Abstract
Stochastic damage to cellular macromolecules and organelles is thought to be a driving force behind aging and associated degenerative changes. However, stress response pathways activated by this damage may also contribute to aging. The IKK/NF-κB signaling pathway has been proposed to be one of the key mediators of aging. It is activated by genotoxic, oxidative, and inflammatory stresses and regulates expression of cytokines, growth factors, and genes that regulate apoptosis, cell cycle progression, cell senescence, and inflammation. Transcriptional activity of NF-κB is increased in a variety of tissues with aging and is associated with numerous age-related degenerative diseases including Alzheimer’s, diabetes and osteoporosis. In mouse models, inhibition of NF-κB leads to delayed onset of age-related symptoms and pathologies. In addition, NF-κB activation is linked with many of the known lifespan regulators including insulin/IGF-1, FOXO, SIRT, mTOR, and DNA damage. Thus NF-κB represents a possible therapeutic target for extending mammalian healthspan.
Keywords: Aging, NF-κB, Inflammation, Senescence
With aging there is an inevitable loss of tissue homeostasis leading to an impaired ability of an organism to respond to stress and as a consequence dramatically increased risk of morbidity and mortality. The cause of aging and its sequelae are hotly debated. The primary debate is whether aging is determined genetically or is a consequence of time-dependent accumulation of stochastic damage [1]. In fact, there is abundant evidence, in particular from Caenorhabditis elegans, that mutation of single genes can extend lifespan as much as six-fold. Likewise, rare diseases called progerias reveal that DNA damage can drive rapid aging in humans. Yet these two theories are not mutually exclusive and in fact converge if one considers that the cellular response to damage is dictated by intricate signaling networks, which are genetically determined. Many types of cellular damage may contribute to aging including DNA damage, mitochondrial damage, telomere attrition and accumulation of macromolecular waste [2]. These various types of damage promote aging by driving cellular senescence, apoptosis, or dysfunction. Given the variety of types damage that can promote aging and the relatively limited number of outcomes to these numerous insults, this argues strongly that damage/stress response pathways play an important role in mediating aging.
Genotoxic, inflammatory, and oxidative stresses all stimulate the NF-κB family of transcription factors. Thus, as a common responder to varied stress stimuli, NF-κB is well positioned to play a key role in driving aging. Indeed, NF-κB has been directly implicated in the aging process. For example, using motif mapping, NF-κB was determined to be the transcription factor most associated with aging [3]. Furthermore, biologic pathways implicated in aging, including immune responses, cell senescence, apoptosis and metabolism are all regulated at least in part by NF-κB. Additionally, other cellular processes implicated in regulating lifespan, including insulin/IGF-1 and growth hormone pathways, SIRT, FoxO and mTOR, are all interconnected with NF-κB signaling. Finally, the role of aberrant NF-κB signaling is well documented in numerous age-associated diseases including neurodegeneration, osteoporosis, diabetes, sarcopenia and atherosclerosis. Here we will review NF-κB signaling and the evidence that it is linked to aging.
The NF-κB transcription factor
NF-κB refers to the Rel family of transcription factors consisting of five family members: p65/relA, relB, c-rel, p50, and p52 (Figure 1). NF-κB was discovered in 1980 as the pro-inflammatory factor that confers LPS signaling [4, 5]. Thus, NF-κB traditionally has been considered as an immunologic transcription factor, involved in the activation of inflammatory cells and gene expression regulation of numerous cytokines and chemokines. However, more recently, NF-κB activity has been implicated in diverse disease pathologies and biologic processes [6, 7].
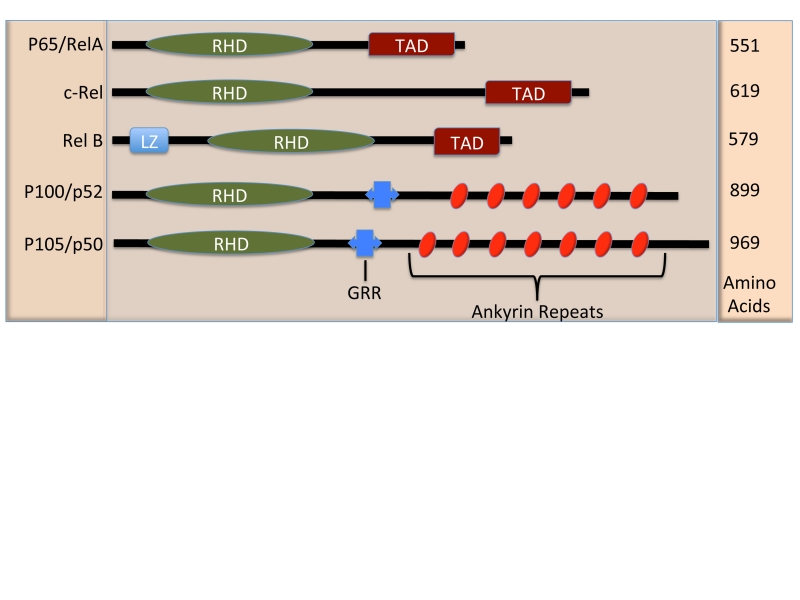
Figure 1. Schematic Diagram of the NF-κB family members.
The NF-κB family members are defined by the n-terminal, Rel Homology Domain (RHD) responsible for DNA binding and dimerization. The p65, c-rel, and RelB family members contain a Transactivation Domain (TAD) which confers positive regulation of gene expression. The transcriptional suppressor family members p52 and p50 contain glycine rich regions (GRR) which are necessary for their proteolytic cleavage and ankyrin repeats similar to those found with IκB proteins, thus acting as cytoplasmic inhibitors of NF-κB. Additionally RelB contains a leucine zipper motif (LZ).
NF-κB family members are defined by a short rel-homology domain (RHD), which is responsible for dimerization as well as DNA binding to the NF-κB consensus sequence (GGGRNNYYCC). Additionally there are two subgroups within the NF-κB family, three of the subunits, p65, relB, and c-rel, contain transcription activation domains (TAD) at the c-terminus. The p50 and p52 family members are proteolytically cleaved from larger proteins p105 and p100, respectively, and do not contain TADs, acting instead as transcriptional suppressors (Figure 1) [8]. These two different roles are exemplified by p65/p50 heterodimer, the prototypical NF-κB dimer, which is known to lead to the transcription of a variety of genes, and the p50/p50 homodimer, which also binds to the NF-κB consensus sequence with a higher affinity than p65/p50 [9], that inhibits transcription.
Activation of NF-κB
There are a large number of activators of NF-κB. The toll like receptor (TLR) ligands, a subgroup of pattern recognition receptors (PRR) that recognize conserved attributes found in bacterial, viral and parasitic pathogens, are potent NF-κB activators. TLR ligands signal through several PRR, for example, lipopolysaccaride (LPS) via TLR4 [10], CPG via TLR9 [11], Flagellin via TLR2 [12] and muramyl dipeptides (MDP) via NOD2 [13]. Additionally, tumor necrosis factors (TNF) and interleukin-1 (IL-1), two major proinflammatory cytokines, are both activators as well as transcriptional targets of NF-κB. Other NF-κB activators include the antigen receptors found on the adaptive immune cells, specifically the T-cell receptor and B-cell receptor (TCR and BCR) and receptors found on antigen presenting cells TLRs and CD40R. Additionally, growth factors such as hepatocyte growth factor (HGF), follicle stimulating hormone (FSH), granulocyte macrophage-colony stimulating factor (GM-CSF), and nerve growth factor (NGF) activate NF-κB [7].
Of specific relevance to the stochastic model of aging, NF-κB can be activated secondary to DNA damage via the DNA response protein ataxia telangiectasia mutated (ATM) [14, 15]. The regulation of NF-κB by ATM is mediated by a pathway involving NEMO (see below), PARP-1 and PIASy that activates cytoplasmic IKK. Additionally, numerous studies suggest that ROS can activate and/or suppress NF-κB [16], depending upon the cell type and conditions evaluated.
Activation of NF-κB through the IKK complex
NF-κB is activated through two different routes: the canonical/activated and non-canonical pathways. However, damage/stress-mediated stimulation of NF-κB, as described above, likely contributes to aging via the canonical pathway. The IKK complex lies at the confluence of the different NF-κB signaling cascades: TLRs, TCR or ATM. It is composed of two catalytic subunits, IKKα and IKKβ, as well as a regulatory subunit IKKγ also known as the NF-κB essential modulator (NEMO) [17]. Many of the signaling cascades, which activate NF-κB share signaling molecules upstream of the IKK complex. In particular, LPS activated TLR4 binds MyD88 and IRAK, which then signal through TRAF6, leading to activation of the IKK complex. Other common signaling proteins involved in TLR, IL-1, TNF, and TCR signaling pathways include MyD88, RIP, NIK and TRAF [8] (Figure 2).
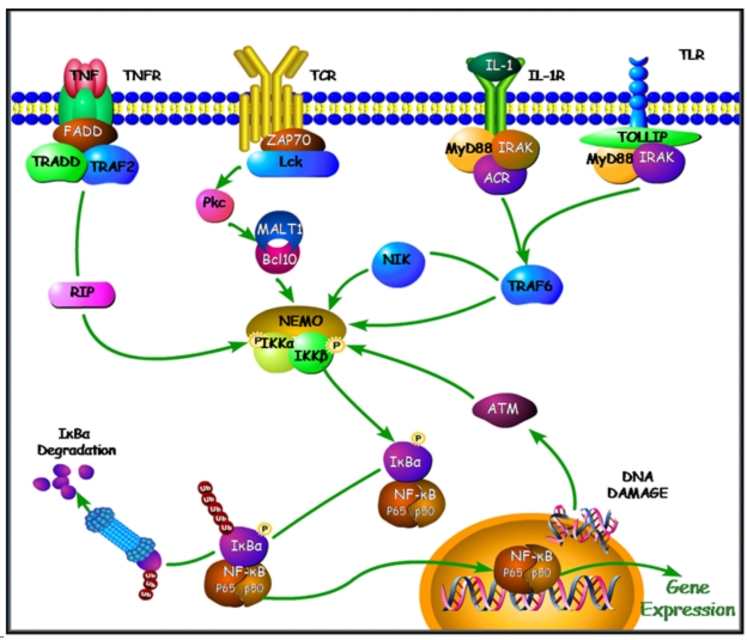
Figure 2. Signaling via the IKK/NF-κB Classical Pathway.
The IKK complex (NEMO, IKK1 (IKKα), and IKK2 (IKKβ)) can be activated by numerous stimuli and via shared signaling components. Extracellular receptors bind to their ligands and signal via TRAF/RIP/NIK molecules leading to phosphorylation of IKK subunits, which subsequently phosphorylate IκBα and lead to its ubiquitination and proteosomal degredation. This then releases NF-κB into the nucleus where it acts as a transcription factor. In addition, ATM responds to DNA damage and can also activate the IKK complex. (Figure adapted from [8, 146, 147])
IKKγ interacts with the upstream RIP, NIK or TRAF proteins, which results in the oligomerization of IKKγ. IKKγ then induces phosphorylation or possible auto-phosphorylation of the IKKα and β subunits. The catalytic subunits are then released from IKKγ and act to phosphorylate IκB at Ser32 and Ser36. After phosphorylation, IκBα is poly-ubiquitinated at Lys22 and degraded by the 26S proteosome. This degradation leads to the loss of the nuclear export signal provided by IκB and reveals the nuclear localization signals on the NF-κB subunits. NF-κB can then migrate to the nucleus [18] where it functions to promote gene expression of various pro-inflammatory, cell growth and other regulated genes (Figure 2/3).
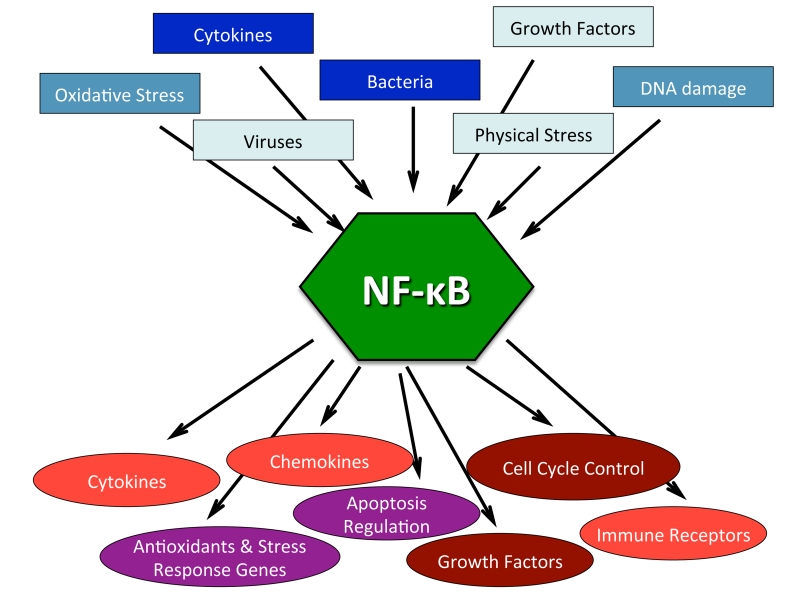
Figure 3. NF-κB is a central regulator in stress response.
The NF-κB signaling pathway can be activated by numerous stimuli as listed in the blue boxes (summarized in Subsection entitled: Activation of NF-κB). In response to these different stimuli NF-κB transcriptionally regulates hundreds of genes, the generalized categories of which are listed in the red circles (summarized in subsection entitled: Genes under NF-κB transcriptional control). A compilation of citations with regards to NF-κB activators and transcriptionally regulated genes can be found at (www.nf-kb.org) [7].
Non-canonical NF-κB signaling
The non-canonical NF-κB signaling pathway is largely involved in lymphoid organ development, which is required for B and T-cell development via activation of the p52/RelB heterodimer. This signaling pathway is activated by a limited number of receptors (Lymphotoxin B, B-cell activating factor, and CD40), which subsequently stimulate an IKKα homodimer. This complex then phosphorylates p100, leading to its subsequent processing to p52, and translocation of the p52/RelB complex to the nucleus [19]. In addition to lymphoid organ development, studies evaluating IKKα−/− mice suggest a role of the non-canonical pathway in epidermal and skeletal development [20]. Whereas the role of the non-canonical pathway appears to be important during development, it is the canonical pathway that appears to be the most relevant in the pathogenesis of mammalian disease and aging.
IKK and IκB signaling components
The IκB and IKK proteins are the central regulators of activated NF-κB signaling. The IKK complex is comprised of a trimer of IKKα/β/γ, but may be found as a higher order complex with numerous IKK trimers. The IKK kinases α and β share a 52% sequence homology [21] with even greater homology in their catalytic and kinase domains. However, genetic knockout studies suggest differential and non-redundant roles for these two proteins [22, 23]. For example, IKKα has been implicated in both canonical and non-canonical signaling.
The major targets of the IKK proteins are the IκB cytoplasmic inhibitors. The common component of the IκB proteins is the ankyrin repeats, which are found on IκBα, β, γ, δ, ɛ, bcl-3 and the uncleaved p50 and p52 subunits p100 and p105 proteins. These ankyrin repeats act to bind to the Rel portion of NF-κB to block their nuclear localization signal (NLS). IκBα is the best characterized of the typical IκBs proteins, α, β and ɛ, and is the predominant inhibitor of the canonically activated p65/p50 NF-κB heterodimer. In contrast, the non-typical IκBs, bcl-3 and IκBδ are thought to inhibit NF-κB transcription by binding to NF-κB in the nucleus. For instance, Bcl-3 stabilizes p50 homodimers on DNA, blocking the NF-κB promoter regions from other NF-κB subunits and preventing TAD positive NF-κB subunits from binding the consensus sequence [24].
Genes under NF-κB transcriptional control
Hundreds of genes have been shown to be transcriptionally regulated by NF-κB [7]. These include genes encoding cytokines, chemokines, and immunoreceptors, as well as proteins involved in antigen presentation, cell adhesion, the acute phase and stress responses, growth factors and their receptors, early response genes, and other transcription factors (www.nf-kb.org) [7] (Figure 3).
The majority of the genes under NF-κB transcriptional control are involved in immune signaling and inflammatory responses. Indeed, transcriptional control of cytokine expression by NF-κB is likely one of the most important factors when evaluating the role of NF-κB in pathologic states. Some of these cytokines include TNFα, IL-1α/β, IL-2, 3, 6, 12, GM-CSF, M-CSF, and G-CSF. NF-κB also regulates expression of chemokines (MCP-1, KC, MIP-1 and several CCLs) and adhesion molecules (ICAM-1, E-selectin, and VCAM-1), which allow for the recruitment and attachment of immune cells to sites of inflammation. Furthermore, NF-κB upregulates the expression of receptors (CD80/81, IL-2Rα chain, TLR-2) and proteins involved in antigen presentation (MHC class I and β2 microglobulin) on immune cells, allowing for proper innate and adaptive immune responses.
In addition to regulating immune response genes, NF-κB regulates several additional biologic processes. Interestingly, NF-κB transcriptionally regulates both pro-apoptotic (Bim, Bax, Fas and Fas-ligand, and caspase 11) and anti-apoptotic (XIAP, bcl-2, A1/bfl-1, and c-Flip) genes. NF-κB blocks apoptosis in a number of inflammatory cells including macrophages, DC, T-cells, B-cells, and neutrophils and is a pro-survival factor in several types of malignancies especially lymphomas. In contrast, the inflammatory response can induce apoptotic cell death. This inflammatory cell death response is initiated by the production of cell death receptors (Fas and FasL) and intracellular apoptosis inducing proteins. This apoptotic death is further assisted by activated immune cells, which secrete granzyme, perforin and nitric oxide, all apoptosis inducing factors, which are regulated by NF-κB.
Another category of NF-κB transcriptionally regulated genes includes growth factors such as nerve growth factor (NGF), vascular endothelial growth factor (VEGF), insulin-like growth factor binding protein (IGFBP), bone morphogenic protein (BMP), and fibroblast growth factor (FGF). Many of the receptors for these growth factors are involved in the expansion and maturation of varying cell types. Of note, many other pathways associated with aging phenotypes are also cellular growth and expansion mediators including insulin/IGF-1, mTOR, and SIRT and will be discussed in more detail below. Thus, the majority NF-κB controlled genes are considered cell stress responders and lead to inflammation, apoptosis, and cellular growth/expansion.
Additional IKK substrates
While it is likely that the actions attributed to IKK exert the majority of their effects due to suppression of the NF-κB canonical signaling pathway, there are number of other IKK substrates including Bcl-10, β-catenin, cyclinD1, FOXO3a, p53, and ERα. These proteins are known to affect cell growth and proliferation [25–27]. NEMO also promotes transcriptional activity of HIF1α and HIF2α, two anti-oxidant proteins [25, 28]. Thus, while the majority of the effects observed after IKK inhibition are likely mediated by NF-κB suppression, it is important to note that other pathways also are affected by IKK suppression, specifically those regarding cell growth and proliferation.
NF-κB and Aging
The NF-κB signaling pathway is one of several signaling response pathways implicated in aging, which include IGF-1, mTOR, SIRT1 and p53 [29]. A cross species study, using motif mapping in promoters of genes upregulated with aging, suggested that NF-κB is the transcription factor most associated with aging [30]. In addition, overexpression of either of two NF-κB subunits, c-rel and RelA/p65, induced a senescent phenotype in cultured cells [31–33]. Furthermore, loss of p65, but not p50, gives mouse embryonic fibroblasts the ability to escape senescence, in part due to its role in DNA repair [34]. Consistent with these findings, evaluation of skin-derived human fibroblasts from aged individuals (aged 72–93), and HGPS progeria patients (8–14) showed increased levels of NF-κB activation and increased inflammatory gene expression when compared with cells derived from young individuals (aged 22–33 and 8–14 respectively) [3, 35]. In addition, NF-κB/p65 DNA binding increases with chronologic age in several tissues including skin, liver, kidney, cerebellum, cardiac muscle, and gastric mucosa [36–41]. Genetic reduction of NF-κB in aged mouse skin reversed age-related pathology and gene expression changes, suggesting a beneficial role for NF-κB inhibition in reversing age-related degeneration [30]. In sirt6−/− mice, which exhibit degenerative changes and an accelerated aging phenotype, haploinsufficiency of p65/relA resulted in 40% of the sirt6−/−p65+/− mice exhibiting improved growth and longer lifespan than their sirt6−/− littermates [42]. However, it is important to note that the sirt6−/− mouse model exhibits a severe colitis phenotype, suggesting that these mice may have chronic colonic infection leading to NF-κB activation and a degenerative rather than aging phenotype [43].
Recently, we have demonstrated that NF-κB transcriptional activity is up-regulated in an increased percentage of cells within a variety of tissues with both natural aging, and in a progeroid mouse model of a human progeroid syndrome caused by defective repair of DNA damage (ERCC1-deficient mice). Genetic reduction in the level of the NF-κB subunit p65(RelA) in Ercc1−/− and Ercc1−/Δ mice, with lifespans of 1 and 7 months respectively, delayed the onset of age-related pathology including muscle wasting, osteoporosis, and intervertebral disc degeneration. These results directly demonstrate that NF-κB is upregulated in response to accumulated DNA damage which drives tissue degeneration. Further, we have elucidated a role of NF-κB in stem cells and aging where stem cells haploinsuffient for p65 have improved self renewal as well as differentiation proficiency. These results suggest multiple mechanisms through which NF-κB could regulate the aging process.
Another component contributing to increased NF-κB activity associated with aging is the altered transcriptional phenotype, which occurs in senescent cells. A specific Senescence-associated secretory phenotype (SASP) has been defined in senescent cells, consisting of increased expression of IL-6, IL-8, IL-7, MCP-2, MIP-3, ICAM, Il-1α, and Il-β [44]. While senescence is initially a tumor-suppressive mechanism [45], there likely are numerous deleterious side effects of this anti-growth, pro-inflammatory senescent phenotype. It is important to note that the vast majority of these SASP profile cytokines and chemokines are transcriptionally regulated by NF-κB [46], again implicating this signaling pathway in aging on a cellular level. Our recent results also suggest that NF-κB drives senescence as well as SASP.
Caloric restriction and NF-κB
Caloric restriction (CR) is the most widely recognized and first reproducible mechanism by which life extension was mediated [47, 48]. CR not only extends longevity, but also ameliorates age-associated pathology including diabetes, cardiovascular disease, sarcopenia as well as autoimmune diseases [48]. While CR is efficacious in numerous mouse models and more recently in primates [49], the beneficial effects have been attributed to numerous mechanisms, including suppression of NF-κB and immune response [50]. CR inhibits NF-κB signaling at the level of the IKK complex, possibly through a ROS dependent mechanism [50]. Additionally, CR downregulates expression of 56 inflammatory genes, many of which are transcriptionally regulated by NF-κB [51]. Even short term CR (10 days) resulted in decreased NF-κB activity in kidneys of aged mice [52]. Therefore, CR, a known mediator of improved life and healthspan, functions, at least in part, via NF-κB and inflammatory suppression. In addition to the direct implication of NF-κB as a mediator of caloric restriction, several other pathways integral to the aging process, including SIRT, IGF-1, DNA damage, and mTOR, all interact with NF-κB to a significant degree (Figure 4), and will be discussed individually below.
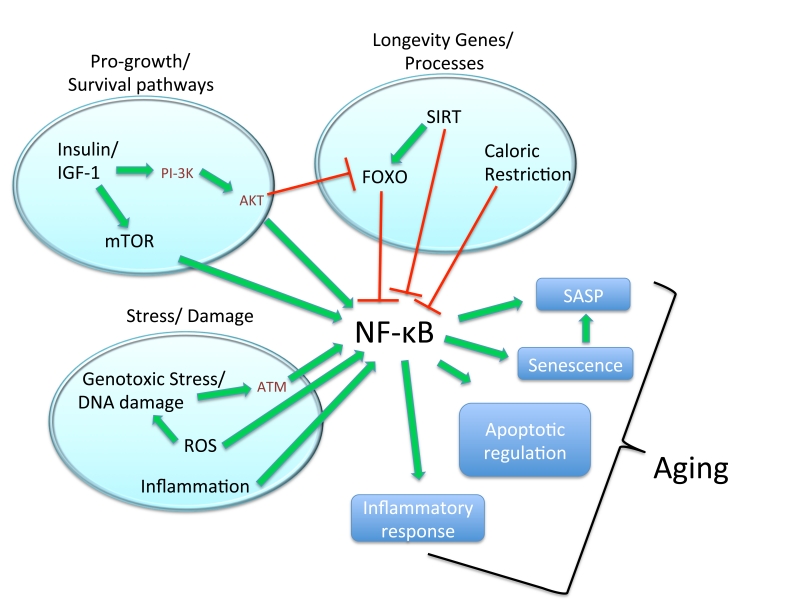
Figure 4. Schematic illustration depicting NF-κB as a central factor in pro-aging and longevity pathways.
Pro-growth survival pathways known to promote aging phenotypes, specifically Insulin/IGF-1 and mTOR are known to stimulate NF-κB as described. Insulin/IGF-1 acts via two mechanisms, AKT and mTOR signaling, to activate NF-κB. However, through AKT, Insulin/IGF-1 signaling also interacts with known longevity processes by inhibiting FOXO. As with the other known longevity factors and signaling components, SIRT and CR, FOXO inhibits NF-κB signaling as described. Additionally stress/damage pathways known promote age-associated changes including genotoxic stress, ROS, and inflammation also activate NF-κB. Secondary to activation of NF-κB by pro-aging pathways, NF-κB then acts to promote aging related changes by contributing to cellular senescence, SASP, apoptotoic signals and inflammatory responses.
Insulin/IGF-1 signaling and NF-κB
The insulin/IGF-1 signaling pathway was the first cellular signaling mechanism clearly shown to influence aging [53]. IGF-1 is a mitogenic peptide produced in response to growth hormone, which binds to its receptor (IGF-1R) to activate pro-survival, proliferation and differentiation signaling cascades. The role of insulin/IGF-1 in aging was initially documented via the analogous Daf2 signaling pathway in C. elegans [54]. Subsequently, the growth hormone (GH) pathway, which signals via IGF1/insulin, has been implicated in mammalian aging. The Ames, Snell, and GHRKO mice all exhibit low levels of IGF-1 and dwarfed growth, but show lifespan extension of 20–70% [55]. Additionally, lifespan extension achieved via CR is associated with reduced levels of IGF-1 and insulin in serum [56]. Given the roles of NF-κB in the stress response and cell survival and proliferation, it is not surprising that the insulin/IGF-1 and NF-κB signaling pathways are linked. The insulin/IGF-1 activates PI-3K/AKT, which in turn stimulates NF-κB signaling via the IKK complex [57, 58]. This upregulation of NF-κB signaling occurs through several mechanisms including stimulating p65 transactivation, as well as phosphorylation and activation of IKKβ. Further studies show that GH, IGF-1 and insulin activate anti-apoptotic responses through NF-κB [59, 60], and that IGF-1R can activate immune responses via NF-κB [61, 62]. These interactions are seen in addition to the interaction between FOXO, a downstream mediator of the IGF-1/insulin pathway, and NF-κB (see below).
SIRT signaling and NF-κB
SIRT1 is a class III histone deacetylase and a member of the sirtuin family, shown in several studies to regulate lifespan. SIRT1 acts, in part, via deacetylation of FOXO, another known longevity factor [55]. Lifespan extension has been achieved through overexpression of SIRT1 in worms and flies [63]. Additionally sirt6−/− mice have an accelerated-aging phenotype, but with the caveats of this finding discussed above. SIRT1 activators, such as resveratrol, are considered cutting edge therapies for treating age-associated diseases. Resveratrol increases healthspan [64], protects against neurodegeneration in models of Alzheimer’s Disease and amyotrophic lateral sclerosis [65], improves osteoporotic changes [66] and reduces health defects occurring secondary to high fat diets [67]. SIRT1 likely acts via numerous mechanisms to alter age-associated changes including: increased mitocondriogenesis through PGC1α deacetylation, improved oxidative stress survival response via FOXO1/4, altered apoptosis and proliferation mediated by p53 deacetylation, and decreased inflammatory response via NF-κB suppression [68]. The specific impact of these varying mechanisms by which SIRT promotes its anti-aging effects is unknown; however, its interaction with the NF-κB signaling pathway has been well documented. SIRT1 interacts directly with p65, leading to deacetylation at lysine 310, culminating in decreased NF-κB associated transcription [69]. SIRT1 further inhibits NF-κB by interacting with TLE1, a transcriptional co-repressor of NF-κB [70]. SIRT6 and p65/RelA interact at the site of the NF-κB promoter region and led to repressed transcriptional activity. While it is difficult to distinguish which beneficial effects of SIRT1 therapy are due to what specific mechanism of action, it is interesting to note the SIRT1 activators and NF-κB inhibitory therapies have been efficacious in the same disease pathologies including diabetes, osteoporosis, neurodegeneration, and inflammatory diseases.
FoxO/Daf16 and NF-κB
FOXO/Daf16 lies downstream of both the Insulin/IGF1 signaling pathway and SIRT signaling [54, 55]. Daf16 and its mammalian homologue FOXO are well documented transcription factors regulating lifespan. Daf-16 has been observed to regulate lifespan extension in C. elegans [71, 72]. In C. elegans, daf16 (−) and daf2 (−) are short lived, however, expression of DAF16 in other tissues can lead to the rescue of this phenotype. Genetic studies show that FOXO3A and certain FOXO1 variants are associated with longevity. The FOXO3A variant associated with longevity in humans was found to inhibit NF-κB activation [73, 74] and FOXO3A deficient mice have overactive NF-κB activation particularly in T-cell populations [74]. In addition, FOXO transciptionally regulates the IκB cytoplasmic inhibitors of NF-κB, thus leading to further suppression of NF-κB.
TOR signaling and NF-κB
The mammalian target of rapamcin (mTOR) kinase, like IGF-1/insulin, is a mediator of stress response. The TOR pathway is responsible for stimulating growth and inhibiting salvage pathways during periods of plentiful food production. mTOR is a PI3-K related kinase which phosphorylates Ser-Thr residues and signals via Raptor, and relies on transcriptional changes as well as translational changes mediated by S6K1 inhibition of 4EBP1 [53]. There is evidence that mTOR is activated via the IGF-1/insulin pathway [75], but acts independently of FOXO/Daf16 [76, 77]. TOR signaling suppression confers lifespan extension in many species. For example, dominant negative forms of TOR and S6K1 lead to lifespan extension in drosophila [78] and deletion of e1F4e which is regulated by TOR/4EBP increases lifespan in C. elegans [79]. Rapamycin, a well-known mTOR inhibitor, conferred increased longevity in mice, thus further supporting the role of mTOR in aging [77]. As with the other aging related pathways discussed, mTOR and NF-κB signaling are associated. IKKα and IKKγ are known to interact directly with mTOR, and additionally, suppression of mTOR and Raptor by siRNA decreased NF-κB binding activity [80]. This mTOR/NF-κB signaling occurs downstream of Akt [80]. In addition to the regulation of NF-κB by mTOR, IKKα/β acts via two mechanisms to regulate mTOR. IKKα/β directly activates mTOR while suppressing TSC1, an mTOR inhibitor [81–83]. These findings suggest that there is an amount of co-regulation between mTOR and NF-κB.
DNA damage, ATM and NF-κB
Transcriptional studies evaluating the aging process show that mice exposed to chronic genotoxic stress, either due to genetic mutation or gamma irradiation, have changes that mimic those observed in natural aging [84]. Similar to naturally aged mammals, DNA repair deficient mice with a progeroid-like syndrome have a suppressed IGF-1/insulin pathway. Although the suppression of insulin/IGF-1 signaling in the progeria mice with accelerated aging appears counterintuitive, it is likely the organism’s response to frailty and an attempt to extend lifespan [85]. Thus factors other than insulin/IGF-1 may play a role in accelerated aging seen in these progeria mice. It was speculated that ATM, a P1–3K kinase, activated in response to stress is a contributing factor to this aging phenotype secondary to DNA damage. ATM is activated by double strand breaks and γ-irradiation and subsequently phosphorylates several proteins including p53 and IKKγ. ATM activates NF-κB via phosphorylation of the ser-85 of IKKγ, and there exists a linked cytoplasmic shuttling of the ATM/IKKγ after genotoxic stress [15]. Additional studies have shown that inducers of replicative stress, hydroxyurea and aphidicolin, also activate ATM/NF-κB signaling. NF-κB signaling after gamma irradiation also can be inhibited completely using the ATM specific inhibitor KU55933 [86]
NF-κB in Age-Associated Disease
Aging is widely considered a physiologic condition; however the majority of aging research focuses on age-associated diseases, which are treated as pathologic conditions. These age-associated diseases include Alzheimer’s, Parkinson’s, type II diabetes, atherosclerosis, sarcopenia, and osteoporosis. One feature that these diseases share is increased inflammation. For example, NF-κB signaling and cytokine secretion are upregulated in atherosclerosis [87], osteoarthritis [88], neurodegeneration (Alzheimer’s and Parkinson’s) [89], osteoporosis [90], and cardiovascular disease [91] as discussed below. The studies conducted using primary cells or cell lines and results from animal model systems or from human patient samples are summarized in Tables 1 and 2.
Table 1.
Summary of studies conducted with primary cells or cell lines
| Disease | Studies in primary cells | Studies with cell lines |
|---|---|---|
| Alzheimer’s Disease | Primary rat neurons [97] Primary rat cerebral granule cells [98] |
C6 rat glioma cells [95] THP-1 cells [96] Microglial BV2 cells [97] SH-SY5Y cells [98] |
| Parkinsons’s Disease | Primary ventral mesencephalic neuron-glia cultures (mouse and rat) [105, 108, 110, 114, 116] Primary ventral mesencephalic neuron-enriched cultures (mouse and rat) [110, 114, 116] Microglia-enriched cultures (mouse and rat) [110, 114] Primary mouse neuron-astroglia cocultures [110] Primary parkin−/− neuronal-enriched mesencephalic primary cultures [115] Primary gp91phox−/− mouse mesencephalic neuron-glia cultures [116] |
Microglial BV2 cells [113] U937 cells [114] PC-12 cells [119] |
| Type II Diabetes | 3T3-L1 adipocytes [123] Fao hepatoma cells [123] |
|
| Atherosclerosis | Monocyte-derived human macrophages [87] Mouse peripheral blood mononuclear cells [87] Mouse peritoneal macrophages [87] Human atherosclerotic plaque cells [130] Rat artery organoid culture [132] Primary rat coronary arterial endothelial cells [132] Primary rat aortic smooth muscle cells [132] |
|
| Sarcopenia | C2C12 cells [136] | |
| Osteoporosis | C2C12 cells [136] |
Table 2.
Summary of studies conduected with model animal systems or human patients
| Disease | Animal models used | Human patients studied |
|---|---|---|
| Alzheimer’s Disease |
apoE−/− mice [94] Sprague-Dawley rat [95] APOE-TR mice [99] |
[93, 101] |
| Parkinsons’s Disease | SIV-infected rhesus monkey [107] MPTP treatment in mice [105, 118] MPTP treatment in cynomolgus monkey [111] Paraquat treatment in mice [112] |
[103, 104, 106, 109, 117, 118] |
| Type II Diabetes | Zucker fa/fa rats [123] ob/ob mice [123, 125, 126, 128] Ikkβ+/−ob/ob mice [123] Hepatocyte-specific constitutive IKKβ mice [124] db/db; agouti; tubby mice [125] CXCL−/−; KKAy mice [126] TNFα−/−; ob/ob p55−/− p75−/− mice [128] |
[122, 127] |
| Atherosclerosis |
apoE−/−; IL-1Ra−/−; IL-1α−/−; IL-1β−/−; IL-4−/−; IL-6−/−; IL-10−/−; IL-12−/−; IL-18−/−; TNFα−/−; IFNγ−/−; Mif−/−; GM-CSF−/− mice reviewed in [131] Fisher 344 rats [132] |
[130] |
| Sarcopenia | MIKK, MISR mice [136] Wistar rats [137] nfkb1−/−; bcl3−/− mice [139] mdx, mdx p65+/−, mdx p50+/−mdx IKKβF/F mice [140] |
[134, 135] |
| Osteoporosis | IKKβ Δ; IKKα−/−; NIK−/−; p50−/− p52−/−; p65−/− TNFR-1−/−; RelB−/−; cRel−/− mice reviewed in: [142] IKKγ dominant-negative mice [145] |
[143, 144] |
Alzheimer’s disease
Alzheimer’s disease (AD) is the most common cause of age-associated dementia, characterized by fibrillary tangles and β-amyloid plaques [92]. AD affects only 0.6% of patients 65–69 years of age, but increases with age to 8.4% of 85+ aged patients. While the causes of AD remain poorly defined, one common characteristic is the increased inflammatory state observed in patients. While an overt inflammatory infiltration is not evident in AD, the NF-κB regulated cytokines IL-1β and TNFα are present at increased levels in the brains of AD patients [92, 93]. Murine studies have confirmed the role of cytokines such as IL-6 and IL-1β in AD [94] and mice that received brain injections of IL-1α or β exhibited increased AD associated plaque formation [95].
A possible mechanism for this increase in cytokines seen in AD is via Aβ stimulation of NF-κB activity in microglia [96]. Suppression of NF-κB in microglia results in decreased neurotoxicity [97] and NF-κB regulatory elements lie upstream of the APP protein, which is necessary for plaque formation [98]. The hypothesis that inflammation and NF-κB activation is the underlying cause of AD is further supported by observations that chronic LPS injections accelerate AD progression [99, 100] and patients with systemic infection exhibit increased rates of cognitive decline [101]. Taken together, the evidence suggests NF-κB plays a role in plaque formation and even a more important role in inflammation and cytokine signaling in AD progression.
Parkinson’s Disease
Parkinson’s disease (PD) is a neurodegenerative disease that results in a movement disorder that is observed in patients over the age of 55 years [102]. Similar to the neurodegeneration in AD patients, there is an increase in inflammatory and cytokine signaling in Parkinson’s patients. TNFα levels are increased in both brain tissue and CSF [103] and IL-1β, IL-2, IL-6 and other cytokines are increased in the CSF of Parkinson’s patients [104]. Furthermore, microglia, the innate immune cells of the brain, are highly active in vitro, in animal models of PD and in PD patients and their suppression in mouse models of PD results in reduced disease [105–116]. There is a 70 fold increase in p65/RelA activation in dopaminergic neurons, the neurons which are central to disease pathology, from PD patients compared with age-matched controls [117]. Further, treatment with a peptide inhibitor of IKK/NF-κB improved motor function and pathologic changes in a murine model of PD [118]. MPP, a chemical compound known to induce a Parkinson-like disease, activated NF-κB signaling in neurons [119]. Also, chronic NF-κB activation contributes either directly or indirectly to neuronal cell death in both PD and AD [120, 121].
Type II Diabetes
Type II Diabetes (T2D) or non insulin-dependent diabetes, is defined by insulin resistance, and is often accompanied by numerous sequela or co-morbidities including dyslipidemia, hypertension, atherosclerosis, central obesity, blindness, end-stage renal disease and non-traumatic loss of limb [122]. NF-κB has been implicated in obesity-induced insulin resistance and glucose metabolism by both pharmacologic and genetic suppression approaches [123]. Upregulation of NF-κB signaling in hepatocytes results in a type II diabetes phenotype[124]. It is further hypothesized that innate immune activation and inflammatory response underlie T2D and its associated features [122]. Macrophages or dendritic cells that reside in adipose tissue and secrete cytokines can contribute to insulin resistance and mediate disease progression [125]. Recent data suggests that mice deficient in DC/macrophage chemoattractant CXCL14 have reduced body mass and are not susceptible to Type II diabetes [126]. As with AD, systemic inflammation and cytokine secretion likely play a significant role in the onset and progression of T2D. IL-1β can induce β-cell cytotoxicity and inhibit β-cell function [127]. Mice deficient in TNFα signaling are resistant to obesity induced insulin resistance [128]. Additionally, IL-6 as well as MCP-1, IL-1β and TNFα levels are found to be increased in T2D patients [129]. Thus aberrant NF-κB activation in numerous tissues including adipose, pancreas, and liver contribute to disease pathology observed in patients with T2D.
Atherosclerosis
Atherosclerosis is a disease of arterial wall thickening and plaque formation associated with increased age and is the leading cause of coronary artery disease. Atherosclerosis results from a combination of endothelial, hematopoietic, T-cell and macrophage dysfunction. Of note, atherosclerotic lesions, specifically unstable coronary plaques have increased levels of NF-κB activity and increased release of cytokines [130]. In an LPS induced model of atherosclerosis, using apoE−/− mice, genetic suppression of NF-κB signaling led to a reduction in the size of atherosclerotic lesions [87]. As with other diseases associated with aging, NF-κB upregulation was accompanied by an increase in cytokine release and inflammatory signaling [131]. TNFα, both an inducer and target of NF-κB, increased ROS formation, apoptosis and endothelial dysfunction in rat carotid arteries, mimicking changes seen in aging arteries [132]. Inhibition of TNFα also has vasculoprotective effects [132], providing additional support for the role of NF-κB activation as a negative regulator of aging associated atherosclerosis.
Sarcopenia
Sarcopenia, defined as the loss of skeletal muscle mass and strength, is highly correlated with advancing age [133]. In hospitalized geriatric patients with increased inflammation, indicated by elevated CRP and IL-6, there was a correlative decrease in grip strength, shoulder extension strength, and an exhibited increase in muscle fatigue [134]. TNFα and IL-6 are inversely related to muscle mass and strength in elderly patients [135]. While there is little research evaluating NF-κB activation in sarcopenia, numerous studies show a significant causal role for NF-κB activation in muscle atrophy. Mice transgenic for an active form of IKKβ exhibit a muscle wasting phenotype [136]. Muscle unloading or loss of muscle innervation led to an 8-fold increase in NF-κB signaling [137–139]. Additionally NF-κB is implicated in the pathology associated with murine muscular dystrophy (mdx mice), a disease of muscle degeneration. Specific suppression of NF-κB activity in macrophages reduced muscle degeneration and systemic treatment with an IKK inhibitor reduced pathologies associated with muscular dystrophy [140].
Osteoporosis
Osteoporosis, another age-associated pathology, is defined by decreased bone density and increased fragility leading to bone breaks [141]. It is known that inflammatory cytokines IL-6, TNFα, and IL-1 [141] signaling via NF-κB are mediators of osteoclastogenesis and osteoclast function [142]. Mice transgenic for a dominant negative IKK expressed in osteoblasts had reduced bone loss after ovariectomy (12%) compared with wild-type mice, which exhibited 40% bone loss. Additionally, the fact that IL-6 and TNFα levels are markers of osteoporotic changes further implicate NF-κB and inflammation in this disease process [143]. Interestingly, patients with overactive inflammatory and NF-κB signaling exhibit increased risk of developing osteoporosis when suffering from diseases, including HIV infection, hyper-IgE syndrome, rheumatoid arthritis, myeloma, and inflammatory bowel disease [144]. Thus it is likely that, as with many age-associated disease, NF-κB acts through numerous mechanisms to promote osteoporotic degeneration [145]. Recent data by our group further supports the notion that NF-κB is directly implicated in osteoporosis, having observed that suppression of NF-κB improves bone density in accelerated aging mice as well as improves the integrity of intervertebral discs.
Concluding Remarks
It appears as if aging is driven, at least in part, by stochastic damage to cells that activates signaling pathways that contribute to age-associated degenerative changes. The NF-κB pathway is well positioned to play a key role in aging as it is activated by genotoxic, oxidative, and inflammatory stresses. The NF-κB pathway is not only activated during aging, but contributes directly to age-related pathologies. This holds true at the cellular level with cells overexpressing NF-κB subunits and also in mouse models where inhibition of NF-κB leads to reversal of skin aging and onset of age-associated pathologies. In addition to the direct evidence for a role of NF-κB in aging, the transcription factor has been linked with many of the pathways linked to lifespan and healthspan determination, including insulin/IGF-1, FOXO, SIRT, mTOR, and DNA repair. Finally there is convincing evidence that aberrant NF-κB is a central feature in many age-associated diseases from Alzheimer’s to diabetes and osteoporosis.
Therefore NF-κB represents a possible therapeutic target for attenuating the sequelae of aging. Currently, the US Federal Drug Administration does not view aging as an indication for treatment. However, as discussed, there are numerous age-associated degenerative diseases, multiple of which commonly occur in a single elderly patient that could potentially be treated with NF-κB inhibitors. In addition there are numerous progeroid syndromes, which may also benefit from similar therapeutics.
Acknowledgements
This work was supported by grants AG024827, AG033907, ES016114, CA103730 and the Ellison Medical Foundation (AG-NS-0303-05). J.S.T was supported by NRSA grant AG032816. C.L.C. is supported by a Biomedical Research Fellowship from the Hartwell Foundation.
References
- 1.Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–8. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–47. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Adler A, Sinha S, Kawahara TLA, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev. 2007:21. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–16. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 5.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–8. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 6.Verma IM. Nuclear factor (NF)-kappaB proteins: therapeutic targets. Ann Rheum Dis. 2004;63(Suppl 2):ii57–ii61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmore T. Rel/NF-κB Transcription Factors. 2009. www.nf-kb.org.
- 8.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, Dhainaut JF, Cavaillon JM. NF-kappaB expression in mononuclear cells of patients with sepsis resembles that observed in lipopolysaccharide tolerance. Am J Respir Crit Care Med. 2000;162:1877–83. doi: 10.1164/ajrccm.162.5.2003058. [DOI] [PubMed] [Google Scholar]
- 10.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, An H, Yu Y, Zhang M, Qi R, Cao X. Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J Biol Chem. 2003;278:36334–40. doi: 10.1074/jbc.M305698200. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 13.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–8. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 14.Huang TT, Wuerzberger-Davis SM, Wu ZH, Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell. 2003;115:565–76. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 15.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–6. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 16.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–48. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 17.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–4. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 18.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–4. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 20.Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–4. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- 21.Hiscott J, Nguyen TL, Arguello M, Nakhaei P, Paz S. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene. 2006;25:6844–67. doi: 10.1038/sj.onc.1209941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 23.Solt LA, Madge LA, Orange JS, May MJ. Interleukin-1-induced NF-kappaB activation is NEMO-dependent but does not require IKKbeta. J Biol Chem. 2007;282:8724–33. doi: 10.1074/jbc.M609613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-kappaB p50 ubiquitination blockade. Science. 2007;317:675–8. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- 25.Lim W, Cho J, Kwon HY, Park Y, Rhyu MR, Lee Y. Hypoxia-inducible factor 1 alpha activates and is inhibited by unoccupied estrogen receptor beta. FEBS Lett. 2009;583:1314–8. doi: 10.1016/j.febslet.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–50. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 28.Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25:6685–705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 29.Niedernhofer LJ, Robbins PD. Signaling mechanisms involved in the response to genotoxic stress and regulating lifespan. Int J Biochem Cell Biol. 2008;40:176–80. doi: 10.1016/j.biocel.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adler A, Sinha S, Kawahara TLA, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev. 2007:21. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernard D, Quatannens B, Begue A, Vandenbunder B, Abbadie C. Antiproliferative and antiapoptotic effects of crel may occur within the same cells via the up-regulation of manganese superoxide dismutase. Cancer Res. 2001;61:2656–64. [PubMed] [Google Scholar]
- 32.Bernard D, Gosselin K, Monte D, Vercamer C, Bouali F, Pourtier A, Vandenbunder B, Abbadie C. Involvement of Rel/nuclear factor-kappaB transcription factors in keratinocyte senescence. Cancer Res. 2004;64:472–81. doi: 10.1158/0008-5472.can-03-0005. [DOI] [PubMed] [Google Scholar]
- 33.Seitz CS, Deng H, Hinata K, Lin Q, Khavari PA. Nuclear factor kappaB subunits induce epithelial cell growth arrest. Cancer Res. 2000;60:4085–92. [PubMed] [Google Scholar]
- 34.Wang J, Jacob NK, Ladner KJ, Beg A, Perko JD, Tanner SM, Liyanarachchi S, Fishel R, Guttridge DC. RelA/p65 functions to maintain cellular senescence by regulating genomic stability and DNA repair. EMBO Rep. 2009;10:1272–8. doi: 10.1038/embor.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriete A, Mayo KL, Yalamanchili N, Beggs W, Bender P, Kari C, Rodeck U. Cell autonomous expression of inflammatory genes in biologically aged fibroblasts associated with elevated NF-kappaB activity. Immun Ageing. 2008;5:5. doi: 10.1186/1742-4933-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helenius M, Hanninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318(Pt 2):603–8. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korhonen P, Helenius M, Salminen A. Age-related changes in the regulation of transcription factor NF-kappa B in rat brain. Neurosci Lett. 1997;225:61–4. doi: 10.1016/s0304-3940(97)00190-0. [DOI] [PubMed] [Google Scholar]
- 38.Bregegere F, Milner Y, Friguet B. The ubiquitin-proteasome system at the crossroads of stress-response and ageing pathways: a handle for skin care? Ageing Res Rev. 2006;5:60–90. doi: 10.1016/j.arr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Giardina C, Hubbard AK. Growing old with nuclear factor-kappaB. Cell Stress Chaperones. 2002;7:207–12. doi: 10.1379/1466-1268(2002)007<0207:gownfb>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao ZQ, Majumdar AP. Induction of transcriptional activity of AP-1 and NF-kappaB in the gastric mucosa during aging. Am J Physiol Gastrointest Liver Physiol. 2000;278:G855–65. doi: 10.1152/ajpgi.2000.278.6.G855. [DOI] [PubMed] [Google Scholar]
- 41.Helenius M, Hanninen M, Lehtinen SK, Salminen A. Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-κB transcription factor in mouse cardiac muscle. J Mol Cell Cardiol. 1996;28:487–98. doi: 10.1006/jmcc.1996.0045. [DOI] [PubMed] [Google Scholar]
- 42.Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Natoli G. When sirtuins and NF-kappaB collide. Cell. 2009;136:19–21. doi: 10.1016/j.cell.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 44.Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour CM, Parrinello S, Hodgson JG, Chin K, Desprez PY, Campisi J. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5:e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends in Cell Biology. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 46.Gosselin K, Abbadie C. Involvement of Rel/NF-kappa B transcription factors in senescence. Exp Gerontol. 2003;38:1271–83. doi: 10.1016/j.exger.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 47.McCay CM, Crowell MF, Maynard LA. The effect of retarded grwoth upon the length of life span and upon the ultimate body size. Nutritions. 1935;5:155–71. [PubMed] [Google Scholar]
- 48.Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–9. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123:1589–95. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 51.Higami Y, Barger JL, Page GP, Allison DB, Smith SR, Prolla TA, Weindruch R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J Nutr. 2006;136:343–52. doi: 10.1093/jn/136.2.343. [DOI] [PubMed] [Google Scholar]
- 52.Jung KJ, Lee EK, Kim JY, Zou Y, Sung B, Heo HS, Kim MK, Lee J, Kim ND, Yu BP, Chung HY. Effect of short term calorie restriction on pro-inflammatory NF-κB and AP-1 in aged rat kidney. Inflamm Res. 2009;58:143–50. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- 53.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 54.Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K. Interaction of aging-associated signaling cascades: inhibition of NF-kappaB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci. 2008;65:1049–58. doi: 10.1007/s00018-008-7461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salminen A, Kaarniranta K. Insulin/IGF-1 paradox of aging: regulation via AKT/IKK/NF-kappaB signaling. Cell Signal. 2010;22:573–7. doi: 10.1016/j.cellsig.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–60. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 57.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–38. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–40. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 59.Bertrand F, Desbois-Mouthon C, Cadoret A, Prunier C, Robin H, Capeau J, Atfi A, Cherqui G. Insulin antiapoptotic signaling involves insulin activation of the nuclear factor kappaB-dependent survival genes encoding tumor necrosis factor receptor-associated factor 2 and manganese-superoxide dismutase. J Biol Chem. 1999;274:30596–602. doi: 10.1074/jbc.274.43.30596. [DOI] [PubMed] [Google Scholar]
- 60.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi NC, Richardson PG, Anderson KC. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–83. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 61.Balaram SK, Agrawal DK, Edwards JD. Insulin like growth factor-1 activates nuclear factor-kappaB and increases transcription of the intercellular adhesion molecule-1 gene in endothelial cells. Cardiovasc Surg. 1999;7:91–7. doi: 10.1016/s0967-2109(98)00044-1. [DOI] [PubMed] [Google Scholar]
- 62.Che W, Lerner-Marmarosh N, Huang Q, Osawa M, Ohta S, Yoshizumi M, Glassman M, Lee JD, Yan C, Berk BC, Abe J. Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: role of Gab1 and MEKK3 in TNF-alpha-induced c-Jun and NF-kappaB activation and adhesion molecule expression. Circ Res. 2002;90:1222–30. doi: 10.1161/01.res.0000021127.83364.7d. [DOI] [PubMed] [Google Scholar]
- 63.Niedernhofer LJ, Robbins PD. Signaling mechanisms involved in the response to genotoxic stress and regulating lifespan. Int J Biochem Cell Biol. 2008;40:176–80. doi: 10.1016/j.biocel.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. Embo J. 2007;26:3169–79. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu ZP, Li WX, Yu B, Huang J, Sun J, Huo JS, Liu CX. Effects of trans-resveratrol from Polygonum cuspidatum on bone loss using the ovariectomized rat model. J Med Food. 2005;8:14–9. doi: 10.1089/jmf.2005.8.14. [DOI] [PubMed] [Google Scholar]
- 67.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–53. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 69.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosh HS, Spencer JV, Ng B, McBurney MW, Robbins PD. Sirt1 interacts with transducin-like enhancer of split-1 to inhibit nuclear factor kappaB-mediated transcription. Biochem J. 2007;408:105–11. doi: 10.1042/BJ20070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinclair D, Mills K, Guarente L. Aging in Saccharomyces cerevisiae. Annu Rev Microbiol. 1998;52:533–60. doi: 10.1146/annurev.micro.52.1.533. [DOI] [PubMed] [Google Scholar]
- 72.Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14:1021–6. [PubMed] [Google Scholar]
- 73.Peng SL. Immune regulation by Foxo transcription factors. Autoimmunity. 2007;40:462–9. doi: 10.1080/08916930701464913. [DOI] [PubMed] [Google Scholar]
- 74.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–13. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 75.Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer. 2006;94:195–9. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 77.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 78.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445:922–6. doi: 10.1038/nature05603. [DOI] [PubMed] [Google Scholar]
- 80.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salminen A, Kaarniranta K. NF-kappaB signaling in the aging process. J Clin Immunol. 2009;29:397–405. doi: 10.1007/s10875-009-9296-6. [DOI] [PubMed] [Google Scholar]
- 82.Dan HC, Baldwin AS. Differential involvement of IkappaB kinases alpha and beta in cytokine- and insulin-induced mammalian target of rapamycin activation determined by Akt. J Immunol. 2008;180:7582–9. doi: 10.4049/jimmunol.180.11.7582. [DOI] [PubMed] [Google Scholar]
- 83.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–55. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 84.Schumacher B, van der Pluijm I, Moorhouse MJ, Rasile Robinson A, Suh Y, Breit TM, van Steeg H, Niedernhofer LJ, van Ijcken W, Bartke A, Spindler SR, Hoeijmakers JHJ, van der Horst GJ, Garinis George A. Parallels in genome-wide expression changes between long-lived and progeroid mice reveal a survival response promoting longevity. PLoS Genet. 2008 (In Press) [Google Scholar]
- 85.Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–43. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 86.Veuger SJ, Durkacz BW. Persistence of unrepaired DNA double strand breaks caused by inhibition of ATM does not lead to radio-sensitisation in the absence of NF-kappaB activation. DNA repair. 2011;10:235–44. doi: 10.1016/j.dnarep.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Cuaz-Perolin C, Billiet L, Bauge E, Copin C, Scott-Algara D, Genze F, Buchele B, Syrovets T, Simmet T, Rouis M. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:272–7. doi: 10.1161/ATVBAHA.107.155606. [DOI] [PubMed] [Google Scholar]
- 88.Berenbaum F. Signaling transduction: target in osteoarthritis. Curr Opin Rheumatol. 2004;16:616–22. doi: 10.1097/01.bor.0000133663.37352.4a. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto Y, Gaynor RB. Role of the NF-kappaB pathway in the pathogenesis of human disease states. Curr Mol Med. 2001;1:287–96. doi: 10.2174/1566524013363816. [DOI] [PubMed] [Google Scholar]
- 90.Kim HJ, Chang EJ, Kim HM, Lee SB, Kim HD, Su Kim G, Kim HH. Antioxidant alpha-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-kappaB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-kappaB ligand and tumor necrosis factor-alpha. Free Radic Biol Med. 2006;40:1483–93. doi: 10.1016/j.freeradbiomed.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 91.Valen G. Signal transduction through nuclear factor kappa B in ischemia-reperfusion and heart failure. Basic Res Cardiol. 2004;99:1–7. doi: 10.1007/s00395-003-0442-7. [DOI] [PubMed] [Google Scholar]
- 92.Steinman L. Nuanced roles of cytokines in three major human brain disorders. J Clin Invest. 2008;118:3557–63. doi: 10.1172/JCI36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–5. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rahman SM, Van Dam AM, Schultzberg M, Crisby M. High cholesterol diet results in increased expression of interleukin-6 and caspase-1 in the brain of apolipoprotein E knockout and wild type mice. J Neuroimmunol. 2005;169:59–67. doi: 10.1016/j.jneuroim.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 95.Sheng JG, Ito K, Skinner RD, Mrak RE, Rovnaghi CR, Van Eldik LJ, Griffin WS. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol Aging. 1996;17:761–6. doi: 10.1016/0197-4580(96)00104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–88. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–74. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 98.Grilli M, Goffi F, Memo M, Spano P. Interleukin-1beta and glutamate activate the NF-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J Biol Chem. 1996;271:15002–7. doi: 10.1074/jbc.271.25.15002. [DOI] [PubMed] [Google Scholar]
- 99.Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE Genotype and an ApoE-mimetic Peptide Modify the Systemic and Central Nervous System Inflammatory Response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- 100.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–15. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 101.Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:788–9. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller RL, James-Kracke M, Sun GY, Sun AY. Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res. 2009;34:55–65. doi: 10.1007/s11064-008-9656-2. [DOI] [PubMed] [Google Scholar]
- 103.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–10. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 104.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–6. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 105.Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide (VIP) in a mouse model of Parkinson’s disease by blocking microglial activation. FASEB J. 2003;17:944–6. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- 106.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 107.Scheller C, Sopper S, Jenuwein M, Neuen-Jacob E, Tatschner T, Grunblatt E, ter Meulen V, Riederer P, Koutsilieri E. Early impairment in dopaminergic neurotransmission in brains of SIV-infected rhesus monkeys due to microglia activation. J Neurochem. 2005;95:377–87. doi: 10.1111/j.1471-4159.2005.03373.x. [DOI] [PubMed] [Google Scholar]
- 108.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong J-S, Zhang J. Aggregated a-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. The FASEB Journal. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 109.Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003;17:1954–6. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- 111.Barcia C, Sanchez Bahillo A, Fernandez-Villalba E, Bautista V, Poza YPM, Fernandez-Barreiro A, Hirsch EC, Herrero MT. Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure. Glia. 2004;46:402–9. doi: 10.1002/glia.20015. [DOI] [PubMed] [Google Scholar]
- 112.McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental Risk Factors and Parkinson’s Disease: Selective Degeneration of Nigral Dopaminergic Neurons Caused by the Herbicide Paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 113.Miller RL, Sun GY, Sun AY. Cytotoxicity of paraquat in microglial cells: Involvement of PKCd- and ERK1/2-dependent NADPH oxidase. Brain Res. 2007;1167:129–139. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–90. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem. 2006;97:934–46. doi: 10.1111/j.1471-4159.2006.03777.x. [DOI] [PubMed] [Google Scholar]
- 116.Li G, Cui G, Tzeng N-S, Wei S-J, Wang T, Block ML, Hong J-S. Femtomolar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. The FASEB Journal. 2005;19:489–496. doi: 10.1096/fj.04-2555com. [DOI] [PubMed] [Google Scholar]
- 117.Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–6. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:18754–9. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Panet H, Barzilai A, Daily D, Melamed E, Offen D. Activation of nuclear transcription factor kappa B (NF-kappaB) is essential for dopamine-induced apoptosis in PC12 cells. J Neurochem. 2001;77:391–8. doi: 10.1046/j.1471-4159.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- 120.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–7. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 121.Gao HM, Liu B, Zhang W, Hong JS. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 122.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 123.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 124.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tanegashima K, Okamoto S, Nakayama Y, Taya C, Shitara H, Ishii R, Yonekawa H, Minokoshi Y, Hara T. CXCL14 deficiency in mice attenuates obesity and inhibits feeding behavior in a novel environment. PLoS One. 2010;5:e10321. doi: 10.1371/journal.pone.0010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD. Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1beta-induced beta-cell impairment and activation of islet cell apoptosis in vitro. Diabetes. 1999;48:1730–6. doi: 10.2337/diabetes.48.9.1730. [DOI] [PubMed] [Google Scholar]
- 128.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 129.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 130.Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A. 2004;101:5634–9. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–76. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-alpha treatment in aging. Am J Pathol. 2007;170:388–98. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol. 1977;43:1001–6. doi: 10.1152/jappl.1977.43.6.1001. [DOI] [PubMed] [Google Scholar]
- 134.Bautmans I, Njemini R, Lambert M, Demanet C, Mets T. Circulating acute phase mediators and skeletal muscle performance in hospitalized geriatric patients. J Gerontol A Biol Sci Med Sci. 2005;60:361–7. doi: 10.1093/gerona/60.3.361. [DOI] [PubMed] [Google Scholar]
- 135.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 136.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 137.Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–38. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 138.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–65. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 139.Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. The Journal of Clinical Investigation. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yun AJ, Lee PY. Maldaptation of the link between inflammation and bone turnover may be a key determinant of osteoporosis. Med Hypotheses. 2004;63:532–7. doi: 10.1016/S0306-9877(03)00326-8. [DOI] [PubMed] [Google Scholar]
- 142.Soysa NS, Alles N. NF-kappaB functions in osteoclasts. Biochem Biophys Res Commun. 2009;378:1–5. doi: 10.1016/j.bbrc.2008.10.146. [DOI] [PubMed] [Google Scholar]
- 143.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: a longitudinal study. J Clin Endocrinol Metab. 2008;93:1952–8. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- 144.Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immun Ageing. 2005;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–9. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Terzic J, Marinovic-Terzic I, Ikeda F, Dikic I. Ubiquitin signals in the NF-kappaB pathway. Biochem Soc Trans. 2007;35:942–5. doi: 10.1042/BST0350942. [DOI] [PubMed] [Google Scholar]
- 147.NF-κB Signaling, in jpeg. 2005. www.proteinlounge.com.