Abstract
Alzheimer’s disease (AD) is a progressive, age-related neurodegenerative disorder which first manifests as profound memory dysfunction. The majority of cases are idiopathic, although advanced age is the greatest risk factor for AD. Recent evidence suggests that pre-fibrillar soluble amyloid-beta (Aβ) underlies an early, progressive loss of synapses that is a hallmark of AD. One of the downstream effects mediated by soluble Aβ aggregates is the hyperactivation of the phosphatase calcineurin (CaN). This important phosphatase is abundant in the nervous system and intimately involved in the mechanisms of memory as well as the immune response. Such a duality places CaN at the crux of neuroimmunomodulation processes. In the present review, we briefly summarize the role of CaN in physiological aging and discuss how CaN hyperactivity could cause the memory impairment, neuroinflammation, and neuronal death that are pathological mechanisms of AD.
Keywords: Alzheimer’s, amyloid beta, calcineurin, inflammation, neurodegeneration
Calcineurin (CaN), or protein phosphatase 2B (PP2B), is a calcium (Ca2+)-sensitive serine/threonine phosphatase originally isolated from mammalian brain [1] and is abundant in the central nervous system (CNS). It is a heteromeric protein consisting of a catalytic subunit (CaNA) and a regulatory subunit (CaNB) [2]. Although the catalytic site is structurally similar to protein phosphatase-1 (PP1) and protein phosphatase-2A (PP2A), the regulatory CaNB shares 30–50% sequence homology with calmodulin (CaM) [3]. This quality makes CaN unique because it is directly activated by CaM, making it the only cellular phosphatase that is exceptionally responsive to intracellular Ca2+ levels [4]. As such, Ca2+ fluctuation is capable of activating powerful cellular processes involving CaN activation that influence cell survival and growth. FK506 is an immunosuppressant drug that binds to endogenous immunophilin (FKBP12), and this complex successfully inhibits CaNs phosphatase activity [5–6]. The use of this compound, coupled with antisense RNA technology and transgenic models have only recently allowed investigation into the roles of CaN in cellular signaling. Given its original purification from bovine brain, it was somewhat surprising to discover that CaN is responsible for a number of important cellular processes in a wide variety of tissues.
CaN is found in many diverse cell types where it responds to the binding of activated CaM in multiple ways, including the modulation of immune responses [7], the formation and remodeling of muscle [8], neuronal plasticity [9–10], and cell death [11–12]. CaN expression is particularly high in neurons – it constitues approximately 1% of total neural protein [2]. Astrocytes and microglia also contain CaN [13–14] Phosphatase substrates include the phosphorylated forms of nuclear factor of activated T- cells (NFAT) [15]; cAMP response element binding (CREB) [4]; PP1 [4, 16]; and Bcl-2 associated death protein (BAD) [4, 11–12]. The effects of CaN hyperactivity on these downstream proteins and the evidence for the involvement of these pathways in AD pathogenesis will be the focus of this review.
I. CALCINEURIN IN THE AGING CNS
The CNS is encumbered with high-energy metabolism requirements and low endogenous antioxidant defenses [17]. Post-mitotic CNS neurons are remarkably long-lived in comparison to other cell types such as epithelial cells. With a few exceptions of continued proliferation in certain brain regions [18–19], the average neuron in the aged brain has been exposed to decades of oxidative insults. The processes of aging exacerbate an already unfavorable environment: evidence of reduced mitochondrial function and increased oxidative stress has been extensively documented in the aged brain [17, 20]. Together, these factors decrease cellular ability to tightly regulate Ca2+. This section will discuss how this impinges on the various cell types found in the CNS, and how the sluggishness of Ca2+ dynamics in the aged brain predisposes it to functional alterations.
Impact on synaptic function
It has been known for many years that neurons from the aged brain have altered Ca2+ currents in comparison to young [21], and evidence suggests this is partly due to increased numbers of voltage gated Ca2+ channels (VGCCs) [22]. More recent studies posit that in aged hippocampal neurons, Ca2+ entry through VGCCs augments a pronounced secondary Ca2+ flux from intracellular stores in endoplasmic reticulum through ryanodine receptor [23]. Such perturbations in ion concentration are troublesome for neurons, as numerous neurotransmission pathways are governed either by intracellular Ca2+ levels or downstream kinases and phosphatases. Strict governance of Ca2+ is especially critical in hippocampal pathways, which employ Ca2+ dynamics to induce term potentiation (LTP) and long-term depression (LTD), synaptic activity-dependent processes widely held to be the molecular correlates of learning and memory [16, 24–25].
Compared to other classical Ca2+ signaling proteins activated by CaM, e.g. calcium/calmodulin dependent protein kinase II (CaMKII), CaN is much more sensitive to subtle rises in intracellular Ca2+ levels. Due to the differential activation of CaMKII and PP2B depending on Ca2+ concentration [26], the elevated resting Ca2+ environment of the aged brain promotes mechanisms of negative plasticity [27]. One of these mechanisms is an increase in CaN expression and activity. CaN is then able to activate additional phosphatases, such as PP1, which further potentiates LTD and decreases neurotransmission and synaptic strength [16, 28]. In the aged rats, impaired performance in the Morris water maze is coincident with elevated CaN expression and activity in the hippocampus [29]. CaN further exacerbates the already dysregulated Ca2+ homeostasis in aged brain by enhancing the activity of VGCCs in hippocampal cultures [30]. Recent work in partially dissociated hippocampal “zipper” slices from young, middle-aged, and old rats show that this phenomenon also occurs in vivo, and suggests that CaN may directly activate VGCCs [31].
Overexpression, inhibition, and knock down of the phosphatase have helped elucidate what the effects of increased CaN activity in the aging brain might be. Targeted overexpression in the forebrain of mice impaires the transition from short-term to long-term memory as well as an intermediate form of LTP [32–33]. Conditional genetic knockout of CaN in mouse forebrain results in impairment within a specific subset of hippocampal-dependent tasks including working and episodic memory [34]. However, knockdown of CaN expression with antisense oligonucleotides results in the facilitation of LTP and improved performance in the Morris water maze [35]. Similarly, partial CaN inhibition with a tunable, inducible rtTA system also facilitates LTP and performance in a hippocampal-dependent behavioral test [36]. Collectively, these studies suggest that equilibrium between positive and negative plasticity is critical for proper cognitive function; and that an appropriate level of CaN activity is imperative. Electrophysiological studies suggest that LTD is more likely than LTP in the aged rat brain, but that this imbalance is corrected by inhibiting CaN [37]. Downstream of LTP, CREB phosphorylation at serine residue 133 induces the translocation of pCREB to the nucleus where it transcribes genes that produce proteins necessary for synaptic maintenance and formation [38]. CaN hyperactivity results in the dephosphorylation of pCREB, precluding protein synthesis that normally occurs during late-stage LTP [4, 39].
Impact on astroglia
The deleterious effects of CaN hyperactivity are not limited to the neurons. Astrocytes and microglia are activated during aging, even in the absence of diagnosed pathology [40]. Astrocytes – the most common non-neuronal cell in the CNS – are responsible for the support and maintenance of an appropriate neuronal environment [41]. Their function may be compromised when Ca2+ is dysregulated, as spatiotemporal Ca2+ waves act as a form of long-range signaling between glial cells [42–43]. In healthy neural tissue CaN is found primarily in neurons, with considerably weaker expression in astrocytes [44]. However, immunohistochemical analyses of aged murine hippocampi show intense CaN reactivity in activated astrocytes [45]. In vitro, overexpression of CaN has been shown to be a causative factor in the activation cascade and phenotype, likely through its regulation of several astrocyte-related growth factors and cytokines [45]. CaN dephosphorylates NFAT, allowing the cytosolic component (NFATc) to translocate to the nucleus where it binds its cognate DNA sequence on promoter regions, thus enhancing the transcription of genes involved in cytokine production and inflammation [15; 46]. NFATc isoforms are rapidly shuttled out of the nucleus, unless intracellular Ca2+ is persistently elevated [47], which is known to occur in aging [27, 30]. In astrocytes, NFAT is involved in mediating neuroinflammatory processes due to injury, disease, and aging [48–50].
There is also evidence that microglia, the resident immune cells of the brain, play an active role in neurodegeneration [51]. Although they account for a small proportion of the CNS cells, they have a major role in inflammation by releasing and responding to a number of cytokines instrumental in astrocyte activation [52] Since microglia also express NFAT [14] it is likely that the microglia inflammation cascade may be initiated, in part, by increased CaN signaling that takes place during aging. Taken together, the evidence suggests that CaN hyperactivity has a possible role in synaptotoxicity, neuronal dysfunction, astrogliosis, and inflammation.
II. CALCINEURIN AND AD PATHOGENESIS
The original “Amyloid Hypothesis” predicted that altered processing of amyloid precursor protein (APP), or clearance of resulting Aβ resulted in plaque deposition and AD symptoms, but this proposal has undergone a revision in recent years. A large body of evidence now indicates that soluble oligomeric Aβ is behind the earliest cognitive deficits [53]. Indeed, the proposition that small Aβ aggregates are able to affect cognition by inducing synaptic dysfunction and loss has received robust experimental confirmation in the last decade. This section will discuss how certain species of Aβ are able to hyperactivate CaN via its impact on intracellular Ca2+ signaling.
Data gathered from in vitro, ex vivo, in vivo, have furthered our mechanistic understanding of how certain species of Aβ perturb Ca2+ dynamics, resulting in hyperactivation of CaN. Together, clinical and basic researches suggest that the subsequent dephosphorylation of CaN substrates can impact gene transcription, cell death, ion channel activity, and synaptic integrity.
Oligomeric Aβ perturbs Ca2+ dynamics
One explanation for Aβ's augmentation of CaN activity is its apparent ability to provoke changes in the level of intracellular Ca2+; certain aggregate species are able to act as Ca2+ channels in synthetic bilayer membranes [54]. Live Ca2+ imaging of SY5Y human neuroblastomas demonstrates that oligomeric Aβ is the only species that appreciably augments the concentration of cytosolic Ca2+ by disrupting the cellular membrane. This increase was reduced but not abolished when the experiment was performed in Ca2+ free conditions, with 30% of the rise coming from internal stores [55]. Whatever the source, these studies suggest that only oligomers should be capable of upregulating CaN activity, via the Ca2+ increase. Indeed, only oligomers raise intracellular Ca2+, CaN hyperactivity, and CaN dependent cell death in cell cultures [56–57]. Multiphoton Ca2+ imaging of AD mice has revealed the extent of Ca2+ dysregulation in vivo. In aged double transgenic mice (APP/PS1) with cortical plaques, 20% of the neurites contained elevated resting Ca2+ levels, much greater than the young double mutants and significantly higher than the 5% increase in aged wild-type mice or single mutants. Observed Ca2+ overload correlated with the proximity to Aβ plaques [58]. Given the importance of Ca2+ signaling in mechanisms of synaptic plasticity [16, 24–25, 59–60], such dysregulation can have negative consequences on neural networks. This may be due in part to the cleavage of CaN by calpain, which is highly active in AD brain [61]. The truncated form maintains the autoinhibitory region, and thus is still dependent on CaM to be activated. In temporal cortex of AD brain, this particularly active, 57-kDa calpain-cleaved isoform is detectable by immunoblot. In the presence of CaM, in vitro phosphatase activity is enhanced following cleavage [62]. A recent publication corroborates these results, reporting a 2-fold increase in the level of a 54-kDa fragment of CaN in the nuclear fraction of AD cortex [63].
Oligomeric Aβ enhances can activity and signaling
Synaptic dysfunction and loss
The outcomes of perturbed Ca2+ dynamics are particularly detrimental for neurotransmission in synaptic spines, which rely on appropriate spatio-temporal Ca2+ entry to make synaptic modifications [64]. These are coincidentally where oligomeric species bind on cultured hippocampal neurons [65–66], where they are able to alter the shape, size, and protein composition of the post-synaptic densities [67]. Aβ disrupts synaptic function as well as structure. Application of oligomeric Aβ counteracts the increase in AMPA phosphorylation that normally occurs following tetanic stimulation of rat hippocampal slices, precluding the expression of early LTP [68]. Synthetic Aβ inhibits late phase LTP in a CaN-dependent fashion during electrophysiological recordings [69–70]. Soluble oligomeric Aβ facilitates electrically evoked LTD and results in a 33% reduction of dendritic spines in organotypic hippocampal cultures. Both outcomes are preventable by inhibiting CaN activity [72]. Collectively, these studies hint that Aβ-mediated activation of CaN promotes LTD over LTP, possibly through a CaN/PP1 phosphatase cascade [16, 71, 59]. Activation of PP1 leads to dephosphorylation of phospho-CaMKII and post-synaptic AMPA receptors, decreasing neurotransmission. As discussed previously, this important balance between positive and negative plasticity is already perturbed in the aged brain [27]. Additional exacerbation by oligomeric Aβ and the resultant increase in synaptic CaN activity could putatively explain the pervasive synaptic loss believed to underlie the early symptoms of AD.
Besides shifting the thresholds of LTP and LTD, CaN hyperactivation alters downstream pathways; such as dephosphorylation/deactivation of the transcription factor CREB. Under normal conditions, LTP expression results in the phosphorylation of CREB and the transcription of genes leading to long-term changes in synaptic strength [38]. In vitro experiments have shown that pCREB levels as well as its transcriptional activity are diminished in a CaN-dependent fashion following treatment with oligomeric Aβ. The same study reported that hippocampal pCREB immunoreactivity is reduced in the Tg2576 murine model of AD, but is restored by treatment with FK506 [57]. This animal model produces high levels of Aβ and first displays behavioral impairments at five months of age, coincident with the onset of elevated CaN activity [73]. Acute inhibition of CaN improved the performance of these animals on a hippocampal-dependent fear conditioning paradigm [73] and novel object recognition as well [74]. Wild-type mice given a single intracerebroventricular injection of oligomeric Aβ exhibited similar deficits in the fear-conditioning paradigm, again this was reversible with FK506 [70]. These studies suggest that some of the behavioral dysfunction in AD mouse models could be explained by CaN hyperactivity and its subsequent effects on pCREB and synaptic plasticity. One small study of autopsy tissue supports this hypothesis – an analysis of CREB and pCREB levels in human tissue show that amounts of pCREB are significantly lower in the AD hippocampus [75]. While this publication did not investigate the possible involvement of CaN, this report of decreased pCREB is circumstantial evidence that fits within the schematic of CaN-mediated cognitive dysfunction in AD.
Neuroinflammation
Inflammatory markers, such as astrogliosis and reactive microglia are found in aged and AD brain [76]. Some of these processes may be mediated by CaN, which dephosphorylates NFAT, allowing its translocation to the nucleus where it promotes the transcription of genes involved in cytokine production and inflammation [15]. Application of oligomeric Aβ increases NFAT activation in primary rat astrocyte cultures. This treatment also results in the loss of dendritic spines, simplification of dendritic arborizations, and neuritic dystrophies through a CaN/NFAT-dependent mechanism [63]. Other in vitro work in astrocytes cultures showed that Aβ oligomers cause a significant reduction in excitatory amino acid transporter 2 (EAAT2) protein levels in astrocyte cultures, theoretically leaving extracellular glutamate levels high and increasing the likelihood of excitotoxic cell death. Inhibition of NFAT prevented Aβ-mediated elevation in glutamate and cell death [50].
Clincally, certain isoforms of NFAT (NFATc1 and 3) are increased in the nuclear fraction from AD hippocampal homogenate. Their localization is an indirect indicator that the protein is active, since they must be dephosphorylated by CaN to access the nucleus. These values of nuclear NFATc correlate with levels of soluble Aβ as well as Mini-Mental State Exam scores (MMSE), a standard measure of cognitive function [50].
Cell death
Application of Aβ is well known to promote apoptosis in neuronal cultures [78]. This rigorously controlled process is distinct from necrotic cell death and is heavily dependent on cellular signaling. One such pathway that leads to an apoptotic outcome is the CaN-mediated dephosphorylation of pBAD. Dephosphorylated BAD is able to dissociate from scaffolding proteins and translocate to the mitochondria, where it forms a dimer with another pro-apoptotic protein (Bcl-x(L)), triggering cytochrome c release and thus initiating programmed cell death [11]. SY5Y human neuroblastoma cells treated with increasing concentrations of oligomeric Aβ exhibit a CaN-dependent dose-dependent decrease in pBAD levels [57]. Treatment of primary cortical neurons with synthetic Aβ peptides increases CaN activity; reduces the level of phosphorylated BAD; and increases the amount of BAD found in the mitochondria [78]. The effects on cortical neurons were also attenuated by CaN inhibition, suggesting that some of the neurodegeneration seen in AD may be due CaN dephosphorylation of BAD.
III. Conclusions
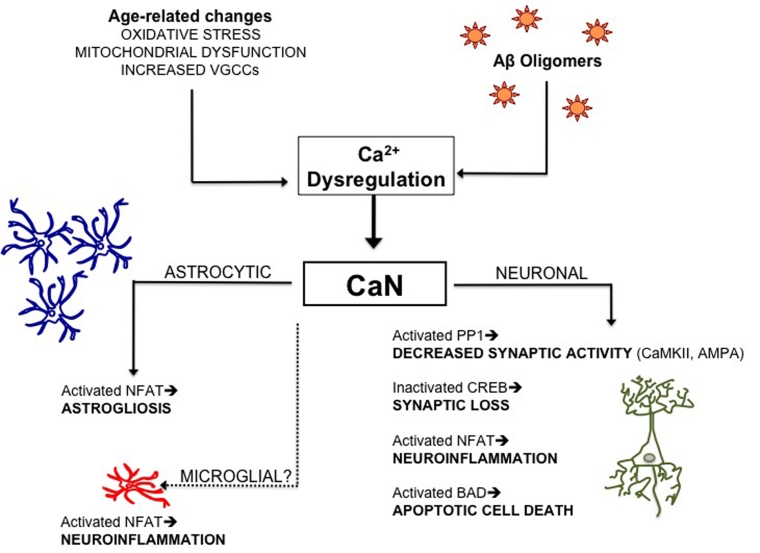
The evidence presented in this review conceptually link Aβ-mediated Ca2+ dysregulation, CaN hyperactivation, decreased synaptic plasticity, cell death, and neuroinflammation. Human studies show that these processes occur in both early- and late-onset AD, and to some degree in ‘normal’ aging. Indeed, the greatest risk factor for developing AD is increasing age. While this is the case for many neurodegenerative conditions and may be a confounded correlation, it does suggest that something about aging neurons renders them especially susceptible to the devastation of AD mechanisms. Aged neurons are unable to tightly control Ca2+ levels, a problem that is exacerbated by the presence of oligomeric Aβ. In vitro, ex vivo, and animal models have elucidated possible reasons for oligomeric Aβ-induced dysfunction and toxicity. CaN hyperactivation would explicate three outcomes seen in AD brain: synaptic loss, neuroinflammation, and cell death (Figure 1).
FIG. 1:
Calcineurin in the aging brain. A number of factors, including oxidative insult, mitochondrial dysfunction, and increased numbers of VGCCs decrease the aged brain’s ability to buffer Ca2+ levels. The additional insult of Aβ oligomers further disrupts Ca2+ homeostasis, resulting in a subtle, prolonged increase in calcium that promotes the hyperactivation of CaN. This important phosphatase mediates the dephosphorylation of four cellular proteins: pCREB, pNFAT, p-PP1 and pBAD. CaN hyperactivation could explain several observations in AD models and pathogenesis; decreased synaptic activity, synaptic loss, neuroinflammation (neuronal and astroglial), and cell death. Therefore, inhibition of CaN in the CNS may be viable therapeutic strategies for combating early stage AD impairment.
To date, some therapeutic regiments delay, but do not halt or reverse the progression of AD. Uncompetitive NMDA-R antagonists, including memantine, are designed to normalize synaptic Ca2+. They have shown some promise at delaying the clinical progression of AD but not preventing the outcome [79]. A number of observational clinical studies have hinted that that regular use of non-steroidal anti-inflammatory drugs (NSAIDS) prevent or delay AD onset [80–82]. This class of drugs, which includes ibuprofen, naproxen, and a number of others, exerts its effects through inhibition of cyclooxygenase-2 (COX-2). Interestingly, CaN plays a central role in the transcriptional regulation of COX-2 via NFAT [83]. Given that CaN is likely involved either downstream (NMDA-R antagonists) or upstream (NSAIDs) of two treatments that have shown some benefit in delaying disease progression, it is appropriate to consider the direct modulation of CaN as a means to prevent and combat AD. Systemic CaN inhibitors FK506 and cyclosporine result in the deleterious side effect of immunosuppression via NFAT-mediated downregulation of interleukin-2. This is an undesirable circumstance for aged patients already contending with compromised immune function. More efficacious therapies might target CaN inhibitors to the CNS so that affected neurons, astrocytes, and microglia can benefit from CaN normalization while avoiding pleiotropic effects.
Acknowledgments
Work in the laboratory of GT is supported by NINDS grant R01NS-38261 and by the Alzheimer Association grant IIRG90755; LCR is the recipient of a pre-doctoral fellowship from NINDS F31NS062558.
References
- [1].Klee CB, Krinks MH. Purification of cyclic 3′5′-nucletoide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to sepharose. Biochemistry. 1978;17:120–126. doi: 10.1021/bi00594a017. [DOI] [PubMed] [Google Scholar]
- [2].Klee CB, Crouch TH, Krinks MH. Calcineurin – calcium-binding and calmodulin-binding protein of the nervous system. PNAS. 1979;76:6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yakel JL. Calcineurin regulation of synaptic function: from ion channels to transmitter release and gene transcription. Trends Pharm Sci. 1997;18:124–134. doi: 10.1016/s0165-6147(97)01046-8. [DOI] [PubMed] [Google Scholar]
- [4].Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- [5].Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear-association of a T-cell transcription factor blocked by FK-506 and cyclosporine-A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- [6].Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporine-A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- [7].Clipstone NA, Crabtree GR. Identification of calcineurin as a key signaling enzyme in lymphocyte-t activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- [8].Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2(+)- and stimulus duration-dependent switch for hippocampcal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- [10].Groth RD, Dunbar RL, Mermelstein PG. Calcineurin regulation of neuronal plasticity. Biochem Biophys Res Commun. 2003;311:1159–1171. doi: 10.1016/j.bbrc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- [11].Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- [12].Asai A, Qiu J, Narita Y, Chi S, Saito N, Shinoura N, Hamada H, Kuchino Y, Kirino T. High level calcineurin activity predisposes neuronal cells to apoptosis. J Biol Chem. 1999;274:34450–34458. doi: 10.1074/jbc.274.48.34450. [DOI] [PubMed] [Google Scholar]
- [13].Matsuda T, Takuma K, Asano S, Kishida Y, Nakamura H, Mori K, Maeda S, Baba A. Involvement of calcineurin in Ca2+ paradox-like injury of cultured rat astrocytes. J Neurochem. 1998;70:2004–2011. doi: 10.1046/j.1471-4159.1998.70052004.x. [DOI] [PubMed] [Google Scholar]
- [14].Nagamoto-Combs K, Combs CK. Microglial phenotype is regulated by activity of the transcription factor NFAT. J Neurosci. 2010;30:9641–9646. doi: 10.1523/JNEUROSCI.0828-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Ann Rev of Immun. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- [16].Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- [17].Reiter RJ. Oxidative processes and anti-oxidative defense mechanisms in the aging brain. FASEB J. 1995;9:526–533. [PubMed] [Google Scholar]
- [18].Altman J, Das DG. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Compar Neurol. 1965;124:319–336. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- [19].Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordberg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- [20].Leslie SW, Chandler LJ, Barr EM, Farrar RP. Reduced calcium-uptake by rat brain mitochondria and synaptosomes in response to aging. Brain Res. 1985;329:177–183. doi: 10.1016/0006-8993(85)90523-2. [DOI] [PubMed] [Google Scholar]
- [21].Landfield PW, Pitler TA. Prolonged Ca2+ dependent after hyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- [22].Landfield PW, Campbell LW, Hao SY, Kerr DS. Aging-related increases in voltage-sensitive, inactivating calcium currents in rat hippocampus. Implications for mechanisms of brain aging and Alzheimer’s disease Ann N Y Acad Sci. 1989;568:95–105. doi: 10.1111/j.1749-6632.1989.tb12495.x. [DOI] [PubMed] [Google Scholar]
- [23].Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bliss TVP, Lømo T. Long-lasting potentiation of synaptic transmission in dentate area of anesthetized rabbit following stimulation of perforant path. J Physiol – London. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bliss TVP, Collingridge GL. A synaptic model of memory – long term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- [26].Stefan MI, Edelstein SJ, Novère NL. An allosteric model of calmodulin explains differential activation of PP2B and CaMKII. Proc Natl Acad Sci U S A. 2008;105:10768–10773. doi: 10.1073/pnas.0804672105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- [28].Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- [29].Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Norris CM, Blalock EM, Chen KC, Porter NM, Landfield PW. Calcineurin enhances L-type Ca(2+) channel activity in hippocampal neurons: increased effect with age in culture. Neuroscience. 2002;110:213–225. doi: 10.1016/s0306-4522(01)00574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Norris CM, Blalock EM, Chen KC, Porter NM, Thibault O, Kraner SD, Landfield PW. Hippocampal ‘zipper’ slice studies reveal a necessary role for calcineurin in the increased activity of Ltype Ca(2+) channels with aging. Neurobiol Aging. 2010;31:328–338. doi: 10.1016/j.neurobiolaging.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted an regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998a;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- [33].Mansuy IM, Winder DG, Moallem TM, Osman M, Mayford M, Hawkins RD, Kandel ER. Inducible and reversible gene expression with the rtTA system for the study of memory. Neuron. 1998b;21:257–265. doi: 10.1016/s0896-6273(00)80533-4. [DOI] [PubMed] [Google Scholar]
- [34].Zeng HK, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- [35].Ikegami S, Inokuchi K. Antisense DNA against calcineurin facilitates memory in contextual fear conditioning by lowering the threshold for hippocampal long-term potentiation. Neuroscience. 2000;98:637–646. doi: 10.1016/s0306-4522(00)00161-5. [DOI] [PubMed] [Google Scholar]
- [36].Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TVP, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. Inducible and reversible enhancement of learning, memory, and longterm potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- [37].Jouvenceau A, Dutar P. A role for the protein phosphatase 2B in altered hippocampal synaptic plasticity in the aged rat. J Physiol Paris. 2006;99:154–161. doi: 10.1016/j.jphysparis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [38].Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- [39].Enslen H, Sun PQ, Brickey D, Soderling SH, Klamo E, Soderling TR. Characterization of Ca2+/calmodulin-dependent protein-kinase-IV role in transcriptional regulation. JBC. 1994;22:15520–15527. [PubMed] [Google Scholar]
- [40].Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol. Aging. 2003;24(Suppl. 1):S123–S127. S131. doi: 10.1016/s0197-4580(03)00051-4. [DOI] [PubMed] [Google Scholar]
- [41].Dong YS, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- [42].Cornellbell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes – long range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- [43].Dani JW, Chernjavsky A, Smith SJ. Neuronal-activity triggers calcium waves in hippocampal astrocytes networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- [44].Goto S, Matsukado Y, Mihara Y, Inoue N, Miyamoto E. The distribution of calcineurin in rat brain by light and electron microscopic immunohistochemistry and enzyme-immunoassay. Brain Res. 1986;397:161–172. doi: 10.1016/0006-8993(86)91381-8. [DOI] [PubMed] [Google Scholar]
- [45].Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V, Porter NM, Landfield PW, Kraner SD. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer's models. J Neurosci. 2005;25:4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- [47].Timmerman LA, Clipstone NA, Ho SN, Northrop JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- [48].Pérez-Ortiz JM, Serrano-Pérez MC, Pastor MD, Martín ED, Calvo S, Rincón M, Tranque P. Mechanical lesion activates newly identified NFATc1 in primary astrocytes: implication of ATP and purinergic receptors. Eur J Neurosci. 2008;27:2453–2465. doi: 10.1111/j.1460-9568.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- [49].Sama MA, Mathis DM, Furman JL, Abdul HM, Artiushin LA, Kraner SD, Norris CM. Interleukin-1 beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. JBC. 2008;283:21953–21964. doi: 10.1074/jbc.M800148200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, Kraner SD, Norris CM. Cognitive decline in Alzheimer's disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- [52].Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia – intrinsic immuneffector cell of the brain. Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- [53].Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- [54].Arispe N, Rojas R, Pollard HB. Alzheimer-disease amyloid beta-protein forms calcium channels in bilayer membranes – blockade by tromethamine and aluminum. PNAS. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- [56].Agostinho P, Oliveira C. Involvement of calcineurin in the neurotoxic effects induced by amyloid-beta and prion peptides. Eur J Neurosci. 2003;17:1189–1196. doi: 10.1046/j.1460-9568.2003.02546.x. [DOI] [PubMed] [Google Scholar]
- [57].Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:824–835. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. A beta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- [61].Saito KI, Elce JS, Hamose JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer-disease – a potential molecular basis for neuronal degeneration. PNAS. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong CX. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem. 2005;280:37755–37762. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- [63].Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkaine A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bi G, Poo M. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gong YS, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Nat Acad Sci USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong YS, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. A beta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhao D, Watson JB, Xie CW. Amyloid β prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol. 2004;92:2853–2858. doi: 10.1152/jn.00485.2004. [DOI] [PubMed] [Google Scholar]
- [69].Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid b-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77:354–371. doi: 10.1006/nlme.2001.4034. [DOI] [PubMed] [Google Scholar]
- [70].Dineley KT, Kayed R, Neugebauer V, Fu Y, Zhang W, Reese LC, Taglialatela G. Amyloid beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J. Neurosci. Res. 2010. ePub ahead of print: 11 June 2010. [DOI] [PMC free article] [PubMed]
- [71].Jouvenceau A, Hédou G, Potier B, Kollen M, Dutar P, Mansuy M. Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. Eur J Neurosci. 2006;24:564–572. doi: 10.1111/j.1460-9568.2006.04938.x. [DOI] [PubMed] [Google Scholar]
- [72].Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDAtype glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dineley KT, Hogan D, Zhang WR, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007;88:217–224. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yamamoto-Sasaki M, Ozawa H, Saito T, Rosler M, Riederer P. Impaired phosphorylation of cyclic AMP response element binding protein in the hippocampus of dementia of the Alzheimer type. Brain Res. 1999;824:300–303. doi: 10.1016/s0006-8993(99)01220-2. [DOI] [PubMed] [Google Scholar]
- [76].Simpson JE, Ince PG, Lace G, Forster G, Shaw PJ, Matthews F, Savva G, Brayne C, Wharton SB. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol of Aging. 2010;31:578–590. doi: 10.1016/j.neurobiolaging.2008.05.015. [DOI] [PubMed] [Google Scholar]
- [77].Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. PNAS. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Agostinho P, Lopes JP, Velez Z, Oliveira CR. Overactivation of calcineurin induced by amyloid-beta and prion proteins. Neurochem Intl. 2008;52:1226–1233. doi: 10.1016/j.neuint.2008.01.005. [DOI] [PubMed] [Google Scholar]
- [79].Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate to severe AD. NEJM. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- [80].McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- [81].Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- [82].Szekely CA, Zandi PP. Non-steroidal anti-inflammatory drugs and Alzheimer’s disease: the epidemiological evidence. CNS & Neurol Disorders – Drug Targets. 2010;9:132–139. doi: 10.2174/187152710791012026. [DOI] [PubMed] [Google Scholar]
- [83].Iniguez MA, Martinez-Martinez S, Punzon C, Redondo JM, Fresno M. An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. JBC. 2000;275:23627–23635. doi: 10.1074/jbc.M001381200. [DOI] [PubMed] [Google Scholar]