Abstract
Reelin is a large extracellular matrix protein essential for mediating proper neuronal positioning during development. Employing the same lipoprotein-mediated signaling cascade, Reelin regulates NMDA receptor homeostasis and modulates synaptic function and plasticity in adult synapses. In line, aging-related reduction in Reelin expression has been shown to contribute to cognitive impairments during normal aging. Although recent experimental evidence suggests an involvement of dysfunctional Reelin in pathological forms of aging, such as late-onset Alzheimer’s disease (AD), the molecular mechanisms by which this conserved extracellular glycoprotein contributes to the pathogenesis of AD remains still largely unknown. In the present review, we briefly summarize the role of Reelin in the developing and adult brain and discuss the implication of loss- or gain-of-functions of developmental programs in the adult brain as putative inducing factors of pathological forms of aging. Finally, we will propose some new concepts on the role of inflammatory cytokines in interfering with Reelin-mediated signaling during neurodevelopment and adult synaptic function, and discuss how this could be translated into a novel non-transgenic mouse model of late-onset AD. Thus, the findings presented in this review are aimed to highlight the important role of Reelin-mediated signaling in maintaining a crucial developmental program in the adult brain that is required to prevent the shift from normal to pathological aging.
Keywords: Aging; Alzheimer’s disease; inflammation; in utero infection; cognitive impairments; polyriboinosinic-polyribocytodilic acid, mid- and late-gestation, APP processing; Tau phosphorylation; neurodegeneration
Reelin is a large glycoprotein and major secretory modulator with important roles during neurodevelopment and adult synaptic plasticity. It is subjected to proteolytic cleavage in the extracellular and/or post-endoplasmic environment at two sites [1, 2]. The physiological functions of the cleaved Reelin fragments are not fully understood. Current evidence suggests that the N-terminal region of Reelin is required for protein homodimerization and full signaling activity [3]; processes shown to be essential for postnatal dendritic maturation of cortical pyramidal neurons [4]. The central region represents the minimal receptor-binding and signaling unit [5, 6] and an APP (amyloid precursor protein) interaction domain [7]. The C-terminal fragment appears to be required for protein secretion, and/or proper protein folding, as well as intracellular activity [8, 9]. Despite the pharmacological data that classified the Reelin proteases into the family of zinc-dependent disintegrin and metalloproteinases with or without thrombospondin motifs (ADAM and ADAMTS [1, 2]) the nature of the involved metalloproteinase is still not identified nor is it known whether it is involved in both the N- and C-terminal cleavage. In addition, although processing of Reelin certainly modulates its signaling in the developing and potentially also in the adult brain; neither the biological significance nor the factors that modulate this process are known.
Role of Reelin in the developing brain
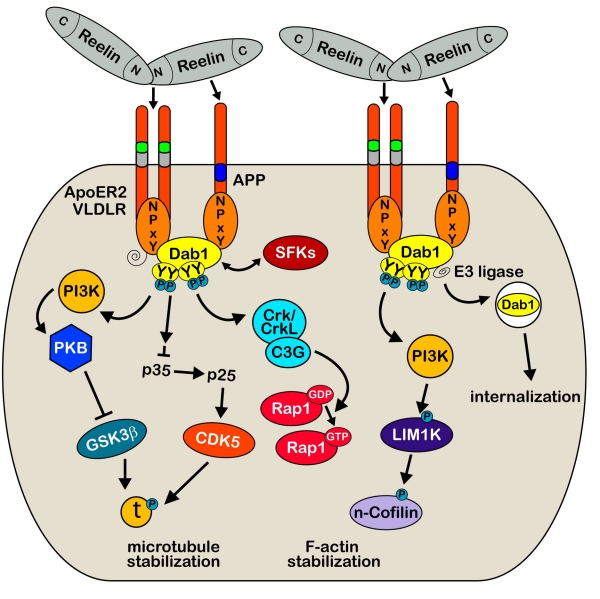
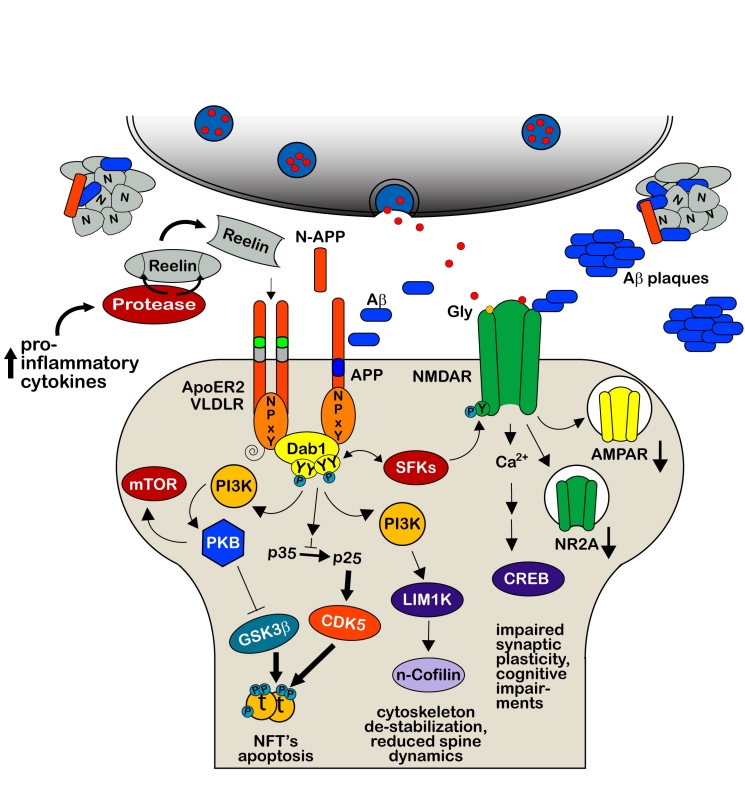
The role of Reelin in the developing brain has been studied in great detail (for recent reviews, see [10, 11]). During neuronal development, Reelin is secreted by Cajal-Retzius (CR) cells located in the marginal zone and is essential for neuronal migration and brain development [12–15]. This is achieved through the activation of the apolipoprotein E receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR, [14, 16–18]), which constitute the major Reelin receptors (Figure 1). Reelin binding to these lipoprotein receptors induces their clustering and results in tyrosine phosphorylation of the adaptor protein Disabled-1 (Dab1) that interacts with conserved NPxY motifs in the cytoplasmic domains of VLDLR, ApoER2 and APP family members [17–22]. The phosphorylation of Dab1 results in the activation of Src family tyrosine kinases (SFKs) and additional non-receptor tyrosine kinases [23], which in turn trigger a downstream cascade allowing the activation of cytosolic kinase pathways involving Phosphatidylinositol-3-kinase (PI3K) and Akt/PKB, and the inhibition of Glycogen synthase kinase 3β (GSK3β) as well as CDK5 ([24, 25]). In line with this modulatory role, Reelin-deficient animals show hyperphosphorylation of the microtubule-associated protein Tau, which represents a major target of both kinases. Recently, Michael Frotscher and his group provided evidence that signaling molecules of the Reelin pathway involving Dab1, SFKs and PI3K are also involved in n-cofilin phosphorylation and actin cytoskeleton regulation [26]. Following phosphorylation of serine3, n-cofilin is unable to depolymerize F-actin and this in turn leads to a stabilization of the actin cytoskeleton. These results suggest a novel role of Reelin in controlling directional neuronal migration processes during development.
Figure 1:
Reelin-mediated signaling during development. Binding of multimeric Reelin to VLDLR and ApoER2 induces their clustering and phosphorylation of the cytoplasmic adapter protein Disabled-1 (Dab1) by the Src family tyrosine (SFKs) kinases Fyn and Src. Dab1 is initially phosphorylated on two (Tyr185, Tyr198) sites which seem to be required to permit transphosphorylation of adjacent Dab1 molecules at all four sites (Tyr185, Tyr198, Tyr220, Tyr232, [131]). This leads to the recruitment and activation of additional activated non-receptor tyrosine kinases allowing the activation of cytosolic kinase cascades, involving Phosphatidylinositol-3-kinase (PI3K), Akt/PKB, and the inhibition of Glycogen synthase kinase 3β (GSK3β) as well as CDK5, two main kinases that phosphorylate the microtubule-stabilizing protein Tau [24, 25]. Phospho-Dab1 recruits also Crk/CrkL-C3G complexes which in turn stimulate Rap1 [131]. The central Reelin domain also directly interacts with the E1 extracellular domain of APP; an interaction that appears to be necessary for proper neurite outgrowth both in vitro and in vivo [7]. Regulation of the actin cytoskeleton through the Reelin signaling cascade also involves activation of LIM1 kinase which results in increased n-cofilin phosphorylation in the leading processes of migrating neurons approaching the marginal zone [26]. Recruitment of ubiquitin ligases to phosphorylated Dab1 results in ubiquitination of the protein, which likely mediates the phosphorylation-dependent endocytosis of the entire Reelin signaling complex [32, 132].
In agreement with the fundamental role of Reelin during neuronal development, impaired Reelin signaling has a devastating effect on the gross morphology of the hippocampus, cerebellum and neocortex, as evident by the abnormal cortical layering and neuronal positioning in Reelin homozygous knockout mice (reeler, [18, 27]). Despite the normal brain morphology, heterozygous reeler mice show significant impairments in synaptic plasticity as well as learning disabilities compared to wild-type littermates [28]. A reeler phenotype has also been described in mice with a loss of both Reelin receptors [18], whereas single-mutants do not show the characteristic cortical disorganization. This indicates that ApoER2 and VLDLR can compensate for each other to a certain extent. However, novel evidence for divergent roles of the Reelin receptors have recently emerged [10, 29]. These different functions might stem from either structural differences in the intracellular domains or selective localizations within distinct sub-domains in the plasma membrane. Support for the latter hypothesis has been provided by the recent study by Duit and colleagues [30]. Investigating the functional difference between the two Reelin receptors using a fibroblast cell culture system, they found that the endocytosis of Reelin is linked to specific receptor sorting to raft versus non-raft domains of the plasma membrane: Duit et al. reported that VLDLR is present in non-raft domains and Reelin binding to this receptor results in fast endocytosis of the complex. Reelin is then targeted to the lysosome and the receptor recycled back to the cell membrane. Binding of Reelin to ApoER2, which is predominantly localized to raft domains of the plasma membrane, however, does not result in significant reduction of extracellular Reelin since this receptor-mediated endocytosis is rather slow. Activation of this pathway results in lysosomal sorting of both ApoER2 and Reelin. Together with the proteosomal degradation of Dab1 following receptor-mediated endocytosis, these differential processes provide not only an essential feedback mechanism and termination signal in the regulation of neuronal positioning [31–33], they likely also contribute to a highly selective and receptor-specific fine-tuning of the Reelin signal that is required for normal synaptic plasticity and learning in the adult brain.
In the following, we will focus on the role and function of Reelin in the mature rodent and human brain and summarize the implication of abnormal Reelin-mediated signaling as potential etiological factor of Alzheimer’s disease (AD).
Reelin-mediated signaling in the adult brain
The studies focusing on developmental functions of Reelin clearly outnumber the investigations addressing the role of Reelin in the mature brain and we are just beginning to understand the implications of reduced Reelin expression during aging. Interestingly, the expression pattern of Reelin changes after the end of the neuronal migration phase [34]. In the adult brain, the Reelin-expressing Cajal-Retzius cells are largely replaced by Reelin-expressing GABAergic interneurons that are dispersed throughout the forebrain. Reelin is also expressed by glutamatergic pyramidal neurons in layer II of the entorhinal cortex, olfactory mitral cells and cerebellar granule cells [12, 35–44]. ApoER2 and VLDLR and the adapter protein Dab1 also remain expressed in the adult brain. The distinct expression pattern of Reelin and its signaling members in brain regions implicated in mediating learning and memory [12, 43, 45–47] suggest that Reelin-mediated signaling in the adult brain is crucial for normal cognitive function.
The role of Reelin in synaptic plasticity
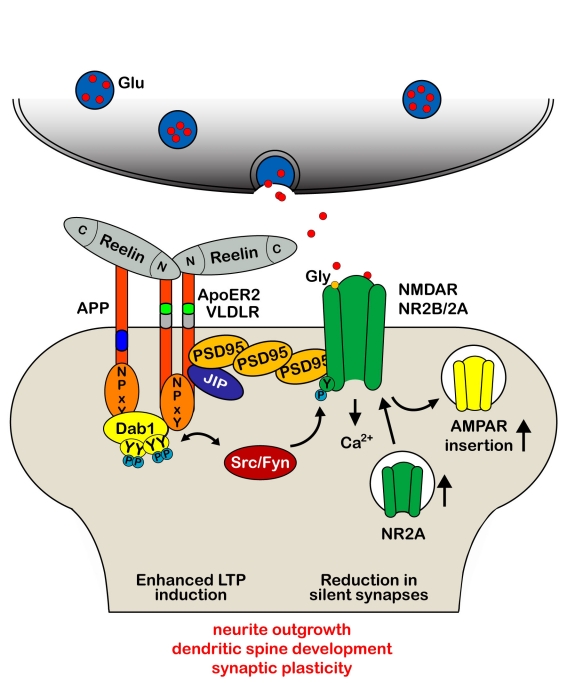
Indeed, first evidence that Reelin plays a role in synaptic plasticity came up in 2002 [28]. The team of Joachim Herz demonstrated in vitro that application of recombinant Reelin can dramatically elevate the magnitude of LTP, a form of synaptic plasticity induced by high frequency stimulations that results in a persisting increase in synaptic efficacy [48]. Using ApoER2- and VLDLR-deficient mice, they verified that high frequency stimulation results in reduced LTP induction in VLDLR-knockout mice and a pronounced decay in late-phase LTP in ApoER2-null mice [28]. These findings are in line with the described hypomorphic phenotype of the single-mutants with developmental defects affecting mainly hippocampus and neocortex (ApoER2) or cerebellum (VLDLR), as well as the reeler phenotype in double-mutants [18]. Overall, the results provided the first experimental evidence for a critical role of Reelin in modulating synaptic function and enabling the formation of long-term memory. Other recent studies have confirmed the significant contribution of the Reelin signaling pathway to synaptic plasticity [45, 49–52] by demonstrating that binding of Reelin to ApoER2 and VLDLR at postsynaptic sites controls glutamatergic neurotransmission through differential modulation of the activity of NMDA and AMPA receptors (Figure 2). Similar to the signaling in the developing brain, the effect was dependent on Reelin-induced activity of Src family kinases that are involved in tyrosine phosphorylation of the NR2 subunits of the NMDA receptors [45, 49]. This in turn results in a potent enhancement of glutamate-stimulated Ca2+ influx that is required for LTP induction and maintenance [53].
Figure 2.
Reelin modulates synaptic plasticity and learning and memory. Dab1 binding site and exon 19-encoded domain of ApoER2 are necessary for the Reelin-mediated increase in synaptic plasticity that allow the coupling of the Reelin receptor and the activated Dab1–Src/Fyn complex to the NR2A and NR2B subunit of the NMDA receptor for its subsequent tyrosine phosphorylation and Ca2+ entry in the postsynaptic density, PSD [45]. Prolonged presence of Reelin at synaptic sites also modulates AMPA receptor-mediated responses, associated with an increased receptor number in the PSD [52] and reduction in the number of silent synapses that facilitated the developmental switch from NR2B to NR2A [133]. Reelin-induced augmentation of Ca2+ entry through NMDA receptors increases phosphorylation and nuclear translocation of the transcription factor cAMP-response element binding protein (CREB), indicating that Reelin may physiologically modulate learning and memory by modulating NMDA receptor functions [49]. These data fit with the observations that in the absence of Reelin or the Reelin receptors, neurons exhibit stunted dendritic growth, a reduction in dendritic branches and significantly fewer spines [18, 58, 59, 133–136].
The Herz lab also established a molecular mechanism by which Reelin, in conjunction with alternatively spliced forms of ApoER2, regulates synaptic function and plasticity in the adult brain. They discovered that adaptor proteins binding to the exon 19-encoded intracellular domain of ApoER2 [54, 55] are necessary for transmitting the Reelin signal that is required to enhance LTP. Together, these results provided the first evidence for a physiological role of Reelin signaling in the control of NMDA receptor function and synaptic strength, suggesting that Reelin can physiologically modulate LTP through regulation of NMDA receptor activity.
Reelin and its impact on dendrite and spine formation, modulation and maintenance:
One critical aspect of synaptic adaptations associated with LTP involves the changes in dendritic spine morphology. Similarly to the situation during development where Reelin-dependent signaling is required for the outgrowth of dendrites and the formation of dendritic spines [4, 56–59], the Reelin signaling cascade is used in adulthood to regulate plasticity-induced morphological changes of dendritic spines. First evidence to support a role for Reelin in this process has been provided by the work of Stanfield and Cowan [60, 61]. They reported altered dendritic morphology and orientation in the cortex and hippocampus of adult reeler mice. In line, heterozygous reeler mice exhibited reduced spine density in the hippocampus and cortex that was accompanied by impaired performance in certain learning and memory tasks [51, 62–64]. In the absence of Reelin or its receptors, Dab1-expressing neurons also exhibited stunted dendritic growth and reduction in dendritic branches [58]. It has been further shown that application of recombinant Reelin can reverse this deficit in neuronal cultures lacking one or both reelin genes [51, 58, 59]. The rescue was again dependent on the activation of the downstream tyrosine kinase-dependent signaling pathway, as shown for the regulation of neuronal migration and adult synaptic plasticity. In addition, recent data showed that stimulation of cultured hippocampal neurons with Reelin leads to enhanced dendritogenesis [57]. This effect was blocked by reduced expression of Crk family proteins, suggesting that Dab1-binding proteins are important downstream components of the Reelin signaling pathway in regulating hippocampal dendritogenesis. Altogether, these studies demonstrate that constitutive levels of Reelin and its receptors are required for correct formation and maintenance of dendritic structures that are crucial for normal information processing in adult synapses.
Recently, the group of Eduardo Soriano addressed for the first time the impact of Reelin overexpression on synaptic plasticity and associated morphological changes in vivo [65]. They reported that adult generated neurons in the dentate gyrus of transgenic mice exhibit an increased complexity in their developing dendrites that was accompanied by a significant enlargement of dendritic spines and an increase in synaptic contacts. Furthermore, the overexpression evoked a dramatic increase in LTP induction and maintenance as compared to controls. They also discovered that overexpression of Reelin leads to dispersion, impaired migration and abnormal positioning of adult-generated neurons, indicating that adequate levels of Reelin above or below a certain threshold are required for successful neuronal migration, dendritic development and morphological adaptations that are associated with processes underlying synaptic plasticity and learning and memory [65]. Taken together, these results suggest that Reelin and its lipoprotein receptors participate in the control of developmental processes that are fundamental for proper synaptic and neuronal functioning in the adult brain.
Reelin expression in humans
In the developing human brain, CR cells in the marginal zone also constitute the main source of Reelin as described in rodents and non-human primates [66, 67]. However, besides the well-characterized human developmental disorder, Lissencephaly, which is caused by dominant mutations in the reelin gene in humans [68], relatively little information is available on the expression and localization of Reelin in the healthy adult and aged human brain. Reelin-positive CR cells can still be detected in the neo- and entorhinal cortex of adult and aged humans [69–72], but they appear to decline during pathological aging [37]. In 2005, Roberts and colleagues reported the first ultrastructural localization of Reelin in postmortem human brain samples [73]. Reelin-immunoreactivity was readily detected in the neocortex; being present both in interneurons and glia cells. In the neuropil, Reelin-immunoreactivity was found in small axons, axon terminals, dendrites and postsynaptic spines. These results are consistent with immunohistochemical studies involving postmortem brain tissue obtained from rodents [39, 44], ferrets [74], primates [39, 75], as well as humans [35, 76–78]. The data fit with the important role of Reelin in normal synaptic function, including synaptic plasticity and dendritic remodeling in the adult brain in several species. However, it is currently unknown whether Reelin expression changes across aging in humans. Recent genome-wide SNP association studies revealed that polymorphisms in the reelin gene (especially in and near the promoter region) are associated with AD neuropathology [79, 80], suggesting that putative alterations in Reelin expression levels may correlate with aging-associated mental decline and contribute to a pathological mechanism involving AD-associated neuropathology.
Reelin and Alzheimer’s disease (AD)
In line with this hypothesis and the critical role of Reelin in synaptic transmission and learning and memory, several recent findings suggest that alterations in Reelin expression and abnormal Reelin signaling may contribute to neuronal dysfunction associated with AD. In addition, several studies have investigated the molecular mechanism by which Reelin, its receptors and downstream signaling proteins may contribute to the pathophysiology of this progressive neurodegenerative disease. The purpose of this section is to summarize the current knowledge and recent findings related to the molecular link between Reelin dysfunction and AD-related neuropathology.
AD is a complex neurodegenerative disease that afflicts an increasing fraction of our aging population. It is characterized by progressive cognitive decline and severe neurodegeneration [81]. Neuropathological hallmarks comprise neurofibrillary tangles (NFTs), consisting of hyperphosphorylated Tau [82] and the formation of senile plaques, primarily composed of amyloid-β (Aβ) peptides [83]; the amyloidogenic cleavage product of APP that results from sequential cleavage by β- and γ-secretases [84, 85]. Abnormal amyloidogenic Aβ processing and formation of amyloid-β plaques have been suggested to lead to synaptic dysfunction, synapse loss, and ultimately to neuronal death. Non-amyloidogenic processing by cleavage through α- and γ-secretase results in the production and release of a large N-terminal extracellular fragment and smaller, membrane-bound C-terminal fragments with putative neuroprotective and transcriptional functions [86–88]. Recently, the team of Tessier-Lavigne discovered an additional disease-modifying, N-terminal fragment of APP that acts as ligand of the death receptor 6 (DR6, a member of the tumor necrosis factor receptor superfamily). This fragment is produced upon growth factor withdrawal by β-secretase plus an additional, as yet unidentified, protease and has been shown to induce Caspase-6-mediated axonal degeneration [89], a process involved in axonal pruning during development. These recent results suggest that abnormal neurodegeneration associated with AD might be due to inappropriate activation or reactivation of a developmental pathway in the aged brain.
Recent insights into the pathophysiology of Aβ peptides suggested a potent negative impact of Aβ oligomers on synaptic functions that may underlie impairments in long-term synaptic plasticity [90–92]. Incubation of hippocampal neurons with Aβ oligomers lead to intracellular trapping or functional impairment of AMPA and NMDA receptors [93–95], thereby decreasing LTP [90, 91, 96–99]. As already mentioned, several studies showed an increase in LTP following Reelin application to hippocampal slices [28], demonstrating that Reelin has an opposite effect on synaptic function compared to Aβ oligomers. A recent study conducted in Joachim Herz’ lab demonstrated that Reelin could indeed antagonize the suppressive effects of Aβ oligomers on synaptic NMDA receptor-mediated neurotransmission [100]. They demonstrated that Reelin signaling in excitatory synapses could restore oligomeric Aβ–induced impairments in synaptic plasticity to normal levels. Activation of SFKs by Reelin through ApoER2 and VLDLR binding was necessary for neutralizing the Aβ-mediated suppression, supporting again the crucial role of Reelin-mediated signaling for normal synaptic function.
The group of Saez-Valero has recently addressed the putative link between abnormal Reelin levels and AD pathogenesis. They analyzed the expression and glycosylation pattern of Reelin in CSF and cortical tissue extracts obtained from AD patients and non-demented controls [101–103]. They reported abnormal glycosylation of Reelin as well as a preferential up-regulation of the N-terminal 180-kDa Reelin fragment in the CSF and frontal cortex of AD patients. Full-length and other proteolytic fragments of Reelin remained unaltered in control and AD subjects [101], suggesting alterations in Reelin processing and signaling in AD. Another recent study showed that the N-terminal fragment of Reelin can be generated within the endosomes after internalization of the full-length form [104], pointing to the possibility that in AD patients the endosomal recycling and re-secretion of this fragment into the extracellular space could also be significantly affected.
Our own studies provide an alternative explanation for the selective increase in the N-terminal Reelin fragment. We have recently observed that Reelin accumulates in oligomeric amyloid-like plaques in the hippocampal formation of several aged species [39], indicating that the N-terminal fragments may preferentially oligomerize and aggregate in the extracellular matrix during aging (Figure 3). Indeed, preliminary biochemical investigations confirmed that these Reelin deposits contain besides the full-length form also significant amounts of N-terminal Reelin fragments (JD, IK unpublished data). Our observations further revealed that Reelin was selectively associated but not completely co-localized with fibrillary Aβ species. Oligomeric Aβ deposits on the other hand showed a very high degree of co-localization with Reelin [105], confirming and extending previous findings involving transgenic AD mouse models [106, 107]. To address the putative functional implications, we have investigated whether early accumulations of extracellular protein deposits in the projection areas of subcortical neurons can act retrogradly to induce degeneration of cholinergic neurons in the basal forebrain [108], known to be affected early in AD pathogenesis and significantly contributing to the progressive hippocampus-dependent memory impairments [109]. Considering the highly consistent finding of aging-related Reelin plaque deposition in target areas of cholinergic neurons, such as the hippocampus, entorhinal and piriform cortices, we reasoned that these plaques could impair the integrity of axonal terminals, potentially resulting in or contributing to the degeneration of basal forebrain projection neurons. We found that the age-related neuropathological changes in the target areas of these neurons were indeed accompanied by abnormal axonal varicosities and altered expression profiles of calcium-binding proteins in plaque dense areas. Moreover, we reported a significant reduction in the number of parvalbumin-positive GABAergic as well as choline acetyltransferase-positive cholinergic projection neurons in several basal forebrain areas. No Reelin deposits were found in these regions, suggesting that the loss of projection neurons was not due to adverse effects of local protein deposition and plaque formation. Altogether, our findings suggest that the elevated Reelin plaque load in the projection areas of afferent subcortical GABAergic and cholinergic neurons affects the axonal integrity and survival of these neurons, potentially also contributing to the cognitive impairments observed in aged wild-type mice.
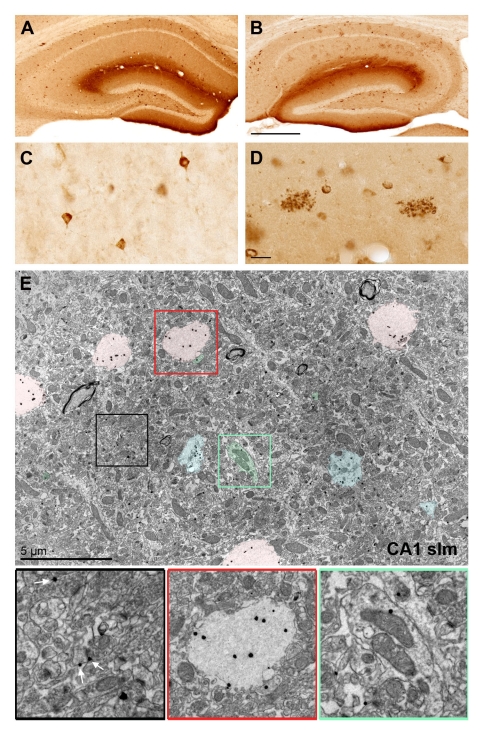
Figure 3:
Reelin accumulates in extracellular deposits in aged individuals. A-B) Anti-Reelin (G10 antibody) immunoreactivity (IR) in the hippocampus of 3 months (A) and 12 months old wild-type mice (B). Note the decrease in intraneuronal IR and concomitant accumulation of Reelin in extracellular deposits. C) Higher magnification images of Reelin-expressing cortical interneurons. D) Enlarged view of two extracellular Reelin-positive deposits in CA1 stratum lacunosum-moleculare. Note their close association with Reelin-positive neurons. E) Immuno-electron microscopy using G10 primary anti-Reelin and fluoronanogold-conjugated secondary antibodies. Representative image taken from area CA1 stratum lacunosum-moleculare (wild-type mouse, 12 months of age) show Reelin-IR as distinct black dots. They are detectable throughout the neuropil; either extrasynaptically at glutamatergic synapses (black box, white arrows) or within fibrillary deposits (pink overlays; enlarged in red box). The green coloring and box show the intracellular localization of Reelin in dendrites and spines. Blue coloring indicates putative intermediate stages of Reelin deposition. Scale bars: B = 500 μm, D = 20 μm, E = 5 μm.
Our findings so far provided important information regarding putative temporal processes underlying the transition from normal to pathological aging. (1) We found that the reduced numerical density of Reelin-expressing interneurons as well as the survival rate of basal forebrain GABAergic neurons appears to be a consistent feature of normal aging. (2) The observation of axonal varicosities selectively in the vicinity of Reelin plaques indicates that the degeneration of GABA- and cholinergic projection neurons is potentially a consequence of the abnormal protein deposition in their target areas. (3) The immunohistochemical findings suggested the presence of several proteins within or associated with Reelin-positive deposits [39].
In order to identify the putative neurotoxic factors within these extracellular Reelin aggregates, we recently initiated a biochemical and proteomic approach to investigate their composition as well as temporal and spatial progression. We developed a new immunohistochemical protocol involving a stringent protease pretreatment to enhance Reelin-immunoreactivity. This procedure allowed a significant increase in Reelin-immunoreactivity within protease-resistant plaques and a parallel reduction in the putative soluble pool of Reelin proteins. Unexpectedly, it also allowed the specific detection of several murine proteolytic APP fragments within the Reelin plaques in the aged hippocampus (Figure 4, [105]). The same treatment in APP knockout mice did not reveal any comparable staining pattern, confirming the specificity of the immunoreactivity. Moreover, our investigations using this adapted immunohistochemical protocol allowed the detection of fibrillary Aβ deposits as seen in human, indicating that similar aging-related pathophysiological changes occur in aged rodents. Ultrastructural investigations confirmed the presence of Reelin in extracellular space, somata of interneurons in young and aged wild-type mice. In aged mice, Reelin- and amyloid-β-immunoreactivity was detected in extracellular, spherical deposits; potentially representing small intermediates or fragments of amyloid fibrils (Figure 3E), confirming our immunohistochemical data and pointing to the usefulness of non-transgenic animals to investigate early molecular mechanisms that underlie the shift from normal to pathological forms of aging. These results confirmed that Reelin itself aggregates into abnormal oligomeric or protofibrillary deposits during aging, potentially creating a precursor condition for senile amyloid-β plaque formation in sporadic AD.
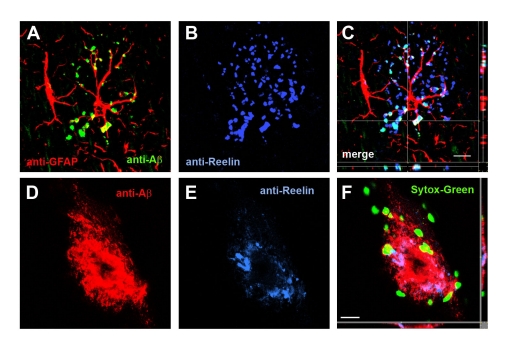
Figure 4:
Reelin-positive plaques are associated with astrocytes as well as proteolytic APP fragments. A–C) Representative confocal images of hippocampal brain sections processed for triple-immunofluorescence stainings using Cy3-GFAP (red), anti-Aβ (1–40/42, green) and anti-Reelin (G10, blue) antibodies. Note the partial overlap between Reelin- and amyloid-β deposits within putative oligomeric, glial-associated plaques. D-F) High magnification image of a fibrillary amyloid-β plaque in the hippocampus of a transgenic AD mouse (9 months old). Only a moderate overlap between anti-Aβ (1–40/42, green) and anti-Reelin (G10, blue) immunoreactivity is observed. Sections have been counterstained with Sytox Green™. Scale bars: C = 10 μm, F = 15 μm. Images A–C are part of a supplementary figure currently in press with J Neuroscience (Kocherhans et al., 2010).
To directly test the role of Reelin in AD pathophysiology, we crossed heterozygous reeler mice into a transgenic AD background [110] to investigate the effect of reduced Reelin-mediated signaling on amyloid-β plaque and neurofibrillary tangle formation, as well as neurodegenerative processes. We provided first biochemical evidence of enhanced amyloidogenic APP processing and accelerated amyloid-β plaque formation in transgenic AD mice with genetically reduced Reelin levels [111], complementing recent in vitro data [7, 19]. Furthermore, we observed that the amyloid-β plaque pathology strongly aggravated in Reelin-deficient AD mice during aging. This was accompanied by significant micro- and astrogliosis and a striking concentric accumulation of phospho-Tau-positive neurons and neurofibrillary tangles (NFTs) around amyloid-β plaques in the aged hippocampal formation. Futhermore, this was associated with a significant ventricular enlargement and cortical shrinkage, again predominantly affecting the entorhinal cortex and hippocampus. Our findings thereby provide the first in vivo support that dysfunctional Reelin-mediated signaling is a critical upstream modulator of amyloidogenic APP processing and Tau hyperphosphorylation, both likely contributing to progressive neurodegeneration observed in Reelin-deficient AD mice. Altogether, these observations add to our understanding of the putative molecular mechanisms that underlie the pathogenesis of AD. Reduced Reelin-dependent signaling during aging appears as crucial driving force able to shift APP processing from non-amyloidogenic to amyloidogenic forms in vivo.
Perspectives and conclusions
Studies performed by us and many other labs have provided exciting new insights into the function of Reelin-mediated signaling in synaptic function in the adult and aging brain. However, the molecular mechanisms how this developmental signaling pathway regulates directly or indirectly AD-associated pathophysiological processes remain to be fully elucidated. Moreover, many of the studies including ours have employed transgenic AD mice that model the genetic, early-onset form of the disease. However, the majority of patients suffer from the age-associated, late-onset form of AD. The question, therefore, is: can the investigations of dysfunctional Reelin-mediated signaling in normal aged wild-type mice provide us some insights into the molecular mechanisms of late-onset AD? In other words, is an impaired Reelin-mediated signaling sufficient to induce AD-like neuropathological changes in aged wild-type rodents that would allow us to study putative early disease-relevant pathophysiological alterations in a more physiological context?
Putative problems related to the work with aged wild-type rodents include both financial (costs for extended animal housing) and technical challenges (i.e. electrophysiological recordings and other investigations using in vitro preparations). Another critical disadvantage is the relative resistance of rodents to develop significant amyloidosis, despite the high sequence homology of the amyloid-β peptides [112, 113]. Our recent experimental approach might provide a potential solution to these issues:
We have recently provided the first evidence that a prenatal immune challenge during late gestation results in significant acceleration of aging-associated neuropathological alterations in non-transgenic wild-type mice. This involved reduced Reelin expression levels, precocious accumulation of Reelin-positive deposits, as well as long-term alterations in inflammatory modulators [39], suggesting inflammatory cytokines as putative regulators of Reelin expression, proteolytic processing, and/or signaling.
The application of our adapted immunohistochemical protocol involving protease-pretreatment allowed for the first time the detection of murine proteolytic APP fragments in extracellular amyloid-like plaques [105], indicating that the experimental conditions contribute more strongly to the observed lack in amyloid deposition in aged rodents than anticipated.
Therefore, the combination of these two experimental paradigms is expected to provide a potent way to address aging-associated neuropathology in non-transgenic mice or rats. In the last chapter of this review we will discuss in more detail the interaction between prenatal inflammation, its impact on development processes both during neurodevelopment and adult brain function and how this could underlie the progression from normal to accelerated and pathological aging.
The role of inflammation in Alzheimer’s disease
Chronic activation of inflammatory pathways can be detrimental and promote degeneration, as suggested by the accumulation of activated microglia and astrocytes in the vicinity of senile amyloid-β plaques and neuronal lesions (for recent review, [114]). It has become apparent that the inflammatory mediators, including cytokines, chemokines, prostaglandins, and complement activation components are not only produced in the circulating leukocytes and peripheral immune organs, but are also generated locally in the brain; most of them by the activated glial cells, but many of them also by neurons [114]. Beside the activation of these modulators, postmortem studies have also shown that the initial pathological stages in the hippocampus and entorhinal cortex in AD include a pronounced up-regulation of cell cycle and cell adhesions proteins [115], hypothesized to reflect an initial regenerative response of neurons to the presence of diffuse Aβ plaques and oligomeric or protofibrillic forms of Aβ peptides [116, 117]. Later stages are characterized by the widespread deposition of amyloid-β plaques and neurofibrillary tangles with their focal complement activation and accumulation of activated glial cells. Under these chronic insulting conditions, the efficiency of the innate immune system appears to fluctuate and it has been suggested that an unwanted potentiation of pro-inflammatory responses could turn the beneficial innate immunity into a negative driving force in AD pathogenesis by inducing autotoxicity [117–119]. On the other hand, a recent study revealed that the formation and maintenance of amyloid-β plaques in transgenic AD mouse models was independent from the presence of microglia [120], challenging our view of the role of inflammatory mediators derived from activated microglia cells. Clearly, more information is needed to understand the role of activated glia cells and to support an adverse, and potentially early-misregulated innate immunity defense in AD pathogenesis.
Prenatal brain inflammation and its impact on Reelin-mediated signaling
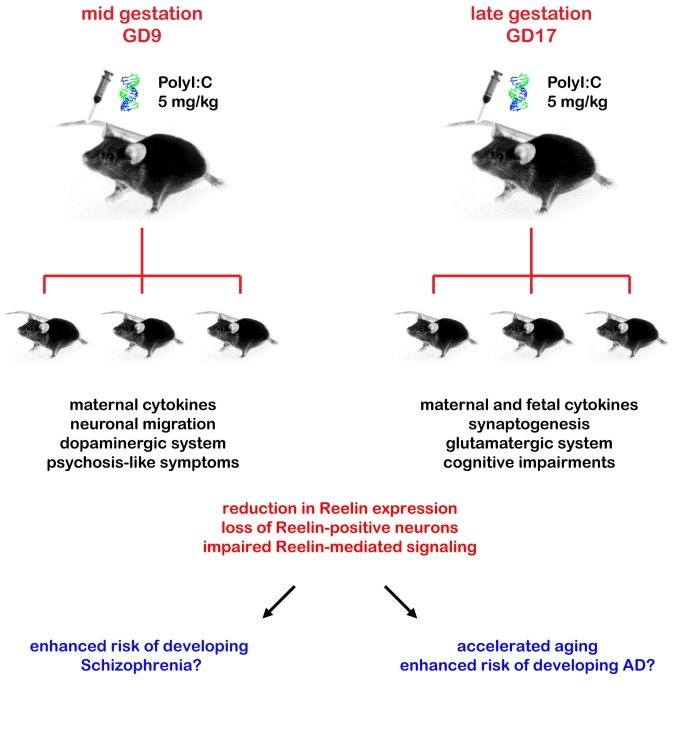
In addition to regulating inflammatory responses induced by neurodegenerative processes, cytokines released from both astrocytes and microglia have also critical effects on synaptogenesis and contribute to the targeting of synapses for elimination, thereby representing important modulators of neuronal activity, differentiation, and survival during neurodevelopment, as well as synaptic strength and plasticity in adulthood (for recent review, [121]). These cytokine-mediated developmental processes are highly susceptible to disruption by immune dysregulation. Overexposure to cytokines, for example, during early brain development in mice has been shown to strongly affect cell migration, neuronal differentiation, and synaptogenesis, leading to multiple morphological and neurochemical defects which result in disturbances of homeostatic neuronal network activity and subsequent behaviour and cognitive functions in the adult offspring [121]. In line with these data obtained from animal studies, elevation of inflammatory cytokines in the maternal serum has been associated with an increased risk factor for neuropsychiatric disorders in the offspring [122, 123]. Our own recent findings provide first support for a comparable relationship for neurodegenerative diseases like AD. We have demonstrated that systemic administration of the synthetic cytokine releaser and viral mimic polyriboinosinic-polyribocytidilic acid (PolyI:C) in mice provides convincing support to the link between maternal infection during pregnancy and behavioral and neurochemical abnormalities in the adult offspring [124–126]. We have shown that the middle and late gestational periods correspond to two windows of enhanced vulnerability to inflammatory cytokines, leading to an acute response in the fetal brain and delayed brain neuropathology, including defective Reelin expression, apoptosis in early adolescence [127], as well as pronounced changes in the GABAergic, glutamatergic, and dopaminergic system in limbic structures in the adult brain (Figure 5, [128–130]). Moreover, we demonstrated for the first time that a viral–like infection in wild-type mice significantly accelerates the reduction in Reelin-expressing interneurons and its accumulation in extracellular amyloid-like plaques, a phenomenon consistently seen in aged subjects across species and correlating with the cognitive performance [39]. These findings suggest that prenatal exposure to infection is not only an important factor in the segregation of symptom clusters in neuropsychiatric disorders but does also represent a critical driving force of aging-related neurodegenerative processes. Moreover, the acceleration of senescence after early brain inflammation confirms the critical role of non-genetic components as major risk factors of late-onset Alzheimer’s disease.
Figure 5.
Animal models of neuropsychiatric and neurodegenerative diseases. The time of maternal immune challenge critically determines the patterns of fetal immunological, juvenile neurochemical, as well as adult behavioural abnormalities displayed by the offspring. An acute systemic challenge of the viral mimic polyriboinosinic-polyribocytodilic acid (PolyI:C), a synthetic analog of double-stranded RNA and cytokine releaser, during mid-gestation (gestational day, GD9) results in a behavioural profile that mimics certain aspects of psychotic symptoms in the adult offspring, potentially linked to the pronounced impact of this treatment on the dopaminergic system. Late-gestational (GD17) immune challenge results in a behavioural phenotype characterized primarily by cognitive impairments, accompanied by a strong imbalance in the glutamatergic neurotransmitter system. The fetal brain can directly contribute to the specific changes in inflammatory cytokine protein levels after late-gestational but not mid-gestational immune challenge, suggesting a close correlation between acute immunological modulators and histopathological changes in the adult offspring. Although both mid- and late gestational PolyI:C treatments are equivalently effective in reducing the number of Reelin-positive neurons, the time of the maternal immune challenge is expected to determine whether the reduction in Reelin-mediated signaling primarily affects proper neuronal migration or impairs synaptogenesis. This might further contribute to the distinct symptom clusters characteristic of the adult offspring born to mid- or late-gestational immune challenged dams.
Conclusions
Accumulating evidence indicates that the same Reelin-mediated signaling pathway required for proper early neurodevelopmental processes is equally relevant for the regulation of adult neuronal function and plasticity. Both a decline and overproduction of Reelin in adulthood is associated with abnormal synaptic function, indicating an important homeostatic regulation of this extracellular glycoprotein (Figure 6). Our investigations showed for the first time that aging across species is accompanied by a reduction in Reelin expression, accompanied by a highly selective layer-specific accumulation of Reelin-positive amyloid-like plaques. Comprehensive ultrastructural and immunohistochemical characterizations indicate that Reelin, likely by self-aggregating into abnormal oligomeric or protofibrillary deposits during aging, is able to induce degenerative axonal varicosities in afferent basal forebrain projection neurons that selectively terminate in plaque dense areas within the hippocampal formation. It is likely that other aggregation-prone proteins that accumulate and associate with these deposits further contribute to degenerative processes. Moreover, our recent findings support the hypothesis that both a genetic or inflammation-induced reduction in Reelin-dependent signaling in the adult brain represents an important upstream factor able to trigger AD-like pathophysiological processes including amyloidogenic APP processing and hyperphosphorylation of Tau, both of which are associated with a strong inflammatory reaction, that potentially contributes to progressive neurodegeneration in aged individuals. Based on these data we propose that dysfunction of the Reelin-mediated signaling represents an important driving force able to induce a shift from normal to pathological aging.
Figure 6.
Hypothetical model how dysfunctional Reelin-mediated signaling may induce pathological aging. In the course of normal aging, Reelin accumulates in the extracellular matrix either as monomers, dimers, or oligomers and/or cleaved fragments, which are efficiently cleared by activated astrocytes and microglia [39, 108]. Long-term imbalances in immune modulators including elevated levels of pro-inflammatory cytokines in old subjects increase the expression of metalloproteinases, resulting in aberrant cleavage of extracellular Reelin and production of an excess of Reelin fragments. While N-terminal fragments might preferentially aggregate in concert with proteolytic APP fragments [105], the central fragments are expected to compete with full-length Reelin homodimers for the available receptors to inhibit Reelin downstream signaling. Dysfunctional Reelin signaling is expected to promote not only amyloidogenic APP processing [7, 19, 111] including the production of N-APP, and Tau hyperphosphorylation [16, 24, 25, 137], it likely also impairs synaptic plasticity [28, 45, 52] via the strong reduction of several Reelin-mediated downstream signaling cascades required for proper neuronal functioning. NFT = Neurofibrillary tangles. Thin arrows represent downregulation; thick arrows indicate an up-regulation compared to the normal signaling pathways described during development and adult synaptic functions.
Acknowledgments
This work was supported by Swiss National Science Foundation (SNF) Grant Nr. 310000-117806 and the Swiss National Competence Centre in Neural Plasticity and Repair (NCCR). We are very grateful to Prof. Dr. Jean-Marc Fritschy for support, mentoring and critical reading of the manuscript.
References
- [1].Kohno S, Kohno T, Nakano Y, Suzuki K, Ishii M, Tagami H, Baba A, Hattori M. Mechanism and significance of specific proteolytic cleavage of Reelin. Biochem Biophys Res Commun. 2009;380:93–7. doi: 10.1016/j.bbrc.2009.01.039. [DOI] [PubMed] [Google Scholar]
- [2].Lambert de Rouvroit C, de Bergeyck V, Cortvrindt C, Bar I, Eeckhout Y, Goffinet AM. Reelin, the extracellular matrix protein deficient in reeler mutant mice, is processed by a metalloproteinase. Exp Neurol. 1999;156:214–7. doi: 10.1006/exnr.1998.7007. [DOI] [PubMed] [Google Scholar]
- [3].Kubo K, Mikoshiba K, Nakajima K. Secreted Reelin molecules form homodimers. Neurosci Res. 2002;43:381–8. doi: 10.1016/s0168-0102(02)00068-8. [DOI] [PubMed] [Google Scholar]
- [4].Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci U S A. 2009;106:7227–32. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jossin Y, Gui L, Goffinet AM. Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J Neurosci. 2007;27:4243–52. doi: 10.1523/JNEUROSCI.0023-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci. 2004;24:514–21. doi: 10.1523/JNEUROSCI.3408-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hoe HS, Lee KJ, Carney RS, Lee J, Markova A, Lee JY, Howell BW, Hyman BT, Pak DT, Bu G, Rebeck GW. Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J Neurosci. 2009;29:7459–73. doi: 10.1523/JNEUROSCI.4872-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Bergeyck V, Nakajima K, Lambert de Rouvroit C, Naerhuyzen B, Goffinet AM, Miyata T, Ogawa M, Mikoshiba K. A truncated Reelin protein is produced but not secreted in the ‘Orleans’ reeler mutation (Reln[rl-Orl]) Brain Res Mol Brain Res. 1997;50:85–90. doi: 10.1016/s0169-328x(97)00166-6. [DOI] [PubMed] [Google Scholar]
- [9].Nakano Y, Kohno T, Hibi T, Kohno S, Baba A, Mikoshiba K, Nakajima K, Hattori M. The extremely conserved C-terminal region of Reelin is not necessary for secretion but is required for efficient activation of downstream signaling. J Biol Chem. 2007;282:20544–52. doi: 10.1074/jbc.M702300200. [DOI] [PubMed] [Google Scholar]
- [10].Förster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S. Emerging topics in Reelin function. Eur J Neurosci. 2010:1–8. doi: 10.1111/j.1460-9568.2010.07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Knuesel I. Reelin-mediated signaling in neuropsychiatric and neurodegenerative diseases. Prog Neurobiol. 2010;91(4):257–74. doi: 10.1016/j.pneurobio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- [12].Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–99. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–9. doi: 10.1016/j.tins.2007.12.003. [DOI] [PubMed] [Google Scholar]
- [14].D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–23. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- [15].Hirotsune S, Takahara T, Sasaki N, Hirose K, Yoshiki A, Ohashi T, Kusakabe M, Murakami Y, Muramatsu M, Watanabe S, et al. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nat Genet. 1995;10:77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- [16].Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–9. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- [17].Howell BW, Lanier LM, Frank R, Gertler FB, Cooper JA. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999;19:5179–88. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- [19].Hoe HS, Tran TS, Matsuoka Y, Howell BW, Rebeck GW. DAB1 and Reelin effects on amyloid precursor protein and ApoE receptor 2 trafficking and processing. J Biol Chem. 2006;281:35176–85. doi: 10.1074/jbc.M602162200. [DOI] [PubMed] [Google Scholar]
- [20].Homayouni R, Rice DS, Sheldon M, Curran T. Disabled-1 binds to the cytoplasmic domain of amyloid precursor-like protein 1. J Neurosci. 1999;19:7507–15. doi: 10.1523/JNEUROSCI.19-17-07507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, Howell B, Schneider WJ, Nimpf J. Receptor clustering is involved in Reelin signaling. Mol Cell Biol. 2004;24:1378–86. doi: 10.1128/MCB.24.3.1378-1386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–60. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- [23].Bock HH, Jossin Y, Liu P, Forster E, May P, Goffinet AM, Herz J. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem. 2003;278:38772–9. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- [24].Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J Biol Chem. 2002;277:49958–64. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- [25].Beffert U, Weeber EJ, Morfini G, Ko J, Brady ST, Tsai LH, Sweatt JD, Herz J. Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission. J Neurosci. 2004;24:1897–906. doi: 10.1523/JNEUROSCI.4084-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chai X, Forster E, Zhao S, Bock HH, Frotscher M. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J Neurosci. 2009;29:288–99. doi: 10.1523/JNEUROSCI.2934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goffinet AM. Events governing organization of postmigratory neurons: studies on brain development in normal and reeler mice. Brain Res. 1984;319:261–96. doi: 10.1016/0165-0173(84)90013-4. [DOI] [PubMed] [Google Scholar]
- [28].Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–52. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- [29].Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, Frotscher M. Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development. 2007;134:3883–91. doi: 10.1242/dev.005447. [DOI] [PubMed] [Google Scholar]
- [30].Duit S, Mayer H, Blake SM, Schneider WJ, Nimpf J. Differential functions of ApoER2 and very low density lipoprotein receptor in Reelin signaling depend on differential sorting of the receptors. J Biol Chem. 2010;285:4896–908. doi: 10.1074/jbc.M109.025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arnaud L, Ballif BA. Cooper JA. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol Cell Biol. 2003;23:9293–302. doi: 10.1128/MCB.23.24.9293-9302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bock HH, Jossin Y, May P, Bergner O, Herz J. Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J Biol Chem. 2004;279:33471–9. doi: 10.1074/jbc.M401770200. [DOI] [PubMed] [Google Scholar]
- [33].Morimura T, Hattori M, Ogawa M, Mikoshiba K. Disabled1 regulates the intracellular trafficking of reelin receptors. J Biol Chem. 2005;280:16901–8. doi: 10.1074/jbc.M409048200. [DOI] [PubMed] [Google Scholar]
- [34].Frotscher M. Cajal-Retzius cells, Reelin, and the formation of layers. Curr Opin Neurobiol. 1998;8:570–5. doi: 10.1016/s0959-4388(98)80082-2. [DOI] [PubMed] [Google Scholar]
- [35].Abraham H, Meyer G. Reelin-expressing neurons in the postnatal and adult human hippocampal formation. Hippocampus. 2003;13:715–27. doi: 10.1002/hipo.10125. [DOI] [PubMed] [Google Scholar]
- [36].Abraham H, Perez-Garcia CG, Meyer G. p73 and Reelin in Cajal-Retzius cells of the developing human hippocampal formation. Cereb Cortex. 2004;14:484–95. doi: 10.1093/cercor/bhh010. [DOI] [PubMed] [Google Scholar]
- [37].Baloyannis SJ. Morphological and morphometric alterations of Cajal-Retzius cells in early cases of Alzheimer’s disease: a Golgi and electron microscope study. Int J Neurosci. 2005;15:965–80. doi: 10.1080/00207450590901396. [DOI] [PubMed] [Google Scholar]
- [38].Chin J, Massaro CM, Palop JJ, Thwin MT, Yu GQ, Bien-Ly N, Bender A, Mucke L. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci. 2007;27:2727–33. doi: 10.1523/JNEUROSCI.3758-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Knuesel I, Nyffeler M, Mormede C, Muhia M, Meyer U, Pietropaolo S, Yee BK, Pryce CR, LaFerla FM, Marighetto A, Feldon J. Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol Aging. 2009;30:697–716. doi: 10.1016/j.neurobiolaging.2007.08.011. [DOI] [PubMed] [Google Scholar]
- [40].Lacor PN, Grayson DR, Auta J, Sugaya I, Costa E, Guidotti A. Reelin secretion from glutamatergic neurons in culture is independent from neurotransmitter regulation. Proc Natl Acad Sci U S A. 2000;97:3556–61. doi: 10.1073/pnas.050589597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Miettinen R, Riedel A, Kalesnykas G, Kettunen HP, Puolivali J, Soininen H, Arendt T. Reelin-immunoreactivity in the hippocampal formation of 9-month-old wildtype mouse: effects of APP/PS1 genotype and ovariectomy. J Chem Neuroanat. 2005;30:105–18. doi: 10.1016/j.jchemneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [42].Pappas GD, Kriho V, Liu WS, Tremolizzo L, Lugli G, Larson J. Immunocytochemical localization of reelin in the olfactory bulb of the heterozygous reeler mouse: an animal model for schizophrenia. Neurol Res. 2003;25:819–30. doi: 10.1179/016164103771953916. [DOI] [PubMed] [Google Scholar]
- [43].Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci U S A. 1998;95:3221–6. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ramos-Moreno T, Galazo MJ, Porrero C, Martinez-Cerdeno V, Clasca F. Extracellular matrix molecules and synaptic plasticity: immunomapping of intracellular and secreted Reelin in the adult rat brain. Eur J Neurosci. 2006;23:401–22. doi: 10.1111/j.1460-9568.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- [45].Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–79. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- [46].Forster E, Tielsch A, Saum B, Weiss KH, Johanssen C, Graus-Porta D, Muller U, Frotscher M. Reelin, Disabled 1, and beta 1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:13178–83. doi: 10.1073/pnas.202035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Frotscher M, Haas CA, Forster E. Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold. Cereb Cortex. 2003;13:634–40. doi: 10.1093/cercor/13.6.634. [DOI] [PubMed] [Google Scholar]
- [48].Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–16. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–9. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- [51].Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–42. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- [52].Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci. 2006;26:12943–55. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Malenka RC. The long-term potential of LTP. Nat Rev Neurosci. 2003;4:923–6. doi: 10.1038/nrn1258. [DOI] [PubMed] [Google Scholar]
- [54].Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–24. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- [55].Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, Herz J, Schneider WJ, Nimpf J. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J Biol Chem. 2000;275:25625–32. doi: 10.1074/jbc.M004119200. [DOI] [PubMed] [Google Scholar]
- [56].Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol Cell Biol. 2007;27:7113–24. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Matsuki T, Pramatarova A, Howell BW. Reduction of Crk and CrkL expression blocks reelin-induced dendritogenesis. J Cell Sci. 2008;121:1869–75. doi: 10.1242/jcs.027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- [59].Niu S, Yabut O, D’Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci. 2008;28:10339–48. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stanfield BB, Cowan WM. The morphology of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979;185:393–422. doi: 10.1002/cne.901850302. [DOI] [PubMed] [Google Scholar]
- [61].Stanfield BB, Cowan WM. The development of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979;185:423–59. doi: 10.1002/cne.901850303. [DOI] [PubMed] [Google Scholar]
- [62].Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, Larson J, Condie BG, Guidotti A, Costa E. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci U S A. 2001;98:3477–82. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pappas GD, Kriho V, Pesold C. Reelin in the extracellular matrix and dendritic spines of the cortex and hippocampus: a comparison between wild type and heterozygous reeler mice by immunoelectron microscopy. J Neurocytol. 2001;30:413–25. doi: 10.1023/a:1015017710332. [DOI] [PubMed] [Google Scholar]
- [64].Tueting P, Costa E, Dwivedi Y, Guidotti A, Impagnatiello F, Manev R, Pesold C. The phenotypic characteristics of heterozygous reeler mouse. Neuroreport. 1999;10:1329–34. doi: 10.1097/00001756-199904260-00032. [DOI] [PubMed] [Google Scholar]
- [65].Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, de Lecea L, Martinez A, Delgado-Garcia JM, Soriano E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci. 2010;30:4636–49. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Meyer G, Goffinet AM. Prenatal development of reelin-immunoreactive neurons in the human neocortex. J Comp Neurol. 1998;397:29–40. [PubMed] [Google Scholar]
- [67].Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- [68].Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26:93–6. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- [69].Belichenko PV, Vogt Weisenhorn DM, Myklossy J, Celio MR. Calretinin-positive Cajal-Retzius cells persist in the adult human neocortex. Neuroreport. 1995;6:1869–74. doi: 10.1097/00001756-199510020-00012. [DOI] [PubMed] [Google Scholar]
- [70].Martin R, Gutierrez A, Penafiel A, Marin-Padilla M, de la Calle A. Persistence of Cajal-Retzius cells in the adult human cerebral cortex. An immunohistochemical study. Histol Histopathol. 1999;14:487–90. doi: 10.14670/HH-14.487. [DOI] [PubMed] [Google Scholar]
- [71].Mikkonen M, Soininen H, Pitkanen A. Distribution of parvalbumin-, calretinin-, and calbindin-D28k-immunoreactive neurons and fibers in the human entorhinal cortex. J Comp Neurol. 1997;388:64–88. [PubMed] [Google Scholar]
- [72].Riedel A, Miettinen R, Stieler J, Mikkonen M, Alafuzoff I, Soininen H, Arendt T. Reelin-immunoreactive Cajal-Retzius cells: the entorhinal cortex in normal aging and Alzheimer’s disease. Acta Neuropathol. 2003;106:291–302. doi: 10.1007/s00401-003-0729-7. [DOI] [PubMed] [Google Scholar]
- [73].Roberts RC, Xu L, Roche JK, Kirkpatrick B. Ultrastructural localization of reelin in the cortex in post-mortem human brain. J Comp Neurol. 2005;482:294–308. doi: 10.1002/cne.20408. [DOI] [PubMed] [Google Scholar]
- [74].Martinez-Cerdeno V, Galazo MJ, Clasca F. Reelin-immunoreactive neurons, axons, and neuropil in the adult ferret brain: evidence for axonal secretion of reelin in long axonal pathways. J Comp Neurol. 2003;463:92–116. doi: 10.1002/cne.10748. [DOI] [PubMed] [Google Scholar]
- [75].Martinez-Cerdeno V, Galazo MJ, Cavada C, Clasca F. Reelin immunoreactivity in the adult primate brain: intracellular localization in projecting and local circuit neurons of the cerebral cortex, hippocampus and subcortical regions. Cereb Cortex. 2002;12:1298–311. doi: 10.1093/cercor/12.12.1298. [DOI] [PubMed] [Google Scholar]
- [76].Deguchi K, Inoue K, Avila WE, Lopez-Terrada D, Antalffy BA, Quattrocchi CC, Sheldon M, Mikoshiba K, D’Arcangelo G, Armstrong DL. Reelin and disabled-1 expression in developing and mature human cortical neurons. J Neuropathol Exp Neurol. 2003;62:676–84. doi: 10.1093/jnen/62.6.676. [DOI] [PubMed] [Google Scholar]
- [77].Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–9. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- [78].Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–23. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kramer PL, Xu H, Woltjer RL, Westaway SK, Clark D, Erten-Lyons D, Kaye JA, Welsh-Bohmer KA, Troncoso JC, Markesbery WR, Petersen RC, Turner RS, Kukull WA, Bennett DA, Galasko D, Morris JC, Ott J. Alzheimer disease pathology in cognitively healthy elderly: A genome-wide study. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Seripa D, Matera MG, Franceschi M, Daniele A, Bizzarro A, Rinaldi M, Panza F, Fazio VM, Gravina C, D’Onofrio G, Solfrizzi V, Masullo C, Pilotto A. The RELN locus in Alzheimer’s disease. J Alzheimers Dis. 2008;14:335–44. doi: 10.3233/jad-2008-14308. [DOI] [PubMed] [Google Scholar]
- [81].Savla GN, Palmer BW. Neuropsychology in Alzheimer’s disease and other dementia research. Curr Opin Psychiatry. 2005;18:621–7. doi: 10.1097/01.yco.0000184413.36706.8e. [DOI] [PubMed] [Google Scholar]
- [82].Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- [84].Estus S, Golde TE, Kunishita T, Blades D, Lowery D, Eisen M, Usiak M, Qu XM, Tabira T, Greenberg BD, et al. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992;255:726–8. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- [85].Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357:500–3. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- [86].Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem. 1996;67:1882–96. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- [87].Gao Y, Pimplikar SW. The gamma - secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc Natl Acad Sci U S A. 2001;98:14979–84. doi: 10.1073/pnas.261463298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].von Rotz RC, Kohli BM, Bosset J, Meier M, Suzuki T, Nitsch RM, Konietzko U. The APP intracellular domain forms nuclear multiprotein complexes and regulates the transcription of its own precursor. J Cell Sci. 2004;117:4435–48. doi: 10.1242/jcs.01323. [DOI] [PubMed] [Google Scholar]
- [89].Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–9. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [90].Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- [93].Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–43. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- [95].Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–8. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- [96].Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–75. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–92. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- [100].Durakoglugil MS, Chen Y, White CL, Kavalali ET, Herz J. Reelin signaling antagonizes beta-amyloid at the synapse. Proc Natl Acad Sci U S A. 2009;106:15938–43. doi: 10.1073/pnas.0908176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Botella-Lopez A, Burgaya F, Gavin R, Garcia-Ayllon MS, Gomez-Tortosa E, Pena-Casanova J, Urena JM, Del Rio JA, Blesa R, Soriano E, Saez-Valero J. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5573–8. doi: 10.1073/pnas.0601279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Botella-Lopez A, Cuchillo-Ibanez I, Cotrufo T, Mok SS, Li QX, Barquero MS, Dierssen M, Soriano E, Saez-Valero J. Beta-amyloid controls altered Reelin expression and processing in Alzheimer’s disease. Neurobiol Dis. 2010;37:682–91. doi: 10.1016/j.nbd.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [103].Saez-Valero J, Costell M, Sjogren M, Andreasen N, Blennow K, Luque JM. Altered levels of cerebrospinal fluid reelin in frontotemporal dementia and Alzheimer’s disease. J Neurosci Res. 2003;72:132–6. doi: 10.1002/jnr.10554. [DOI] [PubMed] [Google Scholar]
- [104].Hibi T, Hattori M. The N-terminal fragment of Reelin is generated after endocytosis and released through the pathway regulated by Rab11. FEBS Lett. 2009;583:1299–303. doi: 10.1016/j.febslet.2009.03.024. [DOI] [PubMed] [Google Scholar]
- [105].Doehner J, Madhusudan A, Konietzko U, Fritschy JM, Knuesel I. Co-localization of Reelin and proteolytic AbetaPP fragments in hippocampal plaques in aged wild-type mice. J Alzheimers Dis. 2010;19:1339–57. doi: 10.3233/JAD-2010-1333. [DOI] [PubMed] [Google Scholar]
- [106].Motoi Y, Itaya M, Mori H, Mizuno Y, Iwasaki T, Hattori H, Haga S, Ikeda K. Apolipoprotein E receptor 2 is involved in neuritic plaque formation in APP sw mice. Neurosci Lett. 2004;368:144–7. doi: 10.1016/j.neulet.2004.06.081. [DOI] [PubMed] [Google Scholar]
- [107].Wirths O, Multhaup G, Czech C, Blanchard V, Tremp G, Pradier L, Beyreuther K, Bayer TA. Reelin in plaques of beta-amyloid precursor protein and presenilin-1 double-transgenic mice. Neurosci Lett. 2001;316:145–8. doi: 10.1016/s0304-3940(01)02399-0. [DOI] [PubMed] [Google Scholar]
- [108].Madhusudan A, Sidler C, Knuesel I. Accumulation of reelin-positive plaques is accompanied by a decline in basal forebrain projection neurons during normal aging. Eur J Neurosci. 2009;30:1064–76. doi: 10.1111/j.1460-9568.2009.06884.x. [DOI] [PubMed] [Google Scholar]
- [109].Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- [110].Knobloch M, Konietzko U, Krebs DC, Nitsch RM. Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol Aging. 2007;28:1297–306. doi: 10.1016/j.neurobiolaging.2006.06.019. [DOI] [PubMed] [Google Scholar]
- [111].Kocherhans S, Madhusudan A, Doehner J, Breu KS, Nitsch RM, Fritschy JM, Knuesel I. Reduced Reelin expression accelerates amyloid-β plaque formation and Tau pathology in transgenic AD mice. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.0418-10.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fung J, Frost D, Chakrabartty A, McLaurin J. Interaction of human and mouse Abeta peptides. J Neurochem. 2004;91:1398–403. doi: 10.1111/j.1471-4159.2004.02828.x. [DOI] [PubMed] [Google Scholar]
- [113].Hilbich C, Kisters-Woike B, Reed J, Masters CL, Beyreuther K. Human and rodent sequence analogs of Alzheimer’s amyloid beta A4 share similar properties and can be solubilized in buffers of pH 7.4. Eur J Biochem. 1991;201:61–9. doi: 10.1111/j.1432-1033.1991.tb16256.x. [DOI] [PubMed] [Google Scholar]
- [114].Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–22. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Parachikova A, Agadjanyan MG, Cribbs DH, Blurton-Jones M, Perreau V, Rogers J, Beach TG, Cotman CW. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol Aging. 2007;28:1821–33. doi: 10.1016/j.neurobiolaging.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. Int J Dev Neurosci. 2006;24:157–65. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- [117].Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–94. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- [118].McGeer PL, McGeer EG. Local neuroinflammation and the progression of Alzheimer’s disease. J Neurovirol. 2002;8:529–38. doi: 10.1080/13550280290100969. [DOI] [PubMed] [Google Scholar]
- [119].Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- [120].Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, Aguzzi A, Staufenbiel M, Mathews PM, Wolburg H, Heppner FL, Jucker M. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1361–3. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- [122].Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–21. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- [123].Penner JD, Brown AS. Prenatal infectious and nutritional factors and risk of adult schizophrenia. Expert Rev Neurother. 2007;7:797–805. doi: 10.1586/14737175.7.7.797. [DOI] [PubMed] [Google Scholar]
- [124].Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–47. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [125].Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–89. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- [127].Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–62. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008;33:441–56. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- [129].Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–86. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- [130].Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143:51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- [131].Park TJ, Curran T. Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway. J Neurosci. 2008;28:13551–62. doi: 10.1523/JNEUROSCI.4323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Suetsugu S, Tezuka T, Morimura T, Hattori M. Mikoshiba K, Yamamoto T, Takenawa T. Regulation of actin cytoskeleton by mDab1 through N-WASP and ubiquitination of mDab1. Biochem J. 2004;384:1–8. doi: 10.1042/BJ20041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Qiu S, Weeber EJ. Reelin signaling facilitates maturation of CA1 glutamatergic synapses. J Neurophysiol. 2007;97:2312–21. doi: 10.1152/jn.00869.2006. [DOI] [PubMed] [Google Scholar]
- [134].Borrell V, Del Rio JA, Alcantara S, Derer M, Martinez A, D’Arcangelo G, Nakajima K, Mikoshiba K, Derer P, Curran T, Soriano E. Reelin regulates the development and synaptogenesis of the layer-specific entorhino-hippocampal connections. J Neurosci. 1999;19:1345–58. doi: 10.1523/JNEUROSCI.19-04-01345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Borrell V, Pujadas L, Simo S, Dura D, Sole M, Cooper JA, Del Rio JA, Soriano E. Reelin and mDab1 regulate the development of hippocampal connections. Mol Cell Neurosci. 2007;36:158–73. doi: 10.1016/j.mcn.2007.06.006. [DOI] [PubMed] [Google Scholar]
- [136].Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature. 1997;385:70–4. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- [137].Ohkubo N, Lee YD, Morishima A, Terashima T, Kikkawa S, Tohyama M, Sakanaka M, Tanaka J, Maeda N, Vitek MP, Mitsuda N. Apolipoprotein E and Reelin ligands modulate tau phosphorylation through an apolipoprotein E receptor/disabled-1/glycogen synthase kinase-3beta cascade. FASEB. 2003;17:295–7. doi: 10.1096/fj.02-0434fje. [DOI] [PubMed] [Google Scholar]