Abstract
Aging is associated with a decline of locomotor, sensory and cognitive performance in humans and experimental animals. The rate and pattern of organismal senescence may be regulated in part by changes in multiple genes involved in multiple processes. While this theory is supported by genetic data in lower organisms, a lack of direct experimental evidence in higher organisms has contributed to a broader acceptance of the “stochastic aging” model, in which accumulating, random damaging biological events play an important role. However, these insults alone cannot account for the inexorable deterioration and loss of function that characterizes aging. The higher the complexity of a system, the less obvious is the effect of genetic regulation on aging and the life span, indicating that epigenetic factors play an important role in aging. Most importantly, we present evidence that aging systems do retain some capacity for regeneration and functional recovery after injuries to the central nervous system like cerebral ischemia.
Keywords: Brain, aging, injuries, regeneration
Aging, a systems-biology approach
Organismal senescence refers to the changes that take place in whole organisms with advancing age. Aging is generally characterized by a declining ability to respond to stress, increasing homeostatic imbalance, and an increased risk of aging-associated diseases that ultimately culminates in death.
Mechanistically, organismal senescence is caused by a gradual, lifelong accumulation of multifaceted molecular and cellular damage. Various types of impairment have been proposed to accumulate with age, either due to an increase in damage, or a decrease in repair or clearance with time [1]. However, no theory satisfactorally explains all of the changes that define the aging process.
Although some changes typically occur with aging, they occur at different rates and to different extents. With the continuing increase in life expectancy in industrialized countries, new approaches are required to illuminate the complexity of the aging process and its implications for age-associated diseases. In the following we review several key aspects of organismal senescence.
Genetic effects on lifespan
Differences in maximum lifes pan among species correspond to different “rates of aging” that are in part due to inherited differences in the rate of aging. These genetic differences affect a variety of physiological processes, including the efficiency of DNA repair, antioxidant enzymes, and rates of free radical production.
One long-standing observation concerning the physiological decline that accompanies aging is its variability—for instance some people age better than others [2] and therefore the rate and pattern of organismal senescence may be regulated in part by changes in multiple genes involved in multiple processes. Therefore one of the current models - the programmed aging model - holds that aging is caused by genetically programmed cell death. While this theory is supported by genetic data in lower organisms, a lack of direct experimental evidence in higher organisms has contributed to a broader acceptance of the “stochastic aging” model, in which random biological events play an important role. Unbiased gene expression profiling of the entire genome is a valuable tool for the study of complex biological phenomena such as aging. Using this technology it is now possible to analyze in detail gene expression at the systems level. In the past decade with the advent of high-throughput technologies, biology has migrated from a descriptive science to a predictive one. A vast amount of information on metabolism has been produced. A large number of specific genetic/metabolic databases and computational systems have been developed. Data concerning fundamental processes like gene regulation, metabolic pathway control, and signal pathway control and cell differentiation are available via the internet, which makes it possible for biologists to perform in silico analysis of metabolism.
Recently it has been reported that the levels of gene expression in individual cardiomyocytes become more variable with age [3]. Furthermore, the tendency toward increased variation is not restricted to a specific set of genes, implying that aging is governed by stochastic events such as genetic mutations [4]. However, increases in expression variation are small, implying that organismal senescence is still not a simple correlate of changes in gene expression at the cellular level in higher organisms.
Genetic components of aging have been identified in model organisms, particularly the budding yeast Saccharomyces cerevisiae, the worm Caenorhabditis elegans and the fruit fly (Drosophila melanogaster). Study of these organisms has revealed the presence of at least two conserved aging pathways. One of these pathways involves the gene Sir2, a NAD+-dependent histone deacetylase. Extra copies of Sir2 are capable of extending the lifespan of both worms and flies, but systemic treatment with an activator of Sir2, resveratrol, does not reproducibly increase lifespan in either species [5]. Caloric restriction increases the activity of Sir2, suggesting that resveratrol treatment might mimic the effects of caloric restriction. More NAD+ is available upon reduction of available glucose by caloric restriction, and this in turn leads to Sir2 activation in the cell. Whether the Sir2 homologues in higher organisms have a role in lifespan is unclear, but the human SIRT1 protein has been shown to deacetylate p53, Ku70, and the forkhead family of transcription factors. SIRT1 also can regulate substrates such as CBP/p300, and deacetylate specific histone residues. RAS1 and RAS2 also affect aging in yeast and have a human homologue, and RAS2 overexpression extends lifespan in yeast. Other genes regulate aging in yeast by increasing the resistance to oxidative stress. Superoxide dismutase, an enzyme that protects against the effects of mitochondria-generated free radicals, can extend yeast lifespan in the stationary phase when overexpressed.
In higher organisms, aging is likely to be regulated in part through the insulin/IGF-1 pathway. Mutations that affect insulin-like signaling in worms and flies, or the growth hormone/IGF1 axis in mice, are associated with extended lifespan. In yeast, Sir2 activity is regulated by the nicotinamidase PNC1. PNC1 is transcriptionally upregulated under stressful conditions such as caloric restriction, heat shock, and osmotic shock. By converting nicotinamide to niacin, the enzyme removes nicotinamide, thereby inhibiting the activity of Sir2. A nicotinamidase found in humans, known as PBEF, may serve a similar function, and a secreted form of PBEF known as visfatin may help to regulate serum insulin levels. It is not known, however, whether these mechanisms also exist in humans, since there are obvious differences in biology between humans and model organisms. Gene expression is imperfectly controlled, and it is possible that random fluctuations in the expression levels of many genes contribute to the aging process, as suggested by a study of such genes in yeast [6]. Furthermore, individual cells that are genetically identical can have substantially different responses to extracellular stimuli, and markedly different life spans, indicating that epigenetic factors play an important role in gene expression.
Stem cells and tissue aging
With age, degenerative changes are accompanied by a gradual decline in the regenerative properties of most tissues.
The question arises as to whether this is due to the intrinsic aging of stem cells or, rather, to the impairment of stem-cell function in the aged tissue environment. Since stem cells are necessary to repair ongoing wear in tissues, their loss of function is an important contributor to degenerative aging. Consequently, inefficient replacement of worn-out cells in adult tissues due to the declining function of stem cells over time may be a primary cause of human aging.
Tissue maintenance in the adult organism requires a constant supply of new cells to replace differentiated cells lost to stress and damage or destroyed as part of the normal cell death program. This replacement is made possible through the activity of tissue-specific dedicated stem cells (exemplified by stem cells for blood, hair follicles, and brain), facultative stem cells (e.g., oval cells of the liver that produce hepatocytes after injury), or fully differentiated cells, which can undergo self-duplication when required for tissue maintenance (e.g., hepatocytes and pancreatic β cells) [7,8].
Tissues with high cellular turnover (such as blood, skin and gut) have a prominent stem-cell compartment and, consequently, have high regenerative capacity. Tissues with low cellular turnover but high regenerative potential might use different strategies to ensure effective repair after an acute injury. In skeletal muscle, for example, differentiated myofibres are unable to proliferate to generate new tissue, so muscle must rely on resident stem cells for all turnover and repair [9]. For the liver, it seems that differentiated hepatocytes can proliferate sufficiently to mediate effective tissue remodelling, repair and replacement under normal conditions [10], whereas stem cells might be recruited after severe injury [11].
Finally, tissues with low turnover and low regenerative potential, such as the heart and brain, might have resident stem cells that mediate only limited tissue repair. Currently there is considerable interest in harnessing the potential of stem cells in the brain [12] and heart [13] for therapeutic purposes.
Cellular aging vs organismal senescence
All cells experience changes with aging. They become larger and are less able to divide and reproduce. Among other changes, there is an increase in pigments and fatty substances inside the cell. Many cells lose their ability to function, or they begin to function abnormally. However, to what extent cellular aging plays a role in organismal senescence is not known and is an active area of investigation. However, mechanisms of aging intrinsic to cells are likely to exist [14].
The evolutionary purpose of cellular senescence is unclear, but a strong hypothesis is that it may be an anti-cancer defence. Somatic cells that have divided many times will have accumulated DNA mutations and would therefore be in danger of becoming cancerous if cell division continued [15]. In 1961 Leonard Hayflick and Paul Moorhead noticed that human cells derived from embryonic tissues can only divide a finite number of times in culture [16]. This phenomenon is known as Hayflick’s limit. In some tissues, such as those of the immune system, decreased proliferative ability may play a role in age-related degeneration [17].
Cancer cells are usually immortal. In about 85% of tumors, this evasion of cellular senescence is the result of up-regulation of telomerase genes [18]. This simple observation suggests that reactivation of telomerases in healthy individuals could greatly increase their cancer risk. Recently, the role of telomeres in cellular senescence has aroused general interest. The successive shortening of the chromosomal telomeres with each cell cycle might provide a mechanism for cellular senescence, thus contributing to aging. Support for this hypothesis comes from studies showing telomere shortening in a variety of human tissues as a consequence of aging and chronic disease. Studies in telomerase-deficient mice have given the first experimental support that telomere shortening limits the replicative potential of organs and tissues in vivo. However in telomerase-deficient mice, telomere shortening also increased tumour incidence due to the induction of chromosomal instability [19].
A long-standing controversy concerns the relevance of cellular senescence to tissue aging in vivo. First, telomere shortening, the principal known mediator of cellular senescence in proliferating cells, does occur in many human tissues. However, it is not clear whether this results in cellular senescence or in some other cell fate (e.g., crisis) [20]. Additionally, mice lacking telomerase do not immediately show accelerated aging [19].
Organismal Aging and Neuroendocrine Integration
Lifespan can be increased by altered endocrine signalling in a group of cells or a single tissue, which indicates that crosstalk between tissues, can coordinate aging of the organism.
Genetic alterations, in some cases in only a subset of cells or tissues, can modulate lifespan through endocrine signals. The insulin/IGF1 signalling pathways of worms and flies are the best-studied models of endocrine regulation of lifespan. The steroid hormone pregnenolone or its metabolites and the bile-acid-like deoxycholic acid have now been added to the list of hormones that are known to regulate lifespan in worms. In mammals, IGF1 is well established as an endocrine regulator of aging, and insulin appears to act in specific tissues to regulate lifespan. KLOTHO might be an anti-aging hormone that modulates insulin/IGF1 signalling and the activity of FGF23 on its receptors [21].
Organismal Aging and Regenerative Medicine
The age-related decline in integrity and function of differentiated adult tissues is a hallmark of aging and may be due to reduction in the number or regenerative potential of resident stem cells.
Rejuvenation of aged progenitor cells by exposure to a young systemic environment
Muscle-specific stem cells
Declining stem cell function during aging contributes to impaired tissue function. Muscle-specific stem cells (satellite cells) are responsible for generating new muscle in response to injury in the adult.
However, aged muscle displays a significant reduction in regenerative abilities and an increased susceptibility to age-related pathologies. Satellite cell activation and cell fate determination are controlled by the Notch signalling pathway that is initiated by the rapid increase in expression of the Notch ligand, Delta, following injury. In aged muscle, this upregulation of Delta is blunted and thus satellite cell activation is markedly diminished.
The extent to which muscle regeneration in aged mice is modulated by changes in the extracellular environment has been recently addressed by several studies. For example, exposing aged mice to factors present in young serum by parabiotic pairing (that is, a shared circulatory system) rejuvenates the satellite cell response and reveals that transplants of aged muscle tissue into a muscle bed regenerate well in a young host but badly in an aged host [22,23].
Most importantly, parabiotic pairings restored the activation of Notch signalling as well as the proliferation and regenerative capacity of aged satellite cells. The exposure of satellite cells from aged mice to serum from young mice enhanced the expression of the Notch ligand Delta, increased Notch activation, and enhanced proliferation in vitro. Furthermore, heterochronic (young-aged) parabiosis increased the proliferation of aged hepatocytes and restored the cEBP-alpha complex to the levels seen in young animals.
Another study addressed the potential of aged satellite cells to proliferate and regenerate when grown in culture. The results show that a minority of cultured aged satellite cells are still capable of generating large clusters of progeny containing both differentiated cells and new cells of a quiescent satellite-cell-like phenotype characteristic of self-renewal. Parallel in vivo engraftment assays showed that, despite the reduction in Pax7(+) cells, the satellite cell population associated with individual aged myofibers could regenerate muscle and self-renew as effectively as the larger population of satellite cells associated with young myofibers.
These results suggest that (i) the age-related decline of progenitor cell activity can be modulated by systemic factors that change with age [23]; and (ii) a subpopulation of stem cells retain their intrinsic potential throughout life. Therefore, it may be that even aged stem cells are capable of maintaining and repairing aged tissues if provided with optimal environmental cues [23, 24,25]
Brain-specific stem cells
There is a growing body of evidence that neural stem cells reside in certain regions of the adult central nervous system where neurogenesis occurs throughout the lifespan. Neurogenesis occurs mainly in two areas of the adult brain: the subgranular zone of the hippocampal dentate gyrus and the subventricular zone.
The addition of new, stem or progenitor cell-derived granule cells to the dentate gyrus declines considerably during aging. In a study addressing age-related alterations in the migration, survival and neuronal fate choice of newly born cells in F344 rats, the extent of new cell production was found to decrease dramatically by 15 months of age, but to exhibit no change thereafter [26].
Systemic stimulation of neurogenesis and its implications for behavioral recovery after brain injury
Systemic exercise and neurogenesis
Neurons are spontaneously recruited in the intact, healthy adult hippocampus, but the variables that affect their survival are not always clear.
Human and other animal studies demonstrate that exercise targets many aspects of brain function and has broad effects on overall brain health [27]. Aging is an important determinant of adult hippocampal neurogenesis as the proliferation of neural stem/precursor cells (NSCs) declines dramatically before 15–18 months of age. Compared with 3-mo-old mice, the numbers of mitotic cells and neuronal progenitor cells decreased dramatically by middle age and remained at low levels after middle age.
Nevertheless, continued exercise reportedly reduces the age-dependent decline in adult neurogenesis [28] and alters the brain chemistry of adult and aging brains toward an environment that is favorable to NSC proliferation, survival, and maturation [29]. Likewise, in a study of young mice, voluntary exercise after radiation-induced injury of the hippocampal stem cell pool restores cellular morphology of neuronal progenitors and ameliorates long-term behavioural changes [30,31]. However, the exercise-related regulation of adult hippocampal neurogenesis is a qualitative rather than a quantitative event. Recently it has been proposed that it is physical activity in the context of cognitive challenges that is of benefit to the brain [30,31]. The mechanisms underlying the modulatory effect of exercise are not yet clarified but exercise was found to have a primary effect on cerebral blood volume (CBV), and the CBV changes were found to selectively correlate with improved cardiopulmonary and cognitive function [33]. Another mechanism implicates running-induced proliferation of transiently amplifying progenitor cells that in turn led to an increase in the number of more mature cells [28].
Stem cell stimulation
Similar to findings on stem cells in aged muscle, there is evidence of a latent precursor and stem cell population in the adult hippocampus and the subventricular wall, which can be activated by neural activity and injuries like stroke.
Brain ischemia in aged animals
Age-related brain injuries including stroke, are a major cause of physical and mental disabilities. Because the latent NSC population also resides in the aged animal [32], studying the basic mechanism underlying functional recovery after stroke in aged subjects it is of considerable clinical interest.
Stroke models using aged animals are clinically more relevant than stroke models in young animals
Aging is associated with a decline of locomotor, sensory and cognitive performance in humans [34, 35] and animals [36–38], part of which is due to age-related functional decline of the brain. Studies of brain ischemia in experimental animals have demonstrated the neuroprotective efficacy of a variety of interventions, but most of the strategies that have been clinically tested failed to show benefit in aged humans. One possible explanation for this discrepancy between experimental and clinical studies may be the role that age plays in the recovery of the brain from insult. Indeed, age-dependent increased conversion of ischemic tissue into infarction suggests that age is a biological marker for the variability in tissue outcome in acute human stroke [39].
Although it is well known that aging is a risk factor for stroke and Alzheimer’s disease [40–43], the majority of experimental studies of stroke have been performed on young animals, and therefore may not fully replicate the effects of ischemia on neural tissue in aged subjects [44–47]. In this light, the aged post-acute animal model is clinically most relevant to stroke rehabilitation and dementia cellular studies, as recommended by the STAIR committee [48] and more recently by the Stroke Progress Review Group [49].
Over the past 10 years suitable models for stroke in aged rats have been established. All are based on middle cerebral artery occlusion (MCAO). MCAO has been produced with permanent [45,46,50–52] or transient occlusion for 30–120 min using a thrombus, or through intraluminal filament occlusion [50,53,54] or by using a hook attached to a micromanipulator to interrupt the blood flow under Laser Doppler control [44], or by permanent occlusion of distal branches of the MCA [55]. Long-term hypoxia-ischemia could also be induced by permanent ligation of only of the common carotid arteries [53].
The nearly universal approach of causing ischemia in young rats with filamentous models of occlusion fails to account for the unique physiology of an aged animal. Although it utilizes a method for induction of ischemia that is facile and reproducible, it differs greatly from the pathophysiology of clinically relevant ischemia [56].
Aged ischemic rats have higher mortality rates but not necessarily larger infarcts
Generally, the mortality rate in aged rats is higher than that of young rats, particularly if the occlusion is produced by an embolus (47% vs. 9%) [55]. The intraluminal filament method or photothrombosis cause, by comparison, lower (20–24%) post-stroke mortality rates [44,49,52,57]
In humans, there is no difference in infarct size with age [58–59]. Data on infarct size in aged rats is contradictory, suggesting that aged rats do not necessarily have larger infarcts. Some studies found an age-related increase in cerebral infarct size [51,57,60], whereas others did not [44,50,55–56, 61].
Aged ischemic animals recover more slowly than young animals and to a more limited extent
Aged individuals recover less well from stroke [58] and rehabilitation aims at improving the physical and cognitive impairments and disabilities of patients with stroke. Therefore studies on behavioral recuperation after stroke in aged animals are necessary.
Various experimental settings have been used to assess the recovery of sensorimotor functions, spontaneous activity and memory after ischemia in aged rats. Overall, the results indicate that aged rats have the capacity to recover behaviorally after cortical infarcts, albeit to a lesser extent than their young counterparts [45,47,49,57,60,61]. It should be kept in mind, however, that before stroke aged rats are already impaired as compared to young animals, and show significantly decreased performance in some tests like spontaneous activity [56] and Morris water-maze [62].
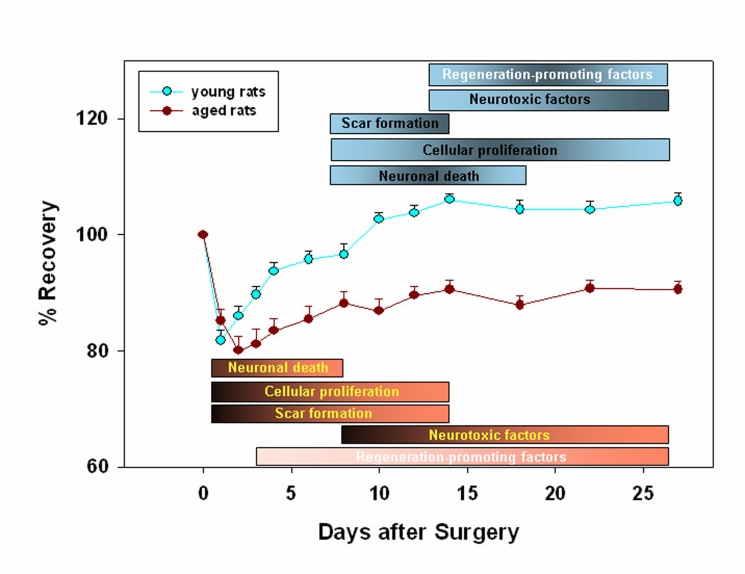
A summary of the time course of recovery from stroke in aged versus young rats is shown in Fig. 1. First, all rats had diminished performance on the first post-surgical day, part of which was attributable to the surgery itself. Second, aged rats started to recover after a delay of 3–4 days, depending on the difficulty of the testing. Similar findings have been reported recently for post-stroke recovery of senescence-accelerated mice [63]. Third, the extent of recovery was dependent on the complexity and difficulty of the test. For example, aged rats had difficulty mastering complex tasks of neurological status (including motor, sensory, reflex, and balance testing), rotarod, adhesive removal test (somatosensory function), and Morris water maze [50,56,62], but were not impaired in simpler tasks such as foot-fault test score and corner test score. Fourth, the performance level in aged rats depends on the infarct size, i.e., functional impairments in animals with the large infarcts (20% tissue loss) were more severe than functional impairments in animals with 4% tissue loss [49].
Figure 1.
Time course for recovery of both aged and young rats. All animals had diminished performance on the first post-surgical day, a state partly attributable to the surgery itself. As expected, young animals showed a more accelerated recovery pattern. The extent of recovery was also dependent on the complexity and difficulty of the behavioral tests assessed. The time course graph is accompanied by a chart depicting the most prominent events occurring at the tissue level during this period, together with the factors promoting these events. Thus, while neuronal death is more extensive in the aged rats, astroglial scar formation seems to dominate when considering young animals group.
Neurobiology of tissue recuperation after stroke in 20 mo-old animals
Poor recovery may reflect the combination of more aggressive activation of factors leading to infarct progression (neuronal degeneration, apoptosis, phagocytosis), factors impeding tissue repair (astroglial scar, neurite inhibitory proteins), and neurotoxic factors.
At the same time, the response of factors promoting brain plasticity and growth may be diminished with age. Growth promoting factors include growth-asssociated proteins, GAP43 and CAP23, the growth-promoting transcription factor c-jun, the growth-promoting cell guidance molecule L1, the CDK5 inhibitor p21, microtubule-associated proteins MAP1B and MAP2, the immature neuron marker doublecortin, and the stem cell marker nestin [64–67]. Tissue damage is due mainly to inflammatory interactions involving cytokines, chemokines, leukocytes, and neurotoxic factors like the C-terminal fragment of ß-amyloid (A-beta) [44,61,65,68–70]. Both timing and magnitude of expression of these factors is dysregulated in the post-ischemic aged rat brain.
The regenerative potential of the brain appears to be competent up to 20 months of age
To explore the potential of aged animals to initiate regenerative processes following cerebral ischemia, we studied the expression of the juvenile-specific cytoskeletal protein, microtubule associated protein 1B (MAP1B); the adult-specific protein, microtubule-associated protein 2 (MAP2); and the axonal growth marker, ßIII-tubulin, in male Sprague-Dawley rats at 3 months and 20 months of age.
Focal cerebral ischemia, produced by reversible occlusion of the right middle cerebral artery, resulted in vigorous expression of both MAP1B in the penumbra of 3 month- and, to a lesser extent, 20 month-old rats, at 14d following stroke [64,65]. Similarly, MAP2 protein and mRNA were upregulated in the peri-infarct area to almost the same extent in young and middle aged rats. Somewhat lower levels of expression were noted for the axonal growth marker, ßIII-tubulin, in the peri-infarct area of middle aged rats as compared to young rats. Collectively, these results suggest that the regenerative potential of the brain at the structural level is competent up to 20 months of age.
Recent studies confirm that mechanisms for self-repair in the young brain also operate in the 20 mo-old brain. For example, stroke causes increased numbers of new striatal neurons despite lower basal cell proliferation in the subventricular zone in the 20 mo-old brain [71–72]. However, despite conserved proliferative activity in the subventricular zone, the number of neurons that reach the injury site is quite modest, as was shown recently for doublecortin-positive neurons in the infacted area of 20 mo-old rats [73]. One possible explanation is that subventricular zone-derived nestin-positive cells do not cross the corpus callosum barrier, and therefore cannot contribute to generation of neurons in the neocortex. Indeed, current evidence indicates that the great majority of newly formed cells in the adult brain are non-neuronal [74–76].
Recent studies also indicate that the molecular profile of growth-promoting genes is very different between 22 to 24 mo-old and 2 to 4 mo-old F334 male rats during the axonal sprouting response to central nervius system lesions. 22 mo-old rodents activate most growth-promoting genes at later timer-point following stroke than do 3 mo-old animals. This includes a delayed induction of GAP43, CAP23 and the growth-promoting transcription factor c-jun. The growth-promoting cell guidance molecule L1 and the CDK5 inhibitor p21 are actually down-regulated during the axonal sprouting process in 22 mo-old rodents compared with a robust and early upregulation of these two molecules in 3 mo-old animals [66–67].
Brain regeneration and stroke therapy using stem cells
The major factors involved in the loss of regenerative ability of the aged brain are (i) the age-related decrease in neurogenesis and (ii) the imposition of inhibitory factors in the local environment which are required in the adult to ensure adequate migration of neuronal precursors toward the damaged region.
In mammals the two main regions of post-natal neurogenesis are the subventricular zone in the wall of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus. For therapeutic purposes, endogenous neurogenesis may need to be supplemented by transplantation of neuronal progenitor cells. However, little is known regarding the effect of the environment on neural stem cell transplants in the aged brain.
The use of stem cells to replace neurons lost after stroke offers a novel approach to treatments aimed at improving recovery of tissue and function [77–78]. Such a treatment might utilize the endogenous reserves of stem cells located in the subventricular zone or the subgranular zone of the hippocampus. One major concern, however, with any therapy designed to boost neurogenesis following stroke is that the capacity of the organism to produce new neurons may be diminished in 18 to 20 mo-old animals [77,79–84].
A countervailing cause for optimism is that a variety of treatments, such as environment enrichment [85], administration of growth factors [86] and epileptic seizures [87] can increase the production of new neurons in 18 mo-old animals, although at a lower level than in younger animals. Even more encouraging is a recent study demonstrating the same degree of post-stroke neurogenesis in the striatum of 15- and 3 mo-old animals [72]. Even though this study reported lower levels of neuron production in 15 mo-old animals in the subgranular and subventricular zones, the report of equivalent levels in the striatum indicates that the potential for self-repair following stroke persists in the 15 mo-old brain. While the use of the organism’s own stem cells has many advantages, this technique is in its infancy, and the field still awaits an unambiguous proof of principle.
Another experimental approach that has received considerably more attention is the use of external sources of stem cells. One important question is the type of cells that should be used. Both fetal [88] and murine stem cell lines [89–90] have been used successfully as grafts to improve functional deficits after experimental stroke in the rat. Adult stem cells, such as those derived from human umbilical cord blood, have also proved efficacious [91–94].
The appropriate route of stem cell administration must also be determined. One approach is transplantation either into the lesioned hemisphere, the contralateral hemisphere, or both. Other possible targets for stem cell administration are the striatum [72,89], the cortical parenchyma, or the cerebral ventricles [90]. Following unilateral stroke, the grafted stem cells appear to be attracted both to the site of damage and to the corresponding contralateral region, suggesting the existence of both local repair processes and those involved in plastic changes in contralateral motor pathways [90].
An additional route of administration of stem cells is via the circulation, either intravenously [92,95–97] or by injection into the carotid artery [55]. Little is known regarding the effect of the environment on differentiation of neural stem cell transplants and transplanted immortalized hippocampal cell lines delivered to the cortex, striatum and hippocampus of 15 to 18 mo-old rats [98]. One study has shown that upon transplantation immortal Maudsley hippocampal stem cell line colonized the aged rat brain and adopted both astrocytic and neuronal morphologies. Functionally, the transplanted stem cells improved cognition which deteriorates with advanced age [98].
In another study [99] human fetal cells were transplanted into the lateral ventricle of aged rats. The injected cells displayed extensive incorporation into the aged host brain with improvement of cognitive score assessed by the Morris water maze at 4 weeks after transplantation. Fetal hippocampal cells transplanted into kainic acid-lesioned CA3 region of the hippocampus of 3 mo-, 15 mo- and 24 mo old animals showed that grafts into both 15 mo- and 24 mo old animals had diminished cell survival (30% of injected cells) compared to similar grafts into the young adult hippocampus (72% cell survival) [100]
However, the extent of cell survival of CA3 grafts pre-treated with a combination of neurotrophic factors (brain-derived neurotrophic factor and neurotrophin-3) and the caspase inhibitor acetyl-tyrosinyl-valyl-alanyl-aspartyl-chloro-methylketone was significantly enhanced in both 15 mo- and 24 mo old animals hippocampus (51–63% cell survival).
The field of stroke therapy using stem cells is a new but promising area and it is hoped that studies to be carried out in the near future may validate a general therapeutic approach.
Neurogenesis after stroke in aged rats
Stroke induces cell proliferation within the subventricular zone, migration of newly born immature neurons into peri-infarct tissues and long-term survival and maturation of a small number of cells with a mature neuronal phenotype and ultrastructural evidence for synapses [101–105].
Post-stroke neurogenesis appears to divert migratory immature neurons from their normal path to the olfactory bulb, the rostral migratory stream (RMS) [102]. Recent research has begun to describe the cellular environments that may lead to post-stroke neurogenesis and immature neuron migration. In the ischemic striatum, immature neurons, identified through their staining for the microtubule-associated protein doublecortin, are found in association with astrocytes. Activated astrocytes in the ischemic striatum secrete stromal-derived factor-1 (SDF-1) and this induces immature neuron migration into this area [106]. SDF-1 induces neuronal migration during development in the hippocampus, cortex and cerebellum [107–109].
Post-stroke neurogenesis also occurs in close association with the vasculature. Newly born immature neurons can be found associated with blood vessels after stroke [102,105,110]. Xenotransplants of stem/progenitor cells also home to the ischemic tissue and associate with blood vessels after stroke [111–112]. In peri-infarct cortex, newly born neurons migrate into the region near the stroke site and form a tight physical association with blood vessels in the first week after stroke in a neurovascular niche in peri-infarct cortex. This vascular/neuroblast association occurs with blood vessels that are actively remodeling after stroke, and undergoing angiogenesis. Pharmacological blockade of angiogenesis after stroke reduces the number of immature neurons that are present in peri-infarct cortex by almost 90% [102]. Thus angiogenesis is causally linked to neurogenesis after stroke. This finding of a neurovascular niche for neurogenesis after stroke is supported by the many growth factors or pharmacological agents that appear capable of inducing both of these processes together, such as VEGF, erythropoietin, FGF2, statins and phosphodieseterase type 5 inhibitors [46,113–115].
Angiogenesis, neurogenesis and axonal sprouting occur in common areas of peri-infarct tissue after stroke and may form a unique regenerative triad that supports neural repair in this disease. Studies in stroke have defined specific receptor-ligand signaling systems that link angiogenesis and neurogenesis. As noted above, blocking angiogenesis severely reduces post-stroke neurogenesis. Angiogenic blood vessels in peri-infarct cortex secrete SDF-1 and angiopoietin-1 in the first week after stroke. Administration of SDF-1 or Ang-1 stimulates neuroblast migration into peri-infarct cortex, and blockade of their receptors, CXCR4 and Tie2, blocks or disperses the migration of immature neurons after stroke [102]. Erythropoietin (EPO) and VEGF also mediate neurovascular coupling of angiogenic blood vessels and migrating neuroblasts. EPO is induced in blood vessels and astrocytes in peri-infarct tissue after stroke [116] and promotes post-stroke neurogenesis [115]. Pharmacological doses of EPO also promote angiogenesis and neurogenesis after stroke [46]. VEGF is induced in peri-infarct tissue after stroke and may be secreted by angiogenic blood vessels. VEGF receptor blockade downregulates and exogenous VEGF promotes post-stroke neurogenesis [113]. VEGF is also produced by neurons and astrocytes in peri-infarct cortex [46,113] and is strongly bound to the extracellular matrix, so the exact cellular communication pattern involved in VEGF signaling in stroke remains to be determined.
Axonal sprouting after stroke in aged rats
Stroke induces a novel pattern of ischemic damage and neural repair in the 20 mo-old compared to the 3 mo-old brain. Using small cortical stroke in the barrel field of the rat, it has been shown that there is no difference in stroke size or degree of apoptotic cell death between young and aged animals [66].
There is also no difference in the number of surviving neurons in the ischemic border zones between aged and young adults. However, aged animals have greater oxidative DNA damage and a reduced expression of the neuroprotective protein HSP70 in peri-infarct cortex [66]. These two findings indicate that peri-infarct tissue in aged individuals has increased cell injury and reduced neuroprotective responses. Peri-infarct tissue may thus be more susceptible to factors in stroke that lead to increased stress or cell injury, such as fever, changes in blood pressure or alterations in blood vessel collaterals. This vulnerability in peri-infarct tissue may explain the controversy between different experimental stroke studies, with some stroke studies finding greater stroke size in 15 to 22 mo-aged individuals and others finding no difference in stroke size between 3 mo-old and aged animals. Minor variations in experimental technique between different stroke models, strains of animals or within laboratories may lead to changes in the stress and extent of cell death within peri-infarct cortex.
On a cellular level there are at least two main processes of neural repair after stroke: post-stroke axonal sprouting and neurogenesis. Both processes contribute to neuronal reorganization in peri-infarct cortex. Axonal sprouting after stroke not only occurs within peri-infarct cortex [66] and between cortical hemispheres on the side of the infarct [117] but also from cortex contralateral to the infarct, into peri-infarct cortex, ipsilateral striatum, red nucleus and cervical spinal cord [102,118–120]. Axonal sprouting after stroke requires a molecular growth program [121–123] in which a neuron must respond to injury, then elaborate a growth cone, extend and axon and establish new synapses. This type of molecular growth program involves cytoskeletal reorganizing proteins, guidance and cell adhesion molecules, intracellular growth cone signaling molecules and specific transcription factors. Recent studies indicate that the molecular profile of growth-promoting genes is very different between aged and young adult during the sprouting response. The environment of peri-infarct cortex also contains molecular systems that exert a powerful negative effect on axonal sprouting [117].
Among these systems, the 22 mo-old brain has a unique upregulation of two molecules compared with the 3 mo-old: ephrin-A5 and myelin associated glycoprotein (MAG) [106]. These two molecules are important for axonal sprouting in cortical development and for the switch in growth inhibition during postnatal maturation. The unique upregulation of ephrin-A5 and MAG in the aged peri-infarct cortex suggest that these molecules may in part mediate the age-associated decrease in axonal sprouting, and provide potential targets for neural repair after stroke.
Gene expression after stroke in aged rats
3 mo-old Sprague Dawley rats recover much better than 18 mo-old rats within 2 weeks after stroke [49] and brain plasticity may underlie the rapid recovery from ischemic damage. Activation of the contralateral hemisphere may help recovery as well [124–125], as has been hypothesized for humans [126–127]. However, other investigators suggest that activation in the contralateral cortex is negatively correlated with functional recovery and may actually contribute to functional impairment as a result of unbalanced inhibition between hemispheres [128–129].
In animals, progressive brain damage accelerated axonal sprouting in the healthy contralateral hemisphere [130]. After unilateral damage to the forelimb representation region in the sensorimotor cortex (SMC) in 3–5 mo-old rats, the cortex contralateral and homotopic to unilateral SMC lesions undergoes a major restructuring of dendrites and synapses [1].
DNA array technology provides insight into the underlying mechanisms of brain repair and regeneration after stroke. Using focal cerebral ischemia as a model, key molecular events and several induced genes have been described [131–135]. These studies provided evidence that focal cerebral ischemia induces transcriptional activity of a variety of genes related to stress response, inflammation, and acute and delayed cell death. Although the contralateral hemisphere could play a pivotal role in recovery after stroke, these studies do not provide data on genes that are regulated following stroke in the contralateral, healthy hemisphere of young and aged rats.
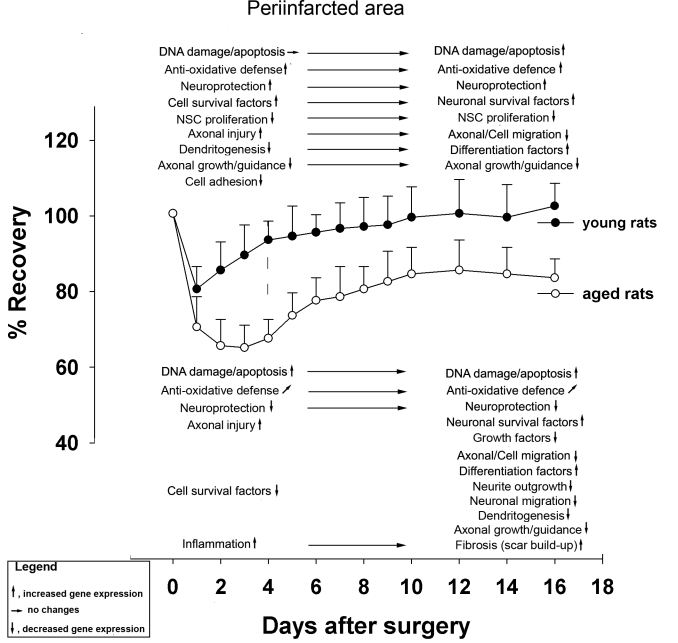
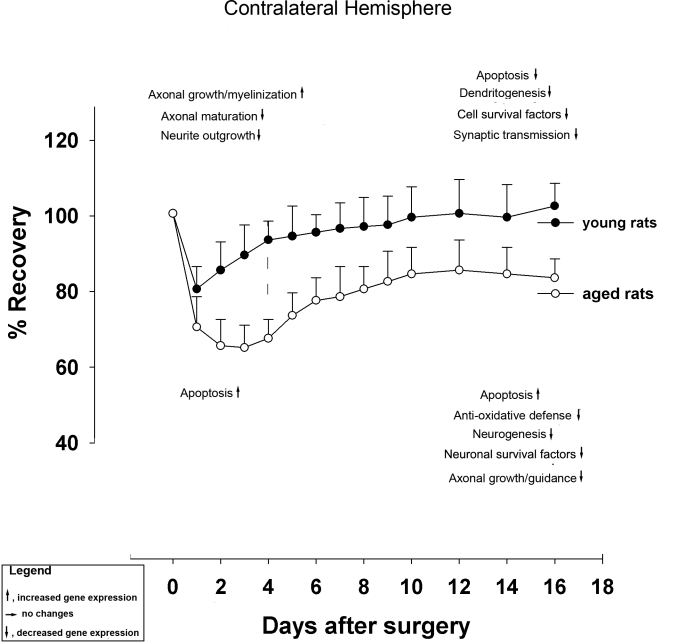
A recent study employed cDNA arrays containing genes coding for DNA damage and apoptosis, cell and axonal injury, inflammation and scar formation, growth and neuroprotective factors, and axono- and dendritogenesis [136]. The major transcriptional events observed in the periinfarct region of 18 mo-old rats after stroke were: (i) upregulation of DNA-damage-related and downregulation of anti-apoptosis-related genes; (ii) strong upregulation of genes associated with inflammation and scar formation; (iii) reduced level of expression of genes important for neuronal survival (iv) reduced or absent expression of genes required for neurogenesis. The major transcriptional events observed in the contralateral (unlesioned) hemisphere of both young and aged after stroke were: (v) own regulation of axonal growth- and dendritogenesis-related genes; (vi) reduced or absent expression of genes required for neurogenesis [136].
To conclude, reduced transcriptional activity in the healthy, contralateral hemisphere in conjunction with an early upregulation of DNA damage-related genes and the early induction of proapoptotic genes in the periinfarct area of 18 mo-old rats are likely to account for poor neurorehabilitation after stroke in aged rats. These conclusions are summarized in Fig 2 and in Fig. 3.
Figure 2.
Overview of major genetic events in the ipsilateral hemisphere associated with functional recovery after stroke in young and aged rats.
Figure 3.
Overview of major genetic events in the contralateral hemisphere associated with functional recovery after stroke in young and aged rats.
REFERENCES
- [1].Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- [2].JW Rowe JW, RL Kahn RL. Human aging: Usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- [3].Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dole ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- [4].Somel M, Khaitovich P, Bahn S, Pääbo S, Lachmann M. Gene expression becomes heterogeneous with age. Curr Biol. 2006;16:R359–366. doi: 10.1016/j.cub.2006.04.024. [DOI] [PubMed] [Google Scholar]
- [5].Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- [6].Ryley J, Pereira-Smith OM. Microfluidics device for single cell gene expression analysis in Saccharomyces cerevisiae. Yeast. 2006;23:1065–1073. doi: 10.1002/yea.1412. [DOI] [PubMed] [Google Scholar]
- [7].Rawlins EL, Hogan BL. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- [8].Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- [9].Rando TA. Stem cells, ageing and the quest for Immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- [10].Sigal SH, Brill S, Fiorino AS, Reid LM. The liver as a stem cell and lineage system. Am J Physiol. 1992;263:G139–G148. doi: 10.1152/ajpgi.1992.263.2.G139. [DOI] [PubMed] [Google Scholar]
- [11].Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gage FH. Stem cells of the central nervous system. Curr Opin Neurobiol. 1998;8:671–676. doi: 10.1016/s0959-4388(98)80098-6. [DOI] [PubMed] [Google Scholar]
- [13].Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardialregeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- [14].de Magalhaes JP, Toussaint O. How bioinformatics can help reverse engineer human aging. Ageing Res Rev. 2004;3:125–41. doi: 10.1016/j.arr.2003.08.006. [DOI] [PubMed] [Google Scholar]
- [15].Wynford-Thomas D. Cellular senescence and cancer. The Journal of pathology. 1999;187:100–11. doi: 10.1002/(SICI)1096-9896(199901)187:1<100::AID-PATH236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- [16].Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- [17].Effros RB. Replicative Senescence in the Immune System: Impact of the Hayflick Limit on T-Cell Function in the Elderly. Am J Hum Genet. 1998;62:1003–07. doi: 10.1086/301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [19].Djojosubroto MW, Choi YS, Lee HW, Rudolph KL. Telomeres and telomerase in aging, regeneration and cancer. Mol Cells. 2003;15:164–75. [PubMed] [Google Scholar]
- [20].Hornby GS, Pollack JB. Creating high-level components with a generatve representation for body-brain evolution. Artif Life. 2002;8:223–246. doi: 10.1162/106454602320991837. [DOI] [PubMed] [Google Scholar]
- [21].Russell SJ, Kahn CR. Endocrine regulation of ageing. Nature Reviews. 2007;8:681–690. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- [22].Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: Age of host determines recovery. Am J Physiol. 1989;2:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- [23].Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- [24].Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- [25].Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- [26].Rao MS, Hattiangady B, Abdel-Rahman A, Stanley DP, Shetty AK. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;13:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- [27].Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- [28].Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- [29].Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, Lo CP, Kuo YM. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- [30].Naylor AS, Bull C, Nilsson MK, Zhu C, BjÃrk-Eriksson T, Eriksson PS, Blomgren K, Kuhn HG. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105:14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008;10:59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- [33].Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grady CL, Craik FI. Changes in memory processing with age. Curr Opin Neurobiol. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- [35].Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- [36].Navarro A, Gomez C, Sanchez-Pino MJ, Gonzalez H, Bandez MJ, Boveris AD, Boveris A. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1392–R1399. doi: 10.1152/ajpregu.00834.2004. [DOI] [PubMed] [Google Scholar]
- [37].Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–324. doi: 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- [39].Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, Menezes N, Lopez CJ, Sorensen AG. Conversion of ischemic brain tissue into infarction increases with age. Stroke. 2005;36:2632–2636. doi: 10.1161/01.STR.0000189991.23918.01. [DOI] [PubMed] [Google Scholar]
- [40].Barnett HJ. Stroke prevention in the elderly. Clin Exp Hypertens. 2002;24:563–571. doi: 10.1081/ceh-120015333. [DOI] [PubMed] [Google Scholar]
- [41].Broderick JP, William M. Feinberg Lecture: stroke therapy in the year 2025: burden, breakthroughs, and barriers to progress. Stroke. 2004;35:205–211. doi: 10.1161/01.STR.0000106160.34316.19. [DOI] [PubMed] [Google Scholar]
- [42].Pluta R. Ischemia-reperfusion factors in sporadic Alzheimer’s disease. In: Welsh EM, editor. New research on Alzheimer’s disease. Nova Science Publishers Inc; 2006. pp. 183–234. [Google Scholar]
- [43].Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- [44].Popa-Wagner A, Schroder E, Walker LC, Kessler C. beta-Amyloid precursor protein and ss-amyloid peptide immunoreactivity in the rat brain after middle cerebral artery occlusion: effect of age. Stroke. 1998;29:2196–2202. doi: 10.1161/01.str.29.10.2196. [DOI] [PubMed] [Google Scholar]
- [45].Brown AW, Marlowe KJ, Bjelke B. Age effect on motor recovery in a post-acute animal stroke model. Neurobiol Aging. 2003;24:607–614. doi: 10.1016/s0197-4580(02)00129-x. [DOI] [PubMed] [Google Scholar]
- [46].Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- [47].Markus TM, Tsai SY, Bollnow MR, Farrer RG, O’Brien TE, Kindler-Baumann DR, Rausch M, Rudin M, Wiessner C, Mir AK, Schwab ME, Kartje GL. Recovery and brain reorganization after stroke in adult and aged rats. Ann Neurol. 2005;58:950–953. doi: 10.1002/ana.20676. [DOI] [PubMed] [Google Scholar]
- [48].Subramanyam B, Pond SM, Eyles DW, Whiteford HA. Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- [49].Lindner MD, Gribkoff VK, Donlan NA, Jones TA. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J Neurosci. 2003;23:10913–10922. doi: 10.1523/JNEUROSCI.23-34-10913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang L, Zhang RL, Wang Y, Zhang C, Zhang ZG, Meng H, Chopp M. Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke. 2005;36:847–852. doi: 10.1161/01.STR.0000158923.19956.73. [DOI] [PubMed] [Google Scholar]
- [51].Davis M, Mendelow AD, Perry RH, Chambers IR, James OF. Experimental stroke and neuroprotection in the aging rat brain. Stroke. 1995;26:1072–1078. doi: 10.1161/01.str.26.6.1072. [DOI] [PubMed] [Google Scholar]
- [52].Futrell N. An improved photochemical model of embolic cerebral infarction in rats. Stroke. 1991;22:225–232. doi: 10.1161/01.str.22.2.225. [DOI] [PubMed] [Google Scholar]
- [53].Macri MA, D’Alessandro N, Di GC, Di IP, Di LS, Giuliani P, Bianchi G, Esposito E. Regional changes in the metabolite profile after long-term hypoxia-ischemia in brains of young and aged rats: a quantitative proton MRS study. Neurobiol Aging. 2006;27:98–104. doi: 10.1016/j.neurobiolaging.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [54].Dinapoli VA, Rosen CL, Nagamine T, Crocco T. Selective MCA occlusion: a precise embolic stroke model. J Neurosci Methods. 2006;154:233–238. doi: 10.1016/j.jneumeth.2005.12.026. [DOI] [PubMed] [Google Scholar]
- [55].Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005b;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- [56].Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–854. doi: 10.1097/01.WCB.0000071883.63724.A7. [DOI] [PubMed] [Google Scholar]
- [57].Rosen CL, Dinapoli VA, Nagamine T, Crocco T. Influence of age on stroke outcome following transient focal ischemia. J Neurosurg. 2005;103:687–694. doi: 10.3171/jns.2005.103.4.0687. [DOI] [PubMed] [Google Scholar]
- [58].Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome, The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- [59].Engelter ST, Provenzale JM, Petrella JR, DeLong DM, Alberts MJ. Infarct volume on apparent diffusion coefficient maps correlates with length of stay and outcome after middle cerebral artery stroke. Cerebrovasc Dis. 2003;15:188–191. doi: 10.1159/000068826. [DOI] [PubMed] [Google Scholar]
- [60].Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27:1663–1667. doi: 10.1161/01.str.27.9.1663. [DOI] [PubMed] [Google Scholar]
- [61].Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Effect of cholinergic medication, before and after focal photothrombotic ischemic cortical injury, on histological and functional outcome in aged and young adult rats. Behav Brain Res. 2005b;156:85–94. doi: 10.1016/j.bbr.2004.05.011. [DOI] [PubMed] [Google Scholar]
- [62].Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Behavioral and histological effects of chronic antipsychotic and antidepressant drug treatment in aged rats with focal ischemic brain injury. Behav Brain Res. 2005a;158:211–220. doi: 10.1016/j.bbr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [63].Lee JC, Cho GS, Choi BO, Kim HC, Kim YS, Kim WK. Intracerebral Hemorrhage-Induced Brain Injury Is Aggravated in Senescence-Accelerated Prone Mice. Stroke. 2006;37:216–222. doi: 10.1161/01.STR.0000195151.46926.7b. [DOI] [PubMed] [Google Scholar]
- [64].Popa-Wagner A, Schroder E, Schmoll H, Walker LC, Kessler C. Upregulation of MAP1B and MAP2 in the rat brain after middle cerebral artery occlusion: effect of age. J. Cereb. Blood Flow Metab. 1999;19:425–434. doi: 10.1097/00004647-199904000-00008. [DOI] [PubMed] [Google Scholar]
- [65].Badan I, Dinca I, Buchhold B, Suofu Y, Walker L, Gratz M, Platt D, Kessler CH, Popa-Wagner A. Accelerated accumulation of N- and C-terminal beta APP fragments and delayed recovery of microtubule-associated protein 1B expression following stroke in aged rats. Eur J Neurosci. 2004;19:2270–2280. doi: 10.1111/j.0953-816X.2004.03323.x. [DOI] [PubMed] [Google Scholar]
- [66].Li S, Zheng J, Carmichael ST. Increased oxidative protein and DNA damage but decreased stress response in the aged brain following experimental stroke. Neurobiol Dis. 2005a;18:432–440. doi: 10.1016/j.nbd.2004.12.014. [DOI] [PubMed] [Google Scholar]
- [67].Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- [68].Brenner D, Labreuche J, Touboul PJ, Schmidt-Petersen K, Poirier O, Perret C, Schonfelder J, Combadiere C, Lathrop M, Cambien F, Brand-Herrmann SM, Amarenco P. Cytokine polymorphisms associated with carotid intima-media thickness in stroke patients. Stroke. 2006;37:1691–1696. doi: 10.1161/01.STR.0000226565.76113.6c. [DOI] [PubMed] [Google Scholar]
- [69].Tang Y, Xu H, Du X, Lit L, Walker W, Lu A, Ran R, Gregg JP, Reilly M, Pancioli A, Khoury JC, Sauerbeck LR, Carrozzella JA, Spilker J, Clark J, Wagner KR, Jauch EC, Chang DJ, Verro P, Broderick JP, Sharp FR. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: a microarray study. J Cereb Blood Flow Metab. 2006;26:1089–1102. doi: 10.1038/sj.jcbfm.9600264. [DOI] [PubMed] [Google Scholar]
- [70].Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell. 2004;3:373–377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- [72].Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36:1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- [73].Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler CH. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol. 2007;113:277–293. doi: 10.1007/s00401-006-0164-7. [DOI] [PubMed] [Google Scholar]
- [74].Hess DC, Hill WD, Carroll JE, Borlongan CV. Do bone marrow cells generate neurons? Arch Neurol. 2004;61:483–485. doi: 10.1001/archneur.61.4.483. [DOI] [PubMed] [Google Scholar]
- [75].Priller J, Persons DA, Klett FF, Kempermann G, Kreutzberg GW, Dirnagl U. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J. Cell Biol. 2001;155:733–738. doi: 10.1083/jcb.200105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Haas S, Weidner N, Winkler J. Adult stem cell therapy in stroke. Curr Opin Neurol. 2005;18:59–64. doi: 10.1097/00019052-200502000-00012. [DOI] [PubMed] [Google Scholar]
- [78].Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38:817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- [79].Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- [80].Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- [81].Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. J Comp Neurol. 2002;454:361–372. doi: 10.1002/cne.10426. [DOI] [PubMed] [Google Scholar]
- [82].Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- [83].Tropepe V, Craig CG, Morshead CM. van der KD. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- [85].Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Decker L, Picard-Riera N, Lachapelle F, Baron-Van EA. Growth factor treatment promotes mobilization of young but not aged adult subventricular zone precursors in response to demyelination. J Neurosci Res. 2002;69:763–771. doi: 10.1002/jnr.10411. [DOI] [PubMed] [Google Scholar]
- [87].Gray WP, May K, Sundstrom LE. Seizure induced dentate neurogenesis does not diminish with age in rats. Neurosci Lett. 2002;330:235–238. doi: 10.1016/s0304-3940(02)00810-8. [DOI] [PubMed] [Google Scholar]
- [88].Sorensen JC, Grabowski M, Zimmer J, Johansson BB. Fetal neocortical tissue blocks implanted in brain infarcts of adult rats interconnect with the host brain. Exp Neurol. 1996;138:227–235. doi: 10.1006/exnr.1996.0061. [DOI] [PubMed] [Google Scholar]
- [89].Wong AM, Hodges H, Horsburgh K. Neural stem cell grafts reduce the extent of neuronal damage in a mouse model of global ischaemia. Brain Res. 2005;1063:140–150. doi: 10.1016/j.brainres.2005.09.049. [DOI] [PubMed] [Google Scholar]
- [90].Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002;33:2270–2278. doi: 10.1161/01.str.0000027693.50675.c5. [DOI] [PubMed] [Google Scholar]
- [91].Xiao J, Nan Z, Motooka Y, Low WC. Transplantation of a novel cell line population of umbilical cord blood stem cells ameliorates neurological deficits associated with ischemic brain injury. Stem Cells Dev. 2005;14:722–733. doi: 10.1089/scd.2005.14.722. [DOI] [PubMed] [Google Scholar]
- [92].Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002b;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Saporta S, Kim JJ, Willing AE, Fu ES, Davis CD, Sanberg PR. Human umbilical cord blood stem cells infusion in spinal cord injury: engraftment and beneficial influence on behavior. J Hematother Stem Cell Res. 2003;12:271–278. doi: 10.1089/152581603322023007. [DOI] [PubMed] [Google Scholar]
- [94].Nan Z, Grande A, Sanberg CD, Sanberg PR, Low WC. Infusion of human umbilical cord blood ameliorates neurologic deficits in rats with hemorrhagic brain injury. Ann N Y Acad Sci. 2005;1049:84–96. doi: 10.1196/annals.1334.009. [DOI] [PubMed] [Google Scholar]
- [95].Willing AE, M Vendrame M, J Mallery J, CJ Cassady CJ, DavisCD CD, J Sanchez-Ramos J, Sanberg PR, PR Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant. 2003;12:449–454.92. doi: 10.3727/000000003108746885. [DOI] [PubMed] [Google Scholar]
- [96].Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hodges H, Veizovic T, Bray N, French SJ, Rashid TP, Chadwick A, Patel S, Gray JA. Conditionally immortal neuroepithelial stem cell grafts reverse age-associated memory impairments in rats. Neuroscience. 2000;101:945–55. doi: 10.1016/s0306-4522(00)00408-5. [DOI] [PubMed] [Google Scholar]
- [99].Qu T, Brannen CL, Kim HM, Sugaya K. Human neural stem cells improve cognitive function of aged brain. Neuroreport. 2001;12:1127–1132. doi: 10.1097/00001756-200105080-00016. [DOI] [PubMed] [Google Scholar]
- [100].Zaman V, Shetty AK. Combined neurotrophic supplementation and caspase inhibition enhances survival of fetal hippocampal CA3 cell grafts in lesioned CA3 region of the aging hippocampus. Neuroscience. 2002;109:537–553. doi: 10.1016/s0306-4522(01)00478-x. [DOI] [PubMed] [Google Scholar]
- [101].A Arvidsson A, Collin T, Kirik D, Z Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- [102].Ohab JJ, Carmichael ST. Post-Stroke Neurogenesis: Emerging Principles of Migration and Localization of Immature Neurons. The Neuroscientist. 2008;14:369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- [103].Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- [104].Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yamashita T, Ninomiya M, Hernandez AP, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- [107].Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002a;11:275–281. [PubMed] [Google Scholar]
- [108].Stumm RK, C Zhou C, T Ara T, F Lazarini F, M Dubois-Dalcq M, T Nagasawa T, V Hollt V, SSchulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Is H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Greenberg DA, Jin K. Growth factors and stroke. NeuroRx. 2006a;3:458–465.113. doi: 10.1016/j.nurx.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Li L, Jiang Q, Zhang L, Ding G, Gang ZZ, Li Q, Ewing JR, Lu M, Panda S, Ledbetter KA, Whitton PA, Chopp M. Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by MRI after administration of sildenafil to rats with embolic stroke. Brain Res. 2007;1132:185–192. doi: 10.1016/j.brainres.2006.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- [117].Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Papadopoulos CM, Tsai SY, Alsbiei T, O’Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- [119].Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002;22:6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Bonilla IE, Tanabe KK, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16–34. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Serrien DJ, Strens LH, Cassidy MJ, Thompson AJ, Brown P. Functional significance of the ipsilateral hemisphere during movement of the affected hand after stroke. Exp Neurol. 2004;190:425. doi: 10.1016/j.expneurol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- [125].Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- [126].Jang SH, You SH, Kwon YH, Hallett M, Lee MY, Ahn SH. Cortical reorganization associated lower extremity motor recovery as evidenced by functional MRI and diffusion tensor tractography in a stroke patient. Restor Neurol Neurosci. 2005;23:325–329. [PubMed] [Google Scholar]
- [127].Kim YH, You SH, Kwon YH, Hallett M, Kim JH, Jang SH. Longitudinal fMRI study for locomotor recovery in patients with stroke. Neurology. 2006;67:330–333. doi: 10.1212/01.wnl.0000225178.85833.0d. [DOI] [PubMed] [Google Scholar]
- [128].Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- [129].Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- [130].Scheff S, Benardo I, Cotman C. Progressive brain damage accelerates axon sprouting in the adult rat. Science. 1977;197:795–797. doi: 10.1126/science.887924. [DOI] [PubMed] [Google Scholar]
- [131].Kim JB, Piao CS, Lee KW, Han PL, Ahn JI, Lee YS, Lee JK. Delayed genomic responses to transient middle cerebral artery occlusion in the rat. J Neurochem. 2004;89:1271–1282. doi: 10.1111/j.1471-4159.2004.02429.x. [DOI] [PubMed] [Google Scholar]
- [132].Soriano MA, Tessier M, Certa U, Gill R. Parallel gene expression monitoring using oligonucleotide probe arrays of multiple transcripts with an animal model of focal ischemia. J Cereb Blood Flow Metab. 2000;20:1045–1055. doi: 10.1097/00004647-200007000-00004. [DOI] [PubMed] [Google Scholar]
- [133].Kury P, Schroeter M, Jander S. Transcriptional response to circumscribed cortical brain ischemia: spatiotemporal patterns in ischemic vs. remote nonischemic cortex. Eur J Neurosci. 2004;19:1708–1720. doi: 10.1111/j.1460-9568.2004.03226.x. [DOI] [PubMed] [Google Scholar]
- [134].Schmidt-Kastner R, Zhang B, Belayev L, Khoutorova L, Amin R, Busto, Busto, Ginsberg MD. DNA microarray analysis of cortical gene expression during early recirculation after focal brain ischemia in rat. Brain Res Mol Brain Res. 2002;108:81–93. doi: 10.1016/s0169-328x(02)00516-8. [DOI] [PubMed] [Google Scholar]
- [135].Kim YD, Sohn NW, Kang C, Soh Y. DNA array reveals altered gene expression in response to focal cerebral ischemia. Brain Res Bull. 2002;58:491–498. doi: 10.1016/s0361-9230(02)00823-7. [DOI] [PubMed] [Google Scholar]
- [136].Buga AM, Sascau M, Mogoanta L, Herndon GH, Kessler C, Popa-Wagner A. The genomic response of the contralateral cortex to stroke is diminished in the aged rats. J Cell Mol Med. 2007;12:1–28. doi: 10.1111/j.1582-4934.2008.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]