Abstract
This work considers reactive oxygen species (ROS) signaling in solid tumors. Most (probably all) cancer cells are characterized by ROS overproduction that is they exist under conditions of incessant oxidative stress. For example ROS overproduction has been shown in prostate, pancreatic, melanoma, and glioma cells. ROS overproduction has been also demonstrated in breast, liver, bladder, colon, and ovarian cancers. Although these examples probably do not incorporate all the described data concerning ROS overproduction in cancer cells, they clearly support a proposal about enhanced oxidative stress in these cells. Therefore the mechanisms of ROS signaling in the survival and death of cancer cells and comparison with ROS signaling in senescent cells ought to be considered. It might be suggested that ROS overproduction in cancer cells is a major origin of their survival and resistance to anticancer treatment while the enhanced oxidative stress responsible for aging development. However it is of particular interest that additional ROS production by prooxidants can induce apoptosis in cancer cells. We suggest that moderate oxidative stress can stimulate proliferation and survival of cancer sells by conditioning mechanism while the enhancement of ROS overproduction by prooxidants under severe oxidative stress results in apoptosis and cell death. Aging development is always characterized by harmful ROS overproduction although the moderate increase in ROS formation in senescent cells might be not dangerous. Similar double-edged sword effects of ROS might be observed during the development of other pathologies for example diabetes mellitus.
Keywords: ROS, signaling, cancer, aging
Role of free radicals and reactive oxygen species (ROS) in cancer and aging is extensively discussed for many years. It seems to be a more explicit in the mechanism of aging development: although there are some findings demonstrating the positive effects of moderate ROS overproduction in the age, ROS signaling under the conditions of severe oxidative stress always enhances cell senescence and organismal aging [1–3]. Role of free radicals and ROS in cancer is much more complicated. In 1965–1967 Professor Emanuel and his co-workers [4] hypothesized that cancer development has many characteristics of free radical chain processes. However, the attempts of authors to treat the cancer patients with antioxidants were unsuccessful. On the one hand it is difficult to imagine free radical chain processes in biological heterogenic systems, on the other hand the present knowledge of free radical-mediated processes indicates an important role of free radicals as signaling species (ROS signaling) which cannot and should not be completely destroyed by antioxidants.
At present it is well established that ROS signaling is an important factor of many gene- and enzyme-catalyzed processes. ROS signaling is responsible for activation or inhibition of numerous processes catalyzed by protein kinases, phosphatases, and many other enzymes although these reactions proceed by heterolytic (non-free radical) mechanisms [5]. Therefore, ROS signaling can initiate both inhibition and activation of tumor formation. This fact might be of utmost importance for the development of anticancer treatment by the drugs possessing both prooxidant and antioxidant properties. In this work we will consider the mechanisms of free radical formation and ROS signaling during cancer development in various human organs.
ROS SIGNALING IN CANCER CELLS
Prostate cancer
It is known that cancer cells are characterized by enhanced oxidative stress (ROS overproduction). Therefore, one might suggest that an increase in ROS signaling is responsible for cancer cell survival. Thus Brar et al. [6] showed that superoxide produced by NADPH oxidase in prostate cancer cells exhibited cancer-promoting effect by facilitating cellular immortality through resistance to programmed cell death. ROS overproduction in prostate cancer cells has also been demonstrated in other works. Kumar et al. [7] found an increased ROS generation in prostate cancer cells compared with normal cells. It was showed that ROS were generated by NADPH oxidase (Nox) and its overproduction characterized the malignant phenotype of prostate cancer cells. Both superoxide and hydrogen peroxide signaling was detected in the enzymatic cascades of extracellular signal-regulated kinases ERK1/2, mitogen-activated protein kinase p38, and protein kinase B (Akt).
Arnold et al. [8] proposed that Nox1 expression caused cellular ROS production and signal transduction in cellular proliferation of prostate cancer cells. It was suggested that Nox1 protein overexpression was an early event in the development of prostate cancer. Tumors have significantly higher Nox1 levels than benign prostate tissue. Thus Nox1 overexpression might function as a reversible signal for cancer cell proliferation.
Veeramani et al. [9] found that in prostate cancer cells ROS might be formed by the p66Shc-depended pathway. These authors showed that the dihydrotestosterone (DHT)-bound androgen receptor (AR) complex stimulated cell proliferation through the p66Shc/ROS pathway in spite of well known fact that the p66Shc protein is a mediator of apoptosis. However in prostate cancer cell lines the level of p66Shc protein positively correlated with their growth rates. It has been suggested that DHT-bound AR increased p66Shc protein levels and its translocation into mitochondria where p66Shc activated superoxide production by the oxidation of cytochrome c. (However, it should be noted that the reduction of dioxygen to superoxide by cytochrome c seems to be thermodynamically prohibited). Increased ROS levels in turn activated tyrosine kinases through the inactivation of phosphatases and promoted cell cycle progression through Cyclin D1 and cell proliferation.
All these findings indicate that an increase in ROS signaling in prostate cancer cells is responsible for prostate cancer development. Paradoxically, it has been found that the treatment of prostate cancer cells with ROS-generating compounds stimulated their apoptosis. For example Singh et al. [10] found that guggulsterone, a constituent of Indian Ayurvedic medicinal plant Commiphora mukul induced apoptosis in prostate cancer cells through ROS generation which led to the activation of ASK1/JNK1/2 signal transduction pathway. It is possible that guggulsterone might target mitochondria to trigger ROS formation; this proposal was supported by the inhibition of JNK1/2 activation and apoptosis induction when mitochondrial MnSOD was overexpressed. Shukla and Gupta [11] showed that the plant flavone apigenin induced ROS generation in prostate cancer cells. This was accompanied by the p53 stabilization through the phosphorylation of its critical serine residue, rapid glutathione depletion, the disruption of mitochondrial membrane potential, cytosolic release of cytochrome c and the induction of apoptosis. Diallyl trisulfide (DATS), a cancer chemopreventive agent induced ROS production and cycle arrest in prostate cancer cells through JNK signaling cascade [12].
Zhao et al. [13] found that superoxide produced by the derivatives of selenomethionine (SeMet) formed in the reaction catalyzed by methioninase (METase) induced prostate cancer cell apoptosis. They suggested that superoxide and p53 initiated apoptosis through mitochondrial pathway. Genipin, the aglycone of geniposide, enhanced NADPH oxidase-depended ROS formation in human prostate cancer cells initiating apoptosis through the phosphorylation of mixed lineage kinase 3 (MLK3) and the downstream activation of JNK [14].
Recently Sun et al. [15] suggested that the enhanced ROS generation by prooxidants or the suppression of antioxidant capacity in cancer cells might selectively increase ROS-depended cancer cell killing, whereas normal cells still might be able to fight relatively moderate oxidative stress in the presence of new ROS producers. They showed that parthenolide, a sesquiterpene lactone, selectively exhibited the radiosensitization effect on prostate cancer cells but not normal prostate epithelial cells. In cancer cells but not in normal ones, parthenolide activated NADPH oxidase, decreased the level of reduced thioredoxin, activated PI3K/Akt kinases, and FOXO3a phosphorylation stimulating downregulation of FOXO3a-depended antioxidant enzymes MnSOD and catalase.
Pancreatic cancer
Vaquero et al. [16] pointed out that one reason why pancreatic cancer is so aggressive and unresponsive to treatments is its resistance to apoptosis. They proposed that ROS are a prosurvival, antiapoptotic factor of pancreatic cancer cells. Human pancreatic adenocarcinoma cells generated ROS, which was stimulated by growth factors (serum, insulin-like growth factor I, or fibroblast growth factor-2). Pancreatic cancer cells were also characterized by enhanced NADPH oxidase activity.
In several works [17,18] Oberley and his co-workers demonstrated a decreased level of antioxidative mitochondrial superoxide dismutase MnSOD in pancreatic cancer cells. Decreased MnSOD levels correlated well with the increased rates of tumor cell proliferation. No correlation was found between cell growth and the levels of CuZnSOD, catalase, or glutathione peroxidase. However, later on it was showed that the overexpression of MnSOD, CuZnSOD, and EcSOD in pancreatic cancer cells decreased superoxide levels and increased hydrogen peroxide levels. The inhibition of ROS formation in cancer cells by SODs resulted in a decrease in vitro tumor growth [19].
It has been suggested that NADPH oxidase (Nox4)-depended generation of ROS is responsible for antiapoptotic activity and therefore is a growth advantage to pancreatic cancer cells. Mochizuki et al. [20] showed that the inhibition of NADPH oxidase diminished the phosphorylation of cell surviving kinase Akt and Akt-stimulated phosphorylation of apoptosis signal-regulating kinase 1 (ASK1) on Ser-83. These findings indicate that ROS generated by Nox4 transmitted cell survival signals through the AKT-ASK1 pathway in pancreatic cancer cells and that its suppression led to apoptosis. On the other hand Lee et al. [21] suggested that ROS produced by NADPH oxidase inhibited key protein tyrosine phosphatases (PTPs) and thus activated kinases of antiapoptotic pathways in pancreatic cancer cells. It was also found that NADPH oxidase promoted pancreatic cancer cell survival via the inhibition of JAK2 dephosphorylation by tyrosine phosphatases.
It has been established that NF-κB transcription factor is contributed to the resistance of pancreatic cancer cells to apoptosis. Lau et al. [22] studied the effect of apoptogenic agent Brucein D on cancer cells. It was found that Brucein D triggered the activation of NADPH oxidase and enhanced the generation of superoxide. Their findings suggested that Brucein D induced apoptosis in pancreatic cancer cells through NADPH oxidase/ROS/p38-MAPK signaling cascade.
Melanoma
Malignant melanoma cells spontaneously generate ROS and promote constitutive activation of the transcription factor nuclear factor NF-κB). Brar et al. [23] found that ROS were produced by NADPH oxidase localized at melanoma plasma membrane. They identified the melanomas p22(phox), gp91(phox), and p67(phox) components of the phagocyte NADPH oxidase and the gp91(phox) homolog of Nox4, while normal human epidermal melanocytes expressed only p22(phox) and Nox4. These findings suggested that the specific NADPH oxidase (the gp91(phox) homolog of Nox4) presented in melanomas which produced ROS together with phagocyte NADPH oxidase that played a role in signaling malignant melanoma growth.
Govindarajan et al. [24] demonstrated that protein kinase Akt stimulated the malignant transformation of radial growth to vertical growth (i.e., noninvasive to invasive melanoma). They suggested that the Akt-depended transformation of melanoma supposedly took place by at least two mechanisms. First, Akt may stabilize ROS-generating cells with extensive mitochondrial DNA mutation. Second, Akt can induce the expression of ROS-generating enzyme Nox4. Akt thus serves as a molecular switch that increases angiogenesis and the generation of superoxide stimulating a more aggressive tumor behavior. Yamaura et al. [25] showed that Nox4 was up-regulated in 13 of 20 melanoma cell lines. Their findings suggested that Nox4-generated ROS were necessary for the transformation of melanoma cells and contribution to melanoma growth through regulation of G2-M cell cycle progression.
Glioma
Human gliomas are aggressive and unresponsive to the treatment supposedly due to their resistance to apoptosis. Shono et al. [26] suggested that the enhanced expression of NADPH oxidase Nox4 might be responsible for proliferation and survival of glioma cells. NADPH oxidase-depended ROS generation can contribute to the resistance of glioma cell to apoptosis through the cascade of antiapoptotic proteins, such as the phosphoinositide-3-kinase (PI3K)/Akt pathway, the mammalian serine/threonine protein kinase target of rapamycin (mTOR), and the transcription factor NF-κB.
It follows that NADPH oxidase-depended ROS production can stimulate the proliferation and survival of glioma cells. However, ROS generation by other pathways might also initiate apoptotic processes in gliomas. Thus Sawada et al. [27] showed that the treatment of human glioma cells with producers of superoxide caused apoptosis in cells expressing transcriptional factor p53. p53 activation was further followed by the formation of superoxide but not hydrogen peroxide or hydroxyl radicals. In subsequent work [28] these authors found that in glioma cells possessing wild-type p53, TNF-α stimulated ceramide (the hydrolyzed product of phospholipid sphingomyelin) formation accompanied by superoxide production, mitochondrial depolarization, and cytochrome c release, whereas p53-deficient cells were partially resistant to TNF-α and lacked superoxide generation. It was concluded that two separate signaling cascades, the p53-mediated superoxide-dependent and -independent pathways, both initiated by caspase-8 activation, resulted in ceramide formation in TNF-α-induced apoptosis of glioma cells.
Breast cancer
Survival of breast cancinoma cells also depended on ROS generation. Lin et al. [29] showed that the interaction between insulin-like growth factor-I (IGF-I) and 17β-estradiol E2 resulted in the proliferation of breast carcinoma cells. Activation of insulin receptor substrate-1 (IRS-1) protein led to the phosphorylation of extracellular signal-related kinases (ERKs) and c-Jun N-terminal kinases (JNKs), but not p38 mitogen-activated protein kinase. An increase in ROS formation was detected in E2/IGF-I-treated cells, and antioxidants NAC and Tiron significantly inhibited E2/IGF-I-induced cell proliferation with blocking phosphorylation of ERKs and JNKs, and the expression of c-Jun protein. Thus E2 and IGF-1 promoted the proliferation of breast carcinoma cells through ROS-dependent MAPK activation and c-Jun protein expression. Choi et al. [30] found that the expression of BLT2, the receptor of leukotriene B4 (LTB4) was significantly upregulated in breast cancer cells. It was proposed that ROS overproduction and expression of Nox1were associated with BLT2-mediated survival effect. These findings indicate that the BLT2-Nox1-ROS signaling cascade is involved in survival of breast cancer cells.
Despite the surviving effects of ROS signaling in breast cancer cells demonstrated above, other ROS-stimulated enzymatic cascades might stimulate apoptosis and cell death. Cao et al. [31] demonstrated that the antibiotic Surfactin inhibited proliferation and induce apoptosis in breast cancer cells. Surfactin initiated ROS formation and the sustained activation of phosphorylated ERK1/2 and JNK, but not p38. It was suggested that Surfactin induced apoptosis of human breast cancer through a ROS/JNK-mediated mitochondrial/caspase pathway.
Rotenone, an inhibitor of the mitochondrial electron transport chain complex I, initiated ROS-dependent apoptosis in breast cancer cells [32]. The treatment of cells with rotenone caused the activation of c-jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases (MAPKs), and the inactivation of extracellular signal-regulated protein kinases 1/2 (ERK1/2). These findings suggested that a ROS/JNK/p38 enzymatic cascade was responsible for apoptosis in breast cancer cells.
Liver cancer
Transforming growth factor-β (TGF-β) is able to induce apoptosis in human hepatocellular carcinoma cells through NADPH oxidase (Nox4)-generated ROS. Caja et al. [33] showed that carcinoma cells can escape from TGF-β proapoptotic effects via the stimulation of the MEK/ERK pathway and the inhibition of Nox4 upregulation, which was required for mitochondria-dependent apoptosis. Huang et al. [34] demonstrated that Oridonin, the diterpenoid isolated from Rabdosia rubescences induced apoptosis in human hepatoma cells by the activation of p53 that initiated ROS formation. ROS-activated p38 kinase in turn enhanced p53-depended ROS generation through a positive feedback loop. It has been proposed that ROS induced apoptosis by classic pathway through the release of cytochrome c from mitochondria and the activation of caspase 9/3. Similarly, synthetic flavonoid LYG-202 induced ROS formation and the downstream signaling pathways including the activation of JNK and p38 MAPKs in the apoptosis of human hepatocellular carcinoma cells [35].
Bladder cancer
Heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF) accumulates in the nucleus in aggressive transitional cell carcinoma (TCC) cells. Kim et al. [36] showed that ROS caused the EGF receptor (EGFR) and Akt1 phosphorylation and mobilization of HB-EGF precursor, proHB-EGF, from the nucleus bladder cancer cells. These findings showed that the nucleus can serve as an intracellular reservoir for a secreted EGFR ligand and contribute to cell proliferation and protection from apoptotic stimuli. Kim et al. [37] found that the leukotriene B(4) receptor BLT2 was overexpressed in advanced malignant bladder cancers (human transitional cell carcinomas (TCCs)). They also showed that BLT2 mediated invasiveness through a signaling pathway dependent on NADPH oxidase-1 and -4 (Nox1/4)-induced generation of ROS and subsequent NF-κB stimulation.
Colon cancer
Park et al. [38] demonstrated the opposite effects of low and high ROS levels on proliferation and apoptosis in colon cancer cells. It was found that ROS (hydrogen peroxide) nontoxic levels stimulated colon cancer cell growth by regulating cyclooxygenase-2 (COX-2), while high ROS concentrations activated apoptosis depending on protein kinase AMPK.
Ovarian cancer
Xia et al. [39] found that high ROS levels were spontaneously produced by ovarian and prostate cancer cells and that ovarian cancer cells had much higher expression of NADPH oxidase Nox4. These authors showed that ROS regulated the hypoxia-inducible factor 1 (HIF-1) and vascular endothelial growth factor (VEGF) expression and induced angiogenesis and tumor growth. Ovarian cancer development probably depended on the activation of ERK1/2 kinase which was inhibited by mitogen-activated protein kinase phosphatase (MKP)-3. Chan et al. [40] suggested that ROS production during ovarian cancer progression may cause the degradation of MKP3, which in turn led to ERK1/2 activation and contributed to tumorigenicity and chemoresistance of human ovarian cancer cells.
Mechanisms of ROS signaling in the survival and death of cancer cells
The data discussed above suggest that the most (probably all) tumors are characterized by ROS overproduction in cancer cells. At the same time the stimulation of ROS formation by external agents and prooxidants results in apoptosis and death of cancer cells. That is a great paradox of ROS signaling in cancer. Now we consider contradictory mechanisms of ROS signaling in cancer cells.
There are different possible origins of ROS contradictory effects in cancer. Two major reactive oxygen species superoxide and hydrogen peroxide participate in ROS signaling. Although both compounds are always formed together (hydrogen peroxide by the dismutation of superoxide), their signaling functions can be different. There are numerous examples of the different effects of O2.− and H2O2 in enzymatic processes [5]. For example Devadas et al. [41] showed that hydrogen peroxide regulated proliferative pathway through ERK phosphorylation while superoxide initiated T cell receptor (TCR)-stimulated activation of apoptosis. Different effects of superoxide and hydrogen peroxide were also demonstrated in cancer cells. Sawada et al. [27] showed that superoxide induced apoptosis in human glioma cells but hydrogen peroxide had no effect. Laurent et al. [42] studied ROS formation in nontransformed and cancer cells. They showed different role of superoxide and hydrogen peroxide in proliferation and death of cancer cells. N-acetylcysteine decreased hydrogen peroxide level and inhibited mitogen-activated protein kinase and normal cell proliferation but increased cancer cell proliferation. In contrast superoxide which formation was inhibited by the SOD mimic initiated cancer cell death.
Mitochondria is a one of ROS major producers in cancer cells. In recent review [43] Schumacker pointed out that mutations in nuclear or mitochondrial genes encoding components of the mitochondrial electron transport chain can stimulate superoxide generation. Petros et al. [44] found that mtDNA mutations which inhibited oxidative phosphorylation in mitochondria increased superoxide formation and contributed to tumorigenicity in prostate cells.
Mitochondria is of course not an only source of reactive oxygen species in cells – another one is NADPH oxidases. It follows from aforementioned data that NADPH oxidase-depended ROS production takes place in prostate [6,7], pancreatic [16,20–22,], melanoma [23], glioma [26], and other cancer cells. Interestingly, two NADPH oxidase isoenzymes Nox1 and Nox4 were found in cancer cells.
There are the other origins of ROS overproduction in cancer cells in addition to mitochondria and NADPH oxidases. It has been shown that hypoxia is an important factor of solid tumor development through the stimulation of mitochondrial ROS (mROS) and activation of hypoxia-inducible transcription factor-1α and nuclear factor-κB (NF-κB). Lluis et al. [45] found that although mROS promoted the hepatoma, neuroblastoma, and colon carcinoma cell survival by NF-κB activation via c-Src, mROS overproduction may stimulate cancer cells death during hypoxia.
Another mechanism involved in the control of death in cancer cells is autophagy (vacuolar lysosomal degradation pathway for organelles and cytoplasmic macromolecules). However, the relations between autophagy and cell death are very complicated because autophagy can be involved in either cell death or survival. It is well established that p53, a tumor suppressor protein inhibits cancer development. Bensaad et al. [46] showed that p53-stimulated TIGAR (the glycolysis and apoptosis regulator) can inhibit ROS and autophagy. Djavaheri-Mergny et al. [47,48] demonstrated the regulation of autophagy and ROS by NF-κB transcription factor. Yuk et al. [49] showed that the cell wall skeleton of Mycobacterium bovis Bacillus Calmette-Guerin (BCG/CWS) might stimulate autophagy and cell death through ROS generation in colon cancer cells.
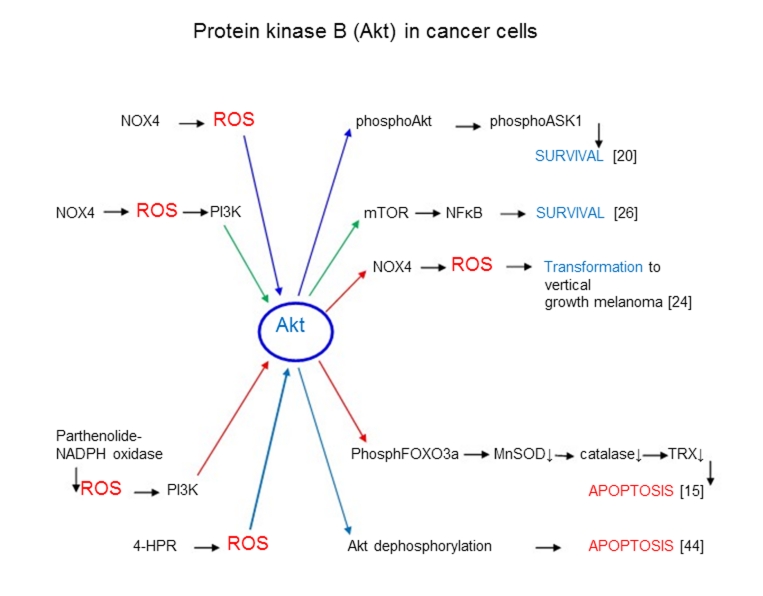
What is an origin of different ROS effects in cancer cells? Is it possible that the different enzymatic cascades initiated by ROS lead to surviving or death of cancer cells? (Major ROS signaling enzymatic cascades are presented in Figure 1–3). It is known that Akt (protein kinase B) is an enzyme which is many cases (but not always) catalyzes cellular surviving pathways. It is seen from Figure 1 that Nox4-depended ROS production activated Akt-catalyzed survival processes in cancer cells through ASK1[20] and NF-κB [26] and stimulated vertical growth melanoma [24]. On the other hand in some cases Akt is apparently able to catalyze apoptotic enzymatic processes in cancer cells by signaling mechanisms distinct from survival ones. For example Sun et al. [15] showed that parthenolide can increase ROS up to toxic levels in cancer cells and stimulate an Akt-depended cascade leading to the inhibition of MnSOD, catalase, reduced thioredoxin, and the stimulation of apoptosis (Figure 1). It is possible that Akt-stimulated apoptosis occurs in enzymatic processes where normal pathways are changed due to the interference or the addition of some additional factors, for example high (toxic) levels of ROS [15]. Cao et al. [50] have studied the mechanism of inhibition of tumor carcinogenesis by the synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR). It was found that ROS were involved in 4-HPR-mediated apoptosis. It was suggested that 4-HPR-mediated ROS induced Akt conformational change by forming an intramolecular disulfide bond that resulted in the dissociation of Akt-Hsp90 complex and enhanced Akt-protein phosphatase (PP2A) interaction. These findings collectively suggest that 4-HPR-induced apoptosis is associated with a ROS-mediated conformational change in Akt that mediated Akt dephosphorylation through the change of Akt-Hsp90 or Akt-PP2A complex formation.
Figure 1.
ROS signaling in processes catalyzed by protein kinase B (Akt) leading to survival or apoptosis of cancer cells.
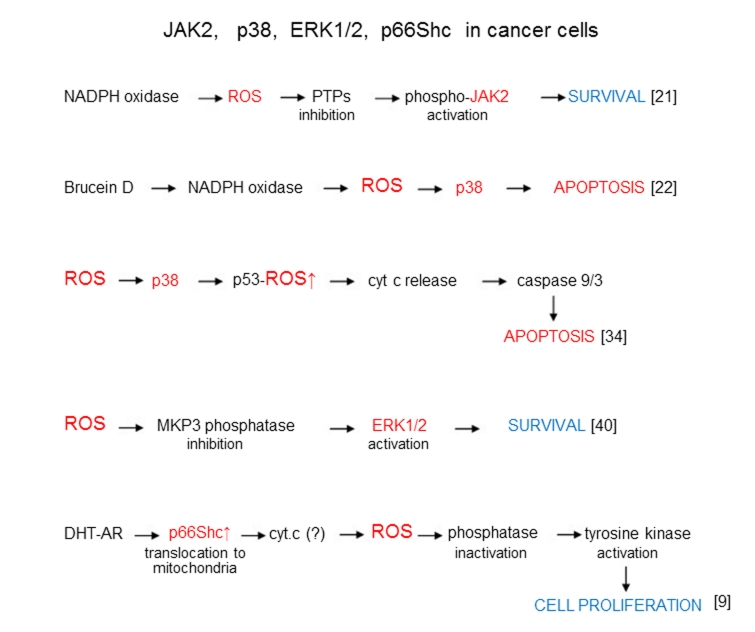
Figure 3.
ROS signaling in survival, apoptosis, and proliferation processes catalyzed by JAK2 p38, ERK1/2, and p66Shc in cancer cells.
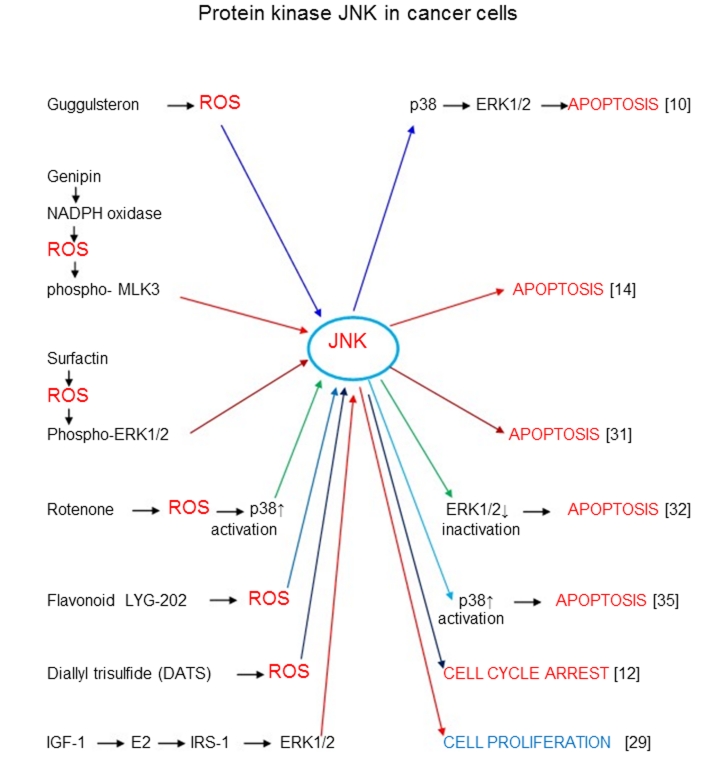
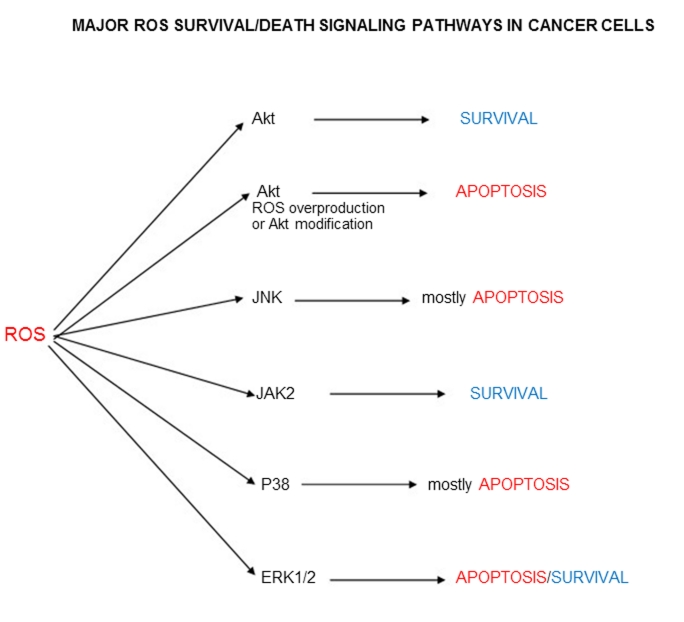
When ROS/Akt signaling generally induces survival pathways in cancer cells (with the exception of some modified pathways), the other enzymes participating in ROS signaling apparently catalyze both the survival and death mechanisms. Thus c-Jun amino-terminal kinase JNK mainly catalyzes apoptosis (Figure 2). At the same time ROS-depended stimulation of breast carcinoma cells through 17beta-estradiol (E2) and insulin-like growth factor-I (IGF-I) pathway led to the proliferation of these cells [29]. Janus kinase Jak2, MAPK kinase p38, and extracellular signal-regulated kinases 1/2 ERK1/2 participate in ROS signaling mechanisms of both survival and death cellular pathways (Figure 3). Unusual effect of p66Shc protein inducing cancer cell proliferation has already been discussed. All major ROS signaling pathways are summarized in Figure 4.
Figure 2.
ROS signaling in processes catalyzed by protein kinase JNK leading to apoptosis, cell cycle arrest, or proliferation of cancer cells.
Figure 4.
Major ROS survival/death signaling pathways in cancer cells.
Surprisingly it was found that the interaction between the sources of ROS can change the pro- and anti-surviving activity of ROS in cancer cells. Desouki et al. [51] found that NADPH oxidase (Nox1) localized in mitochondria was highly expressed in breast (86%) and ovarian (71%) tumors. They suggested that mitochondria controlled Nox1-superoxide generation and that the loss of control of this signaling process contributed to tumorigenesis. Ma et al. [52] found that Rac1 induced NADPH oxidase-dependent superoxide signaling in the development of Kaposi’s sarcoma. Weinberg et al. [53] proposed that mitochondrial ROS generation required for Kras-induced anchorage-independent growth through regulation of the ERK MAPK signaling pathway in lung cancer.
ROS overproduction in cancer cells and comparison with the ROS effects in aging
What is an origin of survival of cancer cells existing under the conditions of enhanced oxidative stress (ROS overproduction)? Could ROS overproduction in cancer cells be a one of major reasons of cancer development and resistance to anticancer treatment? And what is a cause of difference between the positive effects of ROS overproduction in cancer cells and its negative effects during aging development? At present these questions have probably no convincing answers but some cautious proposal might be made.
Aforementioned data suggest that the positive versus negative effects of oxidative stress might depend on the ROS levels: small ROS levels stimulate survival of cells and high ROS levels might result in apoptosis. Such paradoxical effects are not unknown in free radical studies. For example it has been suggested that the increased ROS formation within aged mitochondria causes an adaptive response to increased stress resistance. This type of retrograde response was named mitochondrial hormesis or mitohormesis [54]. Similarly we can suggest that a moderate increase in ROS formation facilitates the transformation of a normal into cancer cell. Such cellular transformation under the conditions of moderate oxidative stress might be realized through the well known induction of antioxidative MnSOD and CuZnSOD enzymes by ROS. Thus one might imagine that small ROS levels stimulate “preconditioning” cancer cells. Similarly Droge [55] proposed that in diabetes mellitus oxidative stress is double-edged phenomenon: the moderate ROS overproduction can enhance insulin signaling while strong ROS overproduction can be destructive. This might be well explained by the different ROS effects on protein tyrosine phosphatases PTPs and the insulin receptor IRS-1. Whereas the oxidation of sulfhydryl groups of PTPs by ROS stimulated the activation of insulin receptor protein tyrosine kinase PTK and enhances insulin signaling, ROS phosphorylation of Ser-307-IRS-1resulted in the inhibition of IRS-1.
Conclusions
Moderate oxidative stress might stimulated proliferation and survival of cancer sells through some kind of conditioning while the enhancement of ROS overproduction by prooxidants under conditions of severe oxidative stress results in apoptosis and cell death. Aging development is always characterized by harmful ROS overproduction although moderate increase in ROS formation in senescent cells might be not dangerous. Similar double-edged sword effects of ROS are observed during the development of other pathologies for example diabetes mellitus.
References
- [1].Afanas’ev I. Superoxide and nitric oxide in senescence and aging. Front Biosci. 2009 Jan 1;14:3899–3912. doi: 10.2741/3499. [DOI] [PubMed] [Google Scholar]
- [2].Afanas’ev I. Reactive oxygen species and age-related genes p66Shc, Sirtuin, Fox03 and Klotho in senescence. Oxid Med Cell Longevity. 2010;3:1–9. doi: 10.4161/oxim.3.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Afanas’ev I. Signaling and damaging functions of free radicals in aging—free radical theory, hormesis, and TOR. Aging and Disease. 2010;1:75–88. [PMC free article] [PubMed] [Google Scholar]
- [4].Saprin AN, Klochko EV, Chibrikin VM, Krugliakova KE, Emanuel’ NM. Kinetic regularities of changes in free radical content during malignant growth and action of inhibitors of free radical processes. Biofizika. 1966;11:443–52. (in Russian). [PubMed] [Google Scholar]
- [5].Afanas’ev IB. Signaling Mechanisms of Oxygen and Nitrogen Free Radicals. CRC Press, Taylor & Francis Group; Boca Raton, Florida: 2009. pp. 1–205. [Google Scholar]
- [6].Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- [7].Kumar B, Koul S, Khandrika L, Measchan RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–85. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- [8].Arnold RS, He J, Remo A, Ritsick D, Yin-Goen Q, Lambeth JD, Datta MW, Young AN, Petros JA. Nox1 expression determines cellular reactive oxygen and modulates c-fos-induced growth factor, interleukin-8, and Cav-1. Am J Pathol. 2007;171:2021–32. doi: 10.2353/ajpath.2007.061144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Veeramani S, Yuan TC, Lin FF, Lin MF. Mitochondrial redox signaling by p66Shc in involved in regulating androgenic growth stimulation of human prostate cancer cell. Oncogene. 2008;27:5057–68. doi: 10.1038/onc.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Singh SV, Choi S, Zeng Y, Haim ER, Xiao D. Guggulsterone-induced apoptosis in human prostate cancer cells is caused by reactive oxygen intermediate dependent activation of c-Jun NH2-terminal kinase. Cancer Res. 2007;67:7439–49. doi: 10.1158/0008-5472.CAN-07-0120. [DOI] [PubMed] [Google Scholar]
- [11].Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med. 2008;44:1833–45. doi: 10.1016/j.freeradbiomed.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Antosiewicz J, Herman-Antociewicz A, Marynowski SW, Singh SV. c-Jun NH2-terminal kinase signaling axis regulates diallyl trisulfide-induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res. 2006;66:5379–86. doi: 10.1158/0008-5472.CAN-06-0356. [DOI] [PubMed] [Google Scholar]
- [13].Zhao R, Domann FE, Zhong W. Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells. Mol Cancer Ther. 2006;5:3275–84. doi: 10.1158/1535-7163.MCT-06-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hong HY, Kim BC. Mixed lineage kinase 3 connects reactive oxygen species to c-Jun NH2-terminal kinase-induced mitochondrial apoptosis in genipin-treated PC3 human prostate cancer cells. Biochem Biophys Res Commun. 2007;362:307–312. doi: 10.1016/j.bbrc.2007.07.165. [DOI] [PubMed] [Google Scholar]
- [15].Sun Y, St Clair DK, Xu Y, Crook PA, St Clair WH. A NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res. 2010;70:2880–90. doi: 10.1158/0008-5472.CAN-09-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–54. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- [17].Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, Oberley LW. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res. 2003;63:1297–303. [PubMed] [Google Scholar]
- [18].Weydert C, Roling B, Liu J, Hinkhouse MM, Ritchie JM, Oberley LW, Cullen JJ. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther. 2003;2:361–69. [PubMed] [Google Scholar]
- [19].Teoh ML, Sun W, Smith BJ, Oberley LW, Cullen JJ. Modulation of reactive oxygen species in pancreatic cancer. Clin Cancer Res. 2007;13:7441–50. doi: 10.1158/1078-0432.CCR-07-0851. [DOI] [PubMed] [Google Scholar]
- [20].Mochizuki N, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayagai J, Konagai A, Hirose K, Kiyosawa K, Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- [21].Lee JK, Edderkaoui M, Truong P, Ohno I, Jang KT, Berti A, Pandol SJ, Gukovsaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–48. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- [22].Lau ST, Lin ZX, Leung PS. Role of reactive oxygen species in brucein D-mediated p38-mitogen-activated protein kinase and nuclear factor-kappa B signaling pathweys in human pancreatic adenocarcinoma cells. Br J Cancer. 2010;102:583–93. doi: 10.1038/sj.bjc.6605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:1212–24. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- [24].Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L, Zhang Y, Slingerland J, Arnold RS, Lambeth JD, Cohen C, Hilenski L, Griendling K, Martínez-Diez M, Cuezva JM, Arbiser JL. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yamaura M, Mitsushita J, Furuta S, Kiniwa Y, Ashida A, Goto Y, Shang WH, Kubodera M, Kato M, Takata M, Saida T, Kamata T. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–2654. doi: 10.1158/0008-5472.CAN-08-3745. [DOI] [PubMed] [Google Scholar]
- [26].Shono T, Yokoyama N, Uesaka T, Kuroda J, Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, Miyamoto K, Akashi K, Iwaki T, Sumimoto H, Sasaki T. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int J Cancer. 2008;123:787–92. doi: 10.1002/ijc.23569. [DOI] [PubMed] [Google Scholar]
- [27].Sawada M, Nakashima S, Kiyono T, Nakagawa M, Yamada J, Yamakawa H, Banno Y, Shinoda J, Nishimura Y, Nozawa Y, Sakai Y. p53 regulates ceramide formation by neutral sphingomyelinase through reactive oxygen species in human glioma cells. Oncogene. 2001;20:1368–78. doi: 10.1038/sj.onc.1204207. [DOI] [PubMed] [Google Scholar]
- [28].Sawada M, Kiyono T, Nakashima S, Shinoda J, Naganawa T, Hara S, Iwama T, Sakai N. Molecular mechanisms of TNF-a-induced ceramide formation in human glioma cells: P53-mediated oxidant stress-dependent and -independent pathways. Cell Death Differ. 2004;11:997–1008. doi: 10.1038/sj.cdd.4401438. [DOI] [PubMed] [Google Scholar]
- [29].Lin CW, Yang LY, Shen SC, Shen YC. IGF-I plus induces proliferation via activation of ROS-dependent ERKs and JNKs in human breast carcinoma cell. J Cell Physiol. 2007;212:666–74. doi: 10.1002/jcp.21061. [DOI] [PubMed] [Google Scholar]
- [30].Choi JA, Lee JW, Kim H, Kim EY, Seo JM, Ko J, Kim JH. Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2-reactive oxygen species-linked signaling pathway. Carcinogenesis. 2010;31:543–51. doi: 10.1093/carcin/bgp203. [DOI] [PubMed] [Google Scholar]
- [31].Cao XH, Wang AH, Wang CL, Mao DZ, Lu MF, Cui YQ, Jiao RZ. Surfactin induces apoptosis in human breast cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase pathway. Chem Biol Interact. 2010;183:357–62. doi: 10.1016/j.cbi.2009.11.027. [DOI] [PubMed] [Google Scholar]
- [32].Deng YT, Huang HC, Lin JK. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol Carcinog. 2010;49:141–51. doi: 10.1002/mc.20583. [DOI] [PubMed] [Google Scholar]
- [33].Caja L, Sancho P, Bertran E, Iglesias-Serret D, Gil J, Fabregat I. Overactivation of the MEK/ERK pathway in liver tumor cells confers resistance to TGF-{beta}-induced cell death through impairing up-regulation of the NADPH oxidase NOX4. Cancer Res. 2009;69:7595–602. doi: 10.1158/0008-5472.CAN-09-1482. [DOI] [PubMed] [Google Scholar]
- [34].Huang J, Wu L, Tashiro S, Onodera S, Ikejima T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J Pharmacol Sci. 2008;107:370–9. doi: 10.1254/jphs.08044fp. [DOI] [PubMed] [Google Scholar]
- [35].Chen FH, Zhang LB, Qiang L, Yang Z, Wu T, Zou MJ, Tao L, You QD, Li ZY, Yang Y, Guo QL. Reactive oxygen species-mitochondria pathway involved in LYG-202-induced apoptosis in human hepatocellular carcinoma HepG(2) cells. Cancer Lett. 2010;296:96–105. doi: 10.1016/j.canlet.2010.04.004. [DOI] [PubMed] [Google Scholar]
- [36].Kim J, Adam RM, Freeman MR. Trafficking of nuclear heparin-binding epidermal growth factor–like growth factor into an epidermal growth factor receptor–dependent autocrine loop in response to oxidative stress. Cancer Res. 2005;65:8242–9. doi: 10.1158/0008-5472.CAN-05-0942. [DOI] [PubMed] [Google Scholar]
- [37].Kim EY, Seo JM, Kim C, Lee JE, Lee KM, Kim JH. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radic Biol Med. 2010;49:1072–81. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- [38].Park IJ, Hwang JT, Kim YM, Ha J, Park OJ. Differential modulation of AMPK signaling pathways by low or high levels of exogenous reactive oxygen species in colon cancer cells. Ann N Y Acad Sci. 2006;1091:102–9. doi: 10.1196/annals.1378.059. [DOI] [PubMed] [Google Scholar]
- [39].Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–30. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- [40].Chan DW, Liu VW, Tsao GS, Yao KM, Furukawa T, Chan KK, Ngan HY. Loss of MK3 mediated by oxidative stress enhances tumorigenicity and chemiluminescence of ovarian cancer cells. Carcinogenesis. 2008;29:1742–50. doi: 10.1093/carcin/bgn167. [DOI] [PubMed] [Google Scholar]
- [41].Devadas S, Zaritskaya L, Rhee SG, Oberlay L, Williams MS. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: Selective regulation of mitogen-activated protein kinase activation and Fas ligand expression. J Exp Med. 2002;195:59–70. doi: 10.1084/jem.20010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, Levy E, Goldwasser F, Panis Y, Soubrane O, Weill B, Batteux F. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–56. [PubMed] [Google Scholar]
- [43].Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–6. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- [44].Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, Marshall FF, Wallace DC. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci USA. 2005;102:719–24. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-κB via c-SRC– and oxidant-dependent dell death. Cancer Res. 2007;67:7368–77. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- [46].Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28:3015–26. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281(41):30373–82. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- [48].Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Codogno P. Regulation of autophagy by NFkappaB transcription factor and reactive oxygen species. Autophagy. 2007;3:390–2. doi: 10.4161/auto.4248. [DOI] [PubMed] [Google Scholar]
- [49].Yuk JM, Shin DM, Song KS, Lim K, Kim KH, Lee SH, Kim JM, Lee JS, Paik TH, Kim JS, Jo EK. Bacillus calmette-guerin cell wall cytoskeleton enhances colon cancer radiosensitivity through autophagy. Autophagy. 2010;6:1–15. doi: 10.4161/auto.6.1.10325. [DOI] [PubMed] [Google Scholar]
- [50].Cao J, Xu D, Wang D, Wu R, Zhang L, Zhu H, He Q, Yang B. ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radic Biol Med. 2009;47(5):536–47. doi: 10.1016/j.freeradbiomed.2009.05.024. [DOI] [PubMed] [Google Scholar]
- [51].Desouki MM, Kulawiec M, Bansal S, Das GM, Singh KK. Cross talk between mitochondria and superoxide generating NADPH Oxidase in breast and ovarian tumors. Cancer Biol Ther. 2005;4:1367–73. doi: 10.4161/cbt.4.12.2233. [DOI] [PubMed] [Google Scholar]
- [52].Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, Wang H, Hale LP, Dong C, Cesarman E, Mesri EA, Goldschmidt-Clermonta PJ. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi’s sarcoma. Proc Natl Acad Sci USA. 2009;106:8683–8. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Scott Budinger GR, Chandela NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA. 2010;107:8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitochormesis) Exp Gerontol. 2010;45:410–8. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [55].Droge W. Oxidative aging and insulin receptor signaling. J Gerontol A Biol Sci Med Sci 1. 2005;60:1378–85. doi: 10.1093/gerona/60.11.1378. [DOI] [PubMed] [Google Scholar]