimer disease there is an increase in GSK3 activity. This increase could impair the neurogenesis process and it could result in the memory deficit found in Alzheimer patients.
Keywords: GSK3, dentate gyrus, Familial Alzheimer’s disease
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder. Today, it affects to nearly 30 million people in the whole world [1].
Aging is the most important risk for AD [2]. No cases have been detected below 20 years old, but there is an incidence about 30% in people aged 85 years or older [1,3]. With each passing year about four million people in the world develop dementia [4]. As the average of population increases, the number of AD patients is expected to rise exponentially and about 110 million of patients are projected for 2050 [5].
Some differences between AD brain and aging brain from non demented people have been indicated [6]. However, there are also some common features suggesting that in Alzheimer disease brain could be an acceleration of some processes occurring in aged brain [7].
In this short review, we will focus on adult neurogenesis occurring in the dentate gyrus (DG), a process that decreases in aged mammals and that could be related with loss of memory, an important feature in Alzheimer disease.
Memory and AD
By studying the consequences of a bilateral hippocampal region resection in a patient with epilepsy [8], it was suggested the existence of at least two memory systems, declarative and non-declarative memory. The declarative memory is involved in remembering facts and events, whereas non declarative memory is related with skill or habit learning [9–11]. The removal of hippocampal region in the patient with epilepsy resulted in the loss of declarative memory without affecting the non declarative memory.
A loss in declarative memory has been found in patients with Alzheimer disease. In these patients, neurodegeneration at the hippocampal region takes place at the first steps of the disease [12]. In normal aging there is a mild cognitive impairment, but this impairment could be accelerated in AD [1,4].
Structure and function of DG
Adult neurogenesis mainly takes place in two brain regions, the subventricular zone and the dentate gyrus, here we will comment on the dentate gyrus. The hippocampal formation comprises the hippocampus (with its CA fields), the dentate gyrus and the entorhinal cortex that provides much of the cortical input to the hippocampus. It has been suggested that the DG plays a role in the formation of new memories [13] in higher vertebrates like mammals. Although a similar structure to dentate gyrus was identified in reptiles [14], the morphological characteristics of dentate gyrus only appears in mammals [15–17]. The dentate gyrus is composed of three layers; the molecular layer, the granule cell layer and the hilus or polymorphic cell layer. The number of granule cells is approximately 6.105 in mouse dentate gyrus [18]. In the DG two different regions the dorsal and ventral regions separated by an intermediate zone, have been described by using different molecular markers like Lct that is preferentially expressed in the dorsal part and Trhr that is expressed specifically in the ventral part [19]. Different functions have been suggested for the dorsal and ventral parts. Dorsal region is mainly involved in cognitive processes like learning and memory, while the ventral region may be more involved in motivational and emotional behavior [20]. Apart of its function in memory, it has been suggested that the dentate gyrus may play a role as a gate for neuronal transmission throughout the hippocampus and that failures on that system could result in the appearance of epileptiform activity [21].
The development of dentate gyrus
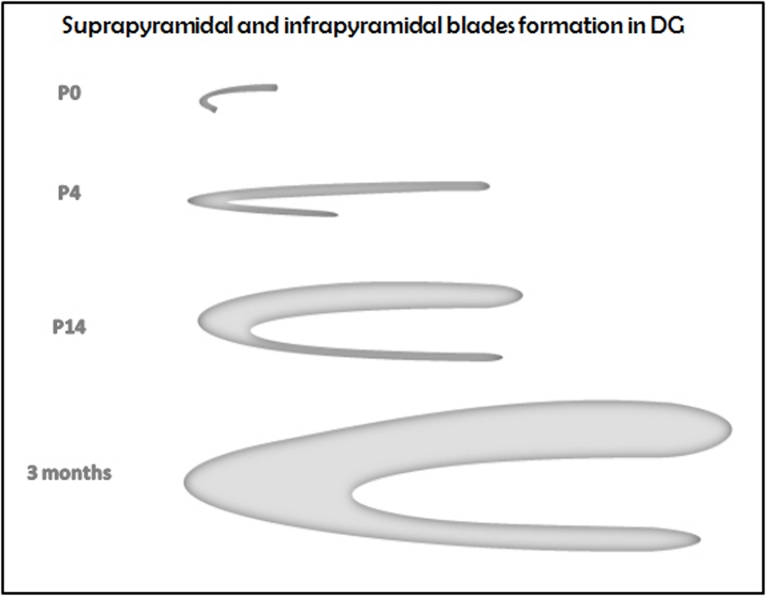
Figure 1 shows a scheme of the formation of mouse DG [22]. A similar picture was found in rat [23]. DG growth can be measured by its increase in volume. Mouse DG raises its highest DG volume at 3 months of age [24].
Figure 1.
Scheme of DG formation in mouse.
In non human primates the number of granule cells is approximately 5 million cells in mature DG [16]. For humans the number of granule cells in DG increases to 10–18 millions in the mature brain [25].
Neurogenesis in adult DG
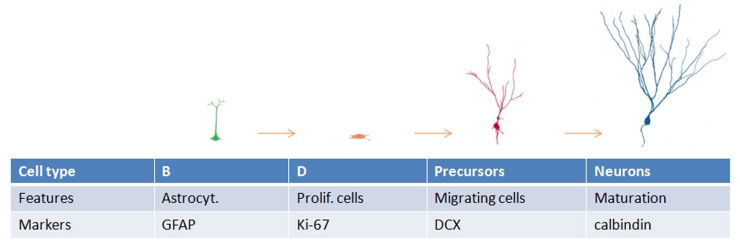
It is now well established that neurogenesis in the brain of adult mammals takes place through the whole life and that one of the localizations where neurogenesis occurs is the subgranular zone (SGZ) of the dentate gyrus [13,26]. Different steps have been characterized for neurogenesis in SGZ: the proliferation of stem like cells that can be recognized because they express GFAP, the formation of neuronal precursors that can be followed by the expression of nestin first and afterwards that of doublecortin (DCX), and, finally, the maturation of the precursors into mature cells [13] (Figure 2).
Figure 2.
Different types of cells are involved in the progression of SGZ neurogenesis.
Neurogenesis rate changes with the age of the organism. In mice, three representative ages have been described: young (from 1 to 3 months old), adult (from 6 to 12 months old) and aged (from 12 to 24 months old) [27]. By taking into account different data, it was calculated that in young mice 9.104 new cells can be formed per day; 2.104 cells in adult and 1.104 cells in aged mice [27–29]. From these results, it is possible to calculate that in the first year of a mouse 6.105 new cells are born in the DG. In other words, almost the same number of cells that compose the whole DG. However, many of the newborns die before becoming mature neurons and only the remaining newborn mature neurons can integrate into the existing neural circuitry and may play a role in learning and memory processes [13,26,30]. It is obvious that in the absence of SGZ neurogenesis those processes should be impaired.
AD, memory and the hippocampus
The enduring anterograde amnesia after bilateral removal of hippocampal region in the patient H.M. was one of the first indications about the importance of that region on declarative memory [8]. Declarative memory is also impaired in Alzheimer disease, being this feature one of the first clinical failures observed in the patients with the disease. During the development of the disease an increase in hippocampal volume loss has been described [31]. The indicated average rate of hippocampal volume loss was 4.4% per year in patients with AD, whereas it was 0.8% in normally elderly subjects [32]. In another study it was estimated that the maximum rate of hippocampal atrophy was reached during mild cognitive impairment, just before the clinical AD [33]. This result may agree with a recent report [34].
In spite of hippocampal atrophy, an increased proliferation of neural precursor cells has been also reported in the hippocampus of AD patients [35,36]. It seems however that immature neurons are not able to mature as an increased expression of mature neuronal markers, such as NeuN and MAP2, has not been observed [35,37]. Studies in mouse models of AD, in particular APP transgenic mice, have yielded conflicting results and both increased and decreased hippocampal neurogenesis have been reported [38,39].
In advanced AD, corresponding to late Braak and Braak stages there is hippocampal degeneration [12], but probably a lack of function of DG can take place at earlier stages [40].
GSK3 and AD
AD could be divided in two types: Familial Alzheimer Disease (FAD) and Sporadic Alzheimer Disease (SAD). In FAD, mutations in three genes (APP, PS-1 and PS-2) promote the onset of the disease [41]. Mutations in APP result in an increase of beta amyloid peptide [42], the major component of an aberrant structure, the senile plaques found in the brain of AD patients [43,44]. Mutations in PS-1 or PS-2 could result in a gain of function of its γ-secretase activity and it will result also in an increase in the amount of beta amyloid peptide [41]. However, there is not a direct relationship between γ-secretase gain of function, increase of beta amyloid peptide and the onset of the disease [45]. Moreover, loss of other PS-1 functions could also result in the onset of neurodegeneration without any increase in the amount of beta amyloid peptide [45].
However, there is a common feature among the different types of PS-1 mutations resulting in the onset of neurodegeneration. This common feature is the activation of GSK3 kinase [46] (Figure 3).
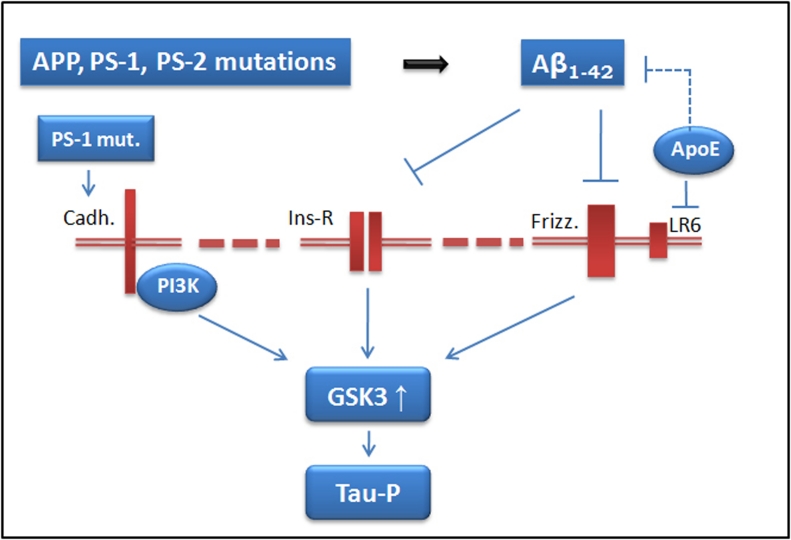
Figure 3.
Possible role of GSK3 in FAD and SAD. Mutations in app, ps1 and ps-2 genes (FAD) may result in an increase of the amount of Aβ1–42 peptide. Aggregates of this peptide could act as antagonist for insulin or wnt (frizzled) receptors and, as consequence, GSK3 could be activated. Also, mutations in ps-1 gene may result in the lack of formation of a complex of that protein with cadherin, resulting in a decrease in PI3K activity and an increase in GSK3 activity. In SAD, ApoE isoforms bind to LR6, a coreceptor of frizzled, interfering in the signaling that results in GSK3 regulation. The presence of ApoE4 could result in a higher GSK3 activity. Also, ApoE could regulate Aβ clearance and, therefore, its amount. Finally, activated GSK3 can phosphorylate tau protein.
For SAD, the main risk (in addition to ageing), is the expression of Apoliprotein E (ApoE) allele 4 [47]. Also, it has been described a preferred activation of GSK3 when this ApoE4, and no other ApoE forms, is expressed [48]. Thus, GSK3 activation could be also a common feature between FAD and SAD (Figure 3).
On the other hand, there is another neuropathological pathognomic feature in Alzheimer disease, the neurofibrillary tangles (NFT) that is a characteristic, together with senile plaques (SP), in the brain of AD patients. The main component of NFT, is the microtubule associated proteins known as tau [49], in phosphorylated form [50]. Although different protein kinases can modify tau protein, the one that can modify more sites in tau molecule [51] is GSK3 [52], also known as tau kinase I [53] (Figure 3).
GSK3 and SGZ neurogenesis
Since there is an activation of GSK3 in FAD and SAD, and that kinase modifies tau protein, a transgenic mouse overexpressing GSK3 in the hippocampus and cerebral cortex (the mouse damaged regions in AD) was raised [54]. The main characteristic of this mouse was the degeneration of its dentate gyrus [55], which appears to result in impairment in learning and memory [56]. In that degeneration, tau protein a well known substrate for GSK3, appears to play a role, because a higher degeneration was found in a transgenic mouse overexpressing both GSK3β and tau than in a transgenic mouse only overexpressing GSK3β [57], suggesting that phosphotau could be toxic for dentate gyrus neurons [58].
Future developments
It has been suggested that not only in AD but also in other tauopathies, DG degeneration could take place [57]. In AD, it appears that a clear feature is a decrease in memory whereas in frontotemporal dementia (FTD) there are other features present, as dementia related to changes in emotional behavior. A working hypothesis could be that degeneration of DG occurring in those tauopathies occurs at different regions of DG. The use of different transgenic mice models in which different DG regions could be damaged, may probably answer this question [59].
Conclusions
Defects in dentate gyrus neurogenesis could be the cause of some of the characteristics observed in aging. These defects could be more evident in some neurodegenerative disorders. In Alzheimer’s disease, studies supporting hippocampal neurogenesis, that is not sufficient however to compensate for the progressive cell loss, suggest that enhancing the generation of mature neurons in this brain region may represent a valuable treatment strategy.
References
- [1].Prince M, Jackson J. Alzheimer’s Disease International World Alzheimer Report 2009. In: Prince M, Jackson J, editors. Alzheimer’s Disease International. Alzheimer’s Disease International; London: 2009. pp. 1–96. [Google Scholar]
- [2].Mucke L. Neuroscience: Alzheimer’s disease. Nature. 2009;461:895–7. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- [3].Barten DM, Albright CF. Therapeutic strategies for Alzheimer’s disease. Mol Neurobiol. 2008;37:171–86. doi: 10.1007/s12035-008-8031-2. [DOI] [PubMed] [Google Scholar]
- [4].Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sleegers K, Lambert JC, Bertram L, Cruts M, Amouyel P, Van Broeckhoven C. The pursuit of susceptibility genes for Alzheimer’s disease: progress and prospects. Trends Genet. 2010;26:84–93. doi: 10.1016/j.tig.2009.12.004. [DOI] [PubMed] [Google Scholar]
- [6].Terry RD. Alzheimer’s disease and the aging brain. J Geriatr Psychiatry Neurol. 2006;19:125–8. doi: 10.1177/0891988706291079. [DOI] [PubMed] [Google Scholar]
- [7].Kazee AM, Johnson EM. Alzheimer’s Disease Pathology in Non-Demented Elderly. J Alzheimers Dis. 1998;1:81–89. doi: 10.3233/jad-1998-1202. [DOI] [PubMed] [Google Scholar]
- [8].Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kandel ER. The biology of memory: a forty-year perspective. J Neurosci. 2009;29:12748–56. doi: 10.1523/JNEUROSCI.3958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Squire LR. The legacy of patient H.M. for neuroscience. Neuron. 2009;61:6–9. doi: 10.1016/j.neuron.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Squire LR. Memory and brain systems: 1969–2009. J Neurosci. 2009;29:12711–6. doi: 10.1523/JNEUROSCI.3575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- [13].Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- [14].Lopez-Garcia C, Martinez-Guijarro FJ. Neurons in the medial cortex give rise to Timm-positive boutons in the cerebral cortex of lizards. Brain Res. 1988;463:205–17. doi: 10.1016/0006-8993(88)90393-9. [DOI] [PubMed] [Google Scholar]
- [15].Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- [16].Seress L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Prog Brain Res. 2007;163:23–41. doi: 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- [17].Striedter GF. Brain homology and function: an uneasy alliance. Brain Res Bull. 2002;57:239–42. doi: 10.1016/s0361-9230(01)00692-x. [DOI] [PubMed] [Google Scholar]
- [18].Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- [19].Fanselow MS, Dong HW. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bast T. Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci. 2007;18:253–81. doi: 10.1515/revneuro.2007.18.3-4.253. [DOI] [PubMed] [Google Scholar]
- [21].Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–80. [PubMed] [Google Scholar]
- [22].Schambra UB, Silver J, Lauder JM. An atlas of the prenatal mouse brain: gestational day 14. Exp Neurol. 1991;114:145–83. doi: 10.1016/0014-4886(91)90034-a. [DOI] [PubMed] [Google Scholar]
- [23].Rahimi O, Claiborne BJ. Morphological development and maturation of granule neuron dendrites in the rat dentate gyrus. Prog Brain Res. 2007;163:167–81. doi: 10.1016/S0079-6123(07)63010-6. [DOI] [PubMed] [Google Scholar]
- [24].Sirerol-Piquer MS, Gomez-Ramos P, Hernandez F, Perez M, Moran MA, Fuster-Matanzo A, Lucas JJ, Avila J, Garcia-Verdugo JM. GSK3β overexpression induces neuronal death and a depletion of the neurogenic niches in the dentate gyrus. Hippocampus. 2010 doi: 10.1002/hipo.20805. in press. [DOI] [PubMed] [Google Scholar]
- [25].West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- [26].Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 11:339–50. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 31:151–61. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- [28].Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- [29].Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- [30].Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26:4546–58. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van der Flier WM, Scheltens P. Alzheimer disease: Hippocampal volume loss and Alzheimer disease progression. Nat Rev Neurol. 2009;5:361–2. doi: 10.1038/nrneurol.2009.94. [DOI] [PubMed] [Google Scholar]
- [32].Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR, Jr, Weiner MW. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–77. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, Scheltens P, Vrenken H, Barkhof F. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Reitz C, Brickman AM, Brown TR, Manly J, DeCarli C, Small SA, Mayeux R. Linking hippocampal structure and function to memory performance in an aging population. Arch Neurol. 2009;66:1385–92. doi: 10.1001/archneurol.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:343–7. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nagy Z, Esiri MM, Cato AM, Smith AD. Cell cycle markers in the hippocampus in Alzheimer’s disease. Acta Neuropathol. 1997;94:6–15. doi: 10.1007/s004010050665. [DOI] [PubMed] [Google Scholar]
- [37].Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, Iqbal K, Grundke-Iqbal I. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol. 2008;67:78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- [39].Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer’s disease transgenic (PDGF-APPSw,Ind) mice. Proc Natl Acad Sci U S A. 2004;101:13363–7. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ohm TG. The dentate gyrus in Alzheimer’s disease. Prog Brain Res. 2007;163:723–40. doi: 10.1016/S0079-6123(07)63039-8. [DOI] [PubMed] [Google Scholar]
- [41].Price DL, Sisodia SS. Mutant genes in familial Alzheimer’s disease and transgenic models. Annu Rev Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- [42].St George-Hyslop PH, Tanzi RE, Polinsky RJ, Haines JL, Nee L, Watkins PC, Myers RH, Feldman RG, Pollen D, Drachman D, et al. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science. 1987;235:885–90. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- [43].Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–5. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- [44].Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. Embo J. 2004;23:2586–96. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hernandez F, de Barreda EG, Fuster-Matanzo A, Goni-Oliver P, Lucas JJ, Avila J. The role of GSK3 in Alzheimer disease. Brain Res Bull. 2009;80:248–50. doi: 10.1016/j.brainresbull.2009.05.017. [DOI] [PubMed] [Google Scholar]
- [47].Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–72. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- [48].Cedazo-Minguez A, Popescu BO, Blanco-Millan JM, Akterin S, Pei JJ, Winblad B, Cowburn RF. Apolipoprotein E and beta-amyloid (1–42) regulation of glycogen synthase kinase-3beta. J Neurochem. 2003;87:1152–64. doi: 10.1046/j.1471-4159.2003.02088.x. [DOI] [PubMed] [Google Scholar]
- [49].Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–62. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995;270:823–9. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- [52].Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112–9. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [53].Ishiguro K, Shiratsuchi A, Sato S, Omori A, Arioka M, Kobayashi S, Uchida T, Imahori K. Glycogen synthase kinase 3 beta is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993;325:167–72. doi: 10.1016/0014-5793(93)81066-9. [DOI] [PubMed] [Google Scholar]
- [54].Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. Embo J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Engel T, Hernandez F, Avila J, Lucas JJ. Full reversal of Alzheimer’s disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J Neurosci. 2006;26:5083–90. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–33. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- [57].Engel T, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J, Hernandez F. Cooexpression of FTDP-17 tau and GSK-3beta in transgenic mice induce tau polymerization and neurodegeneration. Neurobiol Aging. 2006;27:1258–68. doi: 10.1016/j.neurobiolaging.2005.06.010. [DOI] [PubMed] [Google Scholar]
- [58].Gomez de Barreda E, Perez M, Gomez Ramos P, de Cristobal J, Martin-Maestro P, Moran A, Dawson HN, Vitek MP, Lucas JJ, Hernandez F, Avila J. Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiol Dis. 37:622–9. doi: 10.1016/j.nbd.2009.11.017. [DOI] [PubMed] [Google Scholar]
- [59].Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]