Abstract
The past decade or so has witnessed a rekindling of interest in glia requiring a re-evaluation of the early descriptions of astrocytes as merely support cells, and microglia as adopting either a resting state or an activated state in a binary fashion. We now know that both cell types contribute to the optimal functioning of neurons in the healthy brain, and that altered function of either cell impacts on neuronal function and consequently cognitive function. The evidence indicates that both astrocytic and microglial phenotype change with age and that the shift from the resting state is associated with deterioration in synaptic function. In this review, we consider the rapidly-expanding array of functions attributed to these cells and focus on evaluating the changes in cell activation that accompany ageing.
Keywords: Astrocytes, microglia, immune function, T cells, ageing, CD200
With the increasing recent interest in glial cell biology, it is becoming increasingly clear that a reassessment of the traditionally-held views regarding the role of astrocytes and microglial is necessary. Whereas it has been acknowledged that both cell types play neuro-supportive roles, a full appreciation of the extent to which these cells influence neuronal function is currently being uncovered.
Astrocytes have many functions that impact on neurons
Functions of astrocytes
Astrocytes were originally identified as cells which provide nutritional and structural support for neurons, but the finding that these cells were electrically active and capable of releasing so-called gliotransmitters led to the acknowledgement that they are capable of intercellular communication and that they can directly affect the excitability of neurons [1]. Conversely, astrocytes can detect synaptic activity by responding to glutamate released by neurons and, at least under certain circumstances, this feeds back to modulate release of gliotransmitters; this feedback loop can markedly influence the microenvironment and impact on synaptic function [2,3]. Astrocytes regulate the ionic environment and, as well as releasing glutamate, they are actively involved in its uptake. They also respond to calcium [4] and fast communication between cells is facilitated by means of gap junctions [5]. Their end feet make direct contact with capilliaries and arterioles ensuring a constant supply of nutrients, providing for the metabolic needs of neurons and playing a vital role in the formation of the blood brain barrier [6]. Consistent with their role in neuronal survival, regeneration and differentiation, astrocytes secrete an array of growth factors including nerve growth factor, brain-derived growth factor and insulin-like growth factor [7] but, as highlighted in a recent review, there are numerous mechanisms involved in astrocytic modulation of neuronal function [8]. The critical role of astrocytes in neuronal survival was recently highlighted by the finding that co-incubation of neurons with natural killer cells resulted in loss of neurons but that the neuronal loss was secondary to, and triggered by, loss of astrocytes [9].
Astrocytes are generally identified by the expression of glial fibrillary acidic protein (GFAP) although some cells that possess the morphological characteristics of astrocytes do not always express it [10] and ependymal and radial glia also may also express GFAP [11]. For example, astrocytes with end feed that make contact with blood vessels of 8μm or greater in diameter, are GFAP-positive whereas those which make contact with smaller blood vessels are not [12]. GFAP contributes to the modulation of astrocyte motility and shape by providing structural stability to astrocytic processes, although very subtle changes are observed in GFAP-deficient mice suggesting that proteins like vimentin are capable of functionally replacing GFAP [13]. Overexpression of GFAP leads to a fatal encephalopathy and is directly related to a rare type of leukodystrophy [14,15]. The glutamate transporter, GLT-1, is also expressed on astrocytes although regional specificity makes this a less useful marker than GFAP [16] and the calcium binding protein, S100β, is also considered to be glia-specific although its expression in all astrocytic populations is not uniform [17]. Other markers of astrocytes include inward rectifying potassium channels like Kir4.1, the aquaeporin channel 4 and the enzyme aldehyde dehydrogenase 1 family member L1 [18].
A pivotal role for astrocytes in synapse and spine formation has been described, and among the factors involved in the orchestration of these events is the interaction between ephrin ligands and their receptors, specifically the interaction between Eph4A receptors and ephrin-A3, which is expressed on astrocytes [19]. Because of their role in sculpting synapse-spine contacts, it is predictable that ephrins play a role in synaptic plasticity; indeed recent evidence indicates that astrocytes may be involved in processes such as learning and memory [20]. EphA4 and ephrin-A3 are both required for maintenance of long-term potentiation (LTP) and the relative expression of ligand and receptor controls expression of glial glutamate transporters and therefore glutamate concentration at the synapse [21]. Interestingly, a decrease in expression of glutamate transporters, which has been associated with increased synaptic glutamate concentrations and neurodegenerative changes [22], has been observed in the brain of aged mice and also in middle-aged mice which overexpress amyloid precursor protein [23] although an aberrant accumulation has been identified in the hippocampus of individuals with Alzheimer’s Disease (AD) [24].
As well as glutamate, astrocytes release D-serine in response to calcium and this exerts a significant impact on NMDA receptor activation. Indeed LTP is blocked by clamping internal calcium in astrocytes of hippocampal area CA1 because this inhibits D-serine release [25]. An age-related decrease in hippocampal D-serine, coupled with a depression in LTP, has been identified, and the evidence indicates that D-serine reverses the age-related deficit in LTP [26]. The reduction in LTP observed in aged rats has been associated with increased microglial activation (see below) but the evidence indicates that it is also accompanied by an increase in activated astrocytes; thus ladostigil (which also decreases the age-related change in microglial activation) and rosiglitazone attenuate the age-related activation of astrocytes and reverses the deficit in synaptic plasticity [27,28]. Whereas the ladostigil-associated effects were coupled with a reduction in AChE activity [27], the effects of rosiglitazone were coupled with an attenuation of the age-related increases in TNFα and RANTES [28].
Activation of astrocytes
Astrocytes express numerous receptors including G protein-coupled receptors and ionotropic receptors, receptors for growth factors, chemokines and cytokines; interestingly, astrocytes display heterogeneity in their pattern of receptor expression and adjust the pattern according to their microenvironment [16]. Cells become highly reactive in response to any insult to the CNS, often stimulated by inflammatory cytokines, and activation is characterized by hypertrophy and cell proliferation and is associated with rapid GFAP synthesis [13]. Glutamine synthase is also an indicator of astrogliosis [29] while bystin expression is also increased, particularly in response to ischaemia [30]. However incubation of primary astrocytes in a cocktail which contains inflammatory cytokines resulted in modulated expression of 182 genes (on a chip that could identify 480 genes) indicating that activation may have knock-on effects which remain to be uncovered; genes which were upregulated reflected processes involved in antigen presentation and invasion of leukocytes as well as defense against invading organisms [31]. Following an insult which results in neuronal damage, astrocytes appear to surround and isolate dying neurons; this is designed to prevent contact between dying and healthy neurons but impedes any functional recovery [32]. Thus astrogliosis is classified as anisomorphic or isomorphic; in the former case, astrocytes which surround a lesion lead to the formation of a glial scar whereas in the latter case, astrocytes are generally distal to the site of injury and promote neurite outgrowth and facilitate synaptogenesis [33].
Microglia
Functions of resting microglia
Microglia are the primary immune cells of the brain. Under resting conditions, the processes of these cells are constantly motile, with shapes that vary from thin filopodia-like processes to more thickened processes with bulbous protrusions. Microglial processes make contact with astrocytes, neuronal cell bodies and blood vessels and it has been established from time-lapse imaging studies that microglia can sample the parenchymal space in a few hours [34]. Damage to brain tissue results in the immediate recruitment of microglia to the area, and the evidence indicates that when this occurs the microglial processes exhibit spherical protrusions, which is interpreted as an indicator of phagocytic activity [34]. In a recent study involving transcranial imaging of the somatosensory and visual cortices, it was demonstrated that microglial processes made intimate contact with presynaptic terminals and dendritic spines but not dendritic shafts; these contacts were transient, lasting about 5 minutes and it was suggested that individual contacts occurred approximately every hour [35]. Movement of processes has also been assessed in the slice preparation and protrusion/retraction of the processes was estimated to be about 1μm/min [36]; motility of processes in this preparation was decreased by a non selective inhibitor of P2Y receptors, but it is likely that other modulators will be identified in due course. Contact in the healthy brain in situ has been shown to increase with neuronal activity [35] suggesting that the functional state of the neuron plays a role in the monitoring function of microglia. In contrast with the situation in vivo, microglial motility in the hippocampal slices was not altered by neuronal activity, or by application of glutamate or GABA [37] although microglia express both glutamatergic and GABA receptors [38,39]. In one study it was demonstrated that ischemic insult resulted in prolonged contact with synapses after which synaptic loss occurred; this led to the proposal that a balance between expression of repellent and attractant signals exist and that this is altered by insult [35]. Whereas several molecules like chemokines and damage-associated molecular pattern molecules (DAMP), for example ATP, function as chemoattractants, the nature of the repellent signals remains to be identified.
Modulation of microglial function
Microglia become activated following detection of any signal that poses a threat to the integrity of the internal environment of the CNS and these include the presence of bacteria or viruses, a change in the levels of circulating cytokines or antibodies, or the presence of abnormal endogenous proteins. They can also become activated if the so-called ‘off signals’ [40], that help to maintain them in a quiescent state, are interrupted by neuronal damage. These signals include the ligand-receptor pairs, CD200-CD200R, CX3CL1-CS3CR1 and SIRPα-CD47 [40]. However it should be stressed that the term ‘microglial activation’ is not specific, used by different authors to reflect different states. Rather than the originally-perceived all-or-none response which suggested the existence of only one activated state, it is now agreed that microglia are particularly plastic cells that can adopt one of a number of phenotypes when activated [41,42,43]. The shift of microglia from the resting, to the activated, state is associated with several changes; thus the ramified morphology associated with microglia at rest alters and activated cells display an amoeboid morphology characterized by shorter, thicker processes and a larger cell body. Apart from this rather general morphological change, the response of microglia to pathological events is context-dependent and varies as the microenvironment changes [40]. Stimulation can induce microglia to secrete cytokines and chemokines [44], become phagocytic, present antigen, increase expression of cell surface activation markers (Streit et al, 1989), activate the complement system, produce NO and other chemical mediators [40,45,46]. Microglial activation has traditionally been associated with neurotoxic and pro-inflammatory effects [47] but neuroprotective and anti-inflammatory effects have been described by several groups [40,44,48]. However recognition of the activated state of microglia often relies on the expression of cell surface markers that are either absent, or present at very low levels, when microglia are at rest [49,50]. These include CD40, cell adhesion molecules like intercellular adhesion molecule (ICAM)-1 and, importantly, major histocompatability complex (MHC)II and the costimulatory molecules CD80 and CD86, which enable microglia to undertake their role as the main antigen presenting cell in the brain [43,51].
Microglia do not constitutively express cytokine mRNA at any significant level with the exception of IL-6, but mRNA expression of IL-1β, IL-6 and TNF-α is upregulated [52] and release is increased following stimulation with numerous factors including Toll-like receptor (TLR) agonists [53] or with amyloid-β (Aβ) peptides [53,54,55,56].
The immune cells of the brain
Although microglia are the primary immune cells in the brain, astrocytes also play a role in antigen presentation, although the debate concerning their efficiency in this regard continues. Astrocytes express cell surface molecules including MHCII, ICAM-1, CD40, CD80 and CD86 [57]. It has been reported that astrocytes respond to stimulation with TLR agonists including Pam3Cys4 and lipopolysaccharide (LPS) [58] which activate TLR2 and TLR4 respectively, implying that these receptors are present on astrocytes. This is a subject of some debate and it has been proposed that any action of TLR agonists on astrocytes may be secondary to their ability to induce cytokine release from microglia [59]. While some reports indicate that astrocytes express TLR2 [60], others have reported a complete absence of these receptors [61] and similarly there are conflicting results regarding expression of astrocytic expression of TLR4 with some groups demonstrating their presence [60,62,63] and others reporting that TLR4 are not expressed on these cells [64]. It is acknowledged that these contrasting data may be a consequence of the preparations examined [59,65]. In spite of this unresolved issue, the evidence indicates that astrocytes respond to cytokines of the adaptive immune system by increasing expression of MHCII and cell adhesion molecules like ICAM-1 [60,66] and, like microglia, they interact with T cells [66]. For example, it is known that they present antigen to myelin basic protein (MBP)-reactive T cells and this is blocked by neutralizing antibodies to IL-12/IL-23p40 [67], while expression of costimulatory molecules CD80 and CD86 on astrocytes is increased in plaques in multiple sclerosis [68]. A recent review highlighted the role of the noradrenergic system, particularly β2 adrenergic receptors, in modulating MHCII transcription; interestingly a decrease in β2 receptor expression was coupled with increased MHCII expression on astrocytes in multiple sclerosis lesions [69]. The proposal that the loss of these receptors transforms astrocytes into competent antigen presenting cells [70] suggests that these cells may offer a therapeutic target in the treatment of multiple sclerosis. Consistently, fluoxetine appears to reduce the development of new lesions in patients with relapsing-remitting MS [71].
Age-related changes in glia
Ageing is accompanied by a gradual deterioration in function; in the brain, the impairment in neuronal function leads to cognitive impairment. Numerous changes are likely to contribute to this but, in the past decade or so, it has become increasingly clear that the age-related changes in the immune system, typified by a bias towards proinflammatory influences, impact on neuronal function and that, coupled with this, a number of age-related factors lead to the development of neuroinflammatory changes in the brain. The recognition that neuroinflammation is a characteristic of most, if not all, neurodegenerative diseases, and that ameliorating these changes may provide therapeutic promise, has brought into focus the requirement to understand the processes which underlie the development of age-related neuroinflammation. Furthermore, several neurodegenerative diseases are age-related and most, if not all, are associated with inflammatory changes; these observations suggest that, with age, the brain becomes more vulnerable to factors that may trigger, or contribute to, the pathogenesis of these diseases.
Astrocytes and age
Age-related increases in GFAP immunoreactivity and/or GFAP mRNA, as well as proliferation of astrocytes, have been reported in several areas of the brain [72,73,74,75]; specifically, increases in both GFAP immunoreactivity and GFAP mRNA have been observed in the hippocampus (Figure 1a, b). The age-related increases in these measures in the hippocampus were accompanied by increased production of RANTES [28]. Significantly rosiglitazone inhibited these changes suggesting that it targets astrocytes, whereas it exerted no effect on MHCII, CD11b or CD68 [28,76]. Interestingly a correlation between physical property of spin-lattice relaxation time (T1) and astrocytic activation was observed [28] supporting previous findings [77] and age-related changes in both T1 relaxation time and markers of GFAP immunreactivity were attenuated in rosiglitazone treated rats [28].
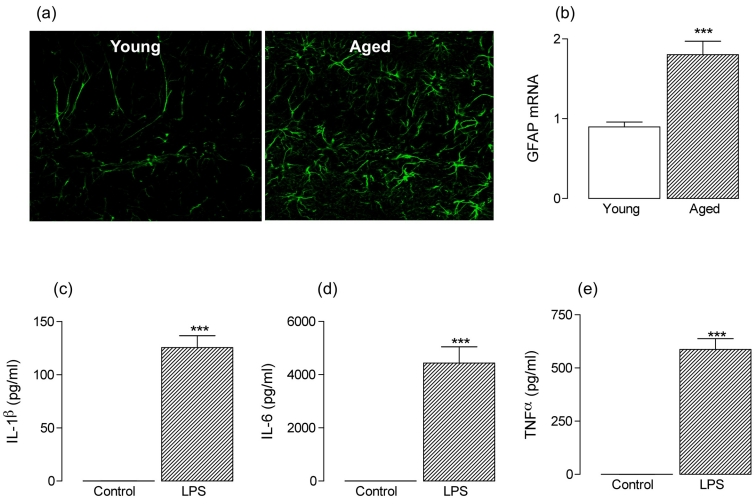
Figure 1:
Astrocytes, which release inflammatory cytokines, are activated in the hippocampus with age (a) GFAP immunoreactivity was markedly increased in sections of hippocampus prepared from aged, compared with young, rats and this was associated with an age-related increase in GFAP mRNA (b). (c)–(e) Purified astrocytes were prepared from 1 day-old rats and incubated in the presence of LPS (1μg/ml) for 24 hours. LPS significantly increased supernatant concentrations of IL-1β, IL-6 and TNFα (***p < 0.001; student’s t-test for independent means).
The functional consequences of the age-related change in astrocytic function, specifically the effects of increased expression of GFAP, are unknown. Deletion or reduction of GFAP in astrocytes has been associated with improved neuronal survival and enhanced LTP [78,79] whereas overexpression causes fatal encephalopathy [14]. It is accepted that microglia are the most likely source of proinflammatory cytokines [80,81], but astrocytes release IL-1β, IL-6 and TNFα in response to stressors [82,83] and the data presented here demonstrate that LPS triggers an increase in IL-1β, IL-6 and TNFα release from purified astrocytes prepared from neonatal rats (***p < 0.001; student’s t-test for independent means; Figure 1c–e) suggesting a direct effect of TLR4 activation. Therefore the observed astrogliosis may contribute to the age-related increases in inflammatory cytokines which have been described [84]. However clear evidence that upregulation of GFAP is directly linked with astrocytic cytokine production is lacking, although it has been shown that both GFAP mRNA and IL-1β mRNA or TNFα are increased in tandem in aged, compared with young, rats [28], in rats treated with quinolinic acid [85], and in animals following ischaemia induced by common carotid artery occlusion, where both changes were blocked in parallel by minocycline [86]. Furthermore, positive staining for these inflammatory cytokines has been observed in GFAP-positive cells, albeit to a greater extent in CD11b-positive cells, in mice that overexpress APP and PS1 [87].
Microglia and age
There is no doubt that age impacts significantly on microglial function [88,89] and numerous groups have reported that there is a shift in microglia from the resting state with age. Microarray analysis on brain tissue prepared from young and aged animals has highlighted upregulation in genes which are indicative of inflammatory and oxidative stress; these include genes which code for inflammatory cytokines, cell surface markers of microglial activation [90], genes which code for proteins involved in microglial migration [91] and activation of astrocytes [92]. A recent study reported that many similar genes, specifically those which play in immunity and defence, especially macrophage-mediated immunity, and also genes involved in interleukin-associated signaling, were upregulated in brain tissue obtained from individuals with AD as well as age [93]. Consistent with the upregulation in genes which code for inflammatory cytokines is the observation that there is an age-related increase in concentration of IL-1β, IL-6 and TNFα [94–100]. This is accompanied by a decrease in concentration of anti-inflammatory cytokines, IL-10 and IL-4 which have been coupled specifically with increases in IL-6 and IL-1β respectively, in the hippocampus [98,100,101]. These findings were confirmed by the observation that microglia prepared from aged mice release greater amounts of IL-6 and TNFα under resting conditions than microglia prepared from young rats [102], while the coupling between the increase in IL-6 and the decrease in IL-10 observed in the hippocampus, extends to the cortex (*p < 0.05; **p < 0.01; student’s t-test for independent means: Figure 2a, b) and these changes were linked with an increase in the chemokine, MCP-1 (***p < 0.001; student’s t-test for independent means: Figure 2c). These changes form an interesting parallel with those in the periphery where age-related changes in the innate and adaptive immune systems are evident, with an overall shift towards an inflammatory phenotype in the mouse [103] and also in humans [104].
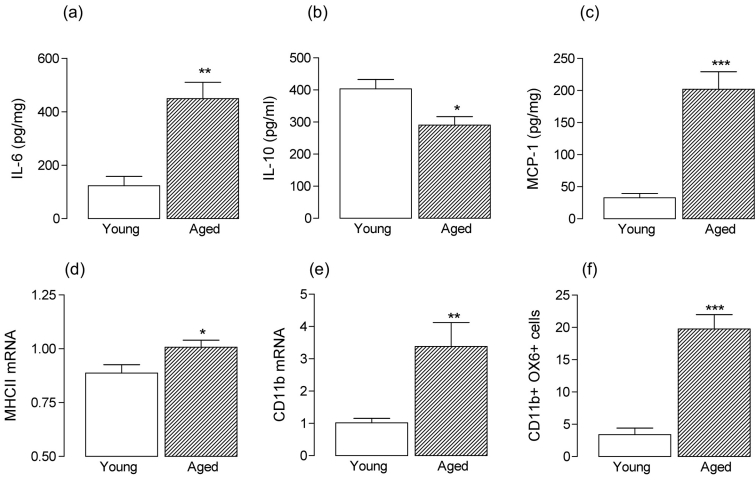
Figure 2:
Age-related changes in cortical tissue are indicative of inflammatory changes The significant age-related increase in IL-6 concentration in rat cortical tissue (a: **p < 0.01; student’s t-test for independent means) was associated with an age-related decrease in cortical concentration of IL-10 (b: *p < 0.05; student’s t-test for independent means) and an age-related increase in MCP-1 concentration was also observed (c: ***p < 0.001; student’s t-test for independent means). MHCII (d) and CD11b (e) mRNA were also increased in cortical tissue prepared from aged, compared with young, rats (*p < 0.05; **p < 0.01; student’s t-test for independent means) and FACS analysis indicated that the number of CD11b-positive cells which were also positive for OX6, were increased in acutely-differentiated cells prepared from cortical tissue of aged, compared with young, rats (f: ***p < 0.001; student’s t-test for independent means).
In addition to the age-related cytokines, there is a wealth of evidence indicating that expression of cell surface markers of activation and co-stimulatory molecules are increased in brain tissue prepared from aged animals, and these changes are consistent with the evidence obtained from microarray analysis. Thus increases in expression of MHCII, CD68, CD40 [43,105,106,107,108] and CD80 and CD86 have been reported [106] in a number of brain areas, notably the hippocampus. However upregulation of at least some of the markers, for example MHCII and CD11b, are also observed in cortical tissue (*p < 0.05; **p < 0.01; student’s t-test for independent means; Figure 2d, e), where FACS analysis indicates that there is an increase in the number of CD11b-positive cells which are also positive for OX6 in samples prepared from cortical tissue (Figure 2f).
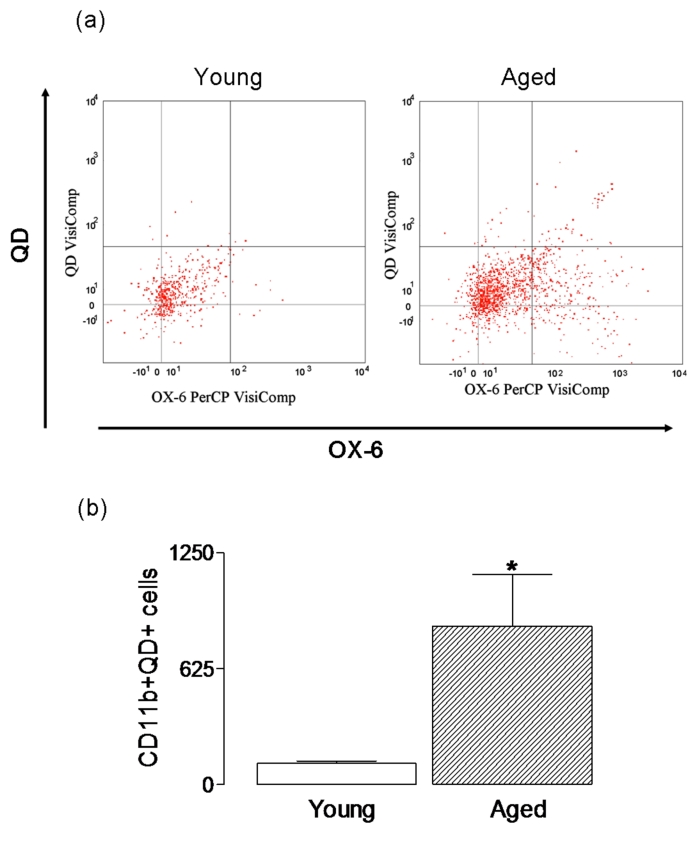
Few studies have directly compared phagocytic activity in microglia prepared from young and aged animals. However the presence of inclusions in microglia in area 17 of aged rhesus monkeys has been interpreted as an indication of an age-related increase in phagocytic activity [109] and this concurs with the data from this laboratory. We evaluated phagocytic activity in CD11b-positive cells prepared from aged, and young, rats using uptake of quantum dots, and the data indicated an age-related increase in their uptake suggesting that phagocytic activity is increased with age (*p < 0.05; student’s t-test for independent means; Figure 3). When analysis of phagocytosis of Aβ is considered, there are marked differences. It is argued that deficits in microglial function, like the function of other cells, occur with age [110]. Consistent with this, is the observation that microglia from aged mice internalize less Aβ than microglia from young mice [102], and the ability of microglia prepared from adult, compared with neonatal, brain to take up fibrillar Aβ is markedly reduced [54]. These findings suggest a decrease in phagocytic activity, at least in terms of Aβ. Similarly an age-related deficit in recruitment and internalization of myelin in a toxin model of demyelination has been described [111], while phagocytosis is ineffective in an animal model of AD [112] though the ability of cells to clear Aβ is restored following immunotherapy. While senescence of microglia is one possible explanation for the age-related reduction in phagocytosis of Aβ, a decrease in expression of a number of cell surface proteins which participate in Aβ phagocytosis including CD36, scavenger receptor A and RAGE has been reported; these changes have been linked with the age-related increase in Aβ accumulation in APP/PS1 transgenic mice, which is interpreted as a decrease in phagocytic activity [113].
Figure 3:
Uptake of quantum dots is increased in acutely-dissociated cells prepared from aged, compared with young, rats. Acutely dissociated cells were incubated in the presence or absence of negatively-charged, thioglycolic acid-capped cadmium-tellurium quantum dots (1 × 10−6M; 4–6 nm; emission wavelength of approximately 620 nm) for 15 minutes and uptake was assessed by FACS analysis. Representative dot plots indicated that the number of CD11b-positive cells, which were also positive for quantum dots, was greater in cells prepared from aged, compared with young, rats (a) and mean values indicated that the age-related change was significant (b; p < 0.05; student’s t-test for independent means).
Many investigators have suggested that microglial activation is detrimental, and certainly the evidence suggests that the deficit in neuronal and synaptic function which is a characteristic of age is attenuated when microglial activation is decreased [76,100,108,114]. However an alternative view is that senescent microglia are dysfunctional and their impaired ability to respond to insults is responsible for neurodegenerative changes. In support of this, degenerating, dystrophic microglia which are morphologically distinct from ‘normal’ microglia because they exhibit short gnarled processes or abnormally-twisted processes, have been detected in the brain of aged individuals and are associated with neurodegeneration [115]; oxidative stress has been suggested as a primary factor leading to degeneration of microglia [116]. Recent evidence has revealed that these dystrophic microglia, as distinct from activated microglia, are found adjacent to tau-positive degenerating neurons and, of particular interest, is the view that their appearance precedes the appearance of tau-associated pathology in AD [117]. This finding was based on analysis of post mortem tissue obtained from individuals at different disease stages. Dystrophic microglia, which were found to be ferritin-positive [118] were not associated with Aβ-containing plaques (in samples prepared from individuals with significant plaque load but no tau pathology) and indeed there was little evidence of microglial activation in these samples. This finding prompted the authors to conclude that Aβ did not cause microglial activation and that microglial activation was not responsible for tau pathology [117].
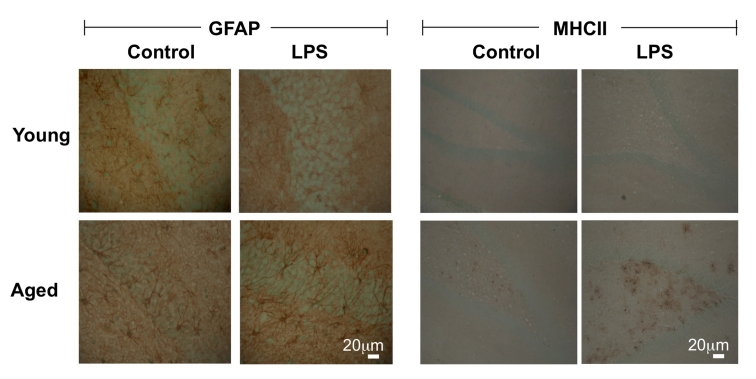
It has been proposed that increased expression of cell surface markers of microglia, without an accompanying increase in proinflammatory cytokine secretion, can occur in the brain with age and, in this case cells are referred to as ‘primed microglia’ [42]. The argument is that, under these circumstances, an activating signal will lead to a more immediate and exaggerated release of inflammatory mediators and that, in this state of heightened reactivity, specific triggers (eg Aβ in the case of AD) causes neurodegenerative changes. A number of observations are consistent with the view that primed microglia are a feature of age. For example, it has been shown by a number of groups that treatment of aged animals with LPS exerts a more profound effect on hippocampal-dependent behavioural tasks than it does in young animals and this has been coupled with increased hippocampal IL-1β concentration [119,120]. Similarly, clinical signs of LPS-induced sickness behavior and depressive behavior are enhanced in aged animals [105,121]. In addition, the LPS-induced increases in activation of microglia and astrocytes, assessed by MHCII and GFAP immunoreactivity respectively, were markedly greater in brain sections prepared from aged, compared with young, rats (Figure 4), and the number of IL-1-positive cells [122] and the production of inflammatory cytokines were similarly increased [123]. Interestingly, activation of TLR2 by PamCSK3 triggered greater release of IL-6 and TNFα from microglia obtained from aged, compared with young, mice; activation of TLR4 by LPS similarly induced an age-related increase in IL-6 but not TNFα [102].
Figure 4:
LPS exerts a greater effect on GFAP and MHCII immunoreactivity in hippocampus of aged, compared with young, rats. Treatment of young rats with LPS (100μg/kg intraperitoneally) increased GFAP and MHCII immunoreactivity in hippocampal tissue but the effect was more marked in aged rats. Age-related increases in immunoreactivity of both markers were also observed.
The effects of infection are more profound in aged, compared with young, animals [120,124] and there is evidence that the course of neurodegenerative diseases in humans is affected by systemic infection, which has been reported to precede ischemic attacks [125], or to precipitate relapse or even onset of MS [126]. Infection has also been reported to accelerate cognitive decline in patients with AD [127]. Indeed there is some evidence that infiltration of Chlamydia pneumonia into the brain may be a trigger in the disease [128]. These observations are consistent with the hypothesis that a single factor does not initiate the pathogenesis of disease but rather disease progression requires the confluence of two or more factors as proposed by Smith and colleagues [129] in the ‘two-hit hypothesis’ model. In this model, it is suggested that the combination of oxidative stress, arising from the age-related upregulation of microglial activation, and mitogenic dysregulation, arising from aberrant expression of cell cycle components in vulnerable cortical neurons, provides the neuropathogenic signal to initiate the disease process. Critically, it is argued, this requires the impaired anti-oxidative strategies which are observed with age.
Activation of glia in neurodegenerative disease
Some neurodegenerative diseases, notably AD and PD are definitively age-related; others, like amyotrophic lateral sclerosis (ALS) and Huntington’s Disease are less so. Yet neuroinflammation and microglial activation, often accompanied by astrocyte activation, appear to be common to most, if not all, neurodegenerative conditions [33,130,131,132]. In AD, activated microglia [133,134,135,136] and GFAP-positive astrocytes [137,138] cluster around amyloid-containing plaques in AD, with activated IL-1-positive microglia accumulated in the centre of this glial knot [139]. However the roles played by these cells in neurodegeneration is far from clear and the important question of whether activation of cells is a response to neurodegenerative processes or plays a role in triggering them remains unanswered. One group has proposed, based on findings in individuals with Down’s syndrome, that activation of astrocytes and microglia occurs in an age-dependent manner and precedes formation of Aβ-containing plaques [139,140]. In contrast, as mentioned above, others have concluded that microglial activation is not responsible for development of tau pathology [117]. In APP/PS1 mice there is evidence indicating that plaque development and the subsequent neuritic changes were followed by microglial activation suggesting that glial activation was a response to the pathogenic changes [141]. At present, there is no consensus regarding the role of glia in neurodegenerative diseases, or in AD in particular, and a fuller understanding of their neurobiology is required before significant progress in this area can be achieved.
Interactions between cells modulate glial function
Microglia express a vast array of receptors on their membrane [142] and therefore have the capacity to respond to a numerous factors including pro-and anti-inflammatory cytokines, growth factors, neurotransmitters and steroids, as well as pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs) which are released from injured cells. Perhaps the most potent activator of microglia in vitro is interferon (IFN)-γ which increases expression of cell surface markers of activation and triggers release of inflammatory cytokines and chemokines [43,53,143–145]; many of these changes are also induced by Aβ peptides [43]. Several inflammatory cytokines also impact on their activation state reflecting the presence of receptors for these cytokines on the microglial membrane [142]. However, microglial function is also influenced by interaction with adjacent cells including astrocytes and neurons, which endow the CNS with an immunosuppressive function, and also with infiltrating cells, particularly T cells [43]. The modulatory effect of astrocytes on microglia has been recognized for over a decade. In addition to their ability to induce ramification of amoeboid microglia [146,147,148], they inhibit LPS-induced upregulation of inducible nitric oxide synthase and NO production [149,150] and reduce microglial expression of MCHII and ICAM in a TGFβ-dependent manner [151]. Since astrocytes are known to release numerous bioactive agents like neurotrophins, anti-inflammatory cytokines and neurotransmitters, and since microglia express receptors for these agents, it is highly likely that the influence of astrocytes on microglia in situ, particularly in circumstances of stress, is a great deal more complex than current data from in vitro experiments suggest. In addition to the effect of astrocyte-derived soluble factors, direct interaction with microglia alters function. Thus recent evidence from this laboratory has also revealed that addition of astrocytes to a microglial culture attenuates the LPS-induced increase in inflammatory cytokine production and release and that this relies on an interaction between CD200 and its receptor (Cox et al. unpublished).
The interaction between neurons and microglia has a profound effect on microglial function. This was clearly demonstrated by the observation that in a mixed neuron-glial culture 10ng/ml LPS induced maximum production of NO, while concentrations of LPS up to 1mg/ml dose-dependently increased TNFα; after 1 month in culture, when almost all neurons had died, 1ng/ml LPS maximally increased both NO and TNFα. These findings indicate that microglial responses are downregulated by their interaction with neurons and that, at least in culture, this disinhibition is reversible [152]. Efforts to understand the mechanisms involved were sparked off by a review in 2002 [153] in which the modulating role played by interaction between cell membrane-associated ligands with their cognate receptors was discussed in myeloid cells. The authors suggested specifically that the interaction between CD200 which is expressed on neurons, and its cognate receptor (CD200R) which is expressed on microglia, helped to maintain microglia in a quiescent state [153]. This explained why mice deficient in CD200 displayed an exaggerated microglial activation in several models of tissue stress including facial nerve transaction [154], experimental autoimmune uveoretinitis[155], experimental autoimmune encephalomyelitis (EAE)[154], and in response to infection with Toxoplasma gondii [156]. In vitro analysis has demonstrated that addition of neurons to LPS-treated or Aβ-treated glial cultures attenuated the expression of cell surface markers of microglia and production of inflammatory cytokines, and that this effect was blocked by anti-CD200 antibody [107,157]. Consistently, an inverse correlation between expression of CD200 and expression of cell surface markers of microglial activation has been observed in hippocampal tissue prepared from aged, compared with young, rats and in tissue prepared from Aβ-treated rats [107,157]. A recent report highlighted the fact that a similar inverse correlation was observed in postmortem brain tissue obtained from individuals with AD. Interestingly CD200Fc has been shown to reduce disease severity in an animal model of multiple sclerosis, EAE [158]
The chemokine fractalkine (CX3CL1) which is expressed primarily on neurons [159,160] interacts with its receptor, which is expressed mainly on microglia [160] and this interaction also downregulates microglial activation in vitro and in vivo [161,162,163]. As has been described for CD200, the absence of fractalkine leads to increased symptomatology in models of neurodegenerative disease including EAE and Parkinson’s Disease and these changes are coupled with striking microglial activation [162]. Interestingly the age-related increase in microglial activation is accompanied by a decrease in expression of fractalkine, whereas intracerebroventricular injection of fractalkine attenuated the age-related increase in MHCII reactivity, MHCII mRNA and CD40 mRNA [163]. While a significant focus has been placed on understanding the impact of the interactions between CD200 and fractalkine and their respective receptors in modulating microglial activation, it is likely that other paired cell surface markers on neurons and microglia. These include the interaction between CD47 and signal regulatory protein (SIRP)-α [164]; expression of SIRPα is relatively restricted and found on neurons but also macrophages and dendritic cells [165] whereas expression of CD47 is widespread and found on both microglia and neurons [166]. The interaction has been shown to decrease phagocytosis of red blood cells by macrophages, while activation of SIRPα by a CD47 fusion protein decreased inflammatory cytokine production by dendritic cells [167] but whether a parallel effect is observed in microglia remains to be assessed. It has recently been suggested that the ability of CD45 to negatively regulate microglial function might be attributed to its interaction with CD22 which is a known ligand, and expressed on neurons [168]. Addition of neurons or conditioned medium from neurons to LPS-treated microglia inhibited production of TNFα and the effect of conditioned medium was blocked by neutralizing antibodies against CD22. Thus in addition to participating in cell-cell interactions, CD22 is secreted from neurons and, like fractalkine which exist in both membrane-bound and soluble forms, must be considered as an ‘off signal’. It is clear that a more detailed understanding of the interaction between microglia and other cells including T cells, astrocytes and neurons will offer possible therapeutic strategies for controlling microglial function.
Conclusion
The importance of glial function, particularly microglial function, in the neurodegenerative changes which occur with age and with disease has triggered a great deal of research in this area. A clear evaluation of the detrimental and protective functions of the different microglial phenotypes is likely to be of significance in identification of new strategies for the treatment of conditions in which neuroinflammatory changes play a significant role. Specifically a detailed dissection of the function of different phenotypes may also provide insights into how age-related detrimental changes might be limited, while an understanding of how ‘off’ signals can be modulated is likely to be important. At present, it appears that glia which release proteases, inflammatory cytokines, reactive oxygen and nitrogen species and DAMPs/PAMPs are probably ultimately damaging to neurons, whereas their contribution to maintenance of homeostasis combined with their ability to release anti-inflammatory cytokines and neurotrophins are beneficial. The significant challenge is identifying agents which can shift the balance between phenotypes and enable restoration of homeostasis.
References
- [1].Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- [2].Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- [3].Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- [4].Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- [5].Kielian T. Glial connexins and gap junctions in CNS inflammation and disease. J Neurochem. 2008;106:1000–1016. doi: 10.1111/j.1471-4159.2008.05405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- [7].Kuno R, Yoshida Y, Nitta A, Nabeshima T, Wang J, et al. The role of TNF-alpha and its receptors in the production of NGF and GDNF by astrocytes. Brain Res. 2006;1116:12–18. doi: 10.1016/j.brainres.2006.07.120. [DOI] [PubMed] [Google Scholar]
- [8].Aremu DA, Meshitsuka S. Some aspects of astroglial functions and aluminum implications for neurodegeneration. Brain Res Rev. 2006;52:193–200. doi: 10.1016/j.brainresrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [9].Darlington PJ, Podjaski C, Horn KE, Costantino S, Blain M, et al. Innate immune-mediated neuronal injury consequent to loss of astrocytes. J Neuropathol Exp Neurol. 2008;67:590–599. doi: 10.1097/NEN.0b013e3181772cf6. [DOI] [PubMed] [Google Scholar]
- [10].Walz W, Lang MK. Immunocytochemical evidence for a distinct GFAP-negative subpopulation of astrocytes in the adult rat hippocampus. Neurosci Lett. 1998;257:127–130. doi: 10.1016/s0304-3940(98)00813-1. [DOI] [PubMed] [Google Scholar]
- [11].Chen HL, Pistollato F, Hoeppner DJ, Ni HT, McKay RD, et al. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- [12].Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- [14].Messing A, Head MW, Galles K, Galbreath EJ, Goldman JE, et al. Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am J Pathol. 1998;152:391–398. [PMC free article] [PubMed] [Google Scholar]
- [15].Hagemann TL, Connor JX, Messing A. Alexander disease-associated glial fibrillary acidic protein mutations in mice induce Rosenthal fiber formation and a white matter stress response. J Neurosci. 2006;26:11162–11173. doi: 10.1523/JNEUROSCI.3260-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Steiner J, Bernstein HG, Bielau H, Berndt A, Brisch R, et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007;8:2. doi: 10.1186/1471-2202-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- [20].Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci Biobehav Rev. 2008;32:927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [21].Filosa A, Paixao S, Honsek SD, Carmona MA, Becker L, et al. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 2009;12:1285–1292. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- [23].Minkeviciene R, Ihalainen J, Malm T, Matilainen O, Keksa-Goldsteine V, et al. Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice. J Neurochem. 2008;105:584–594. doi: 10.1111/j.1471-4159.2007.05147.x. [DOI] [PubMed] [Google Scholar]
- [24].Duerson K, Woltjer RL, Mookherjee P, Leverenz JB, Montine TJ, et al. Detergent-insoluble EAAC1/EAAT3 aberrantly accumulates in hippocampal neurons of Alzheimer's disease patients. Brain Pathol. 2009;19:267–278. doi: 10.1111/j.1750-3639.2008.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Potier B, Turpin FR, Sinet PM, Rouaud E, Mothet JP, et al. Contribution of the d-Serine-Dependent Pathway to the Cellular Mechanisms Underlying Cognitive Aging. Front Aging Neurosci. 2:1. doi: 10.3389/neuro.24.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weinstock M, Luques L, Poltyrev T, Bejar C, Shoham S. Ladostigil prevents age-related glial activation and spatial memory deficits in rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.06.004. In press. [DOI] [PubMed] [Google Scholar]
- [28].Cowley TR, O'Sullivan J, Blau C, Deighan BF, Jones R, et al. Rosiglitazone attenuates the age-related changes in astrocytosis and the deficit in LTP. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [29].Tumani H, Shen G, Peter JB, Bruck W. Glutamine synthetase in cerebrospinal fluid, serum, and brain: a diagnostic marker for Alzheimer disease? Arch Neurol. 1999;56:1241–1246. doi: 10.1001/archneur.56.10.1241. [DOI] [PubMed] [Google Scholar]
- [30].Fang D, Li Z, Zhong-ming Q, Mei WX, Ho YW, et al. Expression of bystin in reactive astrocytes induced by ischemia/reperfusion and chemical hypoxia in vitro. Biochim Biophys Acta. 2008;1782:658–663. doi: 10.1016/j.bbadis.2008.09.007. [DOI] [PubMed] [Google Scholar]
- [31].Falsig J, Porzgen P, Lund S, Schrattenholz A, Leist M. The inflammatory transcriptome of reactive murine astrocytes and implications for their innate immune function. J Neurochem. 2006;96:893–907. doi: 10.1111/j.1471-4159.2005.03622.x. [DOI] [PubMed] [Google Scholar]
- [32].Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- [33].Rodriguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer's disease. Cell Death Differ. 2009;16:378–385. doi: 10.1038/cdd.2008.172. [DOI] [PubMed] [Google Scholar]
- [34].Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- [35].Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55:810–821. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- [37].Wu LJ, Zhuo M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J Neurophysiol. 2008;99:2026–2032. doi: 10.1152/jn.01210.2007. [DOI] [PubMed] [Google Scholar]
- [38].Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:290–300. doi: 10.1097/00004647-199703000-00006. [DOI] [PubMed] [Google Scholar]
- [39].Kuhn SA, van Landeghem FK, Zacharias R, Farber K, Rappert A, et al. Microglia express GABA(B) receptors to modulate interleukin release. Mol Cell Neurosci. 2004;25:312–322. doi: 10.1016/j.mcn.2003.10.023. [DOI] [PubMed] [Google Scholar]
- [40].Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- [41].Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- [43].Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40:139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- [44].Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158:1062–1073. doi: 10.1016/j.neuroscience.2008.07.031. [DOI] [PubMed] [Google Scholar]
- [45].Benveniste EN, Nguyen VT, O'Keefe GM. Immunological aspects of microglia: relevance to Alzheimer's disease. Neurochem Int. 2001;39:381–391. doi: 10.1016/s0197-0186(01)00045-6. [DOI] [PubMed] [Google Scholar]
- [46].Lu DY, Tang CH, Liou HC, Teng CM, Jeng KC, et al. YC-1 attenuates LPS-induced proinflammatory responses and activation of nuclear factor-kappaB in microglia. Br J Pharmacol. 2007;151:396–405. doi: 10.1038/sj.bjp.0707187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Venneti S, Wiley CA, Kofler J. Imaging microglial activation during neuroinflammation and Alzheimer's disease. J Neuroimmune Pharmacol. 2009;4:227–243. doi: 10.1007/s11481-008-9142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- [49].Vidyadaran S, Ooi YY, Subramaiam H, Badiei A, Abdullah M, et al. Effects of macrophage colony-stimulating factor on microglial responses to lipopolysaccharide and beta amyloid. Cell Immunol. 2009;259:105–110. doi: 10.1016/j.cellimm.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [50].Ponomarev ED, Novikova M, Maresz K, Shriver LP, Dittel BN. Development of a culture system that supports adult microglial cell proliferation and maintenance in the resting state. J Immunol Methods. 2005;300:32–46. doi: 10.1016/j.jim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- [51].Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- [52].Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- [53].Lyons A, Griffin RJ, Costelloe CE, Clarke RM, Lynch MA. IL-4 attenuates the neuroinflammation induced by amyloid-beta in vivo and in vitro. J Neurochem. 2007;101:771–781. doi: 10.1111/j.1471-4159.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- [54].Floden AM, Combs CK. Beta-amyloid stimulates murine postnatal and adult microglia cultures in a unique manner. J Neurosci. 2006;26:4644–4648. doi: 10.1523/JNEUROSCI.4822-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Szczepanik AM, Funes S, Petko W, Ringheim GE. IL-4, IL-10 and IL-13 modulate A beta(1--42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J Neuroimmunol. 2001;113:49–62. doi: 10.1016/s0165-5728(00)00404-5. [DOI] [PubMed] [Google Scholar]
- [56].Minogue AM, Lynch AM, Loane DJ, Herron CE, Lynch MA. Modulation of amyloid-beta-induced and age-associated changes in rat hippocampus by eicosapentaenoic acid. J Neurochem. 2007;103:914–926. doi: 10.1111/j.1471-4159.2007.04848.x. [DOI] [PubMed] [Google Scholar]
- [57].Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- [58].Gurley C, Nichols J, Liu S, Phulwani NK, Esen N, et al. Microglia and Astrocyte Activation by Toll-Like Receptor Ligands: Modulation by PPAR-gamma Agonists. PPAR Res. 20082008:453120. doi: 10.1155/2008/453120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Owens T. Toll-like receptors in neurodegeneration. Curr Top Microbiol Immunol. 2009;336:105–120. doi: 10.1007/978-3-642-00549-7_6. [DOI] [PubMed] [Google Scholar]
- [60].Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, et al. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- [61].Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, et al. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. 2006;26:12826–12837. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Salaria S, Badkoobehi H, Rockenstein E, Crews L, Chana G, et al. Toll-like receptor pathway gene expression is associated with human immunodeficiency virus-associated neurodegeneration. J Neurovirol. 2007;13:496–503. doi: 10.1080/13550280701558616. [DOI] [PubMed] [Google Scholar]
- [63].Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- [64].Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- [66].Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21:141–147. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- [67].Constantinescu CS, Tani M, Ransohoff RM, Wysocka M, Hilliard B, et al. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem. 2005;95:331–340. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- [68].Zeinstra E, Wilczak N, De Keyser J. Reactive astrocytes in chronic active lesions of multiple sclerosis express co-stimulatory molecules B7-1 and B7-2. J Neuroimmunol. 2003;135:166–171. doi: 10.1016/s0165-5728(02)00462-9. [DOI] [PubMed] [Google Scholar]
- [69].Laureys G, Clinckers R, Gerlo S, Spooren A, Wilczak N, et al. Astrocytic beta(2)-adrenergic receptors: from physiology to pathology. Prog Neurobiol. 2010;91:189–199. doi: 10.1016/j.pneurobio.2010.01.011. [DOI] [PubMed] [Google Scholar]
- [70].De Keyser J, Laureys G, Demol F, Wilczak N, Mostert J, et al. Astrocytes as potential targets to suppress inflammatory demyelinating lesions in multiple sclerosis. Neurochem Int. 2010;57:446–50. doi: 10.1016/j.neuint.2010.02.012. [DOI] [PubMed] [Google Scholar]
- [71].Mostert JP, Admiraal-Behloul F, Hoogduin JM, Luyendijk J, Heersema DJ, et al. Effects of fluoxetine on disease activity in relapsing multiple sclerosis: a double-blind, placebo-controlled, exploratory study. J Neurol Neurosurg Psychiatry. 2008;79:1027–1031. doi: 10.1136/jnnp.2007.139345. [DOI] [PubMed] [Google Scholar]
- [72].Hayakawa N, Kato H, Araki T. Age-related changes of astorocytes, oligodendrocytes and microglia in the mouse hippocampal CA1 sector. Mech Ageing Dev. 2007;128:311–316. doi: 10.1016/j.mad.2007.01.005. [DOI] [PubMed] [Google Scholar]
- [73].Goss JR, Finch CE, Morgan DG. Age-related changes in glial fibrillary acidic protein mRNA in the mouse brain. Neurobiol Aging. 1991;12:165–170. doi: 10.1016/0197-4580(91)90056-p. [DOI] [PubMed] [Google Scholar]
- [74].Kohama SG, Goss JR, Finch CE, McNeill TH. Increases of glial fibrillary acidic protein in the aging female mouse brain. Neurobiol Aging. 1995;16:59–67. doi: 10.1016/0197-4580(95)80008-f. [DOI] [PubMed] [Google Scholar]
- [75].Cotrina ML, Nedergaard M. Astrocytes in the aging brain. J Neurosci Res. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- [76].Loane DJ, Deighan BF, Clarke RM, Griffin RJ, Lynch AM, et al. Interleukin-4 mediates the neuroprotective effects of rosiglitazone in the aged brain. Neurobiol Aging. 2009;30:920–931. doi: 10.1016/j.neurobiolaging.2007.09.001. [DOI] [PubMed] [Google Scholar]
- [77].Sibson NR, Lowe JP, Blamire AM, Martin MJ, Obrenovitch TP, et al. Acute astrocyte activation in brain detected by MRI: new insights into T(1) hypointensity. J Cereb Blood Flow Metab. 2008;28:621–632. doi: 10.1038/sj.jcbfm.9600549. [DOI] [PubMed] [Google Scholar]
- [78].McCall MA, Gregg RG, Behringer RR, Brenner M, Delaney CL, et al. Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc Natl Acad Sci U S A. 1996;93:6361–6366. doi: 10.1073/pnas.93.13.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Menet V, Gimenez YRM, Sandillon F, Privat A. GFAP null astrocytes are a favorable substrate for neuronal survival and neurite growth. Glia. 2000;31:267–272. doi: 10.1002/1098-1136(200009)31:3<267::aid-glia80>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [80].Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- [81].Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- [82].Yu AC, Lau LT. Expression of interleukin-1 alpha, tumor necrosis factor alpha and interleukin-6 genes in astrocytes under ischemic injury. Neurochem Int. 2000;36:369–377. doi: 10.1016/s0197-0186(99)00145-x. [DOI] [PubMed] [Google Scholar]
- [83].Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- [84].Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010;1:6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ting KK, Brew BJ, Guillemin GJ. Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer's disease. J Neuroinflammation. 2009;6:36. doi: 10.1186/1742-2094-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cai ZY, Yan Y, Chen R. Minocycline reduces astrocytic reactivation and neuroinflammation in the hippocampus of a vascular cognitive impairment rat model. Neurosci Bull. 2010;26:28–36. doi: 10.1007/s12264-010-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ruan L, Kang Z, Pei G, Le Y. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer's disease. Curr Alzheimer Res. 2009;6:531–540. doi: 10.2174/156720509790147070. [DOI] [PubMed] [Google Scholar]
- [88].von Bernhardi R, Tichauer JE, Eugenin J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- [89].Brown DR. Role of microglia in age-related changes to the nervous system. Scientific World Journal. 2009;9:1061–1071. doi: 10.1100/tsw.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Prolla TA. DNA microarray analysis of the aging brain. Chem Senses. 2002;27:299–306. doi: 10.1093/chemse/27.3.299. [DOI] [PubMed] [Google Scholar]
- [91].Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- [92].Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, et al. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Avramopoulos D, Szymanski M, Wang R, Bassett S. Gene expression reveals overlap between normal aging and Alzheimer's disease genes. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Campuzano O, Castillo-Ruiz MM, Acarin L, Castellano B, Gonzalez B. Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J Neurosci Res. 2009;87:2484–2497. doi: 10.1002/jnr.22074. [DOI] [PubMed] [Google Scholar]
- [95].Gelinas DS, McLaurin J. PPAR-alpha expression inversely correlates with inflammatory cytokines IL-1beta and TNF-alpha in aging rats. Neurochem Res. 2005;30:1369–1375. doi: 10.1007/s11064-005-8341-y. [DOI] [PubMed] [Google Scholar]
- [96].Godbout JP, Johnson RW. Interleukin-6 in the aging brain. J Neuroimmunol. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- [97].Lynch AM, Lynch MA. The age-related increase in IL-1 type I receptor in rat hippocampus is coupled with an increase in caspase-3 activation. Eur J Neurosci. 2002;15:1779–1788. doi: 10.1046/j.1460-9568.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- [98].Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [99].Martin DS, Lonergan PE, Boland B, Fogarty MP, Brady M, et al. Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J Biol Chem. 2002;277:34239–34246. doi: 10.1074/jbc.M205289200. [DOI] [PubMed] [Google Scholar]
- [100].Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, et al. Role of interleukin4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem. 2005;280:9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- [101].Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- [102].Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, et al. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Smith P, Dunne DW, Fallon PG. Defective in vivo induction of functional type 2 cytokine responses in aged mice. Eur J Immunol. 2001;31:1495–1502. doi: 10.1002/1521-4141(200105)31:5<1495::AID-IMMU1495>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- [104].Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. J Leukoc Biol. 2010;87:1001–1009. doi: 10.1189/jlb.0809542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- [106].Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, et al. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- [107].Lyons A, McQuillan K, Deighan BF, O'Reilly JA, Downer EJ, et al. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.05.060. [DOI] [PubMed] [Google Scholar]
- [108].Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, et al. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging. 2007;28:845–855. doi: 10.1016/j.neurobiolaging.2006.04.006. [DOI] [PubMed] [Google Scholar]
- [109].Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec. 1991;229:384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- [110].Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [111].Zhao C, Li WW, Franklin RJ. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiol Aging. 2006;27:1298–1307. doi: 10.1016/j.neurobiolaging.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [112].Frautschy SA, Cole GM, Baird A. Phagocytosis and deposition of vascular betaamyloid in rat brains injected with Alzheimer beta-amyloid. Am J Pathol. 1992;140:1389–1399. [PMC free article] [PubMed] [Google Scholar]
- [113].Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective betaamyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Downer EJ, Cowley TR, Lyons A, Mills KH, Berezin V, et al. A novel anti-inflammatory role of NCAM-derived mimetic peptide, FGL. Neurobiol Aging. 31:118–128. doi: 10.1016/j.neurobiolaging.2008.03.017. [DOI] [PubMed] [Google Scholar]
- [115].Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- [116].Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Front Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- [117].Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lopes KO, Sparks DL, Streit WJ. Microglial dystrophy in the aged and Alzheimer's disease brain is associated with ferritin immunoreactivity. Glia. 2008;56:1048–1060. doi: 10.1002/glia.20678. [DOI] [PubMed] [Google Scholar]
- [119].Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [121].Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, et al. Aging Exacerbates Depressive-like Behavior in Mice in Response to Activation of the Peripheral Innate Immune System. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, et al. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, et al. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2009. [DOI] [PMC free article] [PubMed]
- [125].McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Stratton CW, Wheldon DB. Multiple sclerosis: an infectious syndrome involving Chlamydophila pneumoniae. Trends Microbiol. 2006;14:474–479. doi: 10.1016/j.tim.2006.09.002. [DOI] [PubMed] [Google Scholar]
- [127].Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, et al. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Stallings TL. Association of Alzheimer's disease and Chlamydophila pneumoniae. J Infect. 2008;56:423–431. doi: 10.1016/j.jinf.2008.03.013. [DOI] [PubMed] [Google Scholar]
- [129].Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: an update. Biochim Biophys Acta. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- [130].McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- [131].Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [132].Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- [133].McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- [134].Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in Alzheimer's disease. Mol Neurobiol. 41:115–128. doi: 10.1007/s12035-010-8106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- [136].D'Andrea MR, Cole GM, Ard MD. The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol Aging. 2004;25:675–683. doi: 10.1016/j.neurobiolaging.2003.12.026. [DOI] [PubMed] [Google Scholar]
- [137].Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- [138].Koistinaho M, Lin S, Wu X, Esterman M, Koger D, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- [139].Mrak RE, Griffin WS. Potential inflammatory biomarkers in Alzheimer's disease. J Alzheimers Dis. 2005;8:369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- [140].Sheng JG, Mrak RE, Griffin WS. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol (Berl) 1998;95:229–234. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- [141].Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Benveniste EN, Nguyen VT, Wesemann DR. Molecular regulation of CD40 gene expression in macrophages and microglia. Brain Behav Immun. 2004;18:7–12. doi: 10.1016/j.bbi.2003.09.001. [DOI] [PubMed] [Google Scholar]
- [144].Nguyen VT, Benveniste EN. IL-4-activated STAT-6 inhibits IFN-gamma-induced CD40 gene expression in macrophages/microglia. J Immunol. 2000;165:6235–6243. doi: 10.4049/jimmunol.165.11.6235. [DOI] [PubMed] [Google Scholar]
- [145].Gasic-Milenkovic J, Dukic-Stefanovic S, Deuther-Conrad W, Gartner U, Munch G. Beta-amyloid peptide potentiates inflammatory responses induced by lipopolysaccharide, interferon -gamma and 'advanced glycation endproducts' in a murine microglia cell line. Eur J Neurosci. 2003;17:813–821. doi: 10.1046/j.1460-9568.2003.02506.x. [DOI] [PubMed] [Google Scholar]
- [146].Tanaka J, Maeda N. Microglial ramification requires nondiffusible factors derived from astrocytes. Exp Neurol. 1996;137:367–375. doi: 10.1006/exnr.1996.0038. [DOI] [PubMed] [Google Scholar]
- [147].Wirjatijasa F, Dehghani F, Blaheta RA, Korf HW, Hailer NP. Interleukin-4, interleukin-10, and interleukin-1-receptor antagonist but not transforming growth factor-beta induce ramification and reduce adhesion molecule expression of rat microglial cells. J Neurosci Res. 2002;68:579–587. doi: 10.1002/jnr.10254. [DOI] [PubMed] [Google Scholar]
- [148].Eder C, Schilling T, Heinemann U, Haas D, Hailer N, et al. Morphological, immunophenotypical and electrophysiological properties of resting microglia in vitro. Eur J Neurosci. 1999;11:4251–4261. doi: 10.1046/j.1460-9568.1999.00852.x. [DOI] [PubMed] [Google Scholar]
- [149].Vincent VA, Tilders FJ, Van Dam AM. Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: a role for transforming growth factor beta. Glia. 1997;19:190–198. doi: 10.1002/(sici)1098-1136(199703)19:3<190::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [150].Vincent VA, Van Dam AM, Persoons JH, Schotanus K, Steinbusch HW, et al. Gradual inhibition of inducible nitric oxide synthase but not of interleukin-1 beta production in rat microglial cells of endotoxin-treated mixed glial cell cultures. Glia. 1996;17:94–102. doi: 10.1002/(SICI)1098-1136(199606)17:2<94::AID-GLIA2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [151].Hailer NP, Heppner FL, Haas D, Nitsch R. Astrocytic factors deactivate antigen presenting cells that invade the central nervous system. Brain Pathol. 1998;8:459–474. doi: 10.1111/j.1750-3639.1998.tb00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Chang RC, Chen W, Hudson P, Wilson B, Han DS, et al. Neurons reduce glial responses to lipopolysaccharide (LPS) and prevent injury of microglial cells from over-activation by LPS. J Neurochem. 2001;76:1042–1049. doi: 10.1046/j.1471-4159.2001.00111.x. [DOI] [PubMed] [Google Scholar]
- [153].Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- [154].Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- [155].Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, et al. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. 2002;161:1669–1677. doi: 10.1016/S0002-9440(10)64444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Deckert M, Sedgwick JD, Fischer E, Schluter D. Regulation of microglial cell responses in murine Toxoplasma encephalitis by CD200/CD200 receptor interaction. Acta Neuropathol. 2006;111:548–558. doi: 10.1007/s00401-006-0062-z. [DOI] [PubMed] [Google Scholar]
- [157].Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, et al. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Liu Y, Bando Y, Vargas-Lowy D, Elyaman W, Khoury SJ, et al. CD200R1 agonist attenuates mechanisms of chronic disease in a murine model of multiple sclerosis. J Neurosci. 2010;30:2025–2038. doi: 10.1523/JNEUROSCI.4272-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon KB. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol. 1999;163:1628–1635. [PubMed] [Google Scholar]
- [160].Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]
- [162].Ransohoff RM, Liu L, Cardona AE. Chemokines and chemokine receptors: multipurpose players in neuroinflammation. Int Rev Neurobiol. 2007;82:187–204. doi: 10.1016/S0074-7742(07)82010-1. [DOI] [PubMed] [Google Scholar]
- [163].Lyons A, Lynch AM, Downer EJ, Hanley R, O'Sullivan JB, et al. Fractalkineinduced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110:1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- [164].Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- [165].Adams S, van der Laan LJ, Vernon-Wilson E, Renardel de Lavalette C, Dopp EA, et al. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol. 1998;161:1853–1859. [PubMed] [Google Scholar]
- [166].Ohnishi H, Kaneko Y, Okazawa H, Miyashita M, Sato R, et al. Differential localization of Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 and CD47 and its molecular mechanisms in cultured hippocampal neurons. J Neurosci. 2005;25:2702–2711. doi: 10.1523/JNEUROSCI.5173-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Latour S, Tanaka H, Demeure C, Mateo V, Rubio M, et al. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167:2547–2554. doi: 10.4049/jimmunol.167.5.2547. [DOI] [PubMed] [Google Scholar]
- [168].Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, et al. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46:369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]