Abstract
Brain aging has been suggested to be conditioned by an excessive glucocortioid secretion leading to damages on brain areas involved not only in cognitive and emotional processes but also in the control of the activity of the hypothalamic-pituitary adrenal axis. This review describes some of the hypothesis that try to explain the relation between the dysregulation of the stress response and brain aging, focusing on corticosterone but also on neurotransmission in the hippocampus, the prefrontal cortex and the amygdala. Moreover, different molecular factors can account for an enhanced vulnerability of the aged brain to stress exposure, specially for resilience. Among them, good candidates could be those mechanisms determining the levels of corticosterone in the brain, several molecules downstream glucocorticoid receptor activation (ie: heat shock proteins, BAG-1) or even the epigenetic programming of the HPA axis in early stages. In conclusion, genetic and environmental factors (early life stress, chronic stress during adulthood) can produce an enhanced vulnerability and a reduced resilience of the brain to subsequent stress exposures or to metabolic challenges leading, in turn, to an unsuccessful aging of the brain. However, results obtained with the use of the environmental enrichment model in animals, added to several results in humans also described in this review suggest that positive environmental factors (cognitive-demanding tasks or physical exercise) can help to maintain neuronal plasticity during aging and to protect the brain against the damaging effects of stress exposure.
Keywords: Aging, stress, resilience, corticosterone, glucocorticoids, neurotransmitters, hippocampus, prefrontal cortex, amygdalal, rat
Non-pathological or physiological aging is associated with a general decline of cognitive functions, such as declarative memory, verbal fluidity or working memory [1–3]. However, it is clear that aging does not affect everyone in the same way. While some people maintain a good cognitive performance on late ages (successful aging), others show a mild cognitive decline or even dementia (unsuccessful aging). Many different factors have been suggested to cause this enhanced individual vulnerability of the brain to aging. Although some authors suggest a genetic basis for aging [4–6], there is the generalized idea that aging is not a genetically controlled process but an interaction between environment and genes [7]. Among the negative environmental factors enhancing the vulnerability of the brain to aging, the most studied of them is stress. By contrast, diet [8], physical exercise [9], or to be engaged in cognitive-demanding activities [10] can lead to a successful aging of the brain. All of these environmental factors exert their positive or negative actions on the brain through the direct modulation of brain plasticity or through the modulation of other factors involved indirectly in brain plasticity.
This review will focus on the role of the dysregulation of the stress response as an environmental risk factor that negatively affects brain aging. Past and current hypotheses that try to model how the dysregulation of the stress response can lead to unsuccessful aging are described. Some of the molecular factors that could be involved in the dysregulation of the stress response during aging are also depicted. Finally, given that many individuals are able to enjoy a successful aging, this review will also focus on several mechanisms that could be involved in the vulnerability of the brain to stress. Among these mechanisms are resilience, epigenetic mechanisms, and those environmental factors thought to exert positive effects on brain aging, with an special focus on the environmental enrichment model.
1. The response to stress and the HPA axis: effects of aging
The definition of stress can vary depending on the circumstances. This term has been used in an ambiguous way, describing either the set of physiological systems that are activated in response to an actual or potential threat (stressor) or the stimulus itself. In this review, the first of these definitions will be used.
The presence of a stressor triggers different physiological responses. The first response is the activation of the sympathetic nervous system, which promotes the release of adrenaline and noradrenaline from the adrenal glands, allowing the so-called “Fight or Flight” response [11]. Adrenaline and noradrenaline will increase the respiratory rate, the heartbeat, the blood concentration of glucose and the blood flow to the skeletal muscle. This fast response is primarily related to survival. This response is, however, beyond the scope of this review.
The stressor also triggers the activation of the hypothalamo-pituitary-adrenal (HPA) axis, which begins with the activation of the parvocellular neurons in the paraventricular nucleus of the hypothalamus (PVN). The release of corticotrophin releasing hormone (CRH) from the PVN leads in turn to the release of adrenocorticotropic hormone (ACTH) from the hypothalamus. ACTH will be delivered into the bloodstream, reaching the adrenal cortex and promoting the release of glucocorticoids (GCs) into the blood [12]. These GCs are cortisol in most of the mammals, and corticosterone (CORT) in rodents, although it can also be found in the human blood [13]. GCs mobilize energy, either by activating or inhibiting different processes, to cope with the energetic demands triggered by the behavioral response to the stressor. For example, GCs activates proteolysis and lipolysis, and inhibits glycolysis and inmunogenesis in different tissues [14]. These active processes are promoted with the aim to restore homeostasis. When it has been recovered, high levels of GCs are not demanded. In fact, the release of GCs is regulated by a negative feedback mechanism in which CORT can bind to receptors located in the pituitary, the PVN and in different areas of the limbic system [15–19].
There are two types of GCs receptors: mineralocorticoid (MRs) and glucocorticoid receptors (GRs), also called type I and type II receptors, respectively. MRs show a ten-fold higher affinity than GRs for GCs, and the majority of them are occupied under basal conditions. GRs show low affinity for GCs and they are almost unoccupied under basal conditions. Therefore, it is thought that MRs are involved in the maintenance of stress system activity, while GRs drive the steroid control of the recovery from stress [20].
As mentioned, the stress response involves the activation of a set of hormonal and neural responses that contributes to recover the actual or potentially threatened homeostasis. These kind of processes, aimed to actively maintain or re-establish homeostasis are termed allostatic responses. The term allostasis refers to the ability of the body to produce hormones and other mediators (i.e.: neurotransmitters, cytokines) that help an animal adapt to a new situation or challenge [21]. Stress is therefore an adaptive response. However, if this response is maintained for more time than necessary or if it is activated chronically, harmful effects on the body (i.e.: immunosuppression, peripheral muscle dysfunction, neuronal death) can appear. This process is called allostatic load, and it could be considered the basis to explain why stress is an important risk factor for an unsuccessful aging of the brain [22].
The link between stress exposure and aging was begun to be established by the late 70s and the 80s of the last century. In their seminal study Landfield et al. [23] found a positive correlation between blood levels of CORT and the astrocyte reactivity (a marker of brain damage) in the hippocampus (HC) of aged rats. They also observed that adrenalectomy reduced the astrocyte reactivity, which suggested a role for stress hormones on brain aging [24]. A few years later, Sapolsky et al [25] showed that the recovery of stress-induced levels of CORT to basal levels was protracted in the aged rat, a finding that has later been confirmed by other studies [25–29]. These results suggested that aged rats are subjected to an allostatic load mediated by enhanced levels of CORT, which could provide an explanation for some of the age-related changes of the brain.
2. GCs in the brain and the “glucocorticoid cascade hypothesis”
Apart from the PVN, the main site in which CORT release is controlled, MRs and GRs are found in different limbic areas of the brain [30]. While GRs and MRs occur in the (HC) and the amygdala, the prefrontal cortex (PFC) expresses GRs, but the MR expression is sparse in this brain area [15–17]. Importantly, these limbic areas not only mediate the stress response but they also play a key role in the regulation of different cognitive and emotional processes [31–33].
GCs play at least three functions in the brain. The first is, like in the rest of the body, to regulate energy availability during the stress response. For instance, GCs can modulate the glucose transport into the neurons [34]. The second is to promote behavioral adaptation, regulating (either enhancing or impairing) several memory processes [35]. For example, stress can enhance the consolidation of spatial and emotional memories, but it can also impair their retrieval [36–38]. The third function of GCs in the brain is the control of the HPA axis activity through GRs located in the HC, the PFC and the PVN [19]. An impairment in the third of these functions has been in fact closely linked to brain aging.
In the 80s and 90s, several studies showed that lesions of the HC lead to an enhanced increase of CORT under acute stress conditions [39–42] thus suggesting that the HC plays an inhibitory role on the activity of the HPA axis. Moreover, the aged HC was shown to contain lower levels of GCs receptors than the young HC [25,27,43–47]. Added to that it was also found that enhanced levels of CORT leaded to a lower number of neurons in the HC [48] along with an enhanced vulnerability of these neurons to neurotoxic insults [34]. In 1986, Sapolsky et al. [49], proposed the “glucocorticoid cascade hypothesis” (GCH), by which they elegantly linked these findings with those showing a protracted inhibition of the HPA axis in the aged rat. The GCH suggests that an impairment in the negative feedback mechanism to control the release of CORT in the aged rat leads to an enhanced exposure of the brain of aged animals to CORT which, in turn, would reduce the number of neurons in the HC and the consequent age-related cognitive deficits. Moreover, the damage of the HC would reduce the expression of GRs leading to a worsening of the accuracy of the inhibitory control of the CORT release that would potentiates the damages in the HC (Figure 1). Some studies supported this hypothesis. For instance, it was shown that only cognitively-impaired aged rats show a protracted recovery of basal levels of CORT and a reduced neuron density in the CA1 and CA3 regions of the HC [50]. Moreover, a rat model of “successful aging”, which shows a longer expectancy of life does not display the disruption of the GC negative feedback during aging [29,51]. And, importantly, GC scan also damage the human brain. It has been shown that Cushing syndrome patients, who are exposed to high cortisol levels due to an adrenal tumor, are characterized by a reduced hippocampal volume and cognitive deficits [52–54]. Recently, it has also been reported an association between cortisol levels and the thickness of different subregions of the PFC in middle-aged men [55].
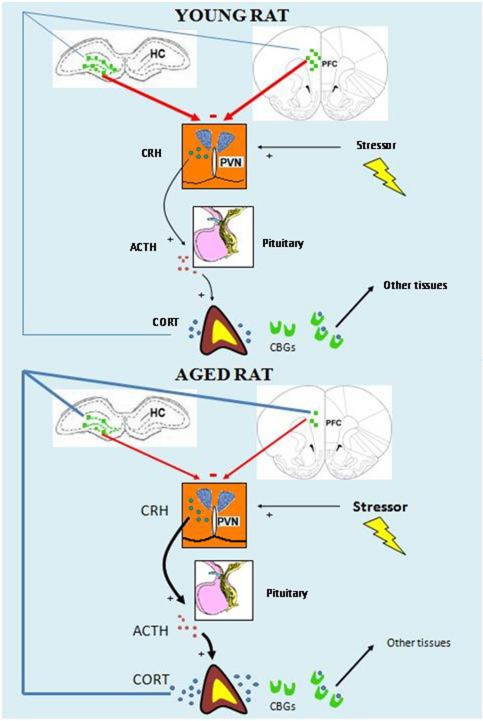
Figure 1.
Schematic representation of the “glucocorticoid cascade hypothesis”. The presence of a stressor triggers the release of CORT by the activation of the HPA axis. CORT can bind to CBGs to act on target tissues, and free CORT can cross the blood-brain-barrier, reaching the GRs (green squares) in the HC and the PFC. In the young rat (up) these brain areas exert a precise inhibitory control on the HPA axis activity in response to the stress-induced levels of CORT. In the aged rat (bottom) a dysregulation in the control of the HPA axis activity leads to enhanced levels of CORT, which in turn will produce damages in the HC and the PFC, thus worsening the inhibitory control of the HPA axis.
However, although by this time the GCH was a convincing and suggestive explanation about the causes of brain aging and the age-related cognitive deficits, subsequent studies have shown that some of the assumptions of this hypothesis were not accurate. The first problem of the GCH is the animal model. Not all strains of rats reproduce the age-impairment of the GCs negative feedback, even although they show a cognitive decline [56,57]. But more importantly, although not many studies have focused on the stress response and aging in humans, it is not clear whether elderly people show a protracted recovery of CORT in response to stress [58–61]. Also, patients suffering Alzheimer’s disease, which is associated with loss of neurons in the HC, do not show impairments in the glucocorticoid negative-feedback elicited by dexamethasone, a synthetic agonist of GRs [62]. Moreover, it is not clear whether GR expression in the primate brain is similar to that observed in the rodent brain. In fact, several studies have shown that, contrary to what is found in the rodent brain, the primate brain shows an enhanced expression of GRs in the PFC compared to the HC [63,64]. The second problem of the GCH refers to the increases of CORT in the brain of aged rats. The GCH made the assumption that higher CORT levels in the blood would lead to higher levels in the brain. However, a recent study has shown that while aged rats show enhanced increases of plasma CORT compared to young rats, they do not show this enhancement in the HC or the PFC [65]. This result suggests that aged animals are not exposed to higher levels of CORT than young animals during the stress response in spite of the differences in the levels of CORT in the blood. The third problem refers to cell death induced by GCs. When neuron-counting techniques were improved it became clear that the aged HC does not show a reduced number of neurons in humans [66] or even in animals showing spatial memory deficits [46,67]. More importantly, chronic stress or chronic treatment with CORT do not produce cell death in the HC of different species, including humans [68–71]. This data suggest that CORT per se is not enough to lead to the death of neurons. Different research groups have shown, however, that these treatments can lead to dendritic atrophy and spine loss in the pyramidal neurons of the CA3 area of the HC, which correlate with deficits in hippocampal-dependent tasks [72–75]. Moreover, it has been shown that CORT may not produce neuronal death but an enhanced vulnerability to metabolic challenges (i.e. hypoxia, ischemia), an effect possibly mediated by an enhanced glutamatergic signal [34]. The fourth problem of the GCH involves the dynamics of the GCs actions during aging. If GCs are the principal cause for age-related changes in the brain, then the changes in different molecular parameters produced by the GCs on the brain should follow the same direction as those produced by aging. However, studies using gene arrays have shown that this is not case [76]. It seems that the interaction between GCs and aging is more complex and, while some actions of the GCs are potentiated during aging, other are inhibited, and these actions could be dependent on the cell type. The fifth problem refers to the initiation of the cascade. In the model of the GCH, an enhanced probability of the aged brain to suffer metabolic challenges leads in turn to an enhanced probability of a concurrent increase of CORT levels together with a metabolic challenge that would produce neuronal damages, a lower expression of GRs and the impaired negative feedback mechanism. At this point, subsequent concurrent events of metabolic challenge and enhanced levels of CORT would be then more probable. However, it has been suggested that a metabolic challenge is not needed at the same time of an increase of CORT to lead to an enhanced vulnerability of the brain. The laboratory of Dr. Cheryl Conrad has shown that a chronic treatment with CORT produces an enhanced vulnerability of the neurons of the HC to the harmful effects of a subsequent treatment with different neurotoxic drugs [77,78].
3. Aging and the “glucocorticoid vulnerability hypothesis”
Several problems of the GCH have been reviewed and tried to be solved by the proposal of the “glucocorticoid vulnerability hypothesis”. This hypothesis suggests that repeated enhanced levels of CORT, such as during chronic stress, would not be the unique determinant for cell loss in the aged HC. It proposes that CORT would play a role priming the HC to show an enhanced vulnerability when exposed to subsequent insults [79]. Although some studies support this hypothesis, this idea has only been tested using neurotoxic drugs as insults [77,78]. Given that it is not easy for the brain to be exposed to neurotoxic drugs, more studies are needed to test whether a period of elevated CORT levels could also produce an enhanced susceptibility to the harmful effects of more realistic insults, such as hypoxia or ischemia. Moreover, as previously mentioned, the aged hippocampus does not show a lower number of neurons in spite of spatial memory impairments and a delayed recovery of basal CORT following the exposure to a stressor [46,67]. For this reason, this hypothesis could not be useful to explain the link between stress and non-pathological aging. The hypothesis suggests, however, that a reduced dendritic arborization may be needed to confer an enhanced vulnerability to the HC, a point that has been sparsely studied [77]. This could be an interesting marker to predict vulnerability to the exposure to chronic stressors or even to GC effects on the aged HC. However, it is not clear whether genetic factors could also contribute to a reduced volume of the HC and, in turn, to a dysregulated HPA axis [80] or whether a prolonged exposure to CORT leads to a reduced volume of the HC and the subsequent HPA axis dysregulation. Some authors suggest that there would be a situation between those two possibilities where different sensitive periods can drive the influence of vulnerability to genetic or environmental factors [81].
Both the GCH and the glucocorticoid vulnerability hypothesis focus on the role of GRs on brain aging. An alternative suggested possibility is that an imbalance between GRs and MRs could be the cause of susceptibility to stress-related brain disorders and brain aging [20]. However, this hypothesis also has its own problems. Thus, while the imbalance of GRs and MRs is a suggestive hypothesis for the changes observed during aging in the HC, it would not be good to explain the changes in other areas of the brain, such as the PFC, in which MRs do not seem to play an important role due to their very low expression levels [15–17].
Another aspect of the relation between aging and the HPA axis is the role of basal levels of CORT on the damages of the HC with aging. The GCH and the “glucocorticoid vulnerability hypothesis” are mostly focused on the harmful effects of the stress-induced levels of CORT than on its basal levels. However, in the case of the “glucocorticoid vulnerability hypothesis” stress levels, as well as high basal levels of CORT could program the HPA axis for an enhanced vulnerability to further insults. GCs are released in a circadian rhythm reaching a peak before the onset of the active phase (dark phase in rodents, light phase in humans). These peak-levels can even be as high as those produced by a mild stressor [82]. Therefore, a dysregulation of the circadian rhythm, or continuously enhanced basal levels of CORT could contribute to the changes of the aged brain and the impaired recovery of basal levels after the exposure to a stressor. Studies focusing on aging show either enhanced levels [25,28,83] or no differences [46,51,56,57,65,84] on basal CORT levels in aged compared to young rats. A more detailed study found that about a 30% of aged rats show hypersecretion of CORT [50], and the only study that has measured CORT in the brain of aged rats in vivo has shown that basal levels of CORT are enhanced in the HC and the PFC of aged compared to young rats [65]. In line with an involvement of basal levels of CORT on brain aging, a study showed that adrenal weight, a marker of HPA axis hyperactivity, correlated negatively with the performance of a spatial memory task and also with the neurogenesis in the dentate gyrus of the aged HC. Adrenalectomy since 10 months of age prevented these effects [28]. In humans, in spite of the lack of differences in basal cortisol levels between young and elderly people [58,59], Lupien et al.[85,86] showed that the dynamics of the basal levels of cortisol during aging can depend on individual differences. In a longitudinal study during 5 years they described three different patterns of variation of cortisol levels on elderly individuals. In the first subgroup, cortisol levels were reduced with time, in the second they remain stable, and in the third they rose. Interestingly, those individuals showing the increasing pattern also showed hippocampal atrophy and memory deficits [86]. These results have been replicated, at least in part, by other research groups [87,88].
Therefore, although the GCH and the “glucocorticoid vulnerability hypothesis” provide a heuristic approach for the link between stress and aging, the scenario seems to be more complex, involving more variables than those mentioned by both hypothesis. Thus, the second half of this review will focus on several factors that could be interesting to study the relation between stress and aging as well as the efficacy of the environmental enrichment model and its application to the human being to attenuate or delay the effects of brain aging related to the exposure to stress.
4. Two new actors: the PFC and the amygdala
The PFC and the amygdala undergo a plethora of changes during aging. The PFC is possibly the only area of the brain in which there is a clear reduction of dendritic branching of the pyramidal neurons with aging, both in humans and rodents [89,90]. The pyramidal neurons of the amygdala, by contrast, become hypertrophic with aging [91]. Under stress conditions, a lower activation of the PFC and the medial amygdaloid nucleus has been also shown in aged rats [84,92]. Moreover, several functions related to the PFC and the amygdala are impaired in aged animals [47,93–98].
The GCH, the “glucocorticoid vulnerability hypothesis” and the hypothesis of an imbalance between GRs and MRs are focused on the effects of GCs on the HC. However, as the HC, the PFC and the amygdala are activated in response to different stressors [99–102], and they modulate the activity of the HPA axis [103–107]. On one hand, the PFC shows a region-specific function: the dorsomedial PFC (prelimbic and cingulate cortices) plays an inhibitory role, while the ventromedial PFC (infralimbic cortex) plays and excitatory role on the activity of the HPA axis [107,108]. On the other hand, the amygdala plays an excitatory role on the HPA axis activity [105]. Furthermore, the dendritic arborization of pyramidal neurons in the PFC and the amygdala is also sensitive to chronic stress or chronic treatment with CORT. While the pyramidal neurons of the PFC show a retraction similar to that observed in the HC [107,109–111], those of the amygdala respond with an increase of their dendritic arborization [112,113]. In both cases, the changes of the dendritic arborization are accompanied by changes on PFC and amygdala dependent behaviors [111,114].
These findings have broadened the point of view about the areas of the brain involved in the regulation of the stress response and its relation to brain aging. Studies regarding the link between the PFC or the amygdala with aging and stress are sparse, although they are progressively increasing. Now, it is thought that the functional interaction between the HC, the amygdala and the PFC could be more important than the sole action of one of these brain areas. For instance, the PFC-basolateral nucleus of the amygdala (BLA) pathway has been suggested to be important for the development of major depression and post-traumatic stress disorder (PTSD) [115]. Changes in this pathway during aging could also have a relevant impact on brain aging and the modulation of the stress response. An imbalance between the activation of the PFC and that of the amygdala could lead to deficits in the response to stress. For instance, as previously described, it has been shown that aged rats show a reduced activation (measured as c-fos expression) in response to an acute stressor of the medial PFC but not of the BLA compared to young animals [84,92]. Since the PFC is thought to inhibit BLA pyramidal neurons [116], the BLA in aged animals could be more active, triggering a disinhibition of the HPA axis. Added to that, and in relation with a lower activity of the PFC of aged rats, in a recent study in our laboratory (P.Garrido., M. De Blas, E.Giné, A.Santos, F. Mora, unpublished observations), we have observed that the activation of the PFC inhibits the release of CORT in response to restraint and also produces an impairment of aversive memory consolidation in young rats, but these effects are not observed in aged animals, which suggest that the aged PFC has a lower capacity to control the HPA axis activity. Finally, a recent study has shown that the PFC-BLA pathway is not affected by a protocol of chronic restraint on adult rats, while other neurons in the PFC and the amygdala showed the usual dendritic retraction and hypertrophy, respectively [115]. The authors suggest that the insensitivity of the PFC-BLA pathway to stress could constitute a mark of health, while the alteration of this specific pathway could be involved in mood disorders. It would be very interesting to know whether this pathway is modified in aged animals showing hypersecretion of CORT and age-related cognitive deficits.
5. The role of neurotransmission on stress and its relevance to aging
While the effects of aging on hormonal stress responses are well characterized, those of the different neurotransmitters in the limbic areas that modulate the HPA axis activity are poorly known. It is known that different acute stressors increase the release of dopamine, noradrenaline, serotonin, acetylcholine or glutamate in different areas of the brain such as the HC, the PFC or the amygdala [117–125]. The precise role of the stress-induced increases of these neurotransmitters in these areas of the brain is not completely understood. This is a relevant issue because the response of neurotransmission is considerably faster than hormonal responses to a stressor, which suggests that changes in neurotransmission in these brain areas can modulate the HPA axis activity [126]. In line with this suggestion, lesions of dopaminergic, cholinergic or noradrenergic neurons in different brain areas produce changes in the HPA axis activity [127–130]. Moreover, some studies have shown local effects of several neurotransmitters on the HPA axis activity acting on the HC, the PFC or the amygdala [131–135].
With regard to aging, the response of dopamine and setotonin to an acute stressor is reduced in the HC and the PFC of aged rats [136,137] and the response of acetylcholine does not change [138,139]. A relevant question is whether changes in these neurotransmitters with aging or an imbalance between them can influence the HPA axis activity and be responsible, at least in part, of the age-related changes on it. They could also be responsible of the age-related cognitive deficits. In relation with this hypothesis, it has been proposed that an imbalance between excitatory and inhibitory signals could be responsible of some of the age-related cognitive deficits [140–142]. This is also in line with the observation that the activation by picrotoxin of the young but not the aged PFC can inhibit the release of CORT in response to an acute stressor (P.Garrido, M. De Blas, E.Giné, A.Santos, F. Mora, unpublished observations). Only a few works have studied the changes of glutamate and GABA subunit receptors in the brain with aging [142,143] and it is not clear whether these changes could contribute to HPA axis dysregulation. Given that glutamatergic signals have been also involved in the harmful effects of CORT on the brain [34], this question is not trivial. Moreover, the contribution of different neuromodulators (mainly the monoamines) to the activation/inhibition imbalance during aging should not be discarded.
The interaction between the HPA axis and neurotransmission could be, however, more complex than the simple action of neurotransmitters on the HPA axis. In fact, it could be established a bi-directional relation between neurotransmitters and CORT. For instance, CORT can modulate the activity of different neurotransmitter systems either at short- or long term-periods. Rapid actions of CORT have been described for the release of glutamate or the trafficking of its AMPA and NMDA receptors [144–148], while long-term effects on the mesocortical dopaminergic system have been observed after ADX in the PFC [149]. Further studies are needed to elucidate the dynamics of the local interaction of CORT and neurotransmitters in different areas of the brain and whether these relations do change with aging.
6. Resilience
Although the continuous exposure to stressors can lead to maladaptive physiological responses (allostatic load), this is not the case for all individuals. For this reason, the ability of an individual to adapt to different stressors and the mechanisms that allow this adaptation are important for the study individual vulnerability for the exposure to GCs. The ability to adapt successfully to different stressors is termed resilience. A resilient individual has thus been tested by adversity and continues to demonstrate adaptive psychological and physiological stress responses, or an adequate allostasic response [150].
A capital question about the changes that stress exposure produces on the brain is the stability of those changes, and whether there is the possibility for a return to normal conditions after a prolonged period of exposure to GCs. The dendritic branching and the number of spines in the HC and the PFC return to normal conditions after several weeks from the end of the exposure to a chronic stressor in young animals [74,151–153]. It has been suggested that the dendritic retraction of the neurons of those brain areas could constitute a protective mechanism against the increased release of glutamate, being reversed when chronic stress conditions have ceased [154]. However, if resilience is lost, those responses activated during the exposure to the chronic stressor would be permanent, leading to a continuous enhanced activity of the HPA axis, along with spatial and working memory impairments produced, at least in part, by the morphological changes in the HC, the PFC and the amygdala.
A lower resilience in response to the changes produced by the exposure to a chronic stressor could explain some of the morphological, hormonal and behavioral changes observed in the aged brain. In accord with this idea, a recent study has shown that the morphological reorganization shown by the PFC of young rats 21 days after the exposure to a chronic stressor is not observed in the PFC of middle-aged or aged rats [90]. Since the aged PFC responds to a chronic stressor, this result suggests that it is resilience but not plasticity which it is impaired in the aged brain. Although the response of the aged HC to a period of chronic stress after a period of recovery is unknown, it is highly probable that the aged HC also shows a similar pattern to that of the PFC. In fact, another study showed that the downregulation of GRs that is usually observed in the HC of young rats after a long period of training in a foot-shock avoidance task is not observed in middle-aged and aged-rats [155]. An important question derived from these findings is whether a chronic exposure to a stressor during middle-age could lead to higher probabilities to develop an unsuccessful aging of the brain. Some studies support this idea in rodents [156,157] although they are still scarce. In humans, different studies show that people exposed to chronic stressors (i.e: high job demands, caregivers of family members with dementia) show accelerated cellular aging [158] and a longer physiological and perceived period of time to recover from the exposure to a situation of stress [159,160]. These evidences suggest that a period of chronic stress during adulthood or at old ages might accelerate the aging of the brain. We can go even further to ask whether suffering stress-related psychopathologies during the adulthood can be considered as a risk factor for brain aging. For instance, elderly people that underwent a situation of PTSD during adulthood show a higher memory decline when they are old [161,162]. In line with this idea and given that depression and aging show some similarities on its effects on the brain, and that both of them are thought to be related to an enhanced cortisol exposure, it has been proposed that mood disorders and cognitive deficits may be considered as a continuum that becomes increasingly manifest with aging [163,164]. Although suggestive, this hypothesis needs further experimental evidences to be evaluated.
The primary cause for the loss of resilience during aging has not been studied. It could be related to the lower levels of brain-derived neurotrophic factor (BDNF) or its receptors in the aged brain [98,165–168], although CORT can also modulate the expression of BDNF in the brain. Moreover, many other molecular parameters may also contribute to the loss of resilience in the aged brain. Some of them are below described.
7. Molecular aspects of the stress response in the aged brain
The morphological, neurochemical and behavioral changes produced by the exposure of the brain to enhanced levels of GCs are obviously driven by underlying molecular mechanisms in response to the binding of CORT to its specific receptors in the neurons. Figure 2 shows some of the intracellular mechanisms that take place when CORT binds to GRs. The “classical” genomic action of the GCs leads to the activation or repression of different genes. In general, many of the genes modulated by GRs are involved in synaptic plasticity and, more specifically, in neuronal remodeling [169]. This observation has sense in view of the plastic changes produced by chronic stressors on the brain [170]. Moreover, there are also GRs located in the membrane of neurons [171]. Membrane GRs mediate genomic actions, but also non-genomic actions, involving the modulation of neurotransmission at synaptic level. For example, GRs have been shown to modulate glutamate [145,146] and GABA signaling [172] in different areas of the brain.
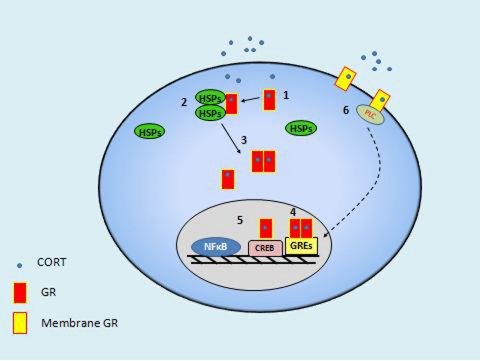
Figure 2.
Schematic view of several intracellular actions promoted by CORT. First, CORT binds to a monomer of GR in the cytosolic region of the neuron (1), and GR in turn rapidly binds to a multiprotein complex of heat-shock proteins (HSPs) (2), which is going to mediate the translocation of GR to the nucleus. Then, the activated GR split up from the HSPs and it can bind to other activated monomer to form a homodimer or remain as a monomer (3). Homodimers and monomers will travel to the nucleus and will bind to specific sequences of DNA in the promoter region on target genes called glucocorticoid response elements (GREs) (4). They can also interact with proteins like the nuclear factor κB (NF-κB) or the CRE-binding protein (CREB), among many others, which also act as transcription factors (5). There are also GR receptors in the membrane that can mediate genomic (6) or non-genomic actions (not shown).
Since many of these molecular mechanisms are still poorly understood there are only a few studies focusing on the role of them on brain aging. In general, different studies have shown reduced levels and binding of GRs in the HC and the PFC of aged animals [27,47,173–176], although these observations seem to be dependent on individual differences [46]. These changes in GR and MR levels would impair or, at least, reduce the accurate cellular response to stress conditions. For instance, it has been shown that the impaired GC negative feedback observed on aged rats following the local injection of dexamethasone in the HC, the PFC or the PVN could be due to a reduced number of GR positive cells in those brain areas [47]. However, it is also possible that further intracellular events produced by the activation of GRs could be impaired in the aged brain. For example, the impairment of the GC inhibitory feedback on aged rats has been related to a decreased translocation of GRs to the cellular nucleus in the HC, the PFC and the PVN of aged rats [47,177]. Related also to the translocation of GRs to the nucleus is the GR-cochaperone protein BAG-1, which could exert a dual action: the attenuation of the translocation of GRs to the nucleus in the case of enhanced CORT levels and the protection against apoptosis [178,179]. Therefore, this could be a good molecular candidate to mediate resilience.
GRs can also regulate gene expression in the mithocondria. CORT binds to Bcl-2, leading to anti-apoptotic responses and to the modulation of calcium influx into the mithocondria [179]. In fact, GRs stimulation enhances Bcl-2 expression and promotes the activity of the mithocondria under acute stress conditions, but these actions are attenuated by high levels of CORT or the exposure to a chronic stressor [179–181]. Could a similar process be observed in the vulnerable neurons of the HC or the PFC with aging? Further studies should address this question.
Another molecular aspect of the stress response is the set of mechanisms that modulates the availability of CORT in the brain. This is a relevant issue since most of the stress studies involving rodents have measured CORT in plasma and not in the brain. However, some studies have shown that CORT levels in the brain could not mirror plasma levels [65,182,182–186]. Therefore, it would be interesting to know the regulation of mechanisms that modulate the availability of CORT in the brain and whether aging changes any of these parameters. When CORT is released from the adrenal cortex, most of it is bound to corticosteroid-binding-globulins (CBGs), proteins that help to carry CORT to its target tissues in the body. A small proportion of CORT (5–10%) is not bound to CBGs [187] and is able to cross the blood brain barrier. Regarding aging, it has been shown that aged rats show either reduced levels of CBGs [174] or reduced binding of CBGs to CORT [27], which suggests that the brain of aged rats would be exposed to higher levels of CORT. This suggestion has been recently confirmed, at least in the case of basal levels of CORT in the HC and the PFC of aged rats [65]. Furthermore, the availability of CORT in the brain could be also modulated by a protein expressed in the blood brain barrier, the multidrug resistance 1 (MDR1) type glycoprotein. Although cortisol levels in the brain are thought to be regulated by this protein, this is not clear in the case of CORT [71,188–191]. Therefore, this mechanism could be even more interesting in the case of humans than in the case of rodents. A third molecule that can also modulate the levels of CORT in the brain is the 11-β-hydroxysteroid dehydrogenase type 1. This enzyme is able to synthesize CORT from 11-keto-derivates [192]. Interestingly, it is enhanced in the CA3 region of the HC and in the cortex of aged mice [193]. Moreover, spatial and working memory impairments are not observed in knock-out mice or mice treated with an inhibitor of this enzyme [83,194,195]. An inhibitor of this enzyme was also able to enhance some cognitive aspects on healthy elderly individuals [196]. Therefore, the mechanisms that modulate the availability of CORT in the brain could be a relevant factor determining the age-related cognitive decline. More studies are needed to define which factors (i.e: chronic stressors, genetic susceptibility, acquired vulnerability) could modulate these molecular mechanisms that mediate the stress response.
8. Epigenetics and individual vulnerability
In the last years we have witnessed a sudden increase in the number of studies related to epigenetics in the field of neuroscience. Epigenetic mechanisms, at a molecular level, refer to biochemical modifications of the DNA and histone proteins that will mediate the silencing or facilitation of gene transcription by modifying the structure of chromatin [197]. Some of these genes are involved in different cognitive processes and also on synaptic plasticity [198–200]. It is also known that acute and chronic stress can either increase or decrease some of those epigenetic marks in the HC or the PFC [201–205]. What is not currently known is whether these mechanisms could be relevant for age-related cognitive deficits and for the changes produced by GCs on the brain. It has been suggested that age-related cognitive deficits could be mediated, at least in part, by a dysregulation of epigenetic control mechanisms and aberrant epigenetic marks [206]. Some studies are in line with this suggestion [206,207]. Interestingly, and regarding the “glucocorticoid vulnerability hypothesis”, epigenetic modifications produced by the enhanced exposure to CORT could be the “priming mechanism” that enhances the vulnerability to subsequent metabolic challenges.
Since epigenetic mechanisms are modulated by the environment, they are excellent candidates to explain the individual vulnerability to stressors and the effects of GCs on the brain during aging. For instance, in a seminal work, Weaver et al. [208] showed that a dysregulation of the HPA axis in rats can be mediated by a reduced maternal care, which leads to an enhanced methylation of the promoter region of the GR gene. These animals show enhanced CORT responses to an acute stressor and reduced levels of GRs in the HC. Interestingly, a similar result has been obtained in the case of prenatal exposure to maternal depression in humans [209]. However, few studies have focused on the effects of negative early life events on aging. One study showed that maternal separation enhances the cognitive deficits observed in aged mice [210]. Another study showed that maternal separation leads to enhanced individual differences in senescent rats (30 minths) [211]. Therefore, negative environmental conditions during early life or during adulthood (above described) not only can modulate brain aging but they can also reveal and expand individual differences produced either by genetic differences or by other environmental factors.
Until now, this review has focused on a negative environmental factor, the exposure to stressors, which can lead to detrimental outcomes during aging. However, some environmental factors could exert a positive influence on brain aging [1,9]. It is therefore paramount to study those environmental factors that not only mediate brain vulnerability to aging, but also those that can attenuate or even reverse the effects of aging on the brain by the modulation of the stress response.
9. Improving environmental conditions: relevance for lifestyles in humans
As it has been shown during this review, most of the changes produced by stress and its consequences on aging are the result of the modulation of brain plasticity. The mechanisms that allow brain plasticity are maintained during the lifespan and they can be considered as essentially bidirectional. Depending on the circumstances, these mechanisms can improve or worsen the brain function [2].
Environmental enrichment (EE) is an experimental model of positive environmental conditions that has been shown to be effective in rodents on the attenuation of age-related-cognitive deficits through the modulation of brain plasticity [212,213]. On EE conditions, laboratory animals are reared in large cages, in which they have enhanced social, sensorial and motor stimulation. Some protocols of EE also include running wheels in their cages to promote voluntary physical exercise.
EE protects against age-related changes on the neuronal remodeling in different areas of the brain [214–217], and the loss of neurogenesis observed during aging in the dentate gyrus of the HC [98,218]. Therefore EE is thought to be a positive factor that could counteract some of the effects of stress on the aged brain. EE on young adult rodents reduces the response of plasma ACTH and CORT to different stressors [219–223]. EE also increases the levels of GRs in the hippocampus, which would facilitate a faster recovery of basal levels of CORT under stress conditions [224]. Moreover, EE has been shown to be effective reducing the effects of chronic stressors and social defeat on different behavioral and molecular parameters [225–227]. However, studies on rodents focusing on EE and the stress response during aging are sparse. It has been shown that the increases of dopamine and acetylcholine in the PFC in response to acute restraint are reduced in aged EE rats [139,228]. Interestingly, EE enhances the levels of BDNF in the aged brain, which could favor resilience to negative effects of chronic stressors in these animals [228–230].
In general, the lower hormonal and neurochemical reactivity of EE animals to different stressors is thought to be due to the “inoculation of stress” [231]. The conditions of EE, which imply continuous changes in the environment and enhanced social interactions would increase the exposure to mild stressors during life that would lead to enhanced levels of neurotrophic factors such as BDNF or GDNF, an enhanced expression of HSPs [232] and better coping with stressors in a similar way to habituation. Interestingly, the PFC could play a main role on this lower reactivity to stressors [233].
The relevant question, however, is if it is possible to reverse or attenuate brain aging in humans. Physiological aging in the human being is associated with a decline of episodic and working memory, linguistic abilities, information processing and speed response [1–3,234]. However, the onset and progression of these cognitive deficits are subjected to individual differences [235]. In fact, it is estimated that at least a 25% individuals diagnosed post-mortem of Alzheimer disease do not show cognitive deficits during their life [3]. These individual differences have lead to the search for factors that could account for a successful aging.
The factors that could predict cognitive decline during aging or dementia are genetic and environmental. One of the most studied genetic factors that could account for the onset of dementia is the presence of the APOEε4 allele [235,236]. However, the fact that not all the individuals carrying the apoEε4 allele develop dementia suggests that environmental factors can also be a relevant variable to account for individual vulnerability to brain aging. Moreover, the discovering of environmental factors related to lifestyles could have a high therapeutic value, given that theoretically lifestyles can be more easily modified than genetic factors. Among the environmental factors that might prevent cognitive decline and dementia are educational levels, engagement in cognitive demanding leisure activities, cognitive demanding jobs during life and physical exercise [235–237]. The positive effects of these factors on brain aging are thought to be mediated by an increase of the so-called cognitive reserve. This term refers to the individual differences in cognitive processes or neural networks that would allow to some individuals to face age-related changes and brain insults in a more effective way than others [3]. This hypothesis proposes that environmental stimuli can change the morphology and function of the brain through brain plasticity mechanisms leading to a lower vulnerability of the brain to aging. Given that stress and GCs can alter the morphology and function of different brain areas involved in cognitive processes, such as the HC and the PFC, they are also good candidates to be modulated by protective factors. However, it is not well-known whether these positive environmental factors prevent cognitive decline by reducing stress levels. For example, higher educational levels do not seem to correlate with lower levels of cortisol [238]. However, in the case of the human being a very important variable regarding the effects of stressors and the subsequent stress response is the feeling of controllability over the stressor [239]. For this reason, variables related to this feeling of control over stress, such as social support, socioeconomic status or hierarchy could also be protective against brain aging [239,240].
10. Conclusions
The main message of this review is that stress plays a key role on brain aging. Changes produced by physiological aging can interact with a certain genetic background and/or negative environmental conditions (i.e: chronic stressors, stressors during infancy), which lead to an allostatic load. The final result of this interaction is an enhanced vulnerability of the brain to enhanced levels of GCs and metabolic challenges, and a reduced resilience to subsequent exposures to stressors. These changes will constitute the features of a unsuccessful aged brain, added to cognitive deficits and neuronal death (Figure 3). However, more efforts should be done to study: 1/the dynamics of the vulnerability to enhanced levels of GCs during the life-span; 2/the influence of stress disorders or the exposure to chronic stressors during adulthood on brain aging; 3/the molecular mechanisms conferring resilience to stress exposure and their changes during aging; 4/the environmental factors that could reduce the levels of stress leading to better coping and to a successful brain aging, and the molecular pathways that mediate this effects.
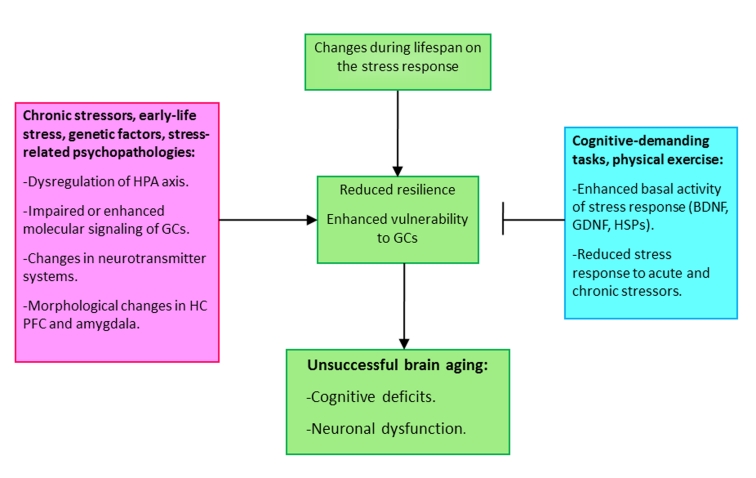
Figure 3.
Scheme depicting the determining factors mediating or preventing unsuccessful aging. Changes during life on the hormonal and neurochemical stress response can lead to a state of enhanced vulnerability to subsequent exposures to stressors or to programmed changes produced by exposure to early life negative events. The interaction between negative environmental factors and changes during life span would lead to cognitive deficits and, in some cases to neuronal dysfunction and dementias. By contrast, positive environmental factors, such as to be engaged in cognitive-demanding tasks during life or physical exercise can attenuate or prevent the effects of aging on the brain.
Acknowledgments
The author deeply appreciates the helpful commentaries of Marta de Blas and Dr.Francisco Mora. The author is recipient of a FPU fellowship from the Ministerio de Educación of Spain.
REFERENCES
- [1].Kramer AF, Bherer L, Colcombe S, Dong W, Greenough WT. Environmental influences on cognitive and brain plasticity during aging. J. Gerontol A Biol. Sci. Med. Sci. 2004;59:M940–957. doi: 10.1093/gerona/59.9.m940. [DOI] [PubMed] [Google Scholar]
- [2].Mahncke HW, Bronstone A, Merzenich MM. Chapter 6 Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. In: Aage RM, editor. Progress in Brain Research Reprogramming of the Brain. Elsevier; 2006. pp. 81–109. [DOI] [PubMed] [Google Scholar]
- [3].Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Martin GM, Bergman A, Barzilai N. Genetic Determinants of Human Health Span and Life Span: Progress and New Opportunities. PLoS Genet. 2007;3:e125. doi: 10.1371/journal.pgen.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Antebi A. Genetics of Aging in Caenorhabditis elegans. PLoS Genet. 2007;3:e129. doi: 10.1371/journal.pgen.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rattan SIS, Singh R. Progress & Prospects: Gene therapy in aging. Gene Ther. 2008;16:3–9. doi: 10.1038/gt.2008.166. [DOI] [PubMed] [Google Scholar]
- [7].Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fontana L, Partridge L, Longo VD. Extending Healthy Life SpanGÇöFrom Yeast to Humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- [10].Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: Mental and physical activity as modulators of brain disorders. Prog. Neurobiol. 2009;89:369–382. doi: 10.1016/j.pneurobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- [11].Cannon W. Bodily changes in pain, hunger, fear, and rage. New York: Appleton-Century-Crofts; 1929. [Google Scholar]
- [12].Armario A. The Hypothalamic-Pituitary-Adrenal Axis: What can it Tell us About Stressors? CNS & Neurological disorders- Drug targets. 2006;5:485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- [13].Bush IE, Sandberg AA. Adrenocortical hormones in human plasma. J. Biol. Chem. 1953;205:783–793. [PubMed] [Google Scholar]
- [14].Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- [15].Reul JMHM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- [16].Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- [17].McEwen BS, de Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol. Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- [18].Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai YM, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- [19].Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- [21].Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. [Google Scholar]
- [22].McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- [23].Landfield PW, Lynch G. Hippocampal aging and adrecorticoids: a quantitative correlation. Science. 1978;202:1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- [24].Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981;214:581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- [25].Sapolsky RM, Krey LC, McEwen BS. The adreno cortical stress-response in the aged male rat: impairment of recovery from stress. Exp. Gerontol. 1983;18:55–64. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- [26].Lorens SA, Hata RJ, Handa RJ, Van de Kar LD, Gusechwan M, Goral J, Lee JM, Hamilton ME, Bethea CL, Clancy J., Jr Neurochemical, endocrine and immunological responses to stress in young and old Fischer 344 male rats. Neurobiol. Aging. 1990;11:139–150. doi: 10.1016/0197-4580(90)90047-4. [DOI] [PubMed] [Google Scholar]
- [27].van Eekelen JAM, Rots NY, Sutanto W, de Kloet ER. The effect of aging on stress responsiveness and central corticosteroid receptors in the Brown Norway rat. Neurobiol. Aging. 1992;13:159–170. doi: 10.1016/0197-4580(92)90024-r. [DOI] [PubMed] [Google Scholar]
- [28].Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol. Aging. 2006;27:645–654. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- [29].Segar TM, Kosckow JV, Welge JA, Herman JP. Heterogeneity of neuroendocrine stress responses in aging rat strains. Physiol. Behav. 2009;96:6–11. doi: 10.1016/j.physbeh.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- [31].Robbins TW, Arnsten AFT. The Neuropsychopharmacology of Fronto-Executive Function: Monoaminergic Modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Squire LR, Zola-Morgan S. Memory: brain systems and behavior. Trends Neurosci. 1988;11:170–175. doi: 10.1016/0166-2236(88)90144-0. [DOI] [PubMed] [Google Scholar]
- [33].Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- [34].Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- [35].Jöels M, Krugers H, Karst H. Stress-induced changes in hippocampal function. Prog Brain Res. 2008;167:3–15. doi: 10.1016/S0079-6123(07)67001-0. [DOI] [PubMed] [Google Scholar]
- [36].Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid Effects on Memory Consolidation Depend on Functional Interactions between the Medial Prefrontal Cortex and Basolateral Amygdala. J. Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sandi C, Pinelo-Nava T. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast. 2007:1–20. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- [39].Kant GF, Meyerhoff JL, Jarrard LE. Biochemical indices of reactivity and habituation in rats with hippocampal lesions. Pharmacol. Biochem. Behav. 1984;20:793–797. doi: 10.1016/0091-3057(84)90201-6. [DOI] [PubMed] [Google Scholar]
- [40].Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc. Natl. Acad. Sci. USA. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J. Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- [43].Rigter H, Veldhuis H, de Kloet ER. Spatial learning and the hippocampal corticosterone receptor system of old rats: effect of the ACTH4-9 analogue ORG 2766. Brain Res. 1984;309:393–398. doi: 10.1016/0006-8993(84)90612-7. [DOI] [PubMed] [Google Scholar]
- [44].Zoli M, Ferraguti F, Gustafsson JA, Toffano G, Fuxe K, Agnati LF. Selective reduction of glucocorticoid receptor immunoreactivity in the hippocampal formation and central amygdaloid nucleus of the aged rat. Brain Res. 1991;545:199–207. doi: 10.1016/0006-8993(91)91287-b. [DOI] [PubMed] [Google Scholar]
- [45].Cizza G, Gold PW, Chrousos GP. Aging is associated in the 344/N Fischer rat with decreased stress responsivity of central and peripheral catecholaminergic systems and impairment of the hypothalamic-pituitary-adrenal axis. Ann. NY Acad. Sci. 1995;771:491–511. doi: 10.1111/j.1749-6632.1995.tb44705.x. [DOI] [PubMed] [Google Scholar]
- [46].Bizon JL, Helm KA, Han JS, Chun HJ, Pucilowska J, Lund PK, Gallagher M. Hypothalamic-pituitary-adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged Long-Evans rats. Eur. J. Neurosci. 2001;14:1739–1751. doi: 10.1046/j.0953-816x.2001.01781.x. [DOI] [PubMed] [Google Scholar]
- [47].Mizoguchi K, Ikeda R, Shoji H, Tanaka Y, Maruyama W, Tabira T. Aging attenuates glucocorticoid negative feedback in rat brain. Neuroscience. 2009;159:259–270. doi: 10.1016/j.neuroscience.2008.12.020. [DOI] [PubMed] [Google Scholar]
- [48].Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J. Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- [50].Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J. Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kasckow JW, Segar TM, Xiao C, Furay AR, Evanson NK, Ostrander MM, Herman JP. Stability of neuroendocrine and behavioral responsiveness in aging fischer 344/brown-norway hybrid rats. Endocrinology. 2005;146:3105–3112. doi: 10.1210/en.2004-1648. [DOI] [PubMed] [Google Scholar]
- [52].Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol. Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- [53].Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol. Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- [54].Michaud K, Forget H, Cohen H. Chronic glucocorticoid hypersecretion in Cushing’s syndrome exacerbates cognitive aging. Brain Cogn. 2009;71:1–8. doi: 10.1016/j.bandc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- [55].Kremen WS, O’Brien RC, Panizzon MS, Prom-Wormley E, Eaves LJ, Eisen SA, Eyler LT, Hauger RL, Fennema-Notestine C, Fischl B, et al. Salivary cortisol and prefrontal cortical thickness in middle-aged men: A twin study. NeuroImage. 2010;53:1093–1102. doi: 10.1016/j.neuroimage.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yau JLW, Morris RGM, Seckl JR. Hippocampal corticosteroid receptor mRNA expression and spatial learning in the aged Wistar rat. Brain Res. 1994;657:59–64. doi: 10.1016/0006-8993(94)90953-9. [DOI] [PubMed] [Google Scholar]
- [57].Meijer OC, Topic B, Steenbergen G, Jocham G, Houston JP, Oitzl MS. Correlations between hypothalamus-pituityary-adrenal axis parameters depend on age and learning capacity. Endocrinology. 2005;146:1372–1381. doi: 10.1210/en.2004-0416. [DOI] [PubMed] [Google Scholar]
- [58].Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Schürmeyer T, Kirschbaum C. Psychosocial stress and HPA functioning: No evidence for a reduced resilience in healthy elderly men. Stress. 2000;3:229–240. doi: 10.3109/10253890009001127. [DOI] [PubMed] [Google Scholar]
- [59].Wolf OT. HPA axis and memory. Best Pract. Res. Clin. Endocrinol. Metab. 2003;17:287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
- [60].Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- [61].Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [62].Swanwick GRJ, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, Lawlor BA. Hypothalamic-Pituitary-Adrenal Axis Dysfunction in Alzheimer’s Disease: Lack of Association Between Longitudinal and Cross-Sectional Findings. Am. J. Psychiatry. 1998;155:286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- [63].Izurieta-Sánchez P, Jonkers N, Sarre S, Ebinger G, Michotte Y. Neostigmine influences the L-dopa-induced extracellular dopamine levels in the striatum. Brain Res. 2000;856:250–253. doi: 10.1016/s0006-8993(99)02398-7. [DOI] [PubMed] [Google Scholar]
- [64].Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J. Psychiatr. Res. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- [65].Garrido P, De Blas M, del Arco A, Segovia G, Mora F. Aging increases basal but not stress-induced levels of corticosterone in the hippocampus and the prefrontal cortex of the awake rat. Neurobiol. Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.02.015. [DOI] [PubMed] [Google Scholar]
- [66].Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- [67].Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatialálearningádeficits. Proc. Natl. Acad. Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J. Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sousa N, Almeida OF, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 1998;2:237–249. doi: 10.3109/10253899809167288. [DOI] [PubMed] [Google Scholar]
- [70].Leverenz JB, Wilkinson CW, Wamble M, Corbin S, Grabber JE, Raskind MA, Peskind ER. Effect of Chronic High-Dose Exogenous Cortisol on Hippocampal Neuronal Number in Aged Nonhuman Primates. J. Neurosci. 1999;19:2356–2361. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Müller MB, Keck ME, Blinder EB, Kresse AE, Hagameyer TP, Landgraf R, Holsboer F, Uhr M. ABCB1 (MDR1)-Type P-Glycoproteins at the blood-brain barrier modulate the activity of the hypothalamic-pituitary-adrenocortical system: implications for affective disorder. Neuropsychopharmacology. 2003;28:1991–1999. doi: 10.1038/sj.npp.1300257. [DOI] [PubMed] [Google Scholar]
- [72].Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- [73].Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- [74].Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- [75].Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur. J. Neurosci. 2003;17:2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- [76].Landfield PW, Blalock EM, Chen KC, Porter NM. A new glucocorticoid hypothesis of brain aging: Implications for Alzheimer’s disease. Curr. Alzheimer Res. 2007;4:205–212. doi: 10.2174/156720507780362083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Conrad CD, Jackson JL, Wise LS. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic Glucocorticoids Increase Hippocampal Vulnerability to Neurotoxicity under Conditions That Produce CA3 Dendritic Retraction But Fail to Impair Spatial Recognition Memory. J. Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nat. Rev. Neurosci. 2009;10:434–444. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- [82].Lightman SL. The Neuroendocrinology of Stress: A Never Ending Story. J. Neuroendocrinol. 2008;20:880–884. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- [83].Yau JLW, Noble J, Kenyon CJ, Hibberd C, Kotelevtsev Y, Mullins JJ, Seckl JR. Lack of tissue glucocorticoid reactivation in 11+¦-hydroxysteroid dehydrogenase type 1 knockout mice ameliorates age-related learning impairments. Proc. Natl. Acad. Sci. USA. 2001;98:4716–4721. doi: 10.1073/pnas.071562698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Shoji H, Mizoguchi K. Acute and repeated stress differentialy regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats. Behav Brain Res. 2010;211:169–177. doi: 10.1016/j.bbr.2010.03.025. [DOI] [PubMed] [Google Scholar]
- [85].Lupien SJ, Lecours AR, Schwartz G, Sharma S, Hauger RL, Meaney MJ, Nair NP. Longitudinal study of basal cortisol levels in healthy elderly subjects: Evidence for subgroups. Neurobiol Aging. 1996;97:95–105. doi: 10.1016/0197-4580(95)02005-5. [DOI] [PubMed] [Google Scholar]
- [86].Lupien SJ, de Leon M, de Santi M, Convit A, Tarsish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat. Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- [87].Seeman TE, Singer B, Wilkinson CW, Bruce M. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- [88].Li G, Cherrier MM, Tsuang DW, Petrie EC, Colasurdo EA, Craft S, Schellenberg GD, Peskind ER, Raskind MA, Wilkinson CW. Salivary cortisol and memory function in human aging. Neurobiol. Aging. 2006;27:1705–1714. doi: 10.1016/j.neurobiolaging.2005.09.031. [DOI] [PubMed] [Google Scholar]
- [89].Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat. Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- [90].Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive Effects of Stress and Aging on Structural Plasticity in the Prefrontal Cortex. J. Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rubinow MJ, Drogos LL, Juraska JM. Age-related dendritic hypertrophy and sexual dimorphism in rat basolateral amygdala. Neurobiol. Aging. 2009;30:137–146. doi: 10.1016/j.neurobiolaging.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nagahara AH, Handa RJ. Age-related changes in c-fos mRNA induction after open-field exposure in the rat brain. Neurobiol. Aging. 2001;18:45–55. doi: 10.1016/s0197-4580(96)00166-2. [DOI] [PubMed] [Google Scholar]
- [93].Zornetzer SF, Thompson R, Rogers J. Rapid forgetting in aged rats. Behav. Neural Biol. 1982;36:49–60. doi: 10.1016/s0163-1047(82)90234-5. [DOI] [PubMed] [Google Scholar]
- [94].Vasquez BJ, Martinez J, Jensen RA, Messing RB, Rigter H, McGaugh JL. Learning and memory in young and aged Fischer 344 rats. Arch. Gerontol. Geriatrics. 1983;2:279–291. doi: 10.1016/0167-4943(83)90001-8. [DOI] [PubMed] [Google Scholar]
- [95].Normile HJ, Altman HJ. Effects of combined acetylcholinesterase inhibition and serotonergic receptor blockade on age-associated memory impairments in rats. Neurobiol. Aging. 1992;13:735–740. doi: 10.1016/0197-4580(92)90097-h. [DOI] [PubMed] [Google Scholar]
- [96].Kikusui T, Tonohiro T, Kaneko T. Age-related working memory deficits in the allocentric place discrimination task: possible involvement in cholinergic dysfunction. Neurobiol Aging. 1999;20:629–636. doi: 10.1016/s0197-4580(99)00096-2. [DOI] [PubMed] [Google Scholar]
- [97].Castner SA, Goldman-Rakic PS. Enhancement of working memory in aged monkeys by a sensitizing regimen of dopamine D1 receptor stimulation. J. Neurosci. 2004;24:1446–1450. doi: 10.1523/JNEUROSCI.3987-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Segovia G, Yagüe AG, García-Verdugo JM, Mora F. Environmental enrichment promotes neurogenesis and changes the extracellular concentrations of glutamate and GABA in the hippocampus of aged rats. Brain Res. Bull. 2006;70:8–14. doi: 10.1016/j.brainresbull.2005.11.005. [DOI] [PubMed] [Google Scholar]
- [99].Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. Stress Selectively Increases Fos Protein in Dopamine Neurons Innervating the Prefrontal Cortex. Cereb. Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- [100].Handa RJ, Cross MK, George M, Gordon BH, Burgess LH, Cabrera TM, Hata N, Campbell DB, Lorens SA. Neuroendocrine and neurochemical responses to novelty stress in young and old male F344 rats: Effects of d-fenfluramine treatment. Pharmacol. Biochem. Behav. 1993;46:101–109. doi: 10.1016/0091-3057(93)90324-m. [DOI] [PubMed] [Google Scholar]
- [101].Ostrander MM, Richtand NM, Herman JP. Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptors. Brain Res. 2003;990:209–214. doi: 10.1016/j.brainres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- [102].Trneckova L, Rotllant D, Hynie S, Armario A. Dynamics of immediate early gene and neuropeptide gene response to prolonged immobilization stress: evidence against a critial role of the termination of exposure to the stressor. J Neurochem. 2007;100:905–914. doi: 10.1111/j.1471-4159.2006.04278.x. [DOI] [PubMed] [Google Scholar]
- [103].Kawakami M, Seto K, Terasawa E, Yoshida K, Miyamoto T, Sekiguchi M, Hattori Y. Influence of electrical stimulation and lesion in limbic structure upon biosynthesis of adrenocorticoid in the rabbit. Neuroendocrinology. 1968;3:337–348. doi: 10.1159/000121722. [DOI] [PubMed] [Google Scholar]
- [104].Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Feldman S, Conforti N, Saphier D. The preoptic area and bed nucleus of the stria terminalis are involved in the effects of the amygdala on adrenocortical secretion. Neuroscience. 1990;37:775–779. doi: 10.1016/0306-4522(90)90107-f. [DOI] [PubMed] [Google Scholar]
- [106].Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J. Neuroendocrinol. 2001;13:625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- [107].Radley JJ, Arias CM, Sawchenko PE. Regional Differentiation of the Medial Prefrontal Cortex in Regulating Adaptive Responses to Acute Emotional Stress. J. Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sullivan RM. Hemispheric Asymmetry in Stress Processing in Rat Prefrontal Cortex and the Role of Mesocortical Dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- [109].Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- [110].Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [111].Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- [112].Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Conrad CD, Millan DD, II, Tsekhanov S, Wright RL, Baran SE, Fuchs RA. Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiol. Learn. Mem. 2004;81:185–199. doi: 10.1016/j.nlm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [115].Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: Effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Sotres-Bayon F, Bush DEA, LeDoux JE. Emotional Perseveration: An Update on Prefrontal-Amygdala Interactions in Fear Extinction. Learn. Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- [117].Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- [118].Abercrombie ED, Keefe KA, Daniel SD, Zigmond MJ. Differential effects of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- [119].Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- [120].Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J. Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol. 1989;165:337–338. doi: 10.1016/0014-2999(89)90735-8. [DOI] [PubMed] [Google Scholar]
- [122].Mark GP, Rada PV, Shors TJ. Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience. 1996;74:767–774. doi: 10.1016/0306-4522(96)00211-4. [DOI] [PubMed] [Google Scholar]
- [123].Thiel CM, Huston JP, Schwarting RKW. Cholinergic activation in frontal cortex and nucleus accumbens related to basic behavioral manipulations: handling, and the role of post-handling experience. Brain Res. 1998;812:121–132. doi: 10.1016/s0006-8993(98)00961-5. [DOI] [PubMed] [Google Scholar]
- [124].Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- [125].Lupinsky D, Moquin L, Gratton A. Interhemispheric Regulation of the Medial Prefrontal Cortical Glutamate Stress Response in Rats. J Neurosci. 2010;30:7624–7633. doi: 10.1523/JNEUROSCI.1187-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Prog. Neuro-Psychopharmacol. & Biol. Psychiat. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- [127].Vermes I, Telegdy G, Lissák K. Effect of midbrain raphe lesion on diurnal and stress-induced changes in serotonin content of discrete regions of the limbic system and in adrenal function in the rat. Acta Physiol Acad Sci Hung. 1974;45:217–224. [PubMed] [Google Scholar]
- [128].Casolini P, Kabbaj M, Leprat F, Piazza PV, Rouge-Pont F, Angelucci L, Simon H, Le Moal M, Maccari S. Basal and stress-induced corticosterone secretion is decreased by lesion of mesencephalic dopaminergic neurons. Brain Res. 1993;622:311–314. doi: 10.1016/0006-8993(93)90836-c. [DOI] [PubMed] [Google Scholar]
- [129].Helm KA, Ziegler DR, Gallagher M. Habituation to stress and dexamethasone suppression in rats with selective basal forebrain cholinergic lesions. Hippocampus. 2004;14:628–635. doi: 10.1002/hipo.10203. [DOI] [PubMed] [Google Scholar]
- [130].Radley JJ, Williams B, Sawchenko P. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J. Neurosci. 2008;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Gabr RW, Birkle DL, Azzaro AJ. Stimulation of the amygdala by glutamate facilitates corticotropin-releasing factor release from the median eminence and activation of the hypothalamic-pituitary-adrenal axis in stressed rats. Neuroendocrinology. 1995;62:333–339. doi: 10.1159/000127022. [DOI] [PubMed] [Google Scholar]
- [132].Bhatnagar S, Costall B, Smythe JW. Hippocampal cholinergic blockade enhances hypothalamic-pituitary-adrenal responses to stress. Brain Res. 1997;766:244–248. doi: 10.1016/s0006-8993(97)00684-7. [DOI] [PubMed] [Google Scholar]
- [133].Ikemoto S, Goeders NE. Microinjections of dopamine agonists and cocaine elevate plasma corticosterone: dissociation effects among the ventral and dorsal striatum and medial prefrontal cortex. Brain Res. 1998;814:171–178. doi: 10.1016/s0006-8993(98)01070-1. [DOI] [PubMed] [Google Scholar]
- [134].Weinberg MS, Johnson DC, Bhatt AP, Spencer RL. Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience. 2010;168:744–758. doi: 10.1016/j.neuroscience.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].del Arco A, Ronzoni G, Cordero I, Mora F. NMDA blokade in the prefrontal cortex increases dialysate concentrations of acetylcholine and corticosterone in the hippocampus and motor activity: Role of prefrontal GABAA stimulation. FENS Abstr. 2010;5 [Google Scholar]
- [136].del Arco A, Segovia G, Mora F. Dopamine release during stress in the prefrontal cortex of the rat decreases with age. NeuroReport. 2001;12:4019–4022. doi: 10.1097/00001756-200112210-00033. [DOI] [PubMed] [Google Scholar]
- [137].Lowy MT, Wittenberg L, Yamamoto BK. Effects of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J. Neurochem. 1995;65:268–274. doi: 10.1046/j.1471-4159.1995.65010268.x. [DOI] [PubMed] [Google Scholar]
- [138].Fischer W, Nilsson OG, Björklund A. In vivo acetylcholine release as measured by microdialysis is unaltered in the hippocampus of cognitively impaired aged rats with degenerative changes in the basal forebrain. Brain Res. 1991;556:44–52. doi: 10.1016/0006-8993(91)90545-7. [DOI] [PubMed] [Google Scholar]
- [139].Segovia G, del Arco A, Garrido P, De Blas M, Mora F. Environmental enrichment reduces the response to stress of the cholinergic system in the prefrontal cortex during aging. Neurochem Int. 2008;52:1198–1203. doi: 10.1016/j.neuint.2007.12.007. [DOI] [PubMed] [Google Scholar]
- [140].Luebke JI, Chang Y-M, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125:277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- [141].Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De Koninck Y. Imbalance towards inhibition as a substrate of aging-associated cognitive impairment. Neurosci. Lett. 2006;397:64–68. doi: 10.1016/j.neulet.2005.11.055. [DOI] [PubMed] [Google Scholar]
- [142].Majdi M, Ribeiro-da-Silva A, Cuello AC. Variations in excitatory and inhibitory postsynaptic protein content in rat cerebral cortex with respect to aging and cognitive status. Neuroscience. 2009;159:896–907. doi: 10.1016/j.neuroscience.2008.11.034. [DOI] [PubMed] [Google Scholar]
- [143].Segovia G, Porras A, del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29. doi: 10.1016/s0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- [144].Abraham I, Juhasz G, Kekesi KA, Kovacs KJ. Effect of intrahippocampal dexamethasone on the levels of amino acid transmitters and neuronal excitability. Brain Res. 1996;733:56–63. doi: 10.1016/0006-8993(96)00538-0. [DOI] [PubMed] [Google Scholar]
- [145].Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- [146].Cho K, Little HJ. Effects of corticosterone on excitatory amino acid responses in dopamine-sensitive neurons in the ventral tegmental area. Neuroscience. 1998;88:837–845. doi: 10.1016/s0306-4522(98)00264-4. [DOI] [PubMed] [Google Scholar]
- [147].Conboy L, Sandi C. Stress at Learning Facilitates Memory Formation by Regulating AMPA Receptor Trafficking Through a Glucocorticoid Action. Neuropsychopharmacology. 2009;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for mantaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav. Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- [152].Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- [153].Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- [155].Eldridge JC, Brodish A, Kute TE, Landfield PW. Apparent age-related resistance of type II hippocampal corticosteroid receptors to down-regulation during chronic escape training. J. Neurosci. 1989;9:3237–3242. doi: 10.1523/JNEUROSCI.09-09-03237.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Sandi C, Touyarot K. Mid-life stress and cognitive deficits during early aging in rats: individual differences and hippocampal correlates. Neurobiol Aging. 2006;27:128–140. doi: 10.1016/j.neurobiolaging.2005.01.006. [DOI] [PubMed] [Google Scholar]
- [157].Borcel E, Pérez-Alvarez L, Herrero AI, Brionne T, Varea E, Berezin V, Bock E, Sandi C, Venero C. Chronic stress in adulthood followed by intermittent stress impairs spatial memory and the survival of newborn hippocampal cells in aging animals: prevention by FGL, a peptide mimetic of neural cell adhesion molecule. Behav. Pharmacol. 2008;19:41–49. doi: 10.1097/FBP.0b013e3282f3fca9. [DOI] [PubMed] [Google Scholar]
- [158].Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Ritvanen T, Louhevaara V, Helin P, VSisSnen S, HSnninen O. Responses of the autonomic nervous system during periods of perceived high and low work stress in younger and older female teachers. Appl Ergon. 2006;37:311–318. doi: 10.1016/j.apergo.2005.06.013. [DOI] [PubMed] [Google Scholar]
- [160].Kiss P, De Meester M, Braeckman L. Differences between younger and older workers in the need for recovery after work. International Archives of Occupational and Environmental Health. 2008;81:311–320. doi: 10.1007/s00420-007-0215-y. [DOI] [PubMed] [Google Scholar]
- [161].Golier JA, Yehuda R, Lupien SJ, Harvey PD, Grossman R, Elkin A. Memory Performance in Holocaust Survivors With Posttraumatic Stress Disorder. Am. J. Psychiatry. 2002;159:1682–1688. doi: 10.1176/appi.ajp.159.10.1682. [DOI] [PubMed] [Google Scholar]
- [162].Golier JA, Harvey PD, Legge J, Yehuda R. Memory Performance in Older Trauma Survivors. Ann. NY Acad. Sci. 2006;1071:54–66. doi: 10.1196/annals.1364.006. [DOI] [PubMed] [Google Scholar]
- [163].Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern Y, Mayeux R. Depressed Mood and the Incidence of Alzheimer’s Disease in the Elderly Living in the Community. Arch. Gen. Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- [164].Sotiropoulos I, Cerqueira JJ, Catania C, Takashima A, Sousa N, Almeida OFX. Stress and glucocorticoid footprints in the brain--The path from depression to Alzheimer’s disease. Neurosci. Biobehav. Rev. 2008;32:1161–1173. doi: 10.1016/j.neubiorev.2008.05.007. [DOI] [PubMed] [Google Scholar]
- [165].Katoh-Semba R, Semba R, Takeuchi IK, Kato K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci. Res. 1998;31:227–234. doi: 10.1016/s0168-0102(98)00040-6. [DOI] [PubMed] [Google Scholar]
- [166].Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett. 2002;328:261–264. doi: 10.1016/s0304-3940(02)00529-3. [DOI] [PubMed] [Google Scholar]
- [167].Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–624. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- [168].Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, et al. Brain-Derived Neurotrophic Factor Is Associated with Age-Related Decline in Hippocampal Volume. J. Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Vreugdenhil E, De Kloet ER, Schaaf M, Datson NA. Genetic dissection of corticosterone receptor function in the rat hippocampus. Eur. Neuropsychopharmacology. 2001;11:423–430. doi: 10.1016/s0924-977x(01)00119-5. [DOI] [PubMed] [Google Scholar]
- [170].McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- [171].Prager EM, Johnson LR. Stress at the Synapse: Signal Transduction Mechanisms of Adrenal Steroids at Neuronal Membranes. Sci. Signal. 2009;2 doi: 10.1126/scisignal.286re5. re5. [DOI] [PubMed] [Google Scholar]
- [172].Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids Regulate Glutamate and GABA Synapse-Specific Retrograde Transmission via Divergent Nongenomic Signaling Pathways. J. Neurosci. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Reul JM, Tonnarer JA, de Kloet ER. Neurotrophic ACTH analogue promotes plasticity of type I corticosteroid receptor in brain of senescent male rats. Neurobiol. Aging. 1988;9:253–260. doi: 10.1016/s0197-4580(88)80062-9. [DOI] [PubMed] [Google Scholar]
- [174].Meaney MJ, Aitken DH, Sharma S, Viau V. Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology. 1992;55:204–213. doi: 10.1159/000126116. [DOI] [PubMed] [Google Scholar]
- [175].Morano MI, Vazquez DM, Akil H. The Role of the Hippocampal Mineralocorticoid and Glucocorticoid Receptors in the Hypothalamo-Pituitary-Adrenal Axis of the Aged Fisher Rat. Mol. Cell. Neurosci. 1994;5:400–412. doi: 10.1006/mcne.1994.1050. [DOI] [PubMed] [Google Scholar]
- [176].Hassan AHS, Patchev VK, von Rosenstiel P, Holsboer F, Almeida OF. Plasticity of hippocampal corticosteroid receptors during aging in the rat. FASEB J. 1999;13:115–122. doi: 10.1096/fasebj.13.1.115. [DOI] [PubMed] [Google Scholar]
- [177].Murphy EK, Spencer RL, Sipe KJ, Herman JP. Decrements in Nuclear Glucocorticoid Receptor (GR) Protein Levels and DNA Binding in Aged Rat Hippocampus. Endocrinology. 2002;143:1362–1370. doi: 10.1210/endo.143.4.8740. [DOI] [PubMed] [Google Scholar]
- [178].Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G, mschroder-Williams P, Du J, Zhang L, Manji HK. The Anti-Apoptotic, Glucocorticoid Receptor Cochaperone Protein BAG-1 Is a Long-Term Target for the Actions of Mood Stabilizers. J. Neurosci. 2005;25:4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, hado-Vieira R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc. Natl. Acad. Sci. USA. 2009;106:3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Adzic M, Djordjevic A, Demonacos C, Krstic-Demonacos M, Radojcic MB. The role of phosphorylated glucocorticoid receptor in mitochondrial functions and apoptotic signalling in brain tissue of stressed Wistar rats. Int. J. Biochem. Cell Biol. 2009;41:2181–2188. doi: 10.1016/j.biocel.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Djordjevic A, Adzic M, Djordjevic J, Radojcic MB. Chronic social isolation suppresses proplastic response and promotes proapoptotic signalling in prefrontal cortex of Wistar rats. J. Neurosci. Res. 2010;88:2524–2533. doi: 10.1002/jnr.22403. [DOI] [PubMed] [Google Scholar]
- [182].Lengvári I, Liposits ZS. Diurnal changes in endogenous corticosterone content of some brain regions of rats. Brain Res. 1977;124:571–575. doi: 10.1016/0006-8993(77)90959-3. [DOI] [PubMed] [Google Scholar]
- [183].Droste SK, Chandramohan Y, Reul JMHM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenal axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- [184].Droste SK, de Groote L, Lightman SL, Reul JMHM, Linthorst ACE. The ultradian and circadian rhythms of free corticosterone in the brain are not affected by gender: an in vivo microdialysis study in Wistar rats. J. Neuroendocrinol. 2009;21:132–140. doi: 10.1111/j.1365-2826.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- [185].Croft AP, O’Callaghan MJ, Shaw SG, Connolly G, Jacquot C, Little HJ. Effects of minor laboratory procedures, adrenalectomy, social defeat or acute alcohol on regional brain concentrations of corticosterone. Brain Res. 2008;1238:12–22. doi: 10.1016/j.brainres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [186].Little HJ, Croft AP, O’Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- [187].Rosner W. The functions of corticosteroid-binding globulin and sex hormone-binding globulin. Endocr Rev. 1990;11:80–91. doi: 10.1210/edrv-11-1-80. [DOI] [PubMed] [Google Scholar]
- [188].Wolf DC, Horwitz SB. P-glycoprotein transports corticosterone and is photoaffinity-labeled by the steroid. Int. J. Cancer. 1992;52:141–146. doi: 10.1002/ijc.2910520125. [DOI] [PubMed] [Google Scholar]
- [189].Meijer OC, Kortekaas R, Oitzl MS, de Kloet ER. Acute rise in corticosterone facilitates 5-HT receptor-mediated behavioural responses. Eur. J. Pharmacol. 1998;351:7–14. doi: 10.1016/s0014-2999(98)00289-1. [DOI] [PubMed] [Google Scholar]
- [190].Uhr M, Holsboer F, Müller MB. Penetration of endogenous steroid hormones corticosterone, cortisol, aldosterone and progesterone into the brain is enhanced in mice deficicent for both mdr1a and mdr1b P-glycoporteins. J. Neuroendocrinol. 2002;14:753–759. doi: 10.1046/j.1365-2826.2002.00836.x. [DOI] [PubMed] [Google Scholar]
- [191].Mason BL, Pariante CM, Thomas SA. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wil-type and ABCB1A/B-deficient mice. Endocrinology. 2008;149:5244–5253. doi: 10.1210/en.2008-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Holmes MC, Yau JLW, Kotelevtsev Y, Mullins JJ, Seckl JR. 11b-hydrosteroid dehydrogenases in the brain. Ann. NY Acad. Sci. 2003;1007:357–366. doi: 10.1196/annals.1286.035. [DOI] [PubMed] [Google Scholar]
- [193].Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated Swim Impairs Serotonin Clearance via a Corticosterone-Sensitive Mechanism: Organic Cation Transporter 3, the Smoking Gun. J. Neurosci. 2010;30:15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Yau JLW, McNair KM, Noble J, Brownstein D, Hibberd C, Morton N, Mullins JJ, Morris RGM, Cobb S, Seckl JR. Enhanced Hippocampal Long-Term Potentiation and Spatial Learning in Aged 11{beta}-Hydroxysteroid Dehydrogenase Type 1 Knock-Out Mice. J. Neurosci. 2007;27:10487–10496. doi: 10.1523/JNEUROSCI.2190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Sooy K, Webster SP, Noble J, Binnie M, Walker BR, Seckl JR, Yau JLW. Partial Deficiency or Short-Term Inhibition of 11{beta}-Hydroxysteroid Dehydrogenase Type 1 Improves Cognitive Function in Aging Mice. J. Neurosci. 2010;30:13867–13872. doi: 10.1523/JNEUROSCI.2783-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Sandeep TC, Yau JLW, MacLullich AMJ, Noble J, Deary IJ, Walker BR, Seckl JR. 11+¦-Hydroxysteroid dehydrogenase inhibition improves cognitive function in healthy elderly men and type 2 diabetics. Proc. Natl. Acad. Sci. USA. 2004;101:6734–6739. doi: 10.1073/pnas.0306996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Jenuwein T, Allis CD. Translating the Histone Code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- [198].GrSff J, Mansuy IM. Epigenetic codes in cognition and behaviour. Behav. Brain Res. 2008;192:70–87. doi: 10.1016/j.bbr.2008.01.021. [DOI] [PubMed] [Google Scholar]
- [199].Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–735. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. Membrane-Associated Glucocorticoid Activity Is Necessary for Modulation of Long-Term Memory via Chromatin Modification. J. Neurosci. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Bilang-Bleuel A, Ulbricht S, Chandramohan Y, De Carli S, Droste SK, Reul JMHM. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioural response. Eur. J. Neurosci. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- [202].Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- [203].Reul JMHM, Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32:S21–S25. doi: 10.1016/j.psyneuen.2007.03.016. [DOI] [PubMed] [Google Scholar]
- [204].Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting Epigenetic Influence of Early-Life Adversity on the BDNF Gene. Biol. Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Hunter RG, McCarthy K, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. PNAS. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Penner MR, Roth TL, Chawla MK, Hoang LT, Roth ED, Lubin FD, Sweatt JD, Worley PF, Barnes CA. Age-related changes in Arc transcription and DNA methylation within the hippocampus. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, gis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, et al. Altered Histone Acetylation Is Associated with Age-Dependent Memory Impairment in Mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- [208].Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- [209].Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- [210].Solas M, Aisa B, Mugueta MC, Del Rio J, Tordera RM, Ramirez MJ. Interactions Between Age, Stress and Insulin on Cognition: Implications for Alzheimer’s Disease. Neuropsychopharmacology. 2010;35:1664–1673. doi: 10.1038/npp.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Oitzl MS, Workel JO, Fluttert M, Fr+Âsch F, De Kloet ER. Maternal deprivation affects behaviour from youth to senescence: amplification of individual differences in spatial learning and memory in senescent Brown Norway rats. Eur. J. Neurosci. 2000;12:3771–3780. doi: 10.1046/j.1460-9568.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- [212].van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- [213].Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- [214].Greenough WT, McDonald JW, Parnisari RM, Camel JE. Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Res. 1986;380:136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- [215].Saito S, Kobayashi S, Ohashi Y, Igarashi M, Komiya Y, Ando S. Decreased synaptic density in aged brains and its prevention by rearing under enriched environment as revealed by synaptophysin contents. J Neurosci Res. 1994;39:57–62. doi: 10.1002/jnr.490390108. [DOI] [PubMed] [Google Scholar]
- [216].Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol. Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- [217].Leal-Galicia P, Castañeda-Bueno M, Quiroz-Baez R, Arias C. Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem. 2008;90:511–518. doi: 10.1016/j.nlm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- [218].Kempermann G, Georg Kuhn H, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- [219].Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J. Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- [220].Mlynarik M, Johansson BB, Jezova D. Enriched environment influences adrenocortical response to immune challenge and glutamate receptor gene expression in rat hippocampus. Ann. NY Acad. Sci. 2004;1018:273–280. doi: 10.1196/annals.1296.032. [DOI] [PubMed] [Google Scholar]
- [221].Meijer MK, Sommer R, Spruijt BM, van Zutphen LFM, Baumans V. Influence of environmental enrichment and handling on the acute stress response in individually housed mice. Lab Anim. 2007;41:161–173. doi: 10.1258/002367707780378168. [DOI] [PubMed] [Google Scholar]
- [222].Peña Y, Prunell M, Rotllant D, Armario A, Escorihuela RM. Enduring effects of environmental enrichment from weaning to adulthood on pituitary-adrenal function, pre-pulse inhibition and learning in male and female rats. Psychoneuroendocrinology. 2009;34:1390–1404. doi: 10.1016/j.psyneuen.2009.04.019. [DOI] [PubMed] [Google Scholar]
- [223].Sztainberg Y, Kuperman Y, Tsoory M, Lebow M, Chen A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol Psychiatry. 2010 doi: 10.1038/mp.2009.151. [DOI] [PubMed] [Google Scholar]
- [224].Olsson T, Mohammed AH, Donaldson LF, Henriksson B, Seckl JR. Glucocorticoid receptor and NGF1-A gene expression are induced in the hippocampus after environmental enruchment in adult rats. Brain Res. Mol. Brain Res. 1994;23:349–353. doi: 10.1016/0169-328x(94)90246-1. [DOI] [PubMed] [Google Scholar]
- [225].Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. PNAS. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Chen Y, Mao Y, Zhou D, Hu X, Wang J, Ma Y. Environmental enrichment and chronic restraint stress in ICR mice: Effects on prepulse inhibition of startle and Y-maze spatial recognition memory. Behav Brain Res. 2010;212:49–55. doi: 10.1016/j.bbr.2010.03.033. [DOI] [PubMed] [Google Scholar]
- [228].Segovia G, del Arco A, De Blas M, Garrido P, Mora F. Effects of an enriched environment on the release of dopamine in the prefrontal cortex produced by stress and on working memory during aging in the awake rat. Behav Brain Res. 2008;187:304–311. doi: 10.1016/j.bbr.2007.09.024. [DOI] [PubMed] [Google Scholar]
- [229].Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. The Journal American Society for Experimental NeuroRx. 2004;1:111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and Physical Activity Differently Modulate Disease Progression in the Amyloid Precursor Protein (APP)-23 Model of Alzheimer’s Disease. Biol. Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [231].Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behav Brain Res. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- [232].Imperato A, Honoré T, Jensen LH. Dopamine release in the nucleus caudatus and in the nucleus accumbens is under glutamatergic control through non-NMDA receptors: a study in freely-moving rats. Brain Res. 1990;530:223–228. doi: 10.1016/0006-8993(90)91286-p. [DOI] [PubMed] [Google Scholar]
- [233].Segovia G, del Arco A, Mora F. Environmental enrichment, prefrontal cortex, stress and aging of the brain. J Neural Transm. 2009;116:1007–1016. doi: 10.1007/s00702-009-0214-0. [DOI] [PubMed] [Google Scholar]
- [234].Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes de Leon CF, Morris MC, Schneider JA, Evans DA. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- [235].Yaffe K, Fiocco A, Lindqvist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, et al. Predictors of maintaining cognitive function in older adults. Neurology. 2009;72:2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Anstey K, Chistensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitve change in old age: a review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- [237].Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- [238].Dowd J, Ranjit N, Do D, Young EA, House J, Kaplan G. Education and Levels of Salivary Cortisol Over the Day in US Adults. Ann Behav Med. 2010 doi: 10.1007./s12160-010-9224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- [240].Kudielka BM, Wüst S. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]