Abstract
Ischemic preconditioning is the effect of brief ischemic episodes which protect the heart from the following more prolonged ischemic episode. This mechanism is effective in younger but not in aged heart. The age-related reduction of ischemic preconditioning has been demonstrated in experimental models and in elderly patients. Preinfarction angina, a clinical equivalent of ischemic preconditioning, reduces mortality in adult but not in elderly patients with acute myocardial infarction. Physical activity or caloric restriction is partially capable to preserve the cardioprotective effect of ischemic preconditioning in the aging heart. More importantly, physical activity and caloric restriction in tandem action completely preserve the protective mechanism of ischemic preconditioning. Accordingly, the protective mechanism of preinfarction angina is preserved in elderly patients with a high grade of physical activity or a low body-mass index. Thus, both physical activity and caloric restriction are confirmed as powerful anti-aging interventions capable to restore age-dependent reduction of a critical endogenous protective mechanism such as ischemic preconditioning.
Keywords: Aged, heart, younger, ischemia, preconditioning, caloric restriction
Ischemic preconditioning is an adaptive mechanism in response to brief episodes of myocardial ischemia able to reduce the cellular damage subsequent to a more prolonged ischemic insult; in other words, a brief period of ischemia and the following reperfusion makes the heart more resistant to successive more prolonged ischemic insult, and therefore ischemic preconditioning is able to reduce the infarct size [1]. Cardiac ischemic preconditioning, the most powerful endogenous protective mechanism, is represented as an anti-ischemic vaccination. In other words, ischemic preconditioning is a classical example of hormetic effect of a mild stress (i.e. brief and multiple ischemic episodes) able to get a protection in the heart against the more prolonged ischemic insult [2–4].
Mechanism of ischemic preconditioning
This mechanism does not depend on collateral vessels: ischemic preconditioning is present in animal models without collateral vessel and in experimental model as in the isolated perfused heart subjected to a global ischemia [2–4]. The protective effect of ischemic preconditioning could be reduced if the time between preconditioning ischemic episode and the prolonged ischemic episode is excessive. Finally, ischemic preconditioning is classified in “early” when the protective effect is manifest immediately from ischemic episode and “delayed” when the protective effect is manifest 24 hours from ischemic episodes [2–4]. Another form of IP is the so-called “remote preconditioning” in which ischemia in one region of the heart causes protection in a remote region of the heart itself or of another organ. This suggests that a circulating humor or perhaps a neural reflex triggers protection in the remote region [4]. Finally, it has been reported that brief episodes of coronary occlusion and reperfusion at the onset of reperfusion after sustained ischemic insult conferred cardioprotection against ischemia-reperfusion injury: this mechanism is defined “post-conditioning”. Interestingly, post-conditioning can be applied at the onset of reperfusion thus being applicable in clinical settings [5]. However, in this review only “early” cardiac ischemic preconditioning will be discussed.
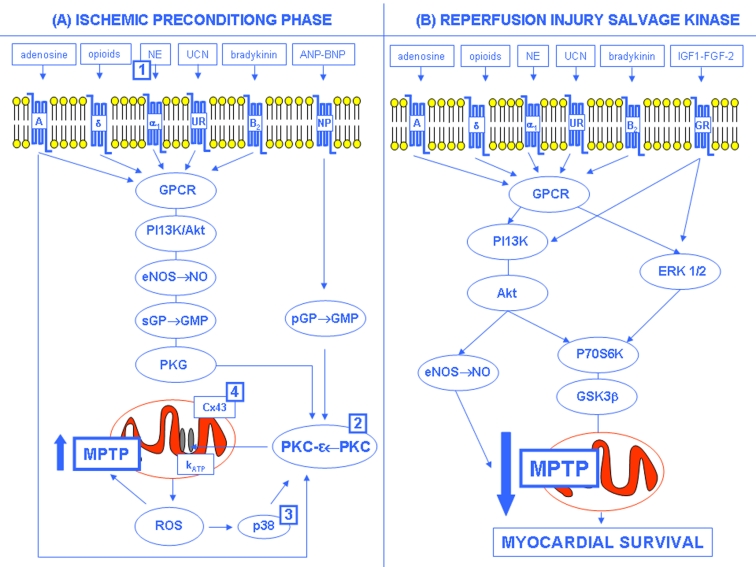
The molecular mechanism of ischemic preconditioning is very complex. From activation of G-protein-coupled receptors (GPCR) by adenosine, norepinephrine, bradykinin, opioids, ect., phosphoinositide-3-kinase (PI3K)/serine/threonine kinase (Akt) is activated with subsequent downstream activation of nitric oxide synthase (NOS) and nitric oxide (NO) formation, and guanylate cyclase, protein kinase G (PKG) and protein kinase C (PKC) activation. Moreover, PKC can be directly activated through adenosine or guanylate cyclase by natriuretic peptide receptor. All these events lead to the opening of the mitochondrial ATP-dependent potassium channels (mito-KATP) through ε-PKC. The opening of mito-KATP channels results in an influx of potassium that causes swelling of the mitochondria which is thought to lead to the production of reactive oxygen species (ROS) [6–8]. In fact, it has been showed that protection conferred by the mito-KATP opener diazoxide can be blocked by ROS scavengers. ROS formation results in p38 mitogen activated kinase and PKC activation and subsequent “priming” of mitochondrial permeability transition pore (MPTP) [6–8]. This mechanism has been proposed as final step of the IP intracellular signaling pathway [9–11]. The MPTPs are multiprotein complexes forming non-selective pores in the inner membrane of the mitochondria. The pore is thought to be formed by alignment of the adenine nucleotide translocator (ANT) on the inner membrane and the voltage-dependent anion channel (VDAC) on the outer membrane. The pore connects the matrix directly to the cytosol and is formed during ischemia reperfusion injury. Once open, this pore allows free passage of any molecule which disrupts the permeability barrier of the inner membrane with devastating consequences related to the uncoupling of the oxidative phosphorylation. MPTP formation results in the collapse of the mitochondrial membrane potential which impairs cells’ ability to produce ATP. Loss of energy for the sodium/potassium ATPase pumps leads to intracellular sodium accumulation along with water. Unless pore closure occurs, these changes will cause irreversible damage to the cell resulting in necrotic death [9–11]. Current evidences suggest that during the preconditioning phase the brief ischemic stimulus causes a reversible MPTP opening and a subsequent mitochondrial depolarization reducing the driving force for Ca2+ uptake into the matrix which would largely occur during reperfusion after a prolonged ischemic period (Fig. 1A) [9–11]. During early reperfusion after the preconditioning stimulus, activation of GPCR or of growth factors receptors results in activation of the reperfusion injury salvage kinase (RISK) program (Fig. 1B). RISK involves the parallel activation of the PI3K/Akt and the extracellular regulated kinase system with downstream p70 ribosomal protein S6 kinase (p70S6K) and glycogen synthase kinase 3b (GSK3b) activation leading to the inhibition of MPTP opening with an increased survival of myocardial cell (Fig. 1B) [6–8].
Fig. 1:
Hypothetical mechanism of IP-induced cardioprotection (see text for details). (A) Ischemic preconditioning phase: NE=Norepinephrine; UCN=Urocortins; ANP=Atrial Natriuretic Peptide; BNP=Brain Natriuretic Peptide; GPCR=G-protein coupled receptor; NPR, natriuretic peptide receptor; PI3K=Phosphatydil-inositol 3 kinase; Akt=serine/threonine kinase; serine/threonine kinase eNOS=endothelial Nitric Oxide Synthase; NO=Nitric Oxide; sGC=soluble Guanylate Cyclase; GMP=Guanosine MonoPhosphate; pGC=particulate Guanylate Cyclase; PKG=Protein Kinase G; PKC=Protein Kinase C; Cx43=Connexin 43; KATP=Mitochondrial potassium ATP-dependent channels; ROS=Reactive Oxygen Species; MPTP=Mitochondrial Permeability Transition Pore; p38=p38 mitogen-activated protein kinase. (B) Reperfusion Injury Salvage Kinase (RISK): IGF-1, insulin-like growth factor; FGF-2, fibroblast growth factor 2; GFR=Growth Factor Receptor; ERK=extracellular regulated kinase; P70S6K=p70 ribosomal S6 protein kinase; GSK3ß=glycogen synthase kinase 3beta. In (A), the numbers in squares indicate the age-related IP impairment sites: 1=Abete et al., 1996; 2=Tani et al., 2001; 3=Fenton et al., 2005; 4=Boengler et al., 2007 (with permission, Abete P et al., 2010) [56].
Clinical evidences of ischemic preconditioning
Clinical observations of ischemic preconditioning are very important because if the mechanism was elucidated, it should become the basis of a new therapeutical approach of coronary artery diseases. Clinical equivalents of ischemic preconditioning are represented by transluminaly coronary angioplasty, preinfarction angina, and walk through angina and warm-up phenomenon [2–4]. In particular, Kloner et al. [12] showed that in patients with preinfarction angina at least 48 hours before myocardial infarction, the incidence of mortality and cardiogenic shock was reduced. Successively, Andreotti et al. [13] have demonstrated that patients with preinfarction angina undergone thrombolytic therapy showed a more rapid reperfusion, and a reduction of infarct size. Finally, three phenomena of clinical relevance should be considered in which the mechanism seems to be the ischemic preconditioning: the first one is represented by a condition of effort angina following physical exercise, which paradoxically disappears when the exercise keeps on going (“walk-through angina”); the second one is characterized by a reduction of clinical and electrocardiographic parameters of effort ischemia following the first exercise test (“Warm-up phenomenon”); the last one is represented by transluminal coronary angioplasty: electrocardiographic, biochemical and clinical signs of ischemia are reduced after the first balloon inflation [2–4].
Age-dependent reduction of ischemic preconditioning
Several investigators have supported the idea that ischemic preconditioning may be reduced with aging. Why? Age is a powerful predictor of mortality for acute myocardial infarction. Mortality for acute myocardial infarction is 80% in coronary heart disease patients older than 65 years, with a frequency three-fold greater compared with adult patients [14–17]. These characteristics have been attributed to several conditions such as myocardial mass increase [18], diastolic dysfunction [19] and reduced angiogenesis [20]. Although the high rate of comorbidity and the reduction of thrombolytic therapy observed in the elderly seem to be the more reasonable explanations [15,16], no factor completely explains the age-related increase of acute myocardial infarction mortality. Thus, the higher mortality and morbidity associated with advancing age could be due to the reduction of some endogenous protective mechanism against myocardial ischemia, a classic example being “ischemic preconditioning”.
Experimental studies have demonstrated that myocardial ischemia may determine a greatest myocardial dysfunction in heart from senescent animals with a less evident recovery during reperfusion when compared to adult ones [21–23]. From these evidences stems the hypothesis that anti-ischemic endogenous mechanisms as ischemic preconditioning may reduce with aging. Thus, we have firstly demonstrated, in the isolated and perfused rat heart, that ischemic preconditioning is reduced in hearts from rats 24 months old that undergone 20 minutes of ischemia and 40 minutes of reperfusion and in rats subjected to a preconditioning protocol with a short period of ischemia (2 minutes) followed by 10 minutes reperfusion. The results obtained showed an improvement of left ventricular function in hearts from adult but not in those from senescent rats. In addition, norepinephrine release from coronary effluent was reduced in senescent hearts in response to preconditioning stimulus when compared to adult ones. In adult animals the pre-treatment with reserpine was able to deplete norepinephrine from adrenergic store and the recovery of left ventricular function during reperfusion was abolished. The study allowed to conclude that ischemic preconditioning is reduced with aging and this reduction is due to a reduction of norepinephrine release in response to preconditioning stimulus [24]. Moreover, the age-related reduction of ischemic preconditioning has been successively confirmed in several studies. Tani et al. [25] demonstrated that hearts became more vulnerable to ischemia with age and that the beneficial effects of preconditioning were reversed in middle-aged rat hearts. Ischemic preconditioning reduced necrosis development and enhanced reperfusion contractile function in young but not in aged hearts [26]. Moreover, not only ischemic stimulus but also pharmacological means such as adenosine A1 agonist, protein kinase C analog, and mitochondrial ATP-sensitive potassium channel opener diazoxide are unable to precondition the aging heart [27]. Bartling et al. [28] have shown that ischemic preconditioning has no positive effect on the postischemic functional recovery of senescent human myocardium in human right atrial trabeculae. Recently, Boengler et al. [29] have demonstrated the age-related reduction of IP in “in vivo” models. In fact, IP by one cycle of 10 min ischemia and 10 min reperfusion reduced infarct size following 30 min regional ischemia and 120 min reperfusion in young but not in old mice hearts.
Mechanism of age-dependent reduction of ischemic preconditioning
A possible mechanism of the age-related reduction of IP may be the decrease of norepinephrine release in response to IP stimulus by α1-adrenoreceptor stimulation. In fact, the abolition of this protective mechanism by prazosin and reserpine strongly suggests that the endogenous release of catecholamines mediates the effect of IP [30]. On the other hand, the age-related decline of catecholamines release due to several mechanisms, including a diminished ability of catecholamine synthesis, has been described [31,32]. Accordingly, it has been shown that norepinephrine release from coronary effluent was reduced in senescent hearts in response to IP stimulus when compared to adult ones. In addition, the depletion of norepinephrine stores by pre-treatment with reserpine blunts the recovery of left ventricular function during reperfusion in hearts from adult animals [24]. These findings allowed to conclude that age-related reduction of IP is due, at least in part, to a reduction of norepinephrine release in response to preconditioning stimulus [24]. Indeed, adenosine failed to induce cardioprotection in aged hearts probably because of an impairment of downstream signaling elements rather than a decreased expression of specific adenosine receptors [33]. PKC is one of the kinase involved in IP-induced cardioprotection [4]. More specifically, IP is related to a translocation of PKC from the cytosol to the particulate fraction and to a consequent activation of mitochondrial potassium ATP channels [4]. PKC translocation in response to IP is impaired in the aging heart and probably is responsible for the loss of age-related reduction of IP cardioprotection [34]. However, it has been reported that in 4- year-old rabbit hearts PKC translocation is impaired but IP is still effective [35]. PKC isoforms may trigger IP by phosphorylating gap junctions and mitochondria connexin-43 which has been recently demonstrated to be reduced in aged mouse heart [29]. Finally, phosphorylation of protein kinases such as ERK1/2 (extracellular signal regulated kinase), Akt, GSK3b (glycogen synthase kinase 3b), or p38 may activate IP cardioprotective effect [10]. Moreover, it has been demonstrated that aging-related loss of IP cardioprotection involves failure to activate p38 MAPK and HSP27 [36]. Since an up-regulation of protein phosphatases such as protein phosphatase 2A was observed in aging rat hearts, an enhanced dephosphorylation by protein phosphatases may be considered another mechanism of age-related IP reduction [37]. Interestingly, ischemic post-conditioning significantly reduced infarct size via up-regulation of ERK signaling in adult but not in old mouse hearts probably as a consequence of the age-related reduction in ERK phosphorylation [38].
Clinical evidence of the age-related reduction of ischemic preconditioning: the preinfarction angina
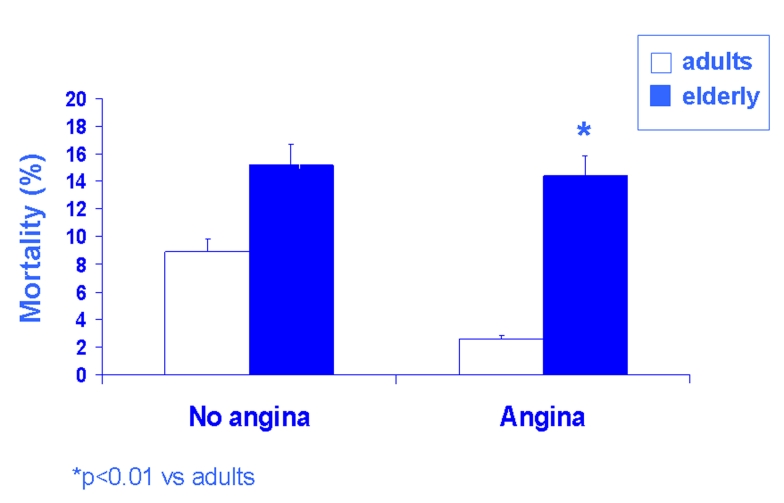
Preinfarction angina, the most evident equivalent of ischemic preconditioning, has been studied in adult and elderly patients in terms of in-hospital primary and secondary events: in adult patients (<65 years), both in-hospital mortality and cardiogenic shock were more frequent in the absence than in the presence of preinfarction angina; CK-MB (creatine kinase myoglobin fraction) peak, transmural infarctions number, the incidence of ventricular tachycardia and fibrillation, and the ventricular dysfunction were significantly higher in the adult patients without than in those with preinfarction angina (Fig. 3B). In elderly patients (≥ 65 years), the protective effect of preinfarction angina seems to be lost: both in-hospital primary (mortality and cardiogenic shock) and secondary (CK-MB peak, transmural infarctions number, the incidence of ventricular tachycardia and fibrillation, and the ventricular dysfunction) end-points were similar in elderly patients with and without preinfarction angina (Fig. 3B). Logistic regression, adjusted for several variables including the use of thrombolytic and anti-anginal therapy, demonstrated that preinfarction angina is a protective variable against mortality and cardiogenic shock in adult but not in elderly patients [39]. Successively, in non-elderly patients, prodromal angina was associated with lower peak creatine kinase levels, lower in-hospital mortality rates, and better 5-year survival rates while in elderly patients there was no significant difference in peak creatine kinase levels, in-hospital mortality rate, and 5-year survival rates. A multivariate analysis showed that prodromal angina in the 24 hours before infarction was associated with 5-year survival rate in non-elderly patients but not in elderly patients [40]. The “warm-up phenomenon” also seems to reduce in elderly patients as demonstrated both with dynamic electrocardiography [41] and effort exercise [42]: with both methods the ischemic episode successive to the first myocardial ischemia was reduced in adult but not in elderly patients. Very recently, the absence of ischemic preconditioning has been demonstrated in elderly patients during coronary angioplasty [43].
Fig. 3:
The age-related reduction of the cardioprotective effect of preinfarction angina, a clinical equivalent of IP, is shown. Bar graphs show that in-hospital mortality was similar in adults and elderly patients. In contrast, in the presence of angina, in-hospital mortality was lower in adults than in elderly patients. Thus, preinfarction angina is protective against in-hospital mortality in adults but not in elderly patients.
Lifestyles and ischemic preconditioning: caloric restriction and exercise training in the aging heart
Caloric restriction has been widely described as an anti-aging intervention [44]. In particular, caloric restriction increases the life span of rodents [45], retards the severity of some age-related diseases [46] and attenuates the physiological decline of several organs, including the heart [44]. Recently, we have demonstrated that ischemic preconditioning improved both mechanical and electrical parameters in adult but not in hearts from “ad libitum” senescent animals and that ischemic preconditioning is preserved in food-restricted senescent animals. In addition, caloric restriction seems to restore ischemic preconditioning in hearts from senescent animals through an involvement of the adrenergic pathway in response to ischemic preconditioning. Norepinephrine release in response to ischemic preconditioning is reduced in the senescent heart and restored in hearts from food-restricted senescent animals [47] (Fig. 4A).
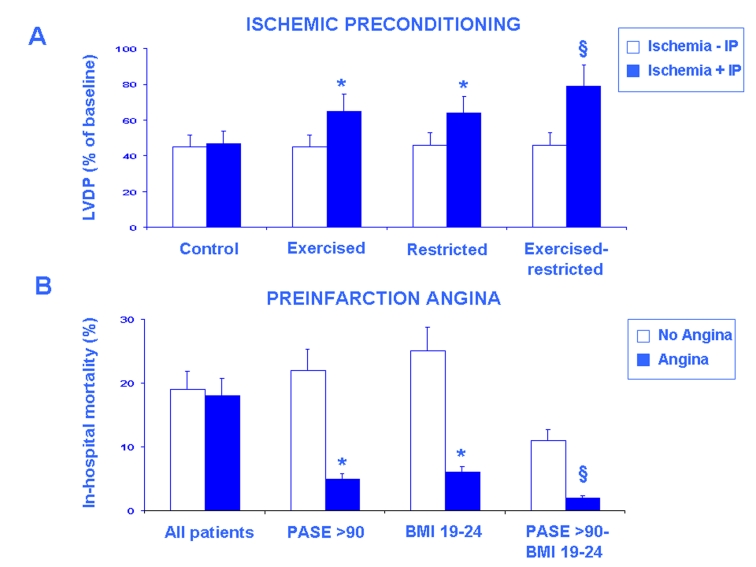
Fig. 4:
The restoration of the age-related of IP by exercise training and caloric restriction in the isolated and perfused rat heart is shown in panel A. Bar graphs show the recovery of left ventricular developed pressure (LVDP) (% of basal) at the end of reperfusion in sedentary ad libitum fed (control), trained ad libitum fed, sedentary food-restricted and trained- and food-restricted senescent hearts subjected to ischemia (20 min) and reperfusion (40 min) (Ischemia - IP) and pre-treated with preconditioning stimulus of 2 min followed by 10 min of reperfusion (Ischemia + IP). LVDP recovery was similar in the absence and the presence of IP. Exercise training and caloric restriction restored IP (p<0.01 vs. Control) and this effect was more evident in hearts from trained- and food-restricted rats (§p<0.001 vs. Control). The preservation of the age-related reduction of the cardio-protective effect of preinfarction angina, a clinical equivalent of IP, by physical activity evaluated by Physical Activity Scale for the Elderly (PASE) score and by a normal body-mass index (BMI) is shown in B. Bar graphs show that in-hospital mortality percentage was similar in elderly patients without and with preinfarction angina but it was lower in elderly patient with preinfarction angina with high PASE score (>90) and normal BMI (19–24) (§p<0.05 vs. all patients). This effect was more evident in elderly patients with the highest PASE (>90) and the normal BMI (19–24) (*p<0.01 vs. all patients) (with permission, Abete P et al., 2010) [56].
Exercise training is able to increase average survival time in rats without increasing their maximal longevity but it might reverse several age-related modification of the heart [48]. The effects on mechanical parameters of ischemic preconditioning against 20 minutes of global ischemia followed by 40 minutes of reperfusion has been investigated in isolated perfused hearts from adult (6 months) and sedentary or trained (6 weeks of graduated swim training) senescent (24 months) rats. The effect of preconditioning on developed pressure recovery was absent in sedentary but present in trained senescent hearts (Fig. 4A). Norepinephrine release significantly increased after preconditioning in adult and in trained but not in sedentary senescent hearts. Thus, in adult and trained but not in sedentary senescent hearts, preconditioning reduces postischemic dysfunction and is associated with an increase in norepinephrine release [49].
“Combined action” of physical activity and caloric restriction can retard the age-related modifications of the heart. In isoproterenol-induced acute myocardial infarction in male rats both exercise or maintenance of body weight are able separately to prevent drug-induced acute myocardial infarction, but additional protection is produced when food restriction is combined with exercise training [50]. Since ischemic preconditioning may be partially corrected by exercise training and food restriction the role of exercise training combined with food restriction on restoring ischemic preconditioning was investigated in isolated hearts [51]. Developed pressure recovery was partial in hearts from trained ad libitum fed and sedentary food-restricted but it was total in adult hearts and in those from trained and food restricted senescent rats (Fig. 4A). Thus, the combined action of exercise training and food restriction is able to completely restore the ischemic preconditioning in the aging heart. In these experimental conditions, cardiac norepinephrine release in response to ischemic preconditioning stimulus is reduced in senescent but not in adult animals and is partially restored by exercise training and by dietary restriction in senescent animals. Interestingly, cardiac norepinephrine release in response to ischemic preconditioning stimulus is completely restored in trained- and food restricted animals, demonstrating that the synergistic action on norepinephrine release might explain how exercise training and food restriction together are able to totally preserve ischemic preconditioning in the aging heart. This hypothesis was supported by the complete abolition of the restoring effect of exercise training and food restriction after reserpine pre-treatment which is able to deplete norepinephrine stores [51].
Effects of physical activity and hypocaloric diet on preinfarction angina, a clinical equivalent of ischemic preconditioning, in the elderly
The effects of physical activity, evaluated by the PASE (Physical Activity Scale for the Elderly) [52], on preinfarction angina, a clinical equivalent of ischemic preconditioning, was investigated in adult and elderly patients with acute myocardial infarction. A high level of physical activity was strongly associated with reduced in-hospital mortality. Moreover, a high level of physical activity reduced in-hospital mortality in elderly patients with but not in those without preinfarction angina (Fig. 4B). Accordingly, regression analysis confirmed that the protective effect of preinfarction angina is preserved in elderly patients with a high level of physical activity [53].
Accordingly, less in-hospital deaths were observed in elderly patients with than in those without preinfarction angina in the subset of patients with the lowest body-mass index (Fig. 4B). Regression analysis demonstrated that preinfarction angina did not protect against in-hospital death when analyzed in all patients independently of body-mass index, whereas it was protective in the subset of patients with the lowest body-mass index [54].
When elderly patients with AMI were stratified in quartiles of physical activity combined with each BMI quartile, in-hospital death progressively decreased as PASE score increased and BMI decreased. In particular the subset of elderly patients with the lowest BMI in hospital death decreased as PASE scores increased (Fig. 4A). In elderly patients without preinfarction angina the combined action of PASE and BMI did not seem to influence the occurrence of in-hospital death (Fig. 4B). In contrast, when elderly patients with preinfarction angina were stratified in quartiles of PASE combined with each BMI quartile, in-hospital death progressively decreased as PASE scores increased and BMI decreased. In particular the subset of elderly patients with the lowest BMI showed in hospital death to decrease as PASE scores increased (Fig. 4C).
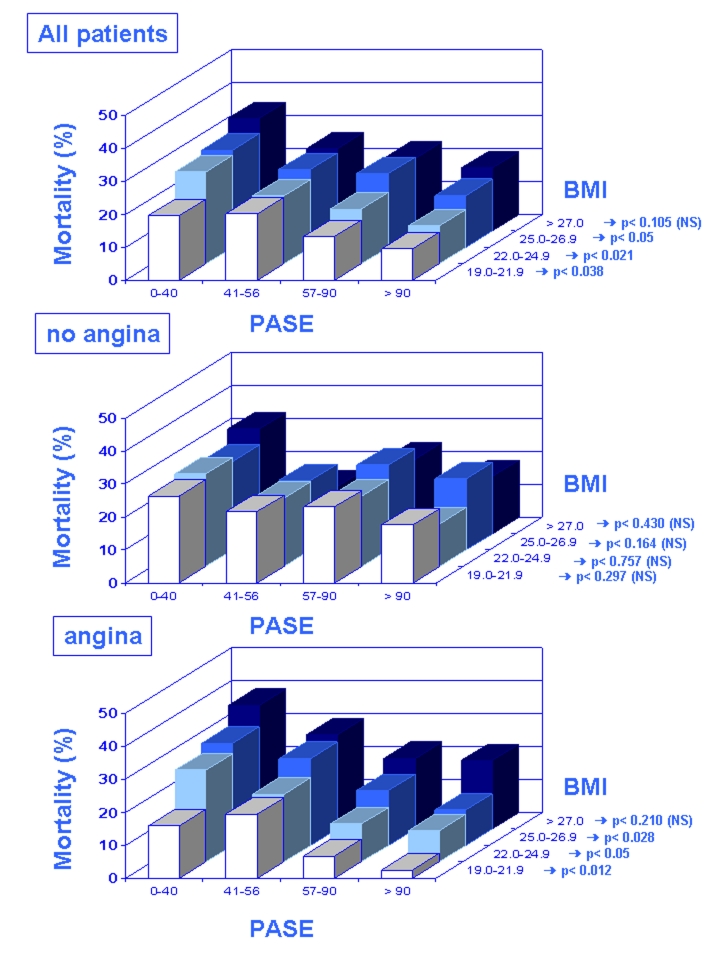
The synergistic action of physical activity and low body mass index is confirmed by the multivariate analysis of preinfarction angina on in-hospital death performed by stratifying for PASE quartiles combined with each body-mass index quartile. Preinfarction angina was protective against in-hospital death in the highest PASE score in all body-mass index subgroups. Interestingly, preinfarction angina reached the maximum at highest PASE and lowest body-mass index score and it was still protective against in-hospital death at 57–90 PASE score but at lowest body-mass index. More importantly, the effect of preinfarction angina is “predictive” of mortality in sedentary and overweight elderly patients, but it becomes “protective” in trained normal weight elderly patients, suggesting a key role of lifestyles in this phenomenon (Fig. 5) [55].
Figure 5:
Mortality for acute myocardial infarction in all elderly patients (A), in those without (no angina, B) and in those with (angina, C) preinfarction angina according to quartiles of physical activity (PASE) combined with each body mass index (BMI) quartile is shown. In-hospital death was lowest in elderly patients “with preinfarction angina” in the highest PASE score and in the lowest BMI subgroups (with permission, Abete P et al., 2009) [55].
Conclusions
We conclude that:
- a brief ischemic episodes may protect the heart from a successive and more prolonged myocardial ischemia (ischemic preconditioning);
- cardiac ischemic preconditioning is reduced with aging in both experimental and clinical settings;
- alterations of mediators release and/or intracellular pathways may be responsible for the age-related ischemic preconditioning reduction;
- exercise training and caloric restriction separately, and more powerfully, taken together, are able to completely preserve and/or restore the age-related reduction of IP in both animal and human studies.
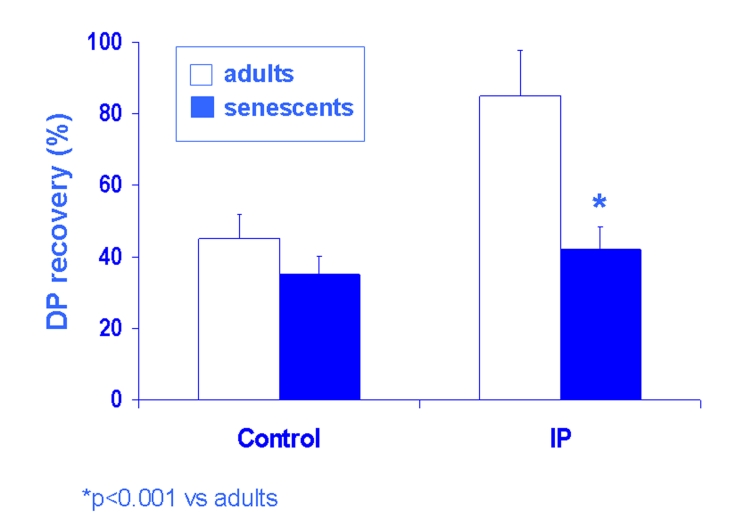
Fig. 2:
The age-related reduction of IP in the isolated and perfused rat heart is shown. In Langendorff experiments left ventricular developed pressure (DP) recovered ≈ 40–50% in controls (standard ischemia [20 min] – reperfusion [40 min] insult) of both adult and senescent rat hearts (A). In preconditioning experiments (ischemia 2 min followed by 10 min of reperfusion and then a standard ischemia – reperfusion insult) (IP), DP recovered ≈ 80% in adult but not in senescent hearts (*p<0.001 vs. adults).
REFERENCES
- [1].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [2].Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation and preconditioning. An NHLBI workshop. Circulation. 1998;97:1848–67. doi: 10.1161/01.cir.97.18.1848. [DOI] [PubMed] [Google Scholar]
- [3].Napoli C, Pinto A, Cirino G. Pharmacological modulation, preclinical studies, and new clinical features of myocardial ischemic preconditioning. Pharmacol Ther. 2000;88:311–31. doi: 10.1016/s0163-7258(00)00093-0. [DOI] [PubMed] [Google Scholar]
- [4].Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–51. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- [5].Vinten-Johansen J, Zhao ZQ, Jiang R, Zatta AJ, Dobson GP. Preconditioning and postconditioning: innate cardioprotection from ischemia-reperfusion injury. J Appl Physiol. 2007;103:1441–8. doi: 10.1152/japplphysiol.00642.2007. [DOI] [PubMed] [Google Scholar]
- [6].Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–44. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- [7].Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–8. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- [8].Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–19. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- [9].Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–31. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–53. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- [11].Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83:213–25. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kloner RA, Shook T, Przyklenk K, Davis VG, Junio L, Matthews RV, Burstein S, Gibson CM, Poole WK, Cannon CP, McCabe CH, Braunwald E. Previous angina alters in-hospital outcome in TIMI 4: a clinical correlate to preconditioning. Circulation. 1995;91:37–47. doi: 10.1161/01.cir.91.1.37. [DOI] [PubMed] [Google Scholar]
- [13].Andreotti F, Pasceri V, Hackett DR, Davies GJ, Haider AW, Maseri A. Preinfarction angina as a predictor of more rapid coronary thrombolysis in patients with acute myocardial infarction. N Engl J Med. 1996;334:7–12. doi: 10.1056/NEJM199601043340102. [DOI] [PubMed] [Google Scholar]
- [14].Tresch DD, Brady WJ, Aufderheide TP, Lawrence SW, Williams KJ. Comparison of elderly and younger patients with out-of-hospital chest pain. Arch Intern Med. 1996;156:1089–93. [PubMed] [Google Scholar]
- [15].Berger AK, Radford MJ, Wang Y, Krumholz HM. Thrombolytic therapy in older patients. J Am Coll Cardiol. 2000;36:366–74. doi: 10.1016/s0735-1097(00)00723-3. [DOI] [PubMed] [Google Scholar]
- [16].Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients The PURSUIT Investigators. Circulation. 2000;101:2557–67. doi: 10.1161/01.cir.101.22.2557. [DOI] [PubMed] [Google Scholar]
- [17].Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, Tognoni G. Age related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. N Engl J Med. 1993;329:1442–8. doi: 10.1056/NEJM199311113292002. [DOI] [PubMed] [Google Scholar]
- [18].Gerstenblith G, Frederiksen J, Yin FC, Fortuin NJ, Lakatta EG, Weisfeldt ML. Echocardiography assessment of a normal adult aging population. Circulation. 1977;56:672–9. doi: 10.1161/01.cir.56.2.273. [DOI] [PubMed] [Google Scholar]
- [19].Tresch DD, McGough MF. Heart failure with normal systolic function: a common disorder in older people. J Am Geriatr Soc. 1995;43:1035–42. doi: 10.1111/j.1532-5415.1995.tb05570.x. [DOI] [PubMed] [Google Scholar]
- [20].Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–20. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- [21].Ataka K, Chen D, Levitsky S, Jimenez E, Feinberg H. Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation. 1992;86(II):371–76. [PubMed] [Google Scholar]
- [22].Abete P, Cioppa A, Ferrara P, Caccese P, Ferrara N, Rengo F. Reduced aerobic metabolic efficiency in postischemic myocardium dysfunction in rats: role of aging. Gerontology. 1995;41:187–94. doi: 10.1159/000213681. [DOI] [PubMed] [Google Scholar]
- [23].Abete P, Cioppa A, Calabrese C, Pascucci I, Cacciatore F, Napoli C, Carnovale V, Ferrara N, Rengo F. Ischemic threshold and myocardial stunning in the aging heart. Experimental Gerontology. 1999;34:875–84. doi: 10.1016/s0531-5565(99)00060-1. [DOI] [PubMed] [Google Scholar]
- [24].Abete P, Calabrese C, Ferrara N, Cioppa A, Pisanelli P, Cacciatore F, Longobardi G, Napoli C, Rengo F. Preconditioning does not prevent post-ischemic dysfunction in aging heart. J Am Coll Cardiol. 1996;27:1777–86. doi: 10.1016/0735-1097(96)00070-8. [DOI] [PubMed] [Google Scholar]
- [25].Tani M, Suganuma Y, Hasegawa H, Shinmura K, Hayashi Y, Guo X, Nakamura Y. Changes in ischemic tolerance and effects of ischemic preconditioning in middle-aged rat hearts. Circulation. 1997;95:2559–66. doi: 10.1161/01.cir.95.11.2559. [DOI] [PubMed] [Google Scholar]
- [26].Fenton RA, Dickson EW, Meyer TE, Dobson JG., Jr Aging reduces the cardioprotective effect of ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 2000;32:1371–75. doi: 10.1006/jmcc.2000.1189. [DOI] [PubMed] [Google Scholar]
- [27].Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281:H1630–6. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- [28].Bartling B, Friedrich I, Silber RE, Simm A. Ischemic preconditioning is not cardioprotective in senescent human myocardium. Ann Thorac Surg. 2003;76:105–11. doi: 10.1016/s0003-4975(03)00186-3. [DOI] [PubMed] [Google Scholar]
- [29].Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, Schulz R, et al. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–9. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- [30].Banerjee A, Locke-Winter C, Rogers KB, Mitchell MB. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an α1-adrenergic mechanism. Circ Res. 1993;73:656–70. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- [31].Mazzeo RS, Horvarth SM. A decline in myocardial and hepatic norepinephrine turnover with age in Fisher 344 rats. Am J Physiol. 1987;252:E762–4. doi: 10.1152/ajpendo.1987.252.6.E762. [DOI] [PubMed] [Google Scholar]
- [32].Dawson R, Meldrum MJ. Norepinephrine content in cardiovascular tissue from the aged Fisher 344 rat. Gerontology. 1992;38:185–91. doi: 10.1159/000213326. [DOI] [PubMed] [Google Scholar]
- [33].Willems L, Ashton KJ, Headrick JP. Adenosine-mediated cardioprotection in the aging myocardium. Cardiovasc Res. 2005;66:245–55. doi: 10.1016/j.cardiores.2004.11.008. [DOI] [PubMed] [Google Scholar]
- [34].Tani M, Honma Y, Hasegawa H, Tamaki K. Direct activation of mitochondrial K [ATP] channels mimics preconditioning but protein kinase C activation is less effective in middle-aged rat hearts. Cardiovasc Res. 2001;49:56–68. doi: 10.1016/s0008-6363(00)00240-6. [DOI] [PubMed] [Google Scholar]
- [35].Przyklenk K, Li G, Simkhovich BZ, Kloner RA. Mechanisms of myocardial ischemic preconditioning are age related: PKC-1 does not play a requisite role in old rabbits. Am J Physiol. 2003;95:2563–9. doi: 10.1152/japplphysiol.00404.2003. [DOI] [PubMed] [Google Scholar]
- [36].Peart JN, Gross ER, Headrick JP, Gross GJ. Impaired p38 MAPK/HSP27 signaling underlies aging-related failure in opioid-mediated cardioprotection. J Mol Cell Cardiol. 2007;42:972–80. doi: 10.1016/j.yjmcc.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fenton RA, Dickson EW, Dobson JG., Jr Inhibition of phosphatase activity enhances preconditioning and limits cell death in the ischemic/reperfused aged rat heart. Life Sci. 2005;77:3375–88. doi: 10.1016/j.lfs.2005.05.047. [DOI] [PubMed] [Google Scholar]
- [38].Przyklenk K, Maynard M, Darling CE, Whittaker P. Aging mouse hearts are refractory to infarct size reduction with post-conditioning. J Am Coll Cardiol. 2008;51:1393–8. doi: 10.1016/j.jacc.2007.11.070. [DOI] [PubMed] [Google Scholar]
- [39].Abete P, Ferrara N, Cacciatore F, Sagnelli E, Manzi M, Carnovale V, Calabrese C, De Santis D, Testa G, Longobardi G, Napoli C, Rengo F. Angina-induced protection against myocardial infarction in adult and senescent patients. A loss of preconditioning mechanism in aging heart. J Am Coll Cardiol. 1997;30:947–54. doi: 10.1016/s0735-1097(97)00256-8. [DOI] [PubMed] [Google Scholar]
- [40].Ishihara M, Sato H, Tateishi H, Kawagoe T, Shimatani Y, Ueda K, Noma K, Yumoto A, Nishioka K. Beneficial effect of prodromal angina pectoris is lost in elderly patients with acute myocardial infarction. Am Heart J. 2000;139:881–8. doi: 10.1016/s0002-8703(00)90021-8. [DOI] [PubMed] [Google Scholar]
- [41].Napoli C, Liguori A, Cacciatore F, Rengo F, Ambrosio G, Abete P. Warm-up phenomenon detected by electrocardiographic ambulatory monitoring in adult and elderly patients. J Am Geriatr Soc. 1999;47:1–4. doi: 10.1111/j.1532-5415.1999.tb05237.x. [DOI] [PubMed] [Google Scholar]
- [42].Longobardi G, Abete P, Ferrara N, Papa A, Rosiello R, Furgi G, Calabrese C, Cacciatore F, Rengo F. Warm-up. Phenomenon in adult and elderly patients with coronary artery disease Further evidence of the loss of ischemic preconditioning in the aging heart. J Gerontol. 2000;55:M124–9. doi: 10.1093/gerona/55.3.m124. [DOI] [PubMed] [Google Scholar]
- [43].Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channel in elderly patients undergoing coronary angioplasty. Circulation. 2002;105:334–40. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- [44].Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- [45].McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- [46].Weindruch R, Walford RL, editors. The retardation of aging and disease by dietary restriction. Springfield, Il: Thomas; 1988. [Google Scholar]
- [47].Abete P, Testa G, Ferrara N, De Santis D, Capaccio P, Viati L, Calabrese C, Cacciatore F, Longobardi G, Condorelli M, Napoli C, Rengo F. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am J Physiol. 2002;282:H1978–87. doi: 10.1152/ajpheart.00929.2001. [DOI] [PubMed] [Google Scholar]
- [48].Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a re-evaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- [49].Abete P, Calabrese C, Ferrara N, Cioppa A, Pisanelli P, Cacciatore F, Longobardi G, Napoli C, Rengo F. Exercise training restores ischemic preconditioning in the aging heart. J Am Coll Cardiol. 2000;36:643–50. doi: 10.1016/s0735-1097(00)00722-1. [DOI] [PubMed] [Google Scholar]
- [50].Crandall DL, Feirer RP, Griffith DR. Relative role of caloric restriction and exercise training upon susceptibility to isoproterenol-induced myocardial infarction in male rats. Am J Clin Nutr. 1981;34:841–7. doi: 10.1093/ajcn/34.5.841. [DOI] [PubMed] [Google Scholar]
- [51].Abete P, Testa G, Galizia G, Mazzella F, Della Morte D, De Santis D, Calabrese C, Cacciatore F, Gargiulo G, Ferrara N, Rengo G, Sica V, Napoli C, Rengo F. Tandem action of exercise training and food restriction completely preserves ischemic preconditioning in the aging heart. Exp Gerontol. 2005;40:43–50. doi: 10.1016/j.exger.2004.10.005. [DOI] [PubMed] [Google Scholar]
- [52].Washburn RA, McAuley E, Katula J, et al. The Physical Activity for the Elderly (PASE): Evidence for validity. J Clin Epidemiol. 1999;52:643–51. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- [53].Abete P, Ferrara N, Cacciatore F, Madrid A, Bianco S, Calabrese C, Napoli C, Scognamiglio P, Bollella O, Cioppa A, Longobardi G, Rengo F. High level of physical activity preserves the cardioprotective effect of preinfarction angina in elderly patients. J Am Coll Cardiol. 2001;38:1357–65. doi: 10.1016/s0735-1097(01)01560-1. [DOI] [PubMed] [Google Scholar]
- [54].Abete P, Cacciatore F, Ferrara N, Calabrese C, De Santis D, Testa G, Galizia G, Del Vecchio S, Leosco D, Condorelli M, Napoli C, Rengo F. Body mass index and preinfarction angina in elderly patients with acute myocardial infarction. Am J Clin Nutr. 2003;78:796–801. doi: 10.1093/ajcn/78.4.796. [DOI] [PubMed] [Google Scholar]
- [55].Abete P, Cacciatore F, Della Morte D, Mazzella F, Testa G, Galizia G, De Santis D, Longobardi G, Ferrara N, Rengo F. Joint effect of physical activity and body mass index on mortality for acute myocardial infarction in the elderly: role of preinfarction angina as equivalent of ischemic preconditioning. Eur J Cardiovasc Prev Rehabil. 2009;16:73–9. doi: 10.1097/HJR.0b013e32831e9525. [DOI] [PubMed] [Google Scholar]
- [56].Abete P, Cacciatore F, Testa G, Della Morte D, Galizia G, De Santis D, Calabrese C, Cioppa A, Ferrara N, Rengo F. Ischemic preconditioning in the aging heart: from bench to bedside. Ageing Res Rev. 2010;9:153–62. doi: 10.1016/j.arr.2009.07.001. [DOI] [PubMed] [Google Scholar]