Abstract
Immune aging is associated with loss of critical immune functions, such as host protection from infection and malignancy. Unexpectedly, immunosenescence also renders the host susceptible to inflammation, which may translate into tissue-damaging disease as the senescent immune system loses its ability to maximize inflammatory protection while minimizing inflammatory injury. On the other hand, chronic inflammation associated with immune-mediated disease represents a profound stress factor for the immune system, affecting cellular turn-over, replication and exhaustion. Immune cell longevity is tightly connected to the functional integrity of telomeres which are regulated by cell multiplication, exposure to oxidative stress and DNA repair mechanisms. Lymphocytes are amongst the few cell types that can actively elongate telomeres through the action of telomerase. In patients with the autoimmune disease rheumatoid arthritis (RA), telomerase deficiency is associated with prematurity of immune aging. Patients with RA have other defects in DNA repair mechanisms, including the kinase Ataxia telangiectasia mutated (ATM), critically involved in the repair of DNA double strand breaks. ATM deficiency in RA shortens lymphocyte survival. Dynamics of telomeric length and structure are beginning to be understood and have distinct patterns in different autoimmune diseases, suggesting a multitude of molecular mechanisms defining the interface between chronic immune stimulation and progressive aging of the immune system.
Keywords: Telomere, Telomere Dysfunction, Autoimmunity, Rheumatoid arthritis, Lupus, Shelterin, Telomerase, Diabetes, Sarcoidosis
Aging can be defined as the progressive decline of tissue function that eventually results in mortality [1]. Aging is a natural occurring process and not a disease state. While aging is inevitable for all humans, the speed of age-related functional deterioration varies considerably amongst individuals. Why some individuals reach frailty earlier than others is not understood and genetic factors as well as environmental exposures are believed to modulate the senescence process. Emerging data suggest that certain autoimmune diseases are associated with prematurity of aging, suggesting that immune homeostasis and aging are tightly interlinked.
Currently, aging is viewed as a generalized process occurring in all organ systems in a parallel fashion [1]. However, recent studies point towards a much higher degree of complexity in the aging process and accumulating data support the notion that diseases (including infectious exposure) incurred during life may accelerate the aging of certain organ systems. Aging of the immune system, often described by the term immunosenescence, has attracted particular attention as the loss of immune function in the aging host is associated with conditions that limit life span, such as, infectious susceptibility and malignancy [2]. Equally important, age-related failure of immune function not only leads to loss of function but also to hyperactivity which clinically manifests as a state of chronic subclinical inflammation. Recent investigative efforts have focused on delineating the relationship between inflammaging and inflammatory disease. Generally, senescence-associated inflammation has been connected to a 2-4-fold increase in the levels of acute-phase reactants, such as C-reactive protein and interleukin-6 [3, 4]. Increased inflammatory activity is now recognized as a hallmark of a number of age-related diseases, such as atherosclerosis and insulin resistance [5, 6]. The connection between inflammation and aging is of particular interest in individuals with autoimmune diseases, conditions typified by chronic smoldering inflammation which affect individuals early in life.
This review will summarize the current knowledge of the interrelationship between immune-mediated disease, immune aging and acceleration of immunosenescence in patients with autoimmunity. Attempts to quantify such complex processes have focused attention on telomeres, structures that function as the cells’ internal clock. Lengths of telomeric repeats are now widely accepted as indices of cellular aging and here we will review recent insights into telomere biology as a molecular approach to aging and autoimmunity.
Telomeres and Aging
Telomeres are the natural ends of linear chromosomes, and function to cap chromosomal ends to prevent them from being recognized as DNA double strand breaks [7]. In human cells, telomeric DNA is composed of G-rich repetitive sequences with the G-rich strand ending in a 3′ single stranded DNA overhang to overcome the “end replication problem” [8]. To protect telomeres from repair activity and fusion, the telomeric region is covered by a complex of specialized proteins, often referred to as the shelterin complex [9]. In addition, proteins involved in repairing DNA double strand breaks contribute to protecting, stabilizing and maintaining telomeres [10]. Finally, the enzyme telomerase can elongate telomeric sequences (Figure 1A). In most somatic cells telomerase is produced at very low levels. In contrast, many malignant cells are able to upregulate this enzyme and extend their survival through continuous telomeric elongation [11]. In essence, in healthy cells maintaining telomeric intactness and securing cellular longevity depends on three molecular processes; telomeric capping by shelterin proteins, sequence elongation by telomerase and repair of strand lesions by DNA repair pathways.
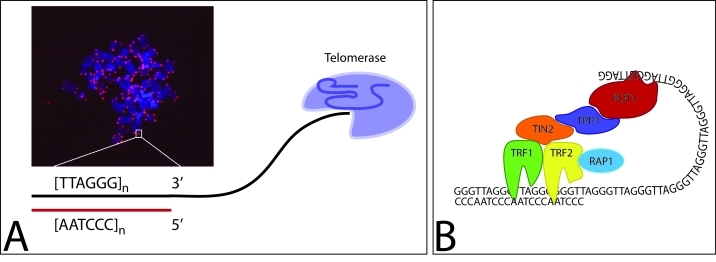
Figure 1. Telomeres and Telomeric Protection.
A) Telomere staining of a CD4 T-cell metaphase spread with telomeres stained in red (PNA probe) and DNA stained in blue (DAPI). Telomeres end in a 3′ overhang that is elongated by telomerase. B) Telomeric ends are protected by a protein complex termed shelterin. The shelterin members TRF1 and TRF2 bind to the double stranded portion of the telomere, POT1 binds to the single stranded part. The three proteins are interconnected by TPP1 and TIN2. RAP1 binds only to TRF2 and modulates its function.
Cells use telomeric ends to monitor their biologic age. Critically short telomeres force the cell either into senescence or apoptotic death. Tissues under permanent replicative stress (e.g. progenitor cells) and organ systems endowed with the ability to build memory (e.g. the immune system) are particularly dependent on cellular survival. They assign a major proportion of cellular energy to the surveillance and repair of DNA, including the telomeric DNA [12]. The average telomere length of peripheral hematopoietic cells appears to be a heritable trait [1]. Stem and progenitor cells are believed to be able to maintain their telomere length whereas somatic cells progressively lose telomeric sequences, a process that ultimately limits their life span [13]. In the hematopoietic system, the early years of life are associated with a high rate of telomeric loss, likely a reflection of the enormous proliferative need generated by the rapidly expanding body size. In donors younger than 10 years, 270–1000 telomeric base pairs are lost in peripheral blood cells each year, during adulthood the rate slows down to 20–60 base pairs [14, 15]. Telomeric attrition is likely not a linear process over lifetime. Studies in CD4 T-cells have demonstrated that adults aged 40–65 years enter a phase of accelerated telomeric shortening [16]; possibly caused by the discontinuation of T cell generation in the failing thymus.
Telomeric length is now recognized as an index of cellular aging and over the last few years measuring of telomeres in peripheral mononuclear cells or in whole blood samples has been applied to patients with a multitude of disease states. Much attention has been given to individuals with atherosclerotic disease and, as a common rule, telomeric length of PBMCs seems to be a strong predictor for disease progression. Patients with myocardial infarction have shorter telomeres compared to controls [17]. Leukocyte telomere length is associated with measures of subclinical atherosclerosis [18] and with HDL cholesterol levels [19]. Cellular aging reflected by shorter leukocyte telomere length is a predictor for advanced atherosclerosis and cardiovascular disease risk [20]. Furthermore, reduced leukocyte telomere length has been linked to increased coronary artery calcium [21] and reduction in left ventricular mass [22]. However, premature loss of telomeres extends beyond blood vessel disease and emerges as a marker of overall functionality. Specifically, telomere length has been connected to cancer incidence and cancer mortality [23].
While the mechanisms underlying excessive erosion of telomeres remain insufficiently understood, there is beginning evidence that environmental stressors play a significant role in shaping the immune aging process. Specifically, virus infection seems to be a contributor to immune cell aging and telomere loss. Epstein-Barr virus infection has been described to be associated with telomeric loss [24]. Another herpes virus, Cytomegalovirus, may have a particular role in driving immune aging. Like other herpes virus infections CMV is characterized by states of latency and reactivation. Cytomegalovirus infection reduces telomere length in the circulating T-cell pool [25] and imposes a signature of immune aging. Chronic hepatitis C virus infection also seems to impact telomeres in immune cells. CD4+ telomere length was reported to correlate with fibrosis stage, clinical outcome and treatment response [26].
The current paradigm holds that chronic infection accelerates erosions of T cell telomeres through increased proliferative stress. In that model, chronic antigenic stimulation imposes constant demand on immune cell production, a demand which involves cellular replication. In a host old enough to lack thymic T cell regeneration this process should eventually use up the telomeric reserve. Whether this is indeed the mechanism that leads to telomere loss in humans remains speculative.
Telomere Protection and Elongation
While replication-dependent telomere loss is one possible mechanism underlying the shortening of telomeric sequences during healthy aging and during chronic inflammatory disease, alternative explanations need to be considered. Specifically, it is possible that structural changes of the telomeric ends results from aberrations in the machinery that maintain the 3-D configuration of telomeres. Chromosomal ends mimic double strand breaks, the most fatal DNA damage lesions and induce repair activity unless such repair activity is specifically suppressed [9, 27]. To protect the telomeric region from constant repair attempts, the telomeric ends are directly covered by specialized telomeric proteins forming a protein complex termed shelterin [9] (Table 1, Figure 1B). The double strand region is bound by TRF1 and TRF2. TRF1 plays a central role in telomere protection; its overexpression is associated with gradual telomere shortening while its removal results in telomere elongation [28]. If TRF2 is compromised, telomeres elicit a robust DNA damage signal. The telomere damage generated by TRF2 loss activates ATM and initiates the well known consequences for the cell [29]. The 3′ overhang of the telomere is bound by POT1 resulting in the characteristic T loop [9]. In contrast to TRF2 deletion, deletion of POT1 activates the ATR pathway [29]. POT1 might act in concert with the WRN 3′ > 5′ helicase on the replicative single G strand [30]. Two proteins function as linker molecules between TRF1, TRF2 and POT1. TIN2 binds to TRF1 and TRF2 and to TPP1 which in turn connects to POT1. The remaining protein RAP1 binds to TRF2 and protects the telomere from unwanted homologous repair pathway activation.
Table 1.
Function of shelterin complex proteins
| Shelterin Complex Molecule | Function |
|---|---|
| TRF1 Telomeric repeat binding factor 1 | Facilitating replication of telomeric DNA Negative regulator of telomeric length Docking protein for non-shelterin proteins (including PINX1, ATM, BLM, DNA-PKcs, Tankyrasel/2) Prevention of telomere replication mistakes Protection of telomeres against fragile telomere phenotype |
| TRF2 Telomeric repeat binding factor 2 | Stabilizing telomeric secondary structure Docking protein for non-shelterin proteins (Including ATM, MRN complex, WRN, BLM, Ku86, ERCC1/XPF, PARP1/2) Inhibition of NHFJ at telomeres Inhibition of HR at telomeres Inhibition of ATM function at telomeres Inhibition of end-to-end chromosome fusion |
| POT1 Protection of Telomere 1 | Regulation of telomeric length Protection of telomeric end zone Regulation of C-strand resection |
| TPP1 | Telomere length maintenance via interaction with telomerase |
| TIN2 TRF1-interacting nuclear protein 2 | Crucial for the stability of the entire shelterin complex Role in anchoring telomeres to the nuclear matrix |
| RAP1 also TRF2 interacting protein | Telomere length regulation Inhibition of end-to-end chromosome fusion Inhibition of HR |
So far, limited data are available about functional consequences of mutations or deregulations of shelterin complex components. TIN2 mutations have been reported for patients with a clinical phenotype of dyskeratosis congenita, a progeroid syndrome characterized by severe aplastic anemia [31]. In a recent report Auguerau and colleagues described shelterin dysregulation in lymphocytic leukemia, expanding the potential role of shelterin proteins in human disease [32]. Considering the central role that telomerase plays in determining the lengths of telomeric sequences the functional activity of this enzyme needs to be assessed whenever telomeres undergo shortening. Critically short telomeres are not compatible with normal cellular function and the cell has to either initiate the senescence program or undergo apoptosis. Escape from these mechanisms enables the cell to proliferate without limitation and is a typical feature of malignancy. Thus, telomerase is often considered a marker of carcinogenesis. However, in few somatic cells, including lymphocytes and stem cells, telomerase actively participates in securing telomeric lengthening and may therefore hold a critical position in the process of immune aging.
To function in telomeric extension telomerase is composed of the telomerase reverse transcriptase enzyme (TERT) which adds telomere (TTAGGG) repeats to the chromosome ends and the telomerase RNA component (TERC), which acts as the template for TERT [12]. The catalytic unit of telomerase contains two copies each of TERT, TERC and dyskerin.
Unequivocal evidence that telomeric maintenance is critically involved in organ function has come from the recognition that mutations in telomerase, TERC or dyskerin have severe consequences for humans. The most severe disease associated with dysfunctional telomere maintenance is dyskeratosis congenita [33]. Family studies have been instrumental in pinpointing mutations in dyskerin. All patients with dyskeratosis congenita have very short telomeres [34]. Patients with the shortest telomeres tend to have the most severe disease [33]. Genetic aberrations in the components of the telomerase complex extend to other diseases than dyskeratosis congenita. Mutations of telomerase or TERC have been discovered in patients with aplastic anemia [35] and telomerase mutations were found in 15% of patients with familial idiopathic pulmonary fibrosis [36]. Interestingly, many more patients with idiopathic pulmonary fibrosis have short telomeres without detectable telomerase mutations [37].
Lymphocytes have a unique requirement for clonal expansion that necessitates robust proliferative capacity. Therefore, immune cells are capable of upregulating telomerase to prevent massive telomere shortening. Telomerase activity is readily detectable after 3 days of in vitro stimulation and is markedly higher in naïve T-cells than in memory T-cells. Interestingly, telomerase might have additional roles in the cell besides its canonical action in adding telomeric sequences. Knockdown of hTERT in naïve CD4 T-cells increases apoptotic susceptibility and restricts clonal expansion during the priming response without significant loss of telomeric length [38].
Cellular Senescence
Cells monitor their individual age by “measuring” the length and structure of the telomeric ends and respond to the gradual loss of telomeric repeats by entering the senescence program. This biologic principle was first described in the 1960s when Hayflick recognized that normal human cells had a limited life span in culture and cannot divide eternally [39]. The limited growth of human cells in culture is partially due to telomere erosion but additional genomic, structural and metabolic changes are equally important in signaling to the cell which stage of its life cycle it has reached. All non-malignant human cells beside stem/progenitor cells and cells of the immune system are incapable of maintaining the telomeric length during proliferation and cell division. Even immune cells, equipped with the ability to upregulate telomeric lengthening mechanisms, eventually enter senescence. Whether there is not enough telomerase to meet the demand of telomere elongation during proliferation of immune cells or whether telomerase itself is regulated in an age-dependent fashion warrants further studies.
Cellular senescence involves slowing and then arrest of cellular division. The senescence growth arrest is essentially permanent. Cells that display a senescent phenotype usually increase in size and express a senescence associated gene signature including induction of beta-galactosidase and p16INK4a [40]. Some cells induce a senescence program due to persistent DNA damage response (DDR) signaling with chromatin alterations reinforcing senescence [40]. However, senescent cells do not stop to be metabolically active and display an array of activities that have profound consequences for the tissue microenvironment and the entire organism. Specifically, senescent cells are actively involved in shaping the cytokine and extracellular matrix environment they are in. The best studied cell type so far is the fibroblast. Senescent fibroblasts were described to express amphiregulin, GRO alpha, IL-6, IL-8, VEGF and various MMPs [41–44].
T-cell Senescence
Senescence is of particular relevance for cells that are long-lived as is the case for T lymphocytes, the carriers of immune memory. T cells are known to be able to live over decades. Nevertheless, they are somatic cells and are not immortal. As humans progress in life they accumulate specific subsets of T-cells that are phenotypically and functionally distinct from those present in the young. Aging T cells have reduced telomeric length and are characterized by the absence of the co-stimulatory receptor CD28 [45]. At birth, virtually all human T-cells express CD28. In the elderly, the expression levels of CD28 drop to around 85%–90% for peripheral CD4 T-cells and 40%–50% for peripheral CD8 T-cells [46, 47]. CD28 is a critical player in T cell regulation and the loss of this co-stimulatory molecule must profoundly reshape adaptive immunity.
The main cause of CD28 loss in T-cells is attributed to repeated antigenic stimulation [45]. In vitro studies have described an almost total loss of CD28+ CD8 cells after subsequent stimulation [48]. This activation induced loss model is further supported by shortened telomeres in CD28− cells compared to the respective CD28+ counterpart [45]. The loss of CD28 is permanent, suggesting transcriptional silencing. The loss of CD28 expression correlates with changes of nuclear protein binding activities to two motifs, site alpha and beta within the CD28 minimal promoter. Complexes binding to the two motifs, expressed only in lymphoid tissue, are coordinately expressed except during replicative senescence. The complete loss of function in both motifs is correlated with a CD28 null phenotype of both CD4+ and CD8+ T cells in vivo [49]. Interestingly, the CD28 gene transcriptional initiator is negatively regulated by TNF-α, a cytokine typically overexpressed during chronic inflammatory disease [50]. Loss of CD28 appears to be part of a broader program of T cell reprogramming. As CD28 is lost, such T cells acquire a number of novel receptors, such as killer-cell immunoglobulin-like receptors (KIR), leukocyte function receptor 1, CD70 and perforin [45]. Whether this is a consequence of epigenetic mechanisms is currently under investigation. Of note, in CD8 cells of the elderly the KIR2DL3 promoter gets partially demethylated rendering CD8 cells more likely to express KIR [51]. Such epigenetic changes may underlie the expansion of CD28− T cells in patients with autoimmune disease implicating environmental and metabolic factors in the immune aging process.
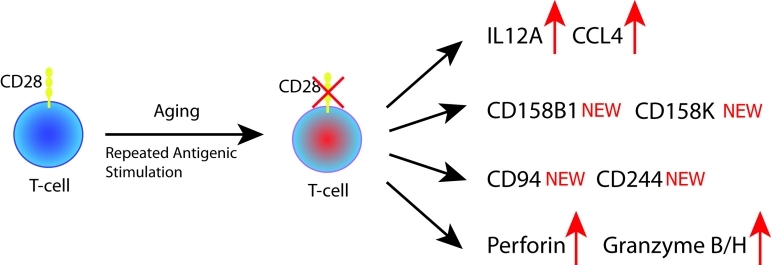
CD28− T-cells have a distinct gene expression profile, including genes generally associated with proinflammatory activities (Figure 2). Aged T cells may thus have a critical role as inflammatory amplificators and represent a major mediator of the aging-associated inflammatory syndromes. They produce IFNγ in higher amounts than their CD28 counterparts [52]. A cardinal feature of CD28− T cells is the expression of receptor molecules that are usually reserved for NK cells, including CD158A, CD158B, CD158J, DAP12 CD94, and CD244 [53–55]. The loss of CD28 and the acquisition of alternate receptors essentially redirects CD28− T cells in terms of cellular partners. Instead of interacting with classical antigen-presenting cells, such as dendritic cells, CD28− T cells are capable of forming productive partnerships with accessory cells that lack classical antigen-presentation function and partner with cells that provide ligands for NK receptors. Often, such cells are of mesenchymal origin and include vascular smooth muscle cells and fibroblast-like cells in the inflamed joint. The reprogramming of CD28− T cells to prefer alternate receptor ligand interactions over CD28−CD80/86 interactions empowers them to receive amplifying signals from cells in peripheral inflammatory lesions [56].
Figure 2. Consequence of CD28 loss in T-cells.
Upon aging or repeated antigenic stimulation, T-cells lose the co-stimulatory receptor CD28. The downregulation of CD28 is associated with multiple changes in T cell surface receptors, including the upregulation of the cytokine receptor IL12A as well as the acquisition of NK-cell receptors and mediators, including CD158B1, CD158K, CD94, CD244 and perforin and granzyme B and H.
Another typical property of CD28− T cells includes their potent cytolytic capability. While this is not unexpected for CD8+CD28− T cells, cytolytic capability may even be more important for CD4+CD28− T cells. CD4+CD28− T cells have been shown to kill endothelial cells [57], a mechanism of potential relevance in tissue injury and instability of the atherosclerotic plaque.
Increased frequencies of CD28− T cells are commonly found in the elderly host and under conditions of chronic viral infection. This finding is compatible with CD28− T cells representing a state of exhaustion. However, accumulation of senescent CD28− T cells is not restricted to the very old or the chronically infected host but rather occurs in a number of autoimmune diseases. CD4+CD28− T cells are expanded in multiple sclerosis, rheumatoid arthritis and Wegener’s disease. CD8+CD28− cells have mostly been associated with the infectious burden from chronic viral infections [45]. Recent studies have examined the possibility that CD4 CD28- T cells in autoimmune patients are a consequence of viral infection. CMV has attracted particular attention. CD4+CD28− T-cell expansion in Wegener’s granulomatosis has been described to be present in CMV infected hosts [58]. CD4+CD28− T cells as a reflection of latent CMV infection has also been investigated in patients with rheumatoid arthritis [59]. T-cell infiltrates in the muscle lesions of patients with Dermatomyositis and Polymyositis have been described to be dominated by CD28− cells and it has been speculated that these cells may actually be CMV reactive [60]. To which extent CMV reactivity may actually contribute to the expansion of CD4+CD28− T cells in patients with autoimmune disease remains insufficiently understood. It has also been proposed that autoantigens may drive the expansion of these senescent T cells, as supported by the observation that CD4+CD28− cells can display reactivity to myelin basic protein [61]. In recent experiments, CD4+CD28− and CD8+CD28− were found to rapidly upregulate the CD80/86 receptor CD152. CTLA-4-Ig-mediated blockade of CD152 enhanced T cell apoptosis, suggesting that survival of CD28− T cells may be majorly regulated through CD152. CTLA-4-Ig is currently used as a potent immunosuppressant therapy and part of its therapeutic effect may be related to interfering with the survival of oligoclonal, autoreactive CD28− T cells [62].
Critical insights into the biology of senescent T cells have derived from studies of vaccine responses in individuals older than 65 years of age. Age above 65 years is the major risk factor to succumb to influenza infection and elderly individuals have been urged to participate in yearly influenza vaccine campaigns. However, only a small fraction of less than 20% of such individuals can actually generate an increased anti-influenza titer following vaccination. Non-responsiveness was closely correlated with the frequency of CD8+CD28− T cells. These in vivo data strongly support the notion that CD8+CD28− T cells are a marker for age-related immunodeficiency. Possible mechanisms include a direct participation of such cells in the immune response, broader immunosuppressive effects and indirect immunomodulatory functions, such as creating a smoldering inflammatory milieu that undermines productive immune responses [63].
In essence, CD28− T cells are profoundly different from their CD28+ counterparts in terms of apoptosis signals, tissue trafficking, and effector functions. CD28− T ells accumulate during the second half of life. They are autoreactive but may possibly originate, at least partially, from anti-viral immune responses. The presence of CD4+CD28− T cells in the old and in patients with autoimmune disease has supported the concept that autoimmune disease may be closely linked to the process of immune aging.
Autoimmunity and Early Immune Aging
Autoimmune diseases are believed to represent the sequel of perpetual immune stimulation; in many cases, the disease-relevant antigens are not known. Since the immune system fails to eliminate the driving antigen, T cells and B cells will continuously encounter antigen and thus proliferate chronically. The end result should be shortening of telomeres. In that model, telomeric stress should selectively affect the memory population as naïve cells have not yet encountered antigen. Eventually, the entire immune system should be composed of memory cells and such memory cells should be prematurely aged. An alternative model considers that telomeric abnormalities and accelerated immune aging may be abnormalities that are not a consequence but a cause of dysfunctional immune responses.
The pool of peripheral T cells is under homeostatic control. Attrition of T cells will thus elicit homeostatic proliferation which will inevitably entail replicative stress. Replacement of T cells after the age of 40 years is particularly problematic because thymic production is minimal and cannot supply sufficient T cells into the peripheral pool [12]. The end result is acceleration of the immune aging process. First reports on altered telomere lengths in patients with autoimmune disease prompted speculation about excessive cellular replication in chronically inflamed hosts. Emerging data, however, question this concept as different autoimmune syndromes appear to have distinct abnormalities in telomere biology. In the recent 5 years molecular lesions have been identified which provided novel insights into why telomeres are prematurely shortened in patients with rheumatoid arthritis. These molecular abnormalities have in common that they impair the maintenance of genome stability and telomeric intactness, rendering T lymphocytes susceptible to apoptosis. In essence, deviations in the immune aging process in patients with rheumatoid arthritis appear to be a complex process that is not simply a consequence of inflammation. Rather, inflammation may be a consequence of hastened immunosenescence.
Here, we will summarize the current understanding of how telomere biology and immune aging deviates in patients with distinct autoimmune disease.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a prototypic autoimmune syndrome characterized by cartilage and bone destructive inflammation affecting approximately 1% of individuals in populations of European origin [64]. In concert with other cell types, lymphocytes hold a critical position in RA [65]. CD4 T-cells dominate the inflammatory infiltrates in the synovial membrane and provide helper function for the production of autoantibodies and proinflammatory cytokines. Memory T-cells in RA patients are characterized by an increased loss of CD28 expression as well as by contraction of the T cell receptor diversity [66, 67]. While abnormalities in CD4 memory T cells may well reflect the chronicity of auto-antigenic stimulation, such a disease model does not account for the abnormalities described in the naïve CD4 T cell population. Unprimed CD4CD45RA+ T-cells from RA patients have shortened telomeres compared to age matched healthy controls [16]. When naïve T-cells of RA patients are driven into clonal expansion, they fail to reach similar clonal sizes as controls. Healthy individuals carrying the HLA-DR4 haplotype share with RA patients the age-inappropriate telomere loss, implicating genetic mechanisms in the premature deterioration of chromosomal ends [68]. In both, RA patients and non-RA HLA-DR4+ controls the loss of telomeric ends amounts to about 1500 kb, equivalent to about 30 cell cycles. Naïve CD4 T-cells have been estimated to divide once per year. Thus, in RA patients the naïve CD4 T-cell pool is about 30 years pre-aged. However, telomeres of RA T-cells are not critically short and should not force T-cells into cell cycle arrest or into apoptotic death.
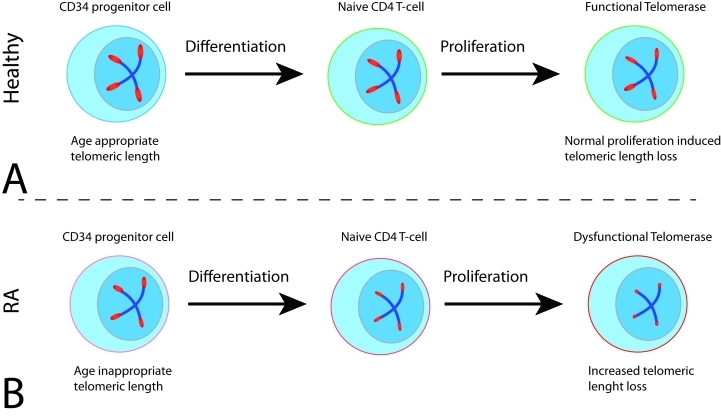
Naïve CD4 T-cells are under low turn-over and their proliferative activity certainly does not explain why they would prematurely use up their telomeric reserve. Recent evidence provides important clues towards a molecular understanding of the abnormal telomere regulation in RA patients (Figure 3). Specifically, RA T cells fail to upregulate sufficient levels of telomerase activity when stimulated through the T cell receptor [38]. Whereas naïve T-cells from healthy controls robustly increase telomerase expression, naïve T-cells from RA patients reach only about half of the peak telomerase activity. Interestingly, this failure in telomerase upregulation is characteristic only for naïve CD4 T-cells; CD34 hematopoietic progenitor cells as well as memory T-cells from RA patients show enhanced or similar telomerase induction when compared to healthy control cells. Proximal and distal events in the T-cell activation cascade remain intact, suggesting a selective defect in telomerase induction.
Figure 3. Telomeric dysfunction in hematopoietic progenitor cells and CD4 naïve T-cells in RA.
A) Healthy CD34 cells differentiate into CD4 naïve cells with a slight loss of telomeric length. Upon stimulation, CD4 naïve T-cells upregulate telomerase and are therefore capable of dampening the impact of massive cellular expansion associated with the priming response. B) CD34+ hematopoietic stem/progenitor cells from patients with RA already have shorter telomeres compared to their healthy counterparts. Thus, the hematopoietic differentiation program starts with a much diminished telomere reserve. Telomeres in RA naïve CD4 cells are shortened by 1500 kb. Upon stimulation, such CD4 naïve cells fail to fully upregulate telomerase leading to an aggravated loss of telomere repeats during clonal expansion.
Telomerase induction appears to be particularly important for naïve T cells, compatible with the need for massive clonal expansion and the necessity to eventually preserve memory of the antigenic encounter. Telomerase functions to maintain and elongate telomeric ends and telomeric dysfunction will preferentially affect highly proliferative tissues. In RA patients, age-inappropriate erosion of telomeres is a feature of three cell types; naïve CD4 T-cell, granulocytes and CD34+ hematopoietic stem/progenitor cells [68, 69]. Whether the premature attrition of telomeric repeats in these three cell types involves identical molecular mechanisms is currently not known. Specifically, why CD34+ hematopoietic stem/progenitor cells lose telomeres is mechanistically unresolved. As they hold a pinnacle position in the hematopoietic tree, their offspring, including granulocytes and lymphocytes, may well start life with a much reduced telomeric reserve. In any way, the finding of abnormal telomeres in CD34+ cells and their progeny raises the interesting possibility that abnormalities in telomere biology occurring in RA can be principally localized to the bone marrow.
In T cells from RA patients, difficulties to maintain integrity of the DNA structure are not limited to the telomeric ends. Besides insufficiencies in keeping telomeres protected and extended, RA T cells have impairments in repairing DNA double strand breaks in non-telomeric DNA [70]. The load of unrepaired DNA in RA T cells is much higher than in age-matched controls, both spontaneously and after genotoxic stress. The underlying defect has been identified as insufficient production of the DNA-damage-inducible protein kinase Ataxia telangiectasia mutated kinase (ATM). Mutations in ATM cause the progerioid syndrome Ataxia telangiectasia (AT) and cells from patients affected with AT have defects in cell cycle progression, abnormalities in responses to DNA breakage and also develop chromosomal end-to-end fusions. In patients with RA, ATM is produced at reduced levels, both on the transciptional and protein level. Forced overexpression of ATM in RA T cells restores DNA repair capability. The accumulation of fragmented DNA is associated with increased susceptibility to cell death. Since naïve T cells are affected, deficiency of ATM will lead to a chronic attrition of such T cells and impose enormous replicative stress upon the RA immune system.
The occurrence of chromosomal end-to-end fusions places ATM at the telomere. Whether ATM deficiency contributes to telomeric dysfunction in RA T cells has not yet been investigated.
T cells from RA patients are well aware of their insufficiency in repairing DNA double strand breaks. This can be concluded from studies that have shown robust induction of DNA-dependent protein kinase catalytic subunit (DNA-PKcs), a member of the phosphatidylinositol-3 kinase-like (PIKK) family of serine/threonine protein kinases. Together with Ku70/80 heterodimers DNA-PKcs forms a synaptic complex that is critically involved in repairing DNA double strand breaks through the non-homologous end-joining (NHEJ) pathway. In naïve T cells from RA patients transcripts and protein levels of DNA-PKcs are elevated, indicating chronic activation of DNA breakage sensing [71]. This chronic genotoxic stress translates into activation of cellular stress kinases, including the JNK signaling pathway. Gene-specific knockdown of DNA-PKcs normalizes the levels of pJNK in RA T-cells, establishing a direct link between cellular stress and DNA damage.
In essence, maintaining integrity of telomeric and non-telomeric DNA is impaired in RA T-cells providing strong evidence that the premature telomeric erosion described more than a decade ago is not only a consequence of enhanced proliferative stress. Several molecules, including hTERT, ATM and DNA-PKcs have been implicated [56], with the common denominator being their functional role in securing genome stability. Thus, telomeric loss needs to be seen as an indicator of broader cellular dysfunction.
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disease preferentially affecting young women that is characterized by multi-organ inflammation leading to significant morbidity and mortality. The pathogenesis of the disease involves the emergence of autoreactive T and B cells, production of autoantibodies, formation and deposition of immune complexes in an array of tissues. For T-cells a broad spectrum of defects has been reported [72]. Although there is constant immune activation in SLE, reports on telomeric length differ and considerable controversy remains regarding the telomere dynamics in SLE. Kurosaka et al and Wu et al described increased telomeric attrition in their Lupus patient cohort [73, 74]. In contrast, Beier et al and Fritsch et al reported essentially normal length measurements when patients and healthy controls were compared [75, 76]. It is possible that such diverging results are a consequence of testing patients at different stages of disease. Activity of telomerase has been examined in SLE patients and matched controls. Fritsch et al described a reduction in telomerase activity in naïve CD4+ T-cells from patients with SLE compared to healthy controls [76]. Disease activity may not have much impact on regulation of telomerase, as telomerase activity has been reported to be similar in active and inactive SLE. Notably, B cell telomerase activity was found to be increased in active SLE patients [73].
Uncertainty remains about the specifics of telomere dynamics in SLE patients and the role of telomerase-dependent telomere repair has been insufficiently examined. Evidence for abnormalities in telomeric maintenance comes from a recent study demonstrating a different expression pattern of shelterin complex molecules in SLE patients, specifically in SLE patients with renal involvement [77].
Sarcoidosis
Sarcoidosis is a systemic granulomatous disorder of unknown etiology. Both, the innate and adaptive immune system have been implicated in driving marked systemic inflammation [78, 79]. Studies determining the selective aging of the immune system have described a significant decrease in the mean telomeric length in patients suffering from sarcoidosis compared to healthy controls. Of note, the investigators describe a significant decrease in long telomeres and a respective increase in short telomeres in patients in their 20s, suggesting a very early onset of intensified immune system turn-over [80]. In addition patients with sarcoidosis have earlier onset of telomeric methylation, again indicating prematurity in the aging process of leukocytes [81].
Type I Diabetes
Type I Diabetes mellitus (DM) results from the immune-mediated destruction of insulin producing islet beta cells with T-cells playing a pinnacle role in the tissue-damaging process [82, 83]. Known autoantigens in DM patients include glutamic acid decarboxylase 65, insulinoma associated protein 2, insulin and proinsulin. These autoantigens are highly antigenic for both T-cells [84–86] and B-cells [87, 88]. Telomeres of blood mononuclear cells of patients with DM have been described to be shorter than those of their age matched controls [89]. A multitude of possible mechanisms have been considered in the attempt to understand the relationship between altered telomere biology and a disease causing B-cell destruction. An obvious model proposes that inflammation has a negative impact on telomeric lengths despite complete lack of understanding how “inflammation” may control the structural integrity, length, repair and maintenance of the chromosomal ends. Analyses of telomeric lengths in CD4 cells from patients with RA have lead to the recognition that the inheritance of the RA disease risk haplotype HLA-DR-B1*04 is sufficient to accelerate telomeric shortening in normal blood bank donors. These studies have separated acceleration of telomeric attrition from disease and have focused attention to alternative mechanisms, such as genetic factors controlling telomeric dynamics. Importantly, HLA-DR-B1*04 has also been associated with higher susceptibility for T1DM. The loss of telomeric ends in patients with T1DM may thus be partially reflective of underlying genetic risk. In that context it is intriguing that patients with T1DM have higher frequency of CD4+CD28− T-cells [90], a phenotype now closely associated with age-inappropriate immune ageing.
Possible mechanisms for accelerated immune aging in T1DM include abnormal cellular turn-over with high proliferative stress, increased oxidative and metabolic toxicities or hyperglycemia itself. Patients suffering from DM have autoantigenic T-cells that are chronically stimulated and have already undergone substantial cell division, but still retain a considerable proliferative capacity [91]. Telomeric shortening is expected in such a cell population. However, recent evidence shows that in patients with DM arterial cells also have a reduction in telomeric length. Notably, this reduction correlated strongly with the HbA1C concentration of the respective person. In addition, telomeric repeats in mononuclear cells of patients with uncontrolled DM were significantly shorter than telomeres in mononuclear cells of patients with well controlled DM [92]. This suggests that diabetes itself might be partly responsible for the loss of telomeric sequences. First evidence that telomeric loss may be reversible has come from studies testing the impact of therapy. A recent report described an increase in telomeric length in naïve T-cells upon long-term insulin therapy in children and adolescents [93].
Outlook
Telomere length and inducibility of telomerase are vital in T cell biology and, thus, in the pathogenic events underlying autoimmunity. Telomeres emerge as critical regulators of T cell survival and thus determine longevity of these already long-lived cells. Immune-mediated diseases have been associated with abnormalities in telomere maintenance and in telomere repair. In several disease states, telomeres are shortened, compatible with excessive proliferative stress within the life cycle of immune cells. Molecular studies in RA have identified alternative defects jeopardizing telomeric stability. Specifically, insufficient production of telomerase has been implicated in weakening telomeric maintenance and altering T cell fate decisions. In RA patients, insufficiencies are not restricted to the handling of the telomeric ends. Rather, T cells accumulate DNA double strand breaks in non-telomeric DNA, pointing towards generalized abnormalities in sensing, repairing and tolerating broken DNA. Based on these studies RA is emerging as a model system for subtle changes in genome stability. Whereas multiple of the inherited progeroid syndromes (e.g. Werner syndrome, Hutchinson-Gilford progeria syndrome, Dyskeratosis congenita, Ataxia telangiectasia) have in common abnormalities in nuclear stability, these recent developments suggest adding RA to this spectrum of diseases. How the molecular changes that cause telomere loss, genome instability, premature cellular senescence and defective stem cell homeostasis in the progeroid syndromes participate in normal human aging warrants further investigation.
Stressed immune cells seem to be pre-aged. It is still unresolved whether chronic stress, e.g. during autoimmunity, causes prematurity of aging or whether entering the senescence program alters the function of immune cells in such a way that they lose their ability to discriminate between self and non-self and cause disease. How antigen-directed immune responses occur in a system that has features of senescence is an important aspect for pathogenic and protective immunity in hosts with autoimmune disease and should give rise to alternative disease models for autoimmune syndromes. The senescence hypothesis of RA pathogenesis will have implications not only for the understanding of the disease process but also for the design of therapies that suppress inflammatory immune responses. Whether the senescence hypothesis is similarly useful in approaching Type 1 DM and sarcoidosis needs to be explored.
Telomeres may come into focus as targets of immunomodulatory therapy. Caution needs to be given to the fact that telomerase is a proto-oncogene, endowing cancer cells with the ability for eternal life. Fine-tuning of telomerase function may still hold promise when it can be directed to selected populations of immune cells. Alternatives include manipulation of other pathways that stabilize the telomere and protect immune cells from premature death. The opportunity to use autoimmune syndromes as model systems to study immune aging should greatly enhance our ability to decipher networks of molecules controlling cellular aging and the overall aging process of the immune system. The development of strategies that slow down the loss of immune function with progressive age has the potential to afford novel therapeutic avenues for a number of age-related maladies.
References
- 1.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–79. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 2.Weyand CM, Fulbright JW, Goronzy JJ. Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp Gerontol. 2003;38:833–41. doi: 10.1016/s0531-5565(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 3.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352:1611–3. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]
- 4.Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–6. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 6.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 7.Mceachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–58. doi: 10.1146/annurev.genet.34.1.331. [DOI] [PubMed] [Google Scholar]
- 8.Lydall D. Taming the tiger by the tail: modulation of DNA damage responses by telomeres. EMBO J. 2009;28:2174–87. doi: 10.1038/emboj.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–52. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain D, Cooper JP. Telomeric strategies: means to an end. Annu Rev Genet. 2010;44:243–69. doi: 10.1146/annurev-genet-102108-134841. [DOI] [PubMed] [Google Scholar]
- 11.Donate LE, Blasco MA. Telomeres in cancer and ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:76–84. doi: 10.1098/rstb.2010.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artandi SE, Depinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–10. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–67. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–8. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maubaret CG, Salpea KD, Jain A, Cooper JA, Hamsten A, Sanders J, Montgomery H, Neil A, Nair D, Humphries SE. Telomeres are shorter in myocardial infarction patients compared to healthy subjects: correlation with environmental risk factors. J Mol Med. 2010;88:785–94. doi: 10.1007/s00109-010-0624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panayiotou AG, Nicolaides AN, Griffin M, Tyllis T, Georgiou N, Bond D, Martin RM, Hoppensteadt D, Fareed J, Humphries SE. Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis. 2010;211:176–81. doi: 10.1016/j.atherosclerosis.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS, Aviv A. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis. 2009;205:620–5. doi: 10.1016/j.atherosclerosis.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F, Kiechl S. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30:1649–56. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- 21.Diaz VA, Mainous AG, 3rd, Everett CJ, Schoepf UJ, Codd V, Samani NJ. Effect of healthy lifestyle behaviors on the association between leukocyte telomere length and coronary artery calcium. Am J Cardiol. 2010;106:659–63. doi: 10.1016/j.amjcard.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsova T, Codd V, Brouilette S, Thijs L, Gonzalez A, Jin Y, Richart T, Van Der Harst P, Diez J, Staessen JA, Samani NJ. Association between left ventricular mass and telomere length in a population study. Am J Epidemiol. 2010;172:440–50. doi: 10.1093/aje/kwq142. [DOI] [PubMed] [Google Scholar]
- 23.Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 24.Hathcock KS, Weng NP, Merica R, Jenkins MK, Hodes R. Cutting edge: antigen-dependent regulation of telomerase activity in murine T cells. J Immunol. 1998;160:5702–6. [PubMed] [Google Scholar]
- 25.Van De Berg PJ, Griffiths SJ, Yong SL, Macaulay R, Bemelman FJ, Jackson S, Henson SM, Ten Berge IJ, Akbar AN, Van Lier RA. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol. 2010;184:3417–23. doi: 10.4049/jimmunol.0903442. [DOI] [PubMed] [Google Scholar]
- 26.Hoare M, Gelson WT, Das A, Fletcher JM, Davies SE, Curran MD, Vowler SL, Maini MK, Akbar AN, Alexander GJ. CD4+ T-lymphocyte telomere length is related to fibrosis stage, clinical outcome and treatment response in chronic hepatitis C virus infection. J Hepatol. 2010;53:252–60. doi: 10.1016/j.jhep.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Lange T. Telomere-related genome instability in cancer. Cold Spring Harb Symp Quant Biol. 2005;70:197–204. doi: 10.1101/sqb.2005.70.032. [DOI] [PubMed] [Google Scholar]
- 28.Sarraf SA, Harper JW. Telomeric TuRF1 wars. Dev Cell. 2010;18:167–8. doi: 10.1016/j.devcel.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Denchi EL, De Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–71. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 30.Arnoult N, Saintome C, Ourliac-Garnier I, Riou JF, Londono-Vallejo A. Human POT1 is required for efficient telomere C-rich strand replication in the absence of WRN. Genes Dev. 2009;23:2915–24. doi: 10.1101/gad.544009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–9. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Augereau A, T'kint De Roodenbeke C, Simonet T, Bauwens S, Horard B, Callanan M, Leroux D, Jallades L, Salles G, Gilson E, Poncet D. Telomeric damage in early stage of chronic lymphocytic leukemia correlates with shelterin dysregulation. Blood. 2011;118:1316–22. doi: 10.1182/blood-2010-07-295774. [DOI] [PubMed] [Google Scholar]
- 33.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–65. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001;27:353–7. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–8. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 36.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, Lansdorp PM, Greider CW, Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 37.Garcia CK. Idiopathic pulmonary fibrosis: update on genetic discoveries. Proc Am Thorac Soc. 2011;8:158–62. doi: 10.1513/pats.201008-056MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–5. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 40.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–56. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 42.Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour CM, Parrinello S, Hodgson JG, Chin K, Desprez PY, Campisi J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang MK, Kameta A, Shin KH, Baluda MA, Kim HR, Park NH. Senescence-associated genes in normal human oral keratinocytes. Exp Cell Res. 2003;287:272–81. doi: 10.1016/s0014-4827(03)00061-2. [DOI] [PubMed] [Google Scholar]
- 44.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–12. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–57. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 47.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–24. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–25. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 49.Vallejo AN, Brandes JC, Weyand CM, Goronzy JJ. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162:6572–9. [PubMed] [Google Scholar]
- 50.Lewis DE, Merched-Sauvage M, Goronzy JJ, Weyand CM, Vallejo AN. Tumor necrosis factor-alpha and CD80 modulate CD28 expression through a similar mechanism of T-cell receptor-independent inhibition of transcription. J Biol Chem. 2004;279:29130–8. doi: 10.1074/jbc.M402194200. [DOI] [PubMed] [Google Scholar]
- 51.Li G, Yu M, Weyand CM, Goronzy JJ. Epigenetic regulation of killer immunoglobulin-like receptor expression in T cells. Blood. 2009;114:3422–30. doi: 10.1182/blood-2009-01-200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–8. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 53.Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+, CD28− T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Fasth AE, Bjorkstrom NK, Anthoni M, Malmberg KJ, Malmstrom V. Activating NK-cell receptors co-stimulate CD4(+)CD28(−) T cells in patients with rheumatoid arthritis. Eur J Immunol. 2010;40:378–87. doi: 10.1002/eji.200939399. [DOI] [PubMed] [Google Scholar]
- 55.Nakajima T, Goek O, Zhang X, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. De novo expression of killer immunoglobulin-like receptors and signaling proteins regulates the cytotoxic function of CD4 T cells in acute coronary syndromes. Circ Res. 2003;93:106–13. doi: 10.1161/01.RES.0000082333.58263.58. [DOI] [PubMed] [Google Scholar]
- 56.Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–8. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 57.Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–5. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 58.Morgan MD, Pachnio A, Begum J, Roberts D, Rasmussen N, Neil DA, Bajema I, Savage CO, Moss PA, Harper L. CD4+CD28− T cell expansion in granulomatosis with polyangiitis (Wegener's) is driven by latent cytomegalovirus infection and is associated with an increased risk of infection and mortality. Arthritis Rheum. 2011;63:2127–37. doi: 10.1002/art.30366. [DOI] [PubMed] [Google Scholar]
- 59.Hooper M, Kallas EG, Coffin D, Campbell D, Evans TG, Looney RJ. Cytomegalovirus seropositivity is associated with the expansion of CD4+CD28− and CD8+CD28− T cells in rheumatoid arthritis. J Rheumatol. 1999;26:1452–7. [PubMed] [Google Scholar]
- 60.Fasth AE, Dastmalchi M, Rahbar A, Salomonsson S, Pandya JM, Lindroos E, Nennesmo I, Malmberg KJ, Soderberg-Naucler C, Trollmo C, Lundberg IE, Malmstrom V. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J Immunol. 2009;183:4792–9. doi: 10.4049/jimmunol.0803688. [DOI] [PubMed] [Google Scholar]
- 61.Markovic-Plese S, Cortese I, Wandinger KP, Mcfarland HF, Martin R. CD4+CD28− costimulation-independent T cells in multiple sclerosis. J Clin Invest. 2001;108:1185–94. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoff H, Knieke K, Cabail Z, Hirseland H, Vratsanos G, Burmester GR, Jorch G, Nadler SG, Broker B, Hebel K, Brunner-Weinzierl MC. Surface CD152 (CTLA-4) expression and signaling dictates longevity of CD28null T cells. J Immunol. 2009;182:5342–51. doi: 10.4049/jimmunol.0801624. [DOI] [PubMed] [Google Scholar]
- 63.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–7. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goronzy JJ, Weyand CM. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res Ther. 2009;11:249. doi: 10.1186/ar2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weyand CM, Goronzy JJ. T-cell-targeted therapies in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2006;2:201–10. doi: 10.1038/ncprheum0142. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–37. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of T-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5:225–34. doi: 10.1186/ar974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schonland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, Weyand CM. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100:13471–6. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colmegna I, Diaz-Borjon A, Fujii H, Schaefer L, Goronzy JJ, Weyand CM. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008;58:990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–49. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2:415–27. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsokos GC, Nambiar MP, Tenbrock K, Juang YT. Rewiring the T-cell: signaling defects and novel prospects for the treatment of SLE. Trends Immunol. 2003;24:259–63. doi: 10.1016/s1471-4906(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 73.Kurosaka D, Yasuda J, Yoshida K, Yoneda A, Yasuda C, Kingetsu I, Toyokawa Y, Yokoyama T, Saito S, Yamada A. Abnormal telomerase activity and telomere length in T and B cells from patients with systemic lupus erythematosus. J Rheumatol. 2006;33:1102–7. [PubMed] [Google Scholar]
- 74.Wu CH, Hsieh SC, Li KJ, Lu MC, Yu CL. Premature telomere shortening in polymorphonuclear neutrophils from patients with systemic lupus erythematosus is related to the lupus disease activity. Lupus. 2007;16:265–72. doi: 10.1177/0961203307077155. [DOI] [PubMed] [Google Scholar]
- 75.Beier F, Balabanov S, Amberger CC, Hartmann U, Manger K, Dietz K, Kotter I, Brummendorf TH. Telomere length analysis in monocytes and lymphocytes from patients with systemic lupus erythematosus using multi-color flow-FISH. Lupus. 2007;16:955–62. doi: 10.1177/0961203307084299. [DOI] [PubMed] [Google Scholar]
- 76.Fritsch RD, Shen X, Illei GG, Yarboro CH, Prussin C, Hathcock KS, Hodes RJ, Lipsky PE. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2184–97. doi: 10.1002/art.21943. [DOI] [PubMed] [Google Scholar]
- 77.Zhou JG, Qing YF, Yang QB, Xie WG, Zhao MC. Changes in the expression of telomere maintenance genes might play a role in the pathogenesis of systemic lupus erythematosus. Lupus. 2011;20:820–8. doi: 10.1177/0961203310397964. [DOI] [PubMed] [Google Scholar]
- 78.Drent M, Van Den Berg R, Haenen GR, Van Den Berg H, Wouters EF, Bast A. NF-kappaB activation in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:50–6. [PubMed] [Google Scholar]
- 79.Psathakis K, Papatheodorou G, Plataki M, Panagou P, Loukides S, Siafakas NM, Bouros D. 8-Isoprostane, a marker of oxidative stress, is increased in the expired breath condensate of patients with pulmonary sarcoidosis. Chest. 2004;125:1005–11. doi: 10.1378/chest.125.3.1005. [DOI] [PubMed] [Google Scholar]
- 80.Guan JZ, Maeda T, Sugano M, Oyama J, Higuchi Y, Suzuki T, Makino N. An analysis of telomere length in sarcoidosis. J Gerontol A Biol Sci Med Sci. 2007;62:1199–203. doi: 10.1093/gerona/62.11.1199. [DOI] [PubMed] [Google Scholar]
- 81.Maeda T, Guan JZ, Higuchi Y, Oyama J, Makino N. Aging-related alterations of subtelomeric methylation in sarcoidosis patients. J Gerontol A Biol Sci Med Sci. 2009;64:752–60. doi: 10.1093/gerona/glp049. [DOI] [PubMed] [Google Scholar]
- 82.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 83.Roep BO. The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia. 2003;46:305–21. doi: 10.1007/s00125-003-1089-5. [DOI] [PubMed] [Google Scholar]
- 84.Atkinson MA, Kaufman DL, Campbell L, Gibbs KA, Shah SC, Bu DF, Erlander MG, Tobin AJ, Maclaren NK. Response of peripheral-blood mononuclear cells to glutamate decarboxylase in insulin-dependent diabetes. Lancet. 1992;339:458–9. doi: 10.1016/0140-6736(92)91061-c. [DOI] [PubMed] [Google Scholar]
- 85.Kelemen K, Gottlieb PA, Putnam AL, Davidson HW, Wegmann DR, Hutton JC. HLA-DQ8-associated T cell responses to the diabetes autoantigen phogrin (IA-2 beta) in human prediabetes. J Immunol. 2004;172:3955–62. doi: 10.4049/jimmunol.172.6.3955. [DOI] [PubMed] [Google Scholar]
- 86.Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, Conlon PJ, Gottlieb PA, Putnam AL, Gaur A. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest. 2001;107:173–80. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114:589–97. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E. Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J Immunol. 1995;155:5419–26. [PubMed] [Google Scholar]
- 89.Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, Warram JH, Aviv A. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47:482–6. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- 90.Giubilato S, Liuzzo G, Brugaletta S, Pitocco D, Graziani F, Smaldone C, Montone RA, Pazzano V, Pedicino D, Biasucci LM, Ghirlanda G, Crea F. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J. 2011;32:1214–26. doi: 10.1093/eurheartj/ehq499. [DOI] [PubMed] [Google Scholar]
- 91.Monti P, Scirpoli M, Rigamonti A, Mayr A, Jaeger A, Bonfanti R, Chiumello G, Ziegler AG, Bonifacio E. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol. 2007;179:5785–92. doi: 10.4049/jimmunol.179.9.5785. [DOI] [PubMed] [Google Scholar]
- 92.Uziel O, Singer JA, Danicek V, Sahar G, Berkov E, Luchansky M, Fraser A, Ram R, Lahav M. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Exp Gerontol. 2007;42:971–8. doi: 10.1016/j.exger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Hofer J, Hofer S, Zlamy M, Jeller V, Koppelstaetter C, Brandstatter A, Kern H, Kohle J, Zimmerhackl LB, Prelog M. Elevated proportions of recent thymic emigrants in children and adolescents with type 1 diabetes. Rejuvenation Res. 2009;12:311–20. doi: 10.1089/rej.2009.0863. [DOI] [PubMed] [Google Scholar]