Abstract
Harman’s Free Radical Theory of Aging has been considered as a major theory of aging for more than 50 years. In 1956 Dr. Harman proposed that the accumulation of free radicals with the age causes the damage of biomolecules by these reactive species and the development of pathological disorders resulting in cell senescence and organismal aging. His hypothesis was supported by numerous experimental studies demonstrated an increase in free radical levels in cells and living organisms with aging. In subsequent years important discoveries of new physiological free radicals superoxide and nitric oxide have been made that led to understanding of other important functions of free radicals. It has been shown that superoxide and nitric oxide together with their diamagnetic reaction products hydrogen peroxide and peroxynitrite (all are now named reactive oxygen and nitrogen species, ROS and RNS) function as signaling species in many physiological enzymatic/gene processes. Furthermore, the disturbance of ROS and RNS physiological signaling can be an origin of various pathologies and aging. These discoveries demanded to widen original free radical theory of aging and to consider the damaging ROS signaling as an important, maybe major route to cell senescence and organismal aging. However, some experimental findings such as the extension of lifespan by calorie restriction of yeast, flies, worms, and mice, and favorable effects of physical exercises stimulated criticism of free radical theory because the expansion of lifespan accompanied in some cases by increasing oxidative stress. On these grounds such theories as Hormesis and Target of rapamycin (mTOR) theories refute the role of ROS and oxidative stress in aging. Accordingly, a major purpose of this review to show that ROS signaling is probably the most important enzyme/gene pathway responsible for the development of cell senescence and organismal aging and that ROS signaling might be considered as further development of free radical theory of aging. In spite of apparent contradictions the Hormesis or TOR theories are also describing processes of aging development regulated by ROS signaling.
Keywords: ROS and RNS signaling, senescence, aging
In early chemical studies (not speaking about biology) free radicals i.e. paramagnetic species with an unpaired electron or in other words, the compounds containing a three-valent carbon atom were always considered the aggressive damaging species. Therefore, it was not surprising that biologists regarded free radicals as life damaging factors because the first free radicals identified in chemical works were reactive short-living alkyl radicals. Furthermore, in the early years biologists could not imagine free radicals to be the metabolites of normal physiological processes and believed that they were only formed due to the action of various environmental factors (contamination by toxic chemicals compounds, irradiation, etc.).
However, discovery of “physiological” radicals i.e. radicals formed in normal physiological processes, superoxide O2.− (McCord and Fridovich, 1968) and nitric oxide NO. (Furchgott, Ignarro, and Murad, 1986) completely changed our views on the role of free radical processes in biological systems. It has been found that these species are inactive free radical agents by themselves but can be precursors of really reactive hydroxyl and peroxy free radicals, which are able to damage biomolecules.
At present free radicals and some their reactive diamagnetic products of mutual interactions are frequently named reactive oxygen species or ROS (superoxide, hydroxyl, and peroxy radicals, and hydrogen peroxide) and reactive nitrogen species or RNS (nitric oxide and peroxynitrite). (Hydrogen peroxide H2O2 and peroxynitrite −OONO are formed in the reactions of physiological radicals superoxide and nitric oxide by following reactions):
Thus mostly harmless superoxide and nitric oxide are capable of producing damaging free radicals and reactive diamagnetic molecules which might be an origin of many pathological disorders and aging.
However, a more important activity of physiological ROS and RNS is their participation in many enzymatic and gene-catalyzed processes. It has been shown that these radicals are produced by mitochondria and such enzymes as xanthine oxidase, NADPH oxidase, and nitric oxide synthase and perform important signaling functions by activating or inhibiting many other enzymes (protein kinases, MAPK kinases, phosphatases, gene-depended cascades, etc.). Therefore, the maintenance of optimal ROS and RNS levels is a critical condition for normal functioning of physiological processes.
However under certain conditions the disturbance of regulation of ROS and RNS signaling might lead to the activation of dangerous enzymatic cascades and stimulation of numerous pathological states including cardiovascular diseases, hypertension, diabetes mellitus, cancer and carcinogenesis, inflammation, and aging. Thus toxic effects of ROS and RNS signaling can depend not only on direct attack of these species on biomolecules (although it might be true for reactive hydroxyl and peroxy free radicals) but to be a consequence of disruption of their physiological levels due to enhancement or reduction of ROS or RNC formation. Below I will consider the necessity of enlargement of free radical theory of aging through incorporation of the effects of ROS and RNS signaling in senescence and aging.
Free radical signaling is a new line of work in aging studies. An important role of free radicals in aging and senescence was proposed in 1956 Dr. Harman’s work “Aging: a theory based on free radical and radiation chemistry” [1] which opened a new era of free radical studies in biology and medicine. This hypothesis led to important practical conclusions pointing out at the possibility of aging regulation by antioxidants, capable of suppressing free radical formation. Now, a more than 50 years after Harman’s proposal free radical studies in aging gather probably a hundred or more works that being a good indicator of the success of his hypothesis. Until now many good reviews on free radical theory of aging have been published. Some of them are cited here [2–5].
One of major predictions of Hartman’s hypothesis was that free radical overproduction (oxidative stress) must shorten lifespan of living life forms characterized by high levels of free radicals and their reactive metabolites. However, at present there are some experimental findings which are not agreed with this early proposal (see below). But it should be stressed that previously all free radicals were considered the toxic species capable of destroying biomolecules and stimulating aging of living organisms. However we now know that ROS and RNS have important signaling functions under both physiological and pathological conditions and therefore the right route to fight aging is the regulation and not simple suppression of dangerous ROS signaling and overproduction in aging and other pathologies.
ROS overproduction in age and senescence
A lot of experimental studies demonstrate free radical and ROS overproduction in organismal aging and cellular senescence. There are some examples of earlier studies: Sawada and Carlson [6] and Sawada et al. [7] showed the enhancement of superoxide production and lipid peroxidation during lifetime of the rat. Similar data has been obtained by Schreiber et al. [8]. Chung et al. [9] found an increase in ROS formation by xanthine oxidase in aging. Hamilton et al. [10] demonstrated superoxide overproduction in hypertension and aging. Moon et al. [11] found free radical overproduction in aged mouse aortic smooth muscle cells. Chen et al. [12] showed the age-related increase in mitochondrial superoxide production in the testosterone-producing cells of Brown Norway rat testes.
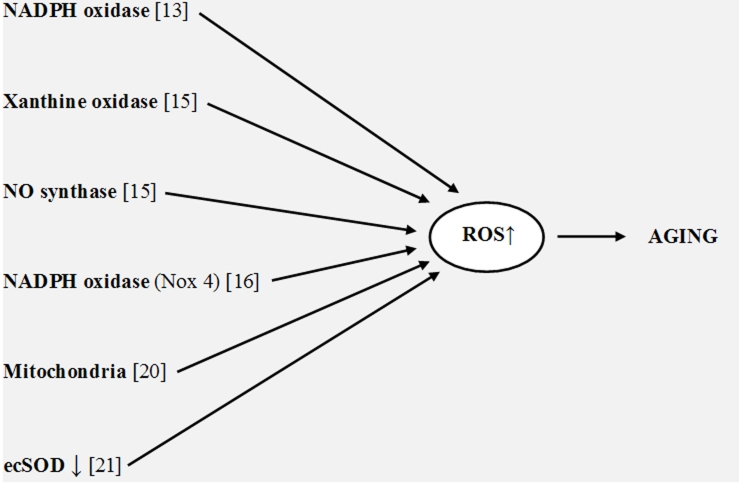
New works support these previous findings. Donato et al. [13] found that endothelial oxidative stress was developed in aged healthy men and was related with reduction in endothelium-depended dilation. In addition these authors observed that aging in humans increased the expression of NADPH oxidase and NF-κB. Choksi et al. [14] investigated the origin of resistance to oxidative stress and longevity in the Ames dwarf (DW) mice. They found that endogenous ROS production in the serum and F2-isoprostanes levels in the liver in DW mice were lower at all ages that apparently was an origin of resistance to oxidative stress. Jacobson et al. [15] showed that superoxide production by xanthine oxidase and NO synthase in mesenteric arteries from aged rats was higher than from young ones. Lener et al. [16] proposed that NADPH oxidase (Nox4) can also be responsible for superoxide overproduction in cell senescence. They showed a significant increase in replicative lifespan of human umbilical vein endothelial cells upon knockdown of Nox4. Rodriguez-Manas et al. [17] suggested that the age-dependent endothelial dysfunction in human vessels was a consequence of oxidative stress and vascular wall inflammation. Sasaki et al. [18] found that the rate of superoxide production increased with age in the mice, Wistar rats, and pigeons being inversely related to the maximum lifespan of animals. Mendoza-Nunez et al. [19] suggested that oxidative stress increased in wealthy humans older than 60 years. Miyazawa et al. [20] found that superoxide overproduction from mitochondria in mice led to premature aging. Lund et al. [21] showed that endothelial vasomotor function decreased in aged mice due to a decrease in extracellular superoxide dismutase (ecSOD). Thus ROS overproduction in aged cells and aged humans and animals is proved by numerous experimental data and must be a source of many destructive processes. It should be noted that aforementioned data show that several enzymes and other sources can increase ROS production in the age and not only mitochondria as it now widely accepted (Figure 1).
Figure 1.
Sources of ROS overproduction in aging
Free radical damaging processes and ROS signaling in aging
ROS overproduction can be harmful through different damaging processes. For example, it has been shown that the overproduction of superoxide and nitric oxide is accompanied by the formation of very active species - hydroxyl radicals (through the Fenton reaction) and peroxynitrite (by combination of O2.− and NO). These really reactive species can destroy biomolecules directly as it has been proposed in original Harman’s theory. An increase in superoxide levels can be consequence of a decrease in nitric oxide with the age [22–24] because the reduction of NO formation diminishes the inhibition by nitric oxide of mitochondrial cytochrome c oxidase that accompanied by superoxide increase [25] (Ref. 26, pp. 171–174). At the same time signaling by overproduced ROS in various enzyme/gene processes leads to cell senescence (as reviewed by Kregel and Zhang [27]). All these processes can be responsible for ROS induced damage in aging and senescence.
Damaging ROS signaling in cellular senescence and organismal aging
ROS signaling plays important role in numerous enzyme and gene catalyzed processes under normal physiological conditions [26]. However in aging these signaling processes can become damaging cascades mostly due to ROS overproduction. It might be suggested that pathological changes in aged cells and tissues initiated by ROS signaling in enzymatic/gene processes can be more important than the direct attack of reactive free radicals on biomolecules during age development. Importance of ROS signaling in aging and age-depended pathologies has already been discussed in several reviews [27–29]. Therefore excessive ROS signaling can be a new confirmation and support of free radical theory of aging.
Importance of ROS signaling functions draws attention to the question, what kind of free radicals are of great significance for aging development. It is now well known that only superoxide and nitric oxide (paramagnetic free radicals) and hydrogen peroxide (diamagnetic product of superoxide dismutation) are real signaling species. (By definition, signaling free radicals cannot be very reactive reactants in typical radical reactions of H-abstraction and the addition to double bonds because in this case they would directly destroy biomolecules without having chance to participate in enzymatic cascades. Therefore such markers of oxidative stress as lipid peroxidation or the formation of carbonyl derivatives formed in the reactions of reactive hydroxyl or peroxy free radicals or in the reaction very active peroxynitrite (the product of reaction between O2.− and NO) might be incorrect characteristics of free radical damage in the age).
Superoxide, nitric oxide, and hydrogen peroxide participate in enzyme/gene processes (see, below) due to their non-radical nucleophilic activity [30–32]. Owing to this they can accelerate reactions of hydrolysis, etherification, and phosphorylation catalyzed by different enzymes [26]. In addition superoxide and hydrogen peroxide can inhibit protein phosphatases by oxidation of their thiol groups and by this activate protein kinases. (It should be noted that if the mechanism of nucleophilic reactions of superoxide was thoroughly studied [33], the mechanism of hydrogen peroxide participation in heterolytic nucleophilic reactions remained uncertain. Iron-catalyzed decomposition of hydrogen peroxide to hydroxyl radicals is a well-established origin of its damaging oxidative activity, but it cannot be a mechanism of its signaling function. Some years ago I suggested that signaling functions of hydrogen peroxide might depend on conversion to superoxide by the reaction with oxidized CuZnSOD [32]).
Mechanisms of major ROS signaling pathways in the age
It has been shown that ROS signaling in the age might occur through the enzyme/gene cascades in which aging-regulating genes p66shc, Sirtuin, FOXO3a, and Klotho play critical role together with some protein kinases particularly protein kinase Akt/B. Akt kinase was frequently considered to be a surviving factor but now it has been shown that this kinase catalyzes cellular senescence in pathways with or without aging-regulating genes.
In addition to Akt kinase other mitogen activated protein kinases (MAPKs) can participate in damaging ROS signaling processes. For example Wu et al. [34] showed that compared with adult (6-month) and aged (27-month) rats, very aged rats (33-month) had higher levels of superoxide, blood glucose, phosphorylation of soleus p38-MAPK and extracellular-regulated kinase 1/2 (ERK1/2), and these changes were associated with decreased soleus Glut4 protein abundance. Chronic acetaminophen treatment (a phenolic drag probably having antioxidant properties) diminished superoxide levels, age-associated increase in blood glucose, and reduced p38-MAPK and ERK1/2 hyperactivation.
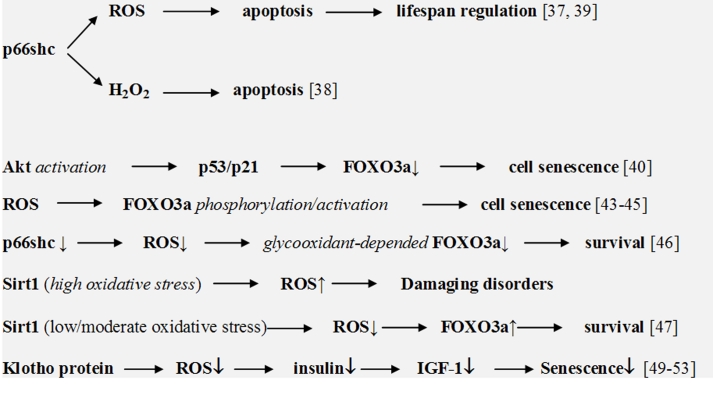
However, the most important factor of aging development and sell senescence is probably ROS signaling in enzyme/gene cascades. Present studies demonstrate importance of p66shc, Sirtuin, FOXO3a, and Klotho genes in the development of various pathologies such as diabetes mellitus, cancer, and aging. Their role in aging has already been widely discussed [25–31]. These genes are able to stimulate or inhibit aging processes. It has been shown [35] that the deletion of p66shc gene induced stress resistance and prolonged lifespan in experimental animals. This study stimulated numerous investigations in which a role of p66shc gene in various pathologies and aging has been studied.
ROS signaling is an important factor of these enzyme/gene cascades. Trinei et al. [36] recently suggested that p66shc is able to stimulate directly ROS overproduction and aging. However, previous studies pointed out that the regulation of lifespan by p66shc depended on the stimulation of apoptosis by oxidative stress [37]. Giorgio et al. [38] also suggested that p66shc initiated apoptosis by the production of hydrogen peroxide through the oxidation of mitochondrial cytochrome c. (It should be noted that electron transfer from reduced cytochrome c to p66shc seems to be thermodynamically unfavorable due to wrong potential difference). Gertz et al. [39] suggested that p66Shc generated ROS and initiated apoptosis when cellular antioxidants were unable to inhibit ROS overproduction.
FOXO3a belong to the O subclass of the forkhead family of transcription factors. There are various examples of ROS signaling in aging processes with participation of FOXO3a. Thus Miyauchi et al. [40] showed that the activation of Akt kinase in human endothelial cells promoted senescence-like arrest of cell growth through a p53/p21-dependent pathway and the inhibition of forkhead transcription factor FOXO3a. FOXO3a influenced p53 activity by the regulation of ROS formation. As it has earlier been shown, superoxide and hydrogen peroxide are the activators of Akt kinase [41, 42]. Therefore ROS can apparently be an initiator and a mediator of enzyme/gene cascades stimulating cell senescence. Several authors also demonstrated that ROS-stimulated phosphorylation/activation of FOXO3a resulted in cellular senescence [43–45]. p66shcA and FOXO3a can mutually interact during aging processes. Thus Chintapalli et al. [46] showed that the inhibition of p66shcA in mesangial cells prevented glycooxidant-dependent FOXO3a regulation and promoted the survival phenotype.
Upregulation of silent information regulator Sirtuin (human Sirt1 and Sirt3) suppresses age-dependent cardiac hypertrophy, apoptosis, cardiac dysfunction, and expression of senescence markers. Although a high level of Sirt1 enhanced damaging disorders, moderate overexpression of Sirt1 protected from ROS overproduction through the FOXO3a-dependent mechanism. Alcendor et al. [47] showed that Sirt1 was significantly upregulated in response to low/moderate oxidative stress in adult mouse hearts. It was suggested that Sirt3 and FOXO1 can comprise a mitochondrial signaling survival cascade.
Klotho gene is another gene which regulates ROS-depended aging processes. Yamamoto et al. [48] showed that cell surface-bound Klotho inhibited FOXO3a phosphorylation and promoted its nuclear translocation. The nuclear FOXO3a then bound to the MnSOD promoter and suppressed ROS formation. It has been shown that the Klotho protein can suppress aging through both the inhibition of insulin-like signaling and an increase in resistance to oxidative stress. Other studies have also demonstrated the suppression of aging processes by Klotho through the inhibition of ROS formation [49–53]. Some pathways of aging-regulated enzyme/gene processes are shown in Figure 2.
Figure 2.
ROS signaling in enzyme/gene processes in aging and cell senescence
Effects of calorie restriction
It has been demonstrated that calorie restriction (CR) exhibits favorable effects on the lifespan of living organisms even though its effects is accompanied by an increase in oxidative stress. Nonetheless numerous data show that CR activity depends also on its antioxidative action. For example, Castello et al. [54] have studied the effect of CR on oxidative damage and its relationship with fibrosis during aging. They found CR suppressed an increase of oxidative stress and fibrosis parameters in the aortae from aged vs. young rats. Ungvari et al. [55] suggested that CR induced pathways responsible for increasing cellular oxidative stress resistance. CR can increase bioavailability of nitric oxide, decrease vascular ROS generation, and activate the Nrf2/ARE antioxidative pathway. These effects of CR might lead to the suppression of vascular disease that accompanies aging. It has also been shown that the transcription factor Nrf2 that binds to the antioxidant response element (ARE) of target genes in response to oxidative stress could be an origin of CR longevity effects [56]. Thus the mechanisms of CR longevity must certainly include an antioxidant component. In recent review Fontana et al. [57] discussed major mechanisms of CR effects on aging and longevity. They showed that in worm, flies, and mammals’ dietary (calorie) restriction influenced various signal pathways through the reduction of insulin-like growth factor I (IGF-1). It was also suggested that antioxidant enzymes SOD and catalase are active ingredients of CR activity. Authors pointed out that the inhibition of this nutrient signaling pathway depended on a decrease in superoxide production through its dismuting by SODs.
Insulin signaling in age
Undoubtedly, insulin signaling pathways are among the important factors of aging development. It has been well documented that the reduction of IGF (insulin growth factor)/insulin signaling cascades led to the extension of lifespan in worms and flies and is probably of importance in mammals [58]. At the same time there are numerous evidences indicating an important role of ROS in insulin signaling processes. For example Koya and King [59] assumed that glucose adverse effects in diabetes and hyperglycemia might depend on ROS-stimulated activation of diacylglycerol (DAG)/PKC enzymatic pathway because the treatment with α-tocopherol prevented glucose-induced vascular dysfunctions and inhibited DAG/PKC activation. Geolotto et al. [60] studied the effect of insulin on ROS formation and the stimulation of PKC-dependent enzymatic cascade in cultured skin fibroblasts from human volunteers. They showed that insulin induced translocation of the p47 (phox) subunit of NADPH oxidase from the cytosol to the membrane and the generation of ROS through a PKC-δ-dependent mechanism. Similar data have been received in many other works (see, for example [61–65].
However damaging effects of ROS in aging and other pathologies can depend on their concentrations. Droge [66] pointed out that the activity of basal insulin receptor tyrosine kinase was strongly increased by small concentrations of hydrogen peroxide or by the oxidation of the intracellular sulfhydryl residues. However, prolonged exposure to hydrogen peroxide inhibited insulin action suggesting that insulin signaling was enhanced only by moderate oxidative conditions and inhibited by excessive exposure to hydrogen peroxide. Goldstein et al. [67] also demonstrated that the weak ROS production can enhance insulin activity through the inhibition of protein tyrosine phosphatases and the activation of protein kinases. Intensification of ROS formation led to the damage of normal enzymatic cascades. Thus levels of ROS production could be critical parameters in favorable or damaging ROS signaling under pathological conditions.
It has been also shown that calorie restriction (CR) can influence insulin signaling in aging in different ways. Thus CR improved insulin sensitivity and increased lifespan in normal mice [68] through an increase in the phosphorylation of cardiac Akt without elevation of Akt protein. This effect might be cardioprotective and thus contribute to increased longevity in response to CR. However insulin signaling cascade was unaffected by CR in the heart of long-lived growth hormone-resistant knockout (GHRKO) mice.
Modifications of free radical theory of aging
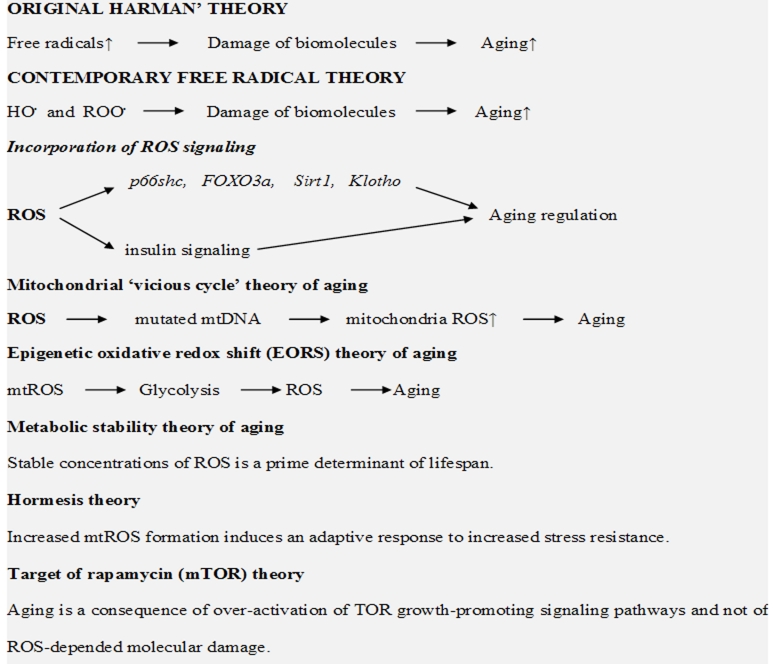
Free radical theory of aging is widely discussed in contemporary studies both supporting and criticizing her major conclusions. A main criticism of this theory is directed at the suggestion that free radicals are responsible for the damage of biomolecules which must be a major reason for cell senescence and organismal aging. This proposal is indeed questionable and been criticized by modern contradictory theories such as the Hormesis theory and the TOR (target of rapamycin) theory (see, below). However, as it has been shown above, free radical activity in aging must not be limited just to ROS damaging effects but should include more important ROS signaling functions. Below we will discuss some interpretations and modifications developed for improvement of free radical theory of aging as well as contradictory theories.
Dioxygen consumption and ROS production
In 2007 Barja [69] discussed an increase in longevity during dietary restriction that can occur together with the enhancement of dioxygen consumption. He concluded that although this phenomenon is frequently interpreted as a contradictory one to the mitochondrial free radical theory of aging, it is erroneous assumption because increasing dioxygen consumption should not always associate with an increase in the rate of mitochondrial oxygen radical generation. Indeed, mitochondrial ROS production must depend not only on mitochondrial respiration but it can be regulated independently of dioxygen consumption in many different physiologic situations, tissues, and animal species.
Mitochondrial ‘vicious cycle’ theory of aging
Mitochondrial “vicious cycle” theory of aging proposes that accumulation of mitochondrial DNA (mtDNA) mutations may lead to the enhanced mitochondrial ROS production and to subsequent increase in oxidative stress in aging. This theory was considered to be a further development of original Harman’s theory of aging. It has been suggested that the mutated mtDNA formed during the ROS attack on mtDNA are able by themselves to generate ROS (apparently by the reduction of dioxygen to superoxide) and by this to enhance oxidative stress (vicious cycle!).
This hypothesis was widely discussed in literature [70–73]. For example Vermulst et al. [71] and Hiona and Leeuwenburgh [72] founded no confirmations that mitochondrial mutations can limit the natural lifespan of animals. On the other hand Poovathingal et al. [73] suggested that improved experimental methods might help to get reliable data.
Unfortunately, it is not clear whatsoever, why mutated mtDNA or their fragments must produced ROS. Opposite case (inhibition of ROS) seems to be equally possible. We also do not know how ROS can induce mtDNA mutations. One of possible directions of ROS attack has been suggested by Sarkar and his co-workers [74]: they proposed that due to the close proximity of the zinc finger to DNA, iron-substituted zinc fingers may generate free radicals formed through the reaction of hydrogen peroxide with ferric ions and by that damage DNA molecules. Thus the source of development of “vicious cycle” in mitochondria remains completely unclear.
Recently Perez et al. [75] showed that among 18 genes coding for antioxidant enzymes only Sod1 gene deletion affected longevity of mice. I believe that it is important fact showing that lifespan depends on the Sod1 gene and correspondingly on the activity of CuZnSOD enzyme performing dismutation of superoxide, a main signaling free radical in enzyme/gene cascades in aging).
Epigenetic oxidative redox shift (EORS) theory of aging
For improvement of free radical theory Brewer [76] proposed to unify free radical theory with the effects of insulin signaling in aging giving this hypothesis the title - the epigenetic oxidative redox shift (EORS) theory of aging. According to EORS, sedentary behavior associated with age triggers an oxidized redox shift and impairs mitochondrial function. (I should comment that the name “oxidized redox shift” is very unfortunate because it means “oxidative reduction/oxidation shift”). Author believes that in contrast to the 2% inefficiency of mitochondrial reduction of dioxygen (to form superoxide) “oxidized redox shift” upregulates aerobic glycolysis and enhances ROS production by 100%. I am uncertain how to estimate an amount of real mitochondrial ROS formation in aging or cell senescence. Even a 2% leak from mitochondrial respiratory chain which must be responsible for superoxide formation is probably seriously overestimated. The same is more true for ROS formation under glycolic conditions. However, aforementioned data show that insulin signaling cascades in aging also regulated by ROS; therefore there is probably no need to add new hypotheses to free radical theory of aging.
Metabolic stability theory of aging
In accord with the metabolic stability theory of aging [77] the ability of cells to maintain the stable concentrations of ROS is a prime determinant of lifespan. The discussion of this theory is too far from the principal topics of this review. However, it is of interest that Brink et al. [77] criticizes Harman’s theory on the basis that it ignores the fact that ROS are not only damaging species but specific signaling molecules which are necessary for maintaining normal cell functions. It is of course true and ROS damaging signaling under pathological conditions including aging is widely discussed in present review.
Theories opposing the significance of free radicals in aging development
Hormesis theory
Despite numerous findings showing ROS overproduction and damaging ROS signaling in cell senescence and organismal ageing some contemporary studies raise objections to importance of these factors in age development. It has been shown that calorie restriction which was proven to result in enhanced lifespan in yeast, worms, flies, and even mice caused simultaneously the enhancement of mitochondrial ROS formation. Similar effect might have physical exercise which is good for healthy life but also accompanied by ROS overproduction. In recent review Ristow and Zarse [78] discussed the effects of calorie restriction and reduced glucose metabolism on the longevity of S. cerevisiae, D. melanogaster, C. elegans, and mice. They suggested that increased ROS formation within the mitochondria caused an adaptive response to increased stress resistance. This type of retrograde response was named mitochondrial hormesis or mitohormesis.
One of findings supporting hormesis theory is an unfavorable effect of antioxidants on aging development when they applied simultaneously with calorie restriction or during physical exercise. In addition, it has been demonstrated [79] that in some case for example in the fungal aging model Podospora anserina the overexpression of antioxidative enzyme MnSOD led to lifespan shortening. Goto and Radak [80] suggested that antioxidant supplementations attenuated beneficial effects of exercise and that hormesis can be caused by ROS in animals. It has been also shown that long term vitamin C supplementation diminished the adaptive response of exercise to oxidants in human lymphocytes [81]. Ristow et al. [82] concluded that exercise increased insulin sensitivity in humans only in the absence of antioxidants in both previously untrained and pretrained individuals. This fact was paralleled by increasing ROS generation.
Do these findings contradict free radicals theory of aging? I believe that there is no contradiction. As it has been proposed above, ascorbic acid (vitamin C) is a free radical scavenger which reacts with reactive free radicals such as HO. or ROO.. Exercise also enhanced the formation of these radicals which were formed, for example during lipid peroxidation. Thus, if aging is regulated by ROS signaling, then antioxidants such as ascorbic acid will not decrease the level of superoxide, a major ROS signaling species. It should be mentioned that ascorbic acid can exhibit both antioxidant and prooxidant properties in in vitro systems and at supplementation to humans [83, 87]. Prooxidant effects of ascorbic acid could depend on iron overloading in various biological systems and can be an origin of free radical overproduction at the supplementation of vitamin C.
To some degree the suggestion about the positive effects of additional antioxidants presented in surrogate vitamin C was supported by data obtained by Thaler et al. [84, 85]. These authors showed that the supplementation of vitamin C to 86 nondiabetic subjects at increased risk for diabetes type 2 had positive effect in lifestyle intervention. However Bluher et al. [86] demonstrated the opposite effect of vitamin C. Therefore it is quite possible this difference might be a consequence of the application of pharmacological vitamin C by Bluher et al. [86] and the surrogate of a healthy lifestyle by Thaler et al. [84, 85]. Surrogate of vitamin C probably contained other antioxidants such flavonoids and natural quinones, which in contrast to ascorbic acid can readily react with superoxide (see, for example Table 29.3, 29.4 in Ref. 87). If we accept this point of view, we might suggest that the use of vitamin C could not affect aging development, while the scavengers of superoxide (flavonoids and quinones) can.
Target of rapamycin (mTOR) theory
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates cell growth, cell proliferation, cell survival, and other cellular functions. It has been recently suggested that aging is a consequence of over-activation of growth-promoting signaling pathways such as the TOR or mTOR and not the consequence of ROS-depended molecular damages [88]. Surprisingly in this paper Blagosklonny presumes that although ROS can induce molecular damage but it is not life-limiting “because the TOR-driven aging terminates life first.”
There is no doubt that the reduction of TOR signaling can result in an extension of lifespan of living organisms. Mechanisms of TOR signaling in aging is not fully understood, although Pan and Shadel [89] documented that the Sch9p kinase is a key downstream effector of oxidative phosphorylation, ROS, and yeast chronological lifespan in the TOR-mitochondria pathway. Thus despite all criticism (see below) many TOR signaling pathways are mediated by ROS. Contemporary free radical theories of aging are summarized in Figure 3.
Figure 3.
Free radical theories of aging
Discussion
As is well known, Dr. Harman developed his free radical theory of aging in 1956. It is interesting to compare our knowledge about free radicals in biological systems there and now. In 1956 Dr. Harman could not see any difference between various free radicals – all of them should seem to him the criminals, the destroyers of living organisms. His theory was really a new leap forward understanding of mechanism of aging.
However, at present, more than 50 years after his discovery our knowledge of free radical-mediated processes in cells and tissues is completely different. We know that major reactive oxygen and nitrogen species (ROS and RNS) physiological free radicals superoxide and nitric oxide and the products of their reactions hydrogen peroxide and peroxynitrite are the obligatory mediators and promoters of numerous enzyme/gene pathways under both physiological and pathophysiological conditions. Yes, of course, reactive free radicals such as hydroxyl and peroxy radicals can damage biomolecules and their activities can be suppressed by some antioxidants (free radical scavengers) such as α-tocopherol (vitamin E) and ascorbic acid (vitamin C). However, ROS and RNS probably play a much more important role as signaling species. Enlargement out knowledge about free radicals in biological systems undoubtedly must broaden and modify free radical theory of aging.
I believe that contemporary interpretation of free radicals theory in aging must incorporate all the damaging activities of ROS and RNS including damaging ROS signaling in enzyme/gene processes. Such damaging signaling probably arises from ROS overproduction – the fact strictly proved in aging. There are many sources of ROS overproduction in aging (see above), the most important source is probably the oxidizible components of diet [90]. From my point of view an increase in ROS during physical exercises or calorie restriction does not contradicted to the role of free radicals in aging because ROS signaling might result in modern oxidative stress, which is not regulated by traditional antioxidants vitamins C and E [66, 67].
Surprisingly critics of free radical theory of aging apparently criticize her at the level of the old Harman’s theory as there was no development in the studies of free radicals and ROS for last fifty years. They demonstrate that scavengers of reactive oxygen radicals do not affect cell senescence and organismal aging – it is true, but they do not look at ROS signaling processes in aging. Blagosklonny’s criticism [88] seems to be particularly inadequate. First of all author claims that all conclusions of free radical theory of aging can be explained by TOR theory. Yes, it could be because TOR signaling is one of the other pathways regulating aging (for example insulin signaling) where ROS participation also plays an important role. I always thought that a new interpretation of old theory is possible if it gives some new benefits. What advantages give Blagosklonny’s interpretation of TOR theory if he agrees that ROS participate in TOR pathways but they are not important because “TOR will kill quicker that ROS.” How can he know this? He writes that “Some opponents have called this point of view extreme, one-sided and unbalanced.” Unfortunately I dare to add – a futile one.
Conclusions
Free radical theory of aging when it takes into account of new developments in free radical studies (ROS and RNS signaling) remains a reliable theory of aging.
References
- [1].Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- [2].Beckman KB, Ames BN. The Free Radical Theory of Aging Matures. Physiol Rev. 1998;78:547–81. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- [3].Loeb LA, Wallace DC, Martin GM. The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc Natl Acad Sci USA. 2005;102:18769–70. doi: 10.1073/pnas.0509776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006;8:582–99. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- [5].Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- [6].Sawada M, Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech Ageing Dev. 1987;41:125–37. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 7.Sawada M, Sester U, Carlson JC. Superoxide radical formation and associated biochemical alterations in the plasma membrane of brain, heart, and liver during the lifetime of the rat. J Cell Biochem. 1992;48:296–304. doi: 10.1002/jcb.240480310. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber SJ, Megow D, Raupach A, Victorov IV, Dirnagl U. Age-related changes of oxygen free radical production in the rat brain slice after hypoxia: on-line measurement using enhanced chemiluminescence. Brain Res. 1995;703:227–30. doi: 10.1016/0006-8993(95)01188-9. [DOI] [PubMed] [Google Scholar]
- 9.Chung HY, Song SH, Kim HJ, Ikeno Y, Yu BP. Modulation of renal xanthine oxidoreductase in aging: gene expression and reactive oxygen species generation. J Nutr Health Aging. 1999;3:19–23. [PubMed] [Google Scholar]
- [10].Hamilton CA, Brosnan MJ, McIntyre M, Graham MD, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–34. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 11.Moon SK, Thompson LJ, Madamanchi N, Ballinger Papaconstantinou SJ, Horaist C, Ruuge MS, Patterson C. Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H2779–88. doi: 10.1152/ajpheart.2001.280.6.H2779. [DOI] [PubMed] [Google Scholar]
- [12].Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36:1361–73. doi: 10.1016/s0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- [13].Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–66. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- [14].Choksi KB, Roberts LJ, 2nd, DeFord JH, Rabek JP, Papaconstantinou J. Lower levels of F2-isoprostanes in serum and livers of long-lived Ames dwarf mice. Biochem Biophys Res Commun. 2007;364:761–4. doi: 10.1016/j.bbrc.2007.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jacobson AK, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol. 2007;293:1344–50. doi: 10.1152/ajpheart.00413.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lener B, Koziel R, Pircher H, Hutter E, Greussing R, Herndler-Brandstetter D, Hermann M, Unterluggauer H, Jansen-Durr P. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem J. 2009;423:363–74. doi: 10.1042/BJ20090666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8:226–38. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- [18].Sasaki T, Unno K, Tahara S, Shimada A, Chiba Y, Hoshino M, Kaneko T. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell. 2008 2008 Apr 14; doi: 10.1111/j.1474-9726.2008.00394.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza-Nunez VM, Ruiz-Ramos M, Sanchez-Rodriguez MA, Retana-Ugalde R, Munoz-Sanchez JL. Aging-related oxidative stress in healthy humans. Tohoku J Exp Med. 2007;213 doi: 10.1620/tjem.213.261. [DOI] [PubMed] [Google Scholar]
- 20.Miyazawa M, Ishii T, Yasuda K, Noda S, Onouchi H, Hartman PS, Ishii N. The role of mitochondrial superoxide anion (O2(−)) on physiological aging in C57BL/6J mice. J Radiat Res. 2009;50:73–83. doi: 10.1269/jrr.08097. [DOI] [PubMed] [Google Scholar]
- [21].Lund DD, Chu Y, Miller JD, Heistad DD. Protective effect of extracellular superoxide dismutase on endothelial function during aging. Am J Physiol Heart Circ Physiol. 2009;296:H1920–5. doi: 10.1152/ajpheart.01342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mollace V, Rodino P, Massoud R, Rotiroti D, Nistico G. Age-dependent changes of NO synthase activity in the rat brain. Biochem Biophys Res Commun. 1995;215:822–827. doi: 10.1006/bbrc.1995.2537. [DOI] [PubMed] [Google Scholar]
- 23.Amrani M, Goodman AT, Gray CC, Yacoub MH. Ageing is associated with reduced basal and stimulated release of nitric oxide by the coronary endothelium. Acta Physiol Scand. 1996;157:79–84. doi: 10.1046/j.1365-201X.1996.451171000.x. [DOI] [PubMed] [Google Scholar]
- 24.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- [25].Afanas’ev IB. Interplay between superoxide and nitric oxide in aging and diseases. Biogerontology. 2004;5:267–270. doi: 10.1023/B:BGEN.0000038047.96106.ad. [DOI] [PubMed] [Google Scholar]
- [26].Afanas’ev IB. Signaling Mechanisms of Oxygen and Nitrogen Free Radicals. CRC Press; Boca Raton: 2009. [Google Scholar]
- [27].Kregel KS, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- [28].Gertz M, Steegborn C. The lifespan-regulator p66Shc in mitochondria – redox enzyme or redox sensor? Antioxid Redox Singal. 2010 Mar 9; doi: 10.1089/ars.2010.3147. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [29].Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Afanas’ev I. Reactive oxygen species and age-related genes p66shc, Sirtuin, FOX03 and Klotho in senescence. Oxid Med Cell Longev. 2010;3:77–85. doi: 10.4161/oxim.3.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Afanas’ev IB. On mechanism of superoxide signaling under physiological and pathophysiological conditions. Med Hypotheses. 2005;64:127–9. doi: 10.1016/j.mehy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- [32].Afanas’ev IB. Competition between superoxide and hydrogen peroxide signaling in heterolytic enzymatic processes. Med Hypotheses. 2006;66:1125–8. doi: 10.1016/j.mehy.2005.11.046. [DOI] [PubMed] [Google Scholar]
- [33].Afanas’ev IB. Superoxide Ion: Chemistry and Biological Implications. Vol. 1. CRC Press; Boca Raton, Florida: 1989. pp. 1–279. [Google Scholar]
- [34].Wu M, Desai DH, Kakarla SK, Katta A, Paturi S, Gutta AK, et al. Acetaminophen prevents aging-associated hyperglycemia in aged rats: effect of aging-associated hyperactivation of p38-MAPK and ERK1/2. Diabetes Metab Res Rev. 2009 Jan 28; doi: 10.1002/dmrr.932. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [35].Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- [36].Trinei M, Berniakovich I, Beltrami E, Migliaccio E, Fassina A, Pelicci P, Giorgio M. P66Shc signals to age. Aging (Albani NY) 2009;1:503–10. doi: 10.18632/aging.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, et al. The life span determinant p66shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689–95. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- [38].Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–33. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- [39].Gertz M, Fischer F, Wolters D, Steegborn C. Activation of the lifespan regulator p66Shc through reversible disulfide bond formation. Proc Natl Acad Sci USA. 2008;105:5705–9. doi: 10.1073/pnas.0800691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–20. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336:241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gorin Y, Ricono JM, Kim N-H, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- [43].Guo J, Gertsberg Z, Ozden N, Steinberg SF. p66shc links {alpha}1-adrenergic receptors to a reactive oxygen species-dependent AKT-FOXO3A phosphorylation pathway in cardiomyocytes. Circ Res. 2009;104:660–9. doi: 10.1161/CIRCRESAHA.108.186288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Purdom S, Chen QM. Linking oxidative stress and genetics of aging with p66Shc signaling and forkhead transcription factors. Biogerontology. 2003;4:181–91. doi: 10.1023/a:1025123413403. [DOI] [PubMed] [Google Scholar]
- [45].Kim HK, Kim YK, Song IH, Baek SH, Lee SR, Kim JH, Kim JR. Down-regulation of a forkhead transcription factor, FOXO3a, accelerates cellular senescence in human dermal fibroblasts. J Gerontol A Biol Sci Med Sci. 2005;60:4–9. doi: 10.1093/gerona/60.1.4. [DOI] [PubMed] [Google Scholar]
- [46].Chintapalli J, Yang S, Opawumi D, Goyal SR, Shamsuddin N, Malhotra A, et al. Inhibition of wild-type p66ShcA in mesangial cells prevents glycooxidant-dependent FOXO3a regulation and promotes the survival phenotype. Am J Physiol Renal Physiol. 2007;292:F523–30. doi: 10.1152/ajprenal.00215.2006. [DOI] [PubMed] [Google Scholar]
- [47].Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- [48].Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone Klotho. J Biol Chem. 2005;280:38029–34. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;2005;309(5742):1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ohta J, Rakugi H, Ishikawa K, Yang J, Ikushima M, Chihara Y, et al. Klotho gene delivery suppresses oxidative stress in vivo. Geriatrics & Gerontology Intern. 2007;7:293–9. [Google Scholar]
- [51].Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–32. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- [52].Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007;31:82–7. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- [53].Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci USA. 2007;104:2331–6. doi: 10.1073/pnas.0611079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto Calorie restriction protects against age-related rat aorta sclerosis. FASEB. 2005;19:1863–5. doi: 10.1096/fj.04-2864fje. [DOI] [PubMed] [Google Scholar]
- [55].Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–28. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Kellie, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA. 2008;105:2325–30. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fontana L, Partridge L, Longo VD. Extending healthy life span from yeast to humans. Science. 2010;328:321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bartke A. Minireview: Role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- 59.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- [60].Geolotto G, Papparella I, Lenzini L, Sartori M, Mazzoni M, Iori E, et al. Insulin generates free radicals in human fibroblasts ex vivo by a protein kinase C-dependent mechanism, which is inhibited by pravastatin. Free Radic Biol Med. 2006;41:473–83. doi: 10.1016/j.freeradbiomed.2006.04.015. [DOI] [PubMed] [Google Scholar]
- [61].Lee HB, Yu MR, Song JS, Ha H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int. 2004;65:1170–9. doi: 10.1111/j.1523-1755.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- [62].Xia L, Wang H, Munk S, Frecker H, Goldberg HJ, Fantus IG, Whiteside CI. Reactive oxygen species, PKC-beta1, and PKC-zeta mediate high-glucose-induced vascular endothelial growth factor expression in mesangial cells. Am J Physiol Endocrinol Metab. 2007;293:E1280–8. doi: 10.1152/ajpendo.00223.2007. [DOI] [PubMed] [Google Scholar]
- [63].Xia L, Wang H, Munk S, Kwan J, Goldberg HJ, Fantus IG, Whiteside CI. High glucose activates PKC-zeta and NADPH oxidase through autocrine TGF-beta1 signaling in mesangial cells. Am J Physiol Renal Physiol. 2008;295:F1705–14. doi: 10.1152/ajprenal.00043.2008. [DOI] [PubMed] [Google Scholar]
- [64].Lavrentyev EN, Malik KU. High glucose-induced Nox1-derived superoxides downregulate PKC-βII, which subsequently decreases ACE2 expression and ANG(1–7) formation in rat VSMCs. Am J Physiol Heart Circ Physiol. 2009;296:H106–18. doi: 10.1152/ajpheart.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ceolotto G, Bevilacqua M, Papparella I, Baritono E, Franco L, Corvaja C, et al. Insulin generates free radicals by an NAD(P)H, phosphatidylinositol 3′-kinase-dependent mechanism in human skin fibroblasts ex vivo. Diabetes. 2004;53:1344–51. doi: 10.2337/diabetes.53.5.1344. [DOI] [PubMed] [Google Scholar]
- [66].Droge W. Oxidative aging and insulin receptor signaling. J Gerontol A Biol Sci Med Sci 1. 2005;60:1378–385. doi: 10.1093/gerona/60.11.1378. [DOI] [PubMed] [Google Scholar]
- [67].Goldstein BJ, Mahadev K, Wu X. Insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–21. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Giani JF, Bonkowski MS, Munoz MC, Masternak MM, Turyn D, Bartke A, Dominici FP. Insulin signaling cascade in the hearts of long-lived growth hormone receptor knockout mice: effects of calorie restriction. J Gerontol A Biol Sci Med Sci. 2008;63:788–797. doi: 10.1093/gerona/63.8.788. [DOI] [PubMed] [Google Scholar]
- [69].Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res. 2007;10:215–24. doi: 10.1089/rej.2006.0516. [DOI] [PubMed] [Google Scholar]
- [70].Loeb LA, Wallace DC, Martin GM. The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc Natl Acad Sci. 2005;102:18769–18770. doi: 10.1073/pnas.0509776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet. 2007;39:540–543. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- [72].Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondria vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Poovathingal SK, Gruber J, Halliwell B, Gunawan R. Stochastic drift in mitochondrial DNA point mutations: a novel perspective ex silico. PLoS Comput Biol. 2009;5:e1000572. doi: 10.1371/journal.pcbi.1000572. Epub 2009 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Conter D, Narindrasorasak S, Sarkar B. In vivo and in vitro iron-replaced zinc finger generates free radicals and causes DNA damage. J Biol Chem. 1996;271:5125–30. doi: 10.1074/jbc.271.9.5125. [DOI] [PubMed] [Google Scholar]
- [75].Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–14. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Brewer GJ. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol. 2010;45:173–9. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Brink TC, Demetrius L, Lehrach H, Adjaye J. Age-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging. Biogerontology. 2009;10:549–64. doi: 10.1007/s10522-008-9197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitochormesis) Exp Gerontol. 2010 Mar 26; doi: 10.1016/j.exger.2010.03.014. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [79].Zintel S, Schwitalla D, Luce K, Hamann A, Osiewacz HD. Increasing mitochondrial superoxide dismutase abundance leads to impairments in protein quality control and ROS scavenging systems and to life shortening. Exp Gerontol. Jan 18; doi: 10.1016/j.exger.2010.01.006. (1010) [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [80].Goto S, Radak Z. Hormetic effects of reactive oxygen species by exercise: a view from animal studies for successful aging in human. Dose Response. 2009;8:68–72. doi: 10.2203/dose-response.09-044.Goto. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol. 2003;549:645–52. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ristow M, Zarsea K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahne CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Carr A, Frey B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999. pp. 1007–1024. [DOI] [PubMed]
- [84].Thamer C, Machicao F, Stefan N, Fritsche A, Häring H-U. High baseline vitamin C levels do not prevent a positive outcome of a lifestyle intervention. Diabetes Care. 2009;32:e112. doi: 10.2337/dc09-0965. [DOI] [PubMed] [Google Scholar]
- [85].Thamer C, Stefan N, Fritsche A, Häring H-U. High baseline vitamin C levels do not prevent a positive outcome of a lifestyle intervention: response to Blüher et al. Diabetes Care. 2010;33:e18. doi: 10.2337/dc09-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bluher M, Stumvoll M, Kahn CR, Ristow M. High baseline vitamin C levels do not prevent a positive outcome of of a lifestyle intervention: response to Thamer et al. Diabetes Care. 2010;33:e17. doi: 10.2337/dc09-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Denisov ET, Afanas’ev IB. Oxidation and Antioxidants in Organic Chemistry and Biology. CRC Press, Taylor & Francis Group; Boca Raton, Florida: 2005. pp. 847–848. [Google Scholar]
- [88].Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–54. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- [89].Pan Y, Shadel GS. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging (Albany NY) 2009;1:131–45. doi: 10.18632/aging.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Afanas’ev IB. Free radical mechanisms of aging processes under physiological conditions. Biogerontology. 2005;6:283–290. doi: 10.1007/s10522-005-2626-z. [DOI] [PubMed] [Google Scholar]