Abstract
Aging is associated with the chronic, low grade, Th-1 type inflammation. The key Th-1 type, pro-inflammatory cytokine, interferon-gamma (IFNG), transcriptionally induces the rate-limiting enzyme of tryptophan (TRY) – kynurenine (KYN) pathway, indoleamine 2,3- dioxygenase (IDO). Activation of IDO shunts TRY metabolism from production of serotonin (substrate of antidepressant effect) and its derivatives: N-acetylserotonin (an agonist to the receptors of brain derived neurotropic factor), and melatonin (regulator of sleep and other circadian rhythms), towards production of KYN and its derivatives (anxiogenic, neurotoxic and pro-oxidant factors). Some of kynurenines up-regulate nitric oxide synthase (NOS). Concurrently with activation of IDO, IFNG induces guanosine triphosphate cyclohydrolase I (GTPCH), the rate limiting enzyme of GTP conversion into BH2 (and increases formation of a stable derivative of BH2, neopterin, at the expense of production of BH4, the mandatory co-factor of NOS). Combination of increased NOS activity (by kynurenines) with decreased formation of BH4 leads to the uncoupling of NOS with consequent shift of arginine metabolism from biosynthesis of NO to formation of superoxide anion and other free radicals, and exacerbation of depression, anxiety and cognitive impairment caused by kynurenines. Polymorphism of IFNG (+874) T/A gene, that encodes production of IFNG protein, impacts the IDO and GTPCH activity that might be assessed in humans by KYN/TRY ratio and neopterin concentrations in biological fluids (e.g., blood, urine and spinal fluid). The hypothesis of IFNG inducible IDO/GTPCH inflammation cascade helps to understand the increased association between aging, inflammation and aging-associated psychiatric and medical (insulin resistance, obesity) disorders. Evaluation of markers of IFNG-inducible inflammation cascade might be used to assess the severity of corresponding behavioral and cognitive changes and the efficacy of pharmacological interventions (e.g., IDO inhibitors).
Keywords: Interferon-gamma, Aging, Inflammation, Neopterin, Kynurenines, Depression, Insulin resistance, Obesity, Metabolic syndrome
Aging and IFNG-induced inflammation
Recent evidences suggest that aging is associated with a chronic low grade inflammation triggered by a shift from the homeostatic balance of pro- and anti-inflammatory mediators to a pro-inflammatory Th-1 (cellular) type state (1); and by increased reactivity upon immune stimulation due to priming of brain microglial cells and peripheral macrophages. Activation of macrophages and microglia requires both a “priming” stimulus (i.e., interferon-gamma, IFNG) and a secondary “triggering” stimulus such as stress [2] or infection (e.g., gram-negative bacterial endotoxin, lipopolysaccharide (LPS) [3]. Most experimental studies of inflammation and aging focused on the role of LPS, and pro-inflammatory factors such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-1b and IL-6. Only very few studies were dedicated to the role of IFNG, the key pro-inflammatory cytokine. Meanwhile, converging evidences point to the involvement of IFNG in mechanisms of aging. Thus, interferon-related genes have been identified among six pathways regulating senescence/immortalization: the cell cycle pRB/p53, cytoskeletal, insulin growth factor-related, MAP kinase and oxidative stress pathway [4]. Gene set analysis suggested up-regulation of IFNG in post-mortem brain tissue samples from Brodmann Area 10 in the prefrontal cortex from psychotropic drug-free persons, with the history of depression [5]. Prolonged treatment with IFNG induces cellular senescence in human vascular endothelial cells via up-regulation of senescence-associated genes [6]. Age-dependent increases in IFNG production have been reported in in vitro and in vivo studies, with minor changes in the remaining evaluated cytokines in senescence-accelerated mice [7]. The frequency of A (low producer) alleles of IFNG(+874) gene that encodes the production of IFNG protein, (see below) increased with aging in line with the other evidences that centenarians are characterized by a higher frequency of genetic markers associated with better control of inflammation [8]. Down’s syndrome, a condition representing an accelerated aging, was associated with higher percentages of IFNG-producing cell in comparison with mentally retarded and healthy controls [2].
Interferones might exert their effects via activation of hundreds of genes [10]. This review focuses only on IFNG-induced concurrent transcriptional activation of indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme of kynurenine (KYN) pathway of tryptophan (TRY) metabolism, and of guanosine triphosphate cyclohydrolase (GTPCH), the rate-limiting enzyme of tetrahydrobiopterin (BH4) formation from guanosine triphosphate (GTP) [11]. Present review of literature and our data suggests that IFNG-induced concurrent activation of the rate-limiting enzymes of TRY and GTP metabolism is one of the mechanisms responsible for the increased association between aging and aging-associated psychiatric conditions such as depression, anxiety, insomnia, and cognitive impairment (e.g., vascular cognitive impairment, post-operative cognitive decline, and Alzheimer’s type dementia).
TRY – KYN metabolism
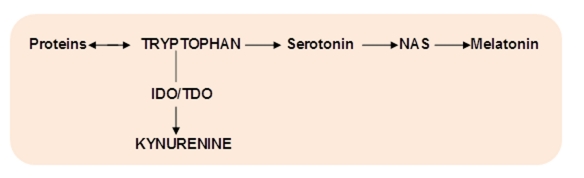
TRY is an essential amino acid (for humans) with two non-protein metabolic pathways: methoxyindoles and kynurenines, competing for the same substrate, TRY (Fig.1).
Figure 1. Major pathways of tryptophan metabolism.
NAS – N-acetylserotonin, IDO - indoleamine 2,3-dioxygenase; TDO – tryptophan 2,3-dioxygenase
Under physiological conditions, about 95% of TRY is metabolized by TRY – KYN pathway. It involves the cleavage of the indole ring of TRY resulted in the formation of N-formylkynurenine followed by KYN in an ensuing step [12] (Fig.1). The rate-limiting enzyme of KYN formation from TRY is IDO, that is ubiquitously (except the liver) distributed in mammals, in neurons, astrocytes, microglia, microvascular endothelial cells and macrophages [13]. Blood KYN/TRY ratio is used as a marker of IDO activity in human studies. Approximately 40% of the kynurenine in brain is synthesized there, the remainder having come from plasma [12].
TRY 2,3-dioxygenase (TDO) is the other enzyme (besides IDO) that catalyzes TRY conversion into kynurenine. TDO is found in the bacteria and liver, kidney and brain of the mammals [14]. In difference with IDO, TDO is activated by stress hormones but not by pro-inflammatory cytokines. However, cortisol and dexamethasone “super-induce” IDO in IFNG- primed human astrocytoma cells and native human astrocytes [15]. Since aging is characterized by elevated cortisol production due to dis-inhibition of the HPA axis [16–19], and by increased IFNG production [7], activation of both IDO and TDO might contribute to high risk of depression in the elderly [20]. On the other hand, decrease of TDO activity during an immune response in human astrocytoma cells and in native human astrocytes occurs concomitantly with IDO induction, suggesting a coordinate shift in TRY metabolism from the liver to extrahepatic tissues [21]. Aging-associated elevation of cortisol might affect life span via up-regulation of TRY - KYN metabolism. We found that life span of Drosophila Melanogaster mutants with deficient TDO (vermillion) or impaired transmembrane transport of TRY (white) was two-fold longer that the life span of wild flies [22].
Post-KYN metabolism
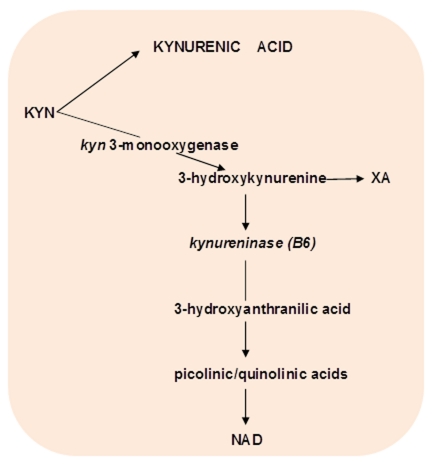
KYN is a substrate for competitive post-KYN metabolic routes: KYN – nicotinamide adenine dinucleotide (NAD), and KYN – kynurenic acid (KYNA), and 3-hydroxyKYN – xanthurenic acid (XA) (Fig.2).
Figure 2. Competitive pathways of post-kynurenine metabolism.
Abbreviations: KYNA – kynureninc acid; XA – xanthurenic acid; NAD - nicotinamide adenine dinucleotide, B6 -vitamin B6
The end product of KYN – NAD pathway is NAD. Among the intermediate metabolites of KYN – NAD pathway are N-methyl-D-aspartate (NMDA) agonists (quinolinic and picolinic acids), free radical generator, 3-hydroxyanthranilic acid, and inducers of T-cell apoptosis and lipid peroxydation (KYN and 3-hydroxyKYN) [23]. One of the major metabolite of this pathway, 3-hydroxyKYN, is a potential neurotoxin in several neurodegenerative disorders [24]. Quinolinic and picolinic acids exerted anxiogenic effects in animal models (25,26), probably, due to effect on benzodiazepines receptors [28].
The end product of KYN – KYNA pathway is KYNA, the only known endogenous antagonist to alpha 7 nicotinic acetylcholine (alpha7nACh) and NMDA receptors [23]. KYNA, as alpha7nACh antagonist, might contribute to cognitive impairment observed in depression, schizophrenia, dementia, and Down’s, Crohn’s, Huntington’s and Parkinson’s diseases and in obesity and hypertension.
The end product of 3-hydroxyKYN – XA pathway is xanthurenic acid (XA) from KYN derivative, 3-hydroxyKYN. Pyridoxal-5′-phosphate (PLP) is a co-factor of kynureninase, the enzyme of the next step converting 3-hydroxyKYN into antranilic acid. Inflammation-associated PLP deficiency inhibits kynureninase, and, consequently shifts 3-hydroxyKYN from formation of antranilic acid towards increased production of XA, that might contribute to the development of insulin resistance [28]. XA reacts with insulin with formation of a complex antigenetically indistinguishable from insulin [29, 30]. Formation of insulin/xanthurenic acid complex might result in decreased sensitivity to insulin rather than in decreased concentrations of insulin, i.e., with insulin resistance [31].
Since IDO inhibitors block the IFNG-induced activation of NOS one might suggest that IFNG effect on NOS is mediated by derivatives of KYN. In fact, two KYN derivatives, quinolinic and picolinic acids are transcriptional inducers of NOS [32, 33].
TRY – methoxyindoles metabolism
Methoxyindoles pathway leads to formation of serotonin (5-HT), N-acetylserotonin (NAS) and melatonin. Under physiological conditions only about 1 – 5% of TRY is metabolized to 5-HT [12]. Up-regulation of TRY – KYN metabolism affects not only TRY – KYN but methoxyindoles pathway as well due to competition for the availability of TRY as a common substrate for both pathways. The rate-limiting enzyme of the 5-HT biosynthesis is TRY-hydroxylase that catalyzes formation of 5-hydroxytryptophan from TRY. The subsequent decarboxylation results in the formation of 5-HT (mainly in the brain raphe nuclei and the gut’s enterochromafin tissues).
5-HT is a substrate of NAS and melatonin biosynthesis that takes place, mostly, in the pineal gland. Our post-mortem studies of human pineal confirmed observation from animal research that NAS biosynthesis depends on the availability of 5-HT (as an initial substrate) and up-regulation of beta-adrenoreceptors that mediates noradrenalin-induced stimulation of N-acetyltransferase (NAT), enzyme converting serotonin into NAS. NAS undergoes O-methylation with the formation of 5-methoxy-N-acetyltryptamine (melatonin) [34, 35].
Interferons are transcriptionally induced IDO in a variety of immune cells (e.g., monocyte-derived macrophages, microglia and 5-HT neurons [13]. IFNG is the strongest among interferones inducer of IDO [14].
TNF-alpha stimulates IDO activity and enhances (up to 300%) IFNG-induced IDO expression [14]. Other proinflammatory molecules such as IL-1, IL-12, Il-18, and PGE2 synergistically with IFNG induce IDO activity [13]. However, the induction of IDO by the bacterial endotoxin LPS might be IFNG-independent [36].
Cross-talk between the methoxyindole and kynurenine pathways
The main impact of IFNG-induced activation of IDO is up-regulation of TRY – KYN pathways and down-regulation of methoxyindoles biosynthesis due to shunt of TRY from 5-HT formation towards KYN pathway. TRY – KYN shunt in depression was originally suggested by Lapin & Oxenkrug [37, 20]. Additionally, increased production of KYN and its derivatives might contribute to down-regulation of methoxyindoles pathway because of KYN ability to inhibit TRY passage via blood-brain-barrier, and to activate IDO and 5-HT transmembrane transport [12, 38, 26]. NMDA agonists stimulate while NMDA antagonist inhibits NAT. Besides IDO, IFNG stimulates both key enzymes of KYN – NAD pathway, kynurenine mono-oxygenase and kynureninase macrophages [39]. Finally, the increased utilization of PLP by IFNG-induced up-regulation of KYN pathways might decrease availability of PLP as a co-factor for 5-HTP and DOPA decarboxylases, and, therefore, inhibit 5-HT and dopamine biosynthesis.
IFNG-induced changes in guanosine triphosphate metabolism
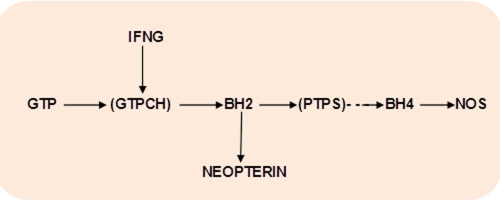
IFNG transcriptionally activates GTPCH, the rate limiting enzyme of biosynthesis of BH4, the obligatory cofactor of NOS [11] (Fig.3).
Figure 3. Interferon-gamma and GTP – BH4 pathway.
Abbreviations: IFNG – interferon-gamma; GTP – guanosine triphosphate; GTPCH – GTPcyclohydrolase 1; BH2 - 7,8-dihydroneopterin; PTPS – pyruvoyl tetrahydropterin synthase; .BH4 – tetrahydrobiopterin; NOS – nitric oxide synthase.
GTPCH catalyzes GTP conversion into 7,8-dihydroneopterin (BH2). Regulation of GTPCH might be tissues and species specific. In wild-type male and female animals the highest levels of GTPCH mRNA expression were observed within 5-HT neurons, followed by noradrenaline and then dopamine neurons. Wild-type female animals were found to express lower levels of GTPCH mRNA in each cell type when compared with levels seen in wild-type males. [40].
In humans, IFNG-induced stimulation of GTPCH does not result in correspondent up-regulation of pyruvoyl tetrahydropterin synthase (PTPS) that becomes the rate-limiting step of pteridines biosynthesis with consequent accumulation of BH2 and its stable metabolite, neopterin [41]. Importantly, IL-1 beta, in difference with IFNG, activates, mainly, PTPS, and partly GTPCH. As a result, IL-1beta increases the amount of BH4 (+40%) but concomitantly reduced the accumulation of BH2 (and neopterin) whereas IFNF-induced stimulation of GTPCH results in accumulation of BH2 (and neopterin) at the expense of BH4 formation [11]. Therefore, elevated neopterin levels in body fluids like cerebrospinal fluid, blood and urine closely reflect activation of GTPCH inducible by IFNG (but not by other pro-inflammatory factors, e.g., TNF-alpha and IL-1beta [42]. Neopterin reflects IFNG-inducible pro-inflammatory immune status and serves as a marker of cellular immune system activation in clinical studies [43]. In addition to its role as a marker of GTPCH activation and IFNG activity, neopterin (as some other pteridines), might modulate oxidative stress [44].
IFNG inducible IDO/GTHCH-inflammation cascade
We suggested that IFNG-induced concurrent up-regulation of production of kynurenines and BH2 leads to formation of an inflammation cascade that combines up-regulated activity of NOS (by kynurenines) with deficient availability of BH4 as NOS cofactor [45]. Such a combination results in an uncoupling of NOS and, consequently, in shifting of arginine metabolism away from the formation of NO and towards production of reactive oxygen species (ROS) such as superoxide anion [46] and hydrogen peroxide [47]. Superoxide is a substrate for formation of one of the most aggressive free radicals, peroxynitrite. Peroxynitrite (as well as 3-hydroxyKYN and 3-hydroxyanthranilic acids, and neopterin and other pteridines), triggers phospholipase A2 – arachidonic acid – cyclooxygenase 2 - arachidonate 5-lipoxygenase metabolic pathway resulting in the increased production of inflammatory factors: prostaglandins, via activation of cycloxygenase (COX) and leucotrienes, via activation of arachidonate 5-lipoxygenase (5-LOX) [48]. The pathway of the inflammatory enzyme, 5-LOX, was suggested as a putative common mechanism that is affected by aging and may link depression and atherosclerosis [49].
IFNG (+874) T/A gene polymorphism and IDO/GTPCH-inflammation cascade
Single-nucleotide polymorphisms (SNPs) in regulatory regions of cytokine genes have affect the levels of expression of some cytokines. IFNG protein production is encoded by polymorphic IFNG (+874) T/A gene [50]. The T to A polymorphism located at position +874 of the intron 1 of IFNG gene (+874 IFNG) directly influences the level of IFNG production associated with the CA microsatellite marker (50). Three possible genotypes are generated by analyzing the sequence mutation for +874 IFNG (either T or A), the A/A, T/A and T/T genotypes, which confer three different phenotypes: low, intermediate and high producers of IFNG respectively [51, 52]. In humans, T (high producer) allele (TA + TT genotypes) is associated with higher IFNG mRNA expression (53) and higher blood levels of IFNG, baseline and released by stimulated peripheral blood mononuclear cells, than AA genotypes [54].
Since IFNG induces IDO activity one might suggest that high producer alleles would be associated with higher IDO activity than low producer alleles. Indeed, in healthy women in Finland high producer T allele of IFNG (+874) gene was associated with increased IDO activity (i.e. elevated plasma KYN/TRY ratios) in comparison with the presence of low producer A allele [55]. This finding indicates that TRY – KYN metabolism is genetically controlled by the IFNG gene via regulation of IDO activity.
We could not find reports on the association of IFNG (+874) T/A genotypes and GTPCH activity. Our study of 174 American Caucasian reveals association of increased GTPCH activity (evaluated by plasma neopterin levels) with IFNG (+874) TT (high producers alleles) in comparison with AA and AT genotypes [56]. Our finding suggests that GTP – BH4 metabolism might be genetically controlled by the IFNG gene via regulation of GTPCH activity.
Therefore, literature and our data suggest that polymorphisms of IFNG (+874) T/A gene might impact the activity of the key enzymes of the IFNG inducible IDO/GTPCH inflammation cascade.
IFNG might be involved in epigenetic regulation of immune response by augmentation of the development of Th-1 cells from Th-17 cells via DNA methylation [57] and of O(2)(-)-generating system via histone modifications [58]. Methylation of IFNG was significantly associated with seniority in shift workers [59].
Psychiatric symptoms associated with IFNG inducible IDO/GTPCH inflammation cascade
Depression- and aging-associated inflammation is associated with behavioral and cognitive impairment. Many of the known biochemical disturbances associated with certain psychiatric conditions might be related to the up-regulated IFNG inducible IDO/GTPCH inflammation cascade.
IFNG inducible IDO/GTPCH inflammation cascade might be clinically characterized by three major markers: IFNG (+874) T/A polymorphism, KYN/TRY ratio (IDO activity), neopterin concentrations (or BH4/neopterin ratio (GTPCH activity) and indexes of NOS uncoupling (e.g., levels of BH4, NO and O2− ).
Psychiatric symptoms associated with IFNG (+874) T/A gene polymorphism
We are not aware of studies of IFNG gene polymorphisms associated with depression. Majority of information on inflammation-associated depression came from the studies of depression developed in 30 – 50% patients during IFN-alpha treatment of hepatitis C virus (HCV) and malignant melanoma [60]. IFN-alpha treatment of HCV patients a) increased the severity of depression and anxiety assessed by a Montgomery–Asberg Depression Rating Scale and HAM-A scores; b). increased blood kynurenine/TRY ratio, indicator of increased IDO activity, and c) reduced plasma TRY and serum serotonin concentrations. There were significant relationships between the IFN-alpha induced changes in the MADRS score and serum KYN (positive) and serotonin (negative) concentrations [60].
The causative role of the elevated IDO activity in mediation of co-morbid depression in inflammatory disorders could be further supported by studies of a depressive-like behavioral syndrome induced in mice by LPS-induced activation of IDO. Blockade of IDO activation by anti-inflammatory tetracycline derivative minocycline [61] or IDO inhibitor, L-methyltryptophan (L-MT), prevented the development of depressive-like symptoms [62]. Administration of KYN to naive mice dose dependently induces depressive-like behavior. These results implicate IDO as a critical molecular mediator of inflammation-induced depressive-like behavior [62]. Systemically administrated IFN-alpha passes the blood–brain barrier and reaches effective concentrations acting on microglial cells as well as macrophage receptors. IFN-alpha has much weaker direct IDO stimulating effect than IFNG but might increase IDO activity by stimulating the production of IFNG and TNF- alpha [14]. Polymorphisms of genes related to 5-HT, IL-6 and IFN-alpha receptors were studied in HCV patients treated with IFN-alpha [60, 62]. However, we found no studies of genetic polymorphisms of IFNG gene that encodes IFNG production. In our retrospective study of 180 HCV patients treated in the past with IFN-alpha we found less frequent A (low producer) allele and more frequent T (high producer) allele among patients who developed depression during treatment with IFN-alpha in comparison with HCV patients who did not experience depression during treatment with IFN-alpha [63]. Our results suggest that T (high producer) allele of IFNG (+874) gene might be a risk factor for the development of depression triggered by interferon-induced inflammation.
Psychiatric symptoms associated with IFNG-induced activation of IDO
Up-regulation of IDO leads to decreased 5-HT, NAS and melatonin production and increased formation of kynurenines due to shunt of TRY metabolism from methoxyindoles toward KYN pathway [37, 20].
“Kynurenine shunt” decreases the availability of TRY as a substrate to biosynthesis of 5-HT, the substrate of depression [37]. IFN-alpha significantly reduces serotonin in the frontal cortex, midbrain, and striatum of rats [13]
Depression and dementia are the symptoms of pellagra, the diet-induced TRY deficiency [64]. TRY depletion has been associated with the induction of depressive relapse in vulnerable patients. Key factors in depression are low levels of 5-HT and its metabolites as well as a reduced sensitivity to 5-HT receptor [65].
IFNG-induced IDO activation leads to deficient production of 5-HT, the substrate of melatonin biosynthesis. Deficiency of melatonin contributes to primary and depression-associated insomnia and circadian rhythms disturbances, especially in elderly population [66].
IDO up-regulation increases production of kynurenine, quinolinic acid and 3-hydroxykynurenine. These kynurenine derivatives exerted an anxiogenic activity in the standard animal models of anxiety [67]. Kynurenine, in its turn, activates IDO, and, thus, a “vicious circle” is formed that supports an elevated level of kynurenines for prolong time [25, 26]. Increased KYN levels were found in patients with anxiety and depression [68] suggesting that aging-associated up-regulation of kynurenines production might contribute to aging-associated increase of anxiety (69).
Depression in elderly is often associated with psychotic features. IDO up-regulation might result in increased production of KYNA, the only known endogenous NMDA antagonist. KYNA activates midbrain dopaminergic neurons similar to the effect of ketamine and MK-801, the exogenous NMDA antagonists and psychotomimetics [39]. KYNA role as psychotomimetic is further stressed by the findings of increased KYNA in brains and CSF in schizophrenia and its ability to inhibit prepulse inhibition (animal model of schizophrenia) [70, 71].
Impaired production of brain derived neurotropic factor (BDNF) is associated with IFN-alpha-induced depression in HCV patients [67]. IFNG-induced IDO activation might lead to decreased formation of BDNF receptors agonists via deficient production of 5-HT and, consequently, of NAS. The general consensus limits NAS role to the intermediate product of melatonin formation from 5-HT. However, recent studies revealed that NAS (but not 5-HT or melatonin) is a specific agonist of BDNF (TrkB) receptors [72]. Neurogenesis is essential for antidepressant effect [73], and BDNF levels were reduced during depressive episodes and recover after treatment [74]. Ability of NAS to stimulate BDNF receptors might explain our observations of anti-aging [75], anti-inflammatory [76], memory-enhancing [77] and anti-hypertensive [78,79,80] effects of NAS and its derivatives. Electroconvulsive shock elevated pineal NAS [81]. Antidepressant-like effect of NAS in mouse tail-suspension test [82], and force swimming test [72] might be mediated (in addition to Trkb receptors) via quinone reductase (QR)/melatonin 2 binding sites [83]. Therefore, NAS deficiency associated with aging [84] and inflammation might contribute to related behavioral and cognitive psychiatric changes.
KYNA antagonism to alpha-7-nicotinic acetylcholine receptors might contribute to impairment of executive function (working memory) in response to IFN-alpha therapy [85]. Increased production of KYNA was observed in Alzheimer’s disease, Down syndrome and Crohn’s disease [9], and aging. While inflammation of white matter microvessels might be one of the causative factor in vascular dementia, it might contribute to cognitive impairment at the late studies of the Alxheimer’s type dementia since beta-amyloid stimulates IFNG production [9, 86,87].
Pro-inflammatory cytokines, including IFNG, might contribute to postoperative cognitive dysfunction that occurs after surgery and can persist long after physical recovery in elderly patients [88].
Psychiatric symptoms associated with IFNG-induced activation of GTPCH
NOS uncoupling (and subsequent activation of lipid peroxidation and arachidonic acid cascade) is a major consequence of the upregulation of IFNG-inducible cascade. Accordingly, baseline levels of both plasma NO and platelet NOS activity were significantly lower in depressed subjects compared to healthy controls [89]. Treatment with paroxetine, selective 5-HT uptake inhibitor, considered a standard for prevention of IFN-alpha induced depression [60] increased plasma NO levels as well [89].
COX-2 activity might contribute to depression while 5-LO activity was suggested with a link depression and atherosclerosis [90].
Interestingly, inflammatory markers, including both IDO (TRY/KYN ratio) and GTPCH (neopterin concentrations) were found to be increased in obese rats [91] and obese subjects [60]. However, only neopterin and KYN/TRY ratios did not normalized after the bariatric surgery and weight loss [92, 93]. These studies suggest that IFNG-induced IDO and GTPCH activities have unique role as the trait (vs state) inflammatory markers in obesity.
Recent analysis of behavior and cognitive changes associated with aging indicated that increased KYNU/TRY ratio was associated with depressive symptoms of lassitude, reduced motivation, anorexia and pessimism while neopterin (and other markers of GTPCH activity were associated with neurovegetative symptoms such as digestive symptoms, fatique, sickness and sleep disturbances [85]. These results suggest that IDO component of IFNG-inducible inflammation cascade is related more to the development of psychiatric symptoms while GTPCH component is related more to the development of medical problems.
Aging and IFNG inducible IDO/GTPCH inflammation cascade
The frequency of A (low producer) alleles of IFNG(+874) gene that encodes the production of IFNG protein, increased with aging [8]. Microglia-derived IFNG was shown to stimulate astrocytes via IFNG receptor in the injured hippocampus of SAMR1 mice [94].
Down’s syndrome, a condition representing an accelerated aging, was associated with higher percentages of IFNG-producing cell in comparison with mentally retarded and healthy controls consistent with observation of an increased KYNA levels in Down’s syndrome patients [9].
Animal and human data suggest the increased activities of IDO and GTPCH with aging. Increased formation of KYN derivative, KYNA, was observed in aged rat brain [95, 96] and in human serum [97]. KYN/TRY ratio positively correlated with increased aging in humans of the three age groups (34–60, 61–71, and 72–93 years) [98] and nonagenarians in comparison with 45 years old subjects [99]. The higher rate of TRY conversion into KYN at the entry into the study was predictive of higher mortality in 10-year prospective study of nonagenarians [100].
Increased plasma levels of neopterin (but not other 33 independent immune parameters) separated the aged and a healthy younger group [101]. Neopterin levels increased with age [44, 102] with no gender differences [42] while age-associated increase of IDO was more prominent in women than in men [55].
Medical diseases associated with IFNG-induced activation of IDO
Elevation of neopterin, as a consequence of up-regulation of IFNG production, has been shown to correlate with several components of inflammation-associated metabolic syndrome, aging, and total and disease-specific mortality in populations of European ancestry [103, 104]. We assessed neopterin correlations with clinical markers of metabolic syndrome and mortality risk in population with a different genetic background, i.e., Puerto Ricans residents of Boston (592 subjects (45 – 75 years of age). Neopterin concentrations correlated with abdominal obesity (waist circumference, r=0.085, p<0.038), HDL cholesterol (r= − 0.15, p<0.0001), and insulin resistance (HOMA-IR, r=0.08, P<0.03). Neopterin concentrations of >16 nmol/L at baseline were associated with the dramatically increased risk of mortality in 113 subjects followed for 6 years [105]. These results together with previously reported data in European subjects suggest a similar pattern of neopterin correlations with metabolic syndrome and mortality risk in population with different genetic backgrounds.
Since inflammation is associated with PLP deficiency [106, 107], we assessed correlations of neopterin with PLP. Neopterin concentrations correlated with plasma (PLP (r= − 0.13, P=0.002) [105]. Our observation is in line with the previously reported decreased serum PLP and increased serum neopterin levels in patients with coronary heart disease (108)”. Since PLP deficiency is associated with the increased production of diabetogenic kynurenine derivative, xanthurenic acid [28], our results suggest that up-regulation of IFNG inducible IDO/GTPCH inflammation cascade, i.e, combination of increased formation of KYN from TRY (e.g., in depression) with PLP deficiency (e.g., in aging-associated inflammation) might contribute to the development of insulin resistance [108], and to increased incidence of diabetes in depressed patients [109]. It is noteworthy that increased uncoupling of NOS (the major consequence of IFNG inducible IDO/GTPCH inflammation cascade) was found to associate with aging and diabetes [110].
Conclusion
The hypothesis of IFNG inducible IDO/GTPCH inflammation cascade helps to understand the increased association between aging, inflammation and aging-associated psychiatric and medical (insulin resistance, obesity) disorders. Evaluation of markers of IFNG-inducible inflammation cascade might be used to assess the severity of corresponding behavioral and cognitive changes and the efficacy of pharmacological interventions (e.g., IDO inhibitors) [61].
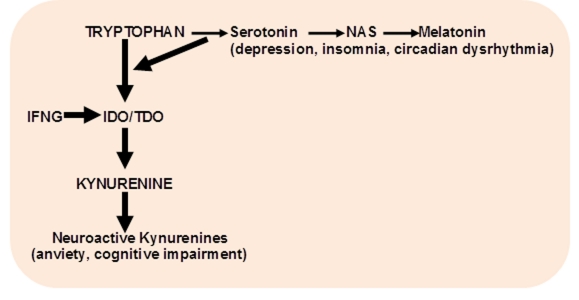
Figure 4. Shift of tryptophan metabolism in depression (Lapin, Oxenkrug, 1969; Oxenkrug, 2010).
IFNG – interferon-gamma; NAS – N-acetylserotonin, IDO-indoleamine 2,3-dioxygenase; TDO – tryptophan 2,3- dioxygenase
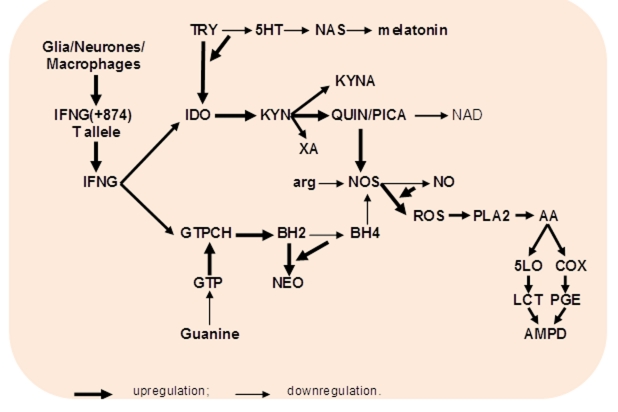
Fig. 5. IFNG-inducible activation of TRY – KYN and GTP – BH4 metabolism in aging and associated medical and psychiatric disorders.
Abbreviations: IFNG – interferon-gamma; TRY- tryptophan; 5HT – serotonin; NAS – N-acetylserotonin; IDO- indoleamine 2,3-dioxygenase; KYN – kynurenine; QUIN/PICA – quinolinic/picolinic acids; XA – xanthurenic acid; NAD - nicotinamide adenine dinucleotide; GTPCH – guanosine triphosphate cyclohydrolase 1; BH2 - 7,8-dihydroneopterin; BH4 – tetrahydrobiopterin; NEO – neopterin; NOS – nitric oxide synthase; arg - arginine; ROS - superoxide anion, hydrogen peroxide; PLA – phospholipase A2; AA – arachidonic acid; COX – cyclooxygenase 2; 5-LO – arachidonate 5-lipoxygenase; PGE – prostaglandins; LCT – leucotriens; AMPD – aging-associated medical and psychiatric disorders
Acknowledgement
Author appreciates the invaluable help of Dr. Karina Tsatourian in preparation of this manuscript.
References
- 1.Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridman AL, Tainsky MA. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27:5975–5987. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2010;16:751–62. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KS, Kang KW, Seu YB, Baek SH, Kim JR. Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells. Mech Ageing Dev. 2009;130:179–188. doi: 10.1016/j.mad.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Rodríguez MI, Escames G, López LC, López A, García JA, Ortiz F, Acuña-Castroviejo D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J Pineal Res. 2007;42:272–279. doi: 10.1111/j.1600-079X.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 8.Lio D, Scola L, Crivello A, Bonafè M, Franceschi C, Olivieri F, Colonna-Romano G, Candore G, Caruso C. Allele frequencies of +874T-A single nucleotide polymorphism at the first intron of interferon-gamma gene in a group Italian centernarians. Experimental Gerontology. 2002;37:315–319. doi: 10.1016/s0531-5565(01)00198-x. [DOI] [PubMed] [Google Scholar]
- 9.Baran H, Cairns N, Lubec B, Lubec G. Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sci. 1996;58:1891–1899. doi: 10.1016/0024-3205(96)00173-7. [DOI] [PubMed] [Google Scholar]
- 10.Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, Heim MH. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci U S A. 2008;105:7034–7039. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoedon G, Troppmair J, Adolf G, Huber C, Niederwieser A. Interferon-gamma enhances biosynthesis of pterins in peripheral blood mononuclear cells by induction of GTP-cyclohydrolase I activity. J Interferon Res. 1986;6:697–703. doi: 10.1089/jir.1986.6.697. [DOI] [PubMed] [Google Scholar]
- 12.Russo S, Kema IP, Fokkema MR, Boon JC, Willemse PH, de Vries EG, den Boer JA, Korf J. Tryptophan as a link between psychopathology and somatic states. Psychosomatic Medicine. 2003;65:665–671. doi: 10.1097/01.psy.0000078188.74020.cc. [DOI] [PubMed] [Google Scholar]
- 13.Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience. 2011;16(184):128–38. doi: 10.1016/j.neuroscience.2011.03.070. [DOI] [PubMed] [Google Scholar]
- 14.Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K, Nakamura T. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents of induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochem. Biophys. Res Commun. 1987;144:1147–1153. doi: 10.1016/0006-291x(87)91431-8. [DOI] [PubMed] [Google Scholar]
- 16.Oxenkrug GF, Pomara N, McIntyre IM, Branconnier RJ, Stanley M, Gershon S. Aging and cortisol resistance to supression by dexamethasone: a positive correlation. Psychiatry Research. 1983;10:125–130. doi: 10.1016/0165-1781(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 17.Oxenkrug GF, McIntyre IM, Stanley M, Gershon S. Dexamethasone suppression test: experimental model in rats and effect of age. Biol. Psychiat. 1984;19:413–416. [PubMed] [Google Scholar]
- 18.Oxenkrug GF, McIntyre IM, Gershon S. Effects of pinealectomy and aging on the serum corticosterone circadian rhythm in rats. J Pineal Research. 1984;1:181–185. doi: 10.1111/j.1600-079x.1984.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 19.Oxenkrug GF, Gershon S. Cognitive function and brain-adrenal axis activity in aging, depression and dementia. In: Altman H, editor. Alzheimer’s Disease and Dementia: Problems, Prospectives and Perspectives. Plenum Press; 1987. pp. 59–66. [Google Scholar]
- 20.Oxenkrug GF. Tryptophan–kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: Serotonin hypothesis revisited 40 years later. Israel J Psychiatry. 2010a;47-1:56–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Rongvaux AF, van Gool Andris F, Oberdan L. Reconstructing eukaryotic NAD metabolism. Bioessays. 2003;25:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- 22.Oxenkrug GF. The extended life span of Drosophila melanogaster eye-color (white and vermilion) mutants with impaired formation of kynurenine. J Neural Transm. 2010b;117:23–26. doi: 10.1007/s00702-009-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 24.Kincses ZT, Toldi J, Vécsei L. Kynurenines, neurodegeneration and Alzheimer’s disease. J Cell Mol Med. 2010 Jul 12; doi: 10.1111/j.1582-4934.2010.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapin IP. Kynurenines and anxiety. Adv Exp Med Biol. 1996;398:191–194. doi: 10.1007/978-1-4613-0381-7_31. [DOI] [PubMed] [Google Scholar]
- 26.Lapin IP. Neurokynurenines (NEKY) as common neurochemical links of stress and anxiety. Adv Exp Med Biol. 2003;527:121–125. doi: 10.1007/978-1-4615-0135-0_14. [DOI] [PubMed] [Google Scholar]
- 27.Guilarte TR, Block LD, Wagner HN., Jr The putative endogenous convulsant 3-hydroxykynurenine decreases benzodiazepine receptor binding affinity: implications to seizures associated with neonatal vitamin B-6 deficiency. Pharmacol Biochem Behav. 1988;30:665–668. doi: 10.1016/0091-3057(88)90080-9. [DOI] [PubMed] [Google Scholar]
- 28.Bender DA, Njagi EN, Danielian PS. Tryptophan metabolism in vitamin B6-deficient mice. Br J Nutr. 1990;63:27–36. doi: 10.1079/bjn19900089. [DOI] [PubMed] [Google Scholar]
- 29.Kotaki Y, Ueda T, Mori T, Igaki S, Hattori M. Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid (XA) Acta Vitaminol Enzymol. 1975;29:236–239. [PubMed] [Google Scholar]
- 30.Meyramov G, Korchin V, Kocheryzkina N. Diabetogenic activity of xanturenic acid determined by its chelating properties? Acta Vitaminol Enzymol. 1984;6:221–228. doi: 10.1016/s0041-1345(98)00788-x. [DOI] [PubMed] [Google Scholar]
- 31.Connick JH, Stone TW. The role of kynurenines in diabetes mellitus. Med Hypotheses. 1985;18:371–376. doi: 10.1016/0306-9877(85)90104-5. [DOI] [PubMed] [Google Scholar]
- 32.Melillo G, Cox DW, Biragyn A, Sheffler LA. Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J Biol Chem. 1994;269:8128–813. [PubMed] [Google Scholar]
- 33.Perez-Severiano F, Escalante, Rios C. Nitric oxide synthase inhibition prevents acute quinolinate-induced striatal neurotoxicity. Neurochem Res. 1998;23:1297–1302. doi: 10.1023/a:1020700401678. [DOI] [PubMed] [Google Scholar]
- 34.Oxenkrug GF, Anderson GF, Dragovic L, Blaivas M, Riederer P. Circadian rhythms of human pineal melatonin, related indoles, and beta adrenoreceptors: post-mortem evaluation, J. Pineal Res. 1990;9:1–11. doi: 10.1111/j.1600-079x.1990.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 35.Oxenkrug GF, Requintina PJ. Stimulation of rat pineal melatonin biosynthesis by N-acetylserotonin. Int. J Neurosci. 1994;77:237–241. doi: 10.3109/00207459408986034. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Lawson MA, Dantzer R, Kelley KW. LPS-induced indoleamine 2,3-dioxygenase is regulated in an interferon-gamma-independent manner by a JNK signaling pathway in primary murine microglia. Brain Beh Immun. 2010;24:201–209. doi: 10.1016/j.bbi.2009.06.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapin IP, Oxenkrug GF. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;1:32–39. doi: 10.1016/s0140-6736(69)91140-4. [DOI] [PubMed] [Google Scholar]
- 38.Kiseleva IP, Lapin IP, Prakh’e IB, Samsonova ML. Different effects of kynurenines on rat liver tryptophan pyrrolase activity. Vopr Med Khim. 1980 Jul-Aug;26(4):458–61. [PubMed] [Google Scholar]
- 39.Zawilska JB, Lorenc A, Berezińska M. Regulation of serotonin N-acetyltransferase activity in the chick pineal gland by UV-A and white light: role of MK-801- and SCH 23390-sensitive retinal signals. Pharmacol Rep. 2007;59:408–413. [PubMed] [Google Scholar]
- 40.Shimoji M, Hirayama K, Hyland K, Kapatos G. GTP cyclohydrolase I gene expression in the brains of male and female hph-1 mice. J Neurochem. 1999;72:757–764. doi: 10.1046/j.1471-4159.1999.0720757.x. [DOI] [PubMed] [Google Scholar]
- 41.Franscini N, Blau N, Walter RB, Schaffner A, Schoedon G. Critical role of interleukin-1beta for transcriptional regulation of endothelial 6-pyruvoyltetrahydropterin synthase. Arterioscler Thromb Vasc Biol. 2003 Nov 1;23(11):e50–53. doi: 10.1161/01.ATV.0000099785.65848.F1. [DOI] [PubMed] [Google Scholar]
- 42.Schennach H, Murr C, Gächter E, Mayersbach P, Schönitzer D, Fuchs D. Factors Influencing Serum Neopterin Concentrations in a Population of Blood Donors. Clinical Chemistry. 2002;48:643–645. [PubMed] [Google Scholar]
- 43.Widner MB, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs D, Avanzas P, Arroyo-Espliguero R, Jenny M, Consuegra-Sanchez L, Kaski JC. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem. 2009;16:4644–4653. doi: 10.2174/092986709789878247. [DOI] [PubMed] [Google Scholar]
- 45.Oxenkrug GF. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated medical and psychiatric disorders. J Neural Transm. 2011;118:75–85. doi: 10.1007/s00702-010-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 47.Heinzel B, John M, Klatt P, Böhme E, Mayer B. Ca+2/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–233. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 49.Qu T, Uz T, Manev H. Inflammatory 5-LOX mRNA and protein are increased in brain of aging rats. Neurbiology of Aging. 2000:647–652. doi: 10.1016/s0197-4580(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 50.Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV. A single nucleotide polymorphism in the first intron of the human INFG gene: absolute correlation with a polymorphic CA micro marker of high INFG gene. Hum Immunol. 2000;61:8333–866. doi: 10.1016/s0198-8859(00)00167-1. [DOI] [PubMed] [Google Scholar]
- 51.Perrey C, Pravica V, Sinnott PJ, Hutchinson IV. Genotyping for polymorphisms in interferon-gamma, interleukin-10, transforming growth factor-beta 1 and tumour necrosis factor-alpha genes: a technical report. Transplant Immunol. 1998;6:193–197. doi: 10.1016/s0966-3274(98)80045-2. [DOI] [PubMed] [Google Scholar]
- 52.Tambur AR, Ortegel JW, Ben-Ari Z, Shabtai E, Klein T, Michowiz R, Tur-Kaspa R, Mor E. Role of cytokine gene polymorphism in hepatitis C recurrence and allograft rejection among liver transplant recipients. Transplantation. 2001;71:1475–1480. doi: 10.1097/00007890-200105270-00020. [DOI] [PubMed] [Google Scholar]
- 53.Biolo G, Amoroso A, Savoldi S, Bosutti A, Martone M, Pirulli D, Bianco F, Ulivi S, Bertok S, Artero M, Barazzoni R, Zanetti M, Grassi G, Guarnieri G, Panzetta G. Association of interferon-gamma +874A polymorphism with reduced long-term inflammatory response in haemodialysis patients. Nephrol Dial Transplant. 2006;21:1317–1322. doi: 10.1093/ndt/gfk033. [DOI] [PubMed] [Google Scholar]
- 54.Anuradha B, Rakh SS, Ishaq M, Murthy KJ, Valluri VL. Interferon-gamma Low producer genotype +874 overrepresented in Bacillus Calmette-Guerin nonresponding children. Pediatr Infect Dis J. 2008;27:325–329. doi: 10.1097/INF.0b013e31816099e6. [DOI] [PubMed] [Google Scholar]
- 55.Raitala A, Pertovaara M, Karjalainen J, Oja SS, Hurme M. Association of interferon-gamma +874(T/A) single nucleotide polymorphism with the rate of tryptophan catabolism in healthy individuals. Scand J Immunol. 2005;61:387–390. doi: 10.1111/j.1365-3083.2005.01586.x. [DOI] [PubMed] [Google Scholar]
- 56.Oxenkrug G, Perianayagam, Summergrad P. Interferon-gamma (+874) single nucleotide polymorphism and human plasma neopterin levels. American Journal of Neuroprotection and Neuroregeneration. 2011 in press. [Google Scholar]
- 57.Bending D, Newland S, Krejcí A, Phillips JM, Bray S, Cooke A. Epigenetic changes at Il12rb2 and Tbx21 in relation to plasticity behavior of Th17 cells. J Immunol. 2011;186:3373–33825. doi: 10.4049/jimmunol.1003216. [DOI] [PubMed] [Google Scholar]
- 58.Kikuchi H, Kuribayashi F, Kiwaki N, Takami Y, Nakayama T. GCN5 regulates the superoxide-generating system in leukocytes via controlling gp91-phox gene expression. J Immunol. 2011;186:3015–3022. doi: 10.4049/jimmunol.1000364. [DOI] [PubMed] [Google Scholar]
- 59.Bollati V, Baccarelli A, Sartori S, Tarantini L, Motta V, Rota F, Costa G. Epigenetic effects of shiftwork on blood DNA methylation. Chronobiol Int. 2010;27:1093–1104. doi: 10.3109/07420528.2010.490065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oxenkrug G, Perianayagam M, Mikolich D, Requintina P, Shick L, Ruthazer R, Zucker D, Summergrad P. Interferon-gamma (+874) T/A genotypes and risk of IFN-alpha-induced depression. J Neural Transm. 2011;118:271–274. doi: 10.1007/s00702-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pitche PT. [Pellagra] Sante. 2005;15:205–208. [PubMed] [Google Scholar]
- 65.Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 66.Zhdanova IV, Masuda K, Quasarano-Kourkoulis C, Rosene DL, Killiany RJ, Wang S. Aging of intrinsic circadian rhythms and sleep in a diurnal nonhuman primate, Macaca mulatta. J Biol Rhythms. 2011;26:149–159. doi: 10.1177/0748730410395849. [DOI] [PubMed] [Google Scholar]
- 67.Kenis G, Prickaerts J, van Os J. Depressive symptoms following interferon-alpha therapy: mediated by immune-induced reductions in brain-derived neurotropic factor. Inter J Neuropsychpharmacol. 2010:1–7. doi: 10.1017/S1461145710000830. [DOI] [PubMed] [Google Scholar]
- 68.Orlikov AB, Prakhye IB, Ryzov IV. Kynurenine in blood and DST in patients with endogenus anxiety and endogenous depression. Biol Psychiatry. 1994;36:97–102. doi: 10.1016/0006-3223(94)91189-4. [DOI] [PubMed] [Google Scholar]
- 69.Dilman VM, Lapin IP, Oxenkrug GF. Serotonin and aging. In: Essman W, editor. Serotonin in health and disease. Vol. 5. Spectrum Press; NY-London: 1979. pp. 111–123. [Google Scholar]
- 70.Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulated cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–37. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 71.Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. 2010;36:211–8. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, Ye K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci U S A. 2010;107:3876–3881. doi: 10.1073/pnas.0912531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 74.Fernandes BS, Gama CS, Ceresér KM, Yatham LN, Fries GR, Colpo G, de Lucena D, Kunz M, Gomes FA, Kapczinski F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: A systematic review and meta-regression analysis. J Psychiatr Res. 2011;45:995–1004. doi: 10.1016/j.jpsychires.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Oxenkrug GF. Anti-Aging Effect of (-) Deprenyl and Inhibition of Lipid Peroxidation by N- acetylserotonin and Mating (a mini-review) In: Torok T, Klebovich I, editors. Monoamine Oxidase inhibitors and their role in neurotransmission (drug development) Medicina Publishing House Co; Budapest: 2004. pp. 309–319. [Google Scholar]
- 76.Oxenkrug GF. Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications. Ann. N Y Acad Sci. 2005;1053:334–347. doi: 10.1196/annals.1344.029. [DOI] [PubMed] [Google Scholar]
- 77.Oxenkrug GF, Requintina PJ, Bachurin S. Antioxidant and anti-aging activity of N-acetylserotonin in the in vivo and in vitro models. Ann. New York Acad Sci. 2001;939:190–199. doi: 10.1111/j.1749-6632.2001.tb03626.x. [DOI] [PubMed] [Google Scholar]
- 78.Oxenkrug GF. N-acetylserotonin and the hypotensive effect of MAO-A inhibition (mini-review) Vopr. Med. Khim. 1998;43:522–526. (Russian) [PubMed] [Google Scholar]
- 79.Oxenkrug GF. Antidepressive and antihypertensive effects of MAO-A inhibition: role of N-acetylserotonin. Neurobiology. 1999;7:213–224. [PubMed] [Google Scholar]
- 80.Oxenkrug GF. Antidepressant effect of N-acetylserotonin in relation to aging, hypertension and cancer. In: Berstein L, editor. Hormones, age and cancer. Nauka; St.Petersburg: 2005. pp. 140–158. [Google Scholar]
- 81.Oxenkrug GF, Requintina PJ, McIntyre IM, Davis R. Stimulation of rat pineal melatonin synthesis by a single electroconvulsive shock: chronobiological effect of antidepressant therapy? In: Sandler M, Coppen A, Harnett S, editors. 5-hydroxytryptamine in Psychiatry: A spectrum of Ideas. Oxford University Press; 1991. pp. 110–115. [Google Scholar]
- 82.Prakhie IV, Oxenkrug GF. The effect of nifedipine on activity of MAO inhibitors, N-acetylserotonin and melatonin in the mouse tail suspension test. Int J Neuropsychopharmacol. 1998;1:35–40. doi: 10.1017/S1461145798001096. [DOI] [PubMed] [Google Scholar]
- 83.Oxenkrug GF, Bachurin SO, Prakhie IV, Zefirov NS. Quinone reductase 2 and antidepressant effect of melatonin derivatives. Ann N Y Acad Sci. 2010;1199:121–124. doi: 10.1111/j.1749-6632.2009.05354.x. [DOI] [PubMed] [Google Scholar]
- 84.McIntyre IM, Oxenkrug GF. Effect of aging on melatonin biosynthesis induced by 5-hydroxytryptophan and constant light on rats. Prog. Neuro-Psychopharmacol.& Biol Psychiat. 1991;15:561–566. doi: 10.1016/0278-5846(91)90031-u. [DOI] [PubMed] [Google Scholar]
- 85.Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Layé S, Fuchs D. Chronic Low-Grade Inflammation in Elderly Persons Is Associated with Altered Tryptophan and Tyrosine Metabolism: Role in Neuropsychiatric Symptoms. Biol Psychiatry. 2011;70:175–82. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 86.Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep. 2002;7:199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- 87.Leonard B. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 88.Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chrapko W, Jurasz P, Radomski MW, Archer SL, Newman SC, Baker G, Lara N, Le Mellédo JM. Alteration of decreased plasma NO metabolites and platelet NO synthase activity by paroxetine in depressed patients. Neuropsychopharmacology. 2006;31:1286–1293. doi: 10.1038/sj.npp.1300961. [DOI] [PubMed] [Google Scholar]
- 90.Manev G, Manev R. 5-lipoxygenase as a possible biological link between depressive symptoms and atherosclerosis. Arch Gen Psychiatry. 2007;64:1333–1334. doi: 10.1001/archpsyc.64.11.1333. [DOI] [PubMed] [Google Scholar]
- 91.Finkelstein JA. Brain serotonergic activity and plasma amino acid levels in genetically obese Zucker rats. Pharmacol Biochem Behav. 1982;17:939–944. doi: 10.1016/0091-3057(82)90476-2. [DOI] [PubMed] [Google Scholar]
- 92.Brandacher G, et al. Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes Surg. 2006a;16:541–548. doi: 10.1381/096089206776945066. [DOI] [PubMed] [Google Scholar]
- 93.Breum L, Rasmussen MH, Hilsted J, Fernstrom JD. Twenty-four-hour plasma tryptophan concentrations and ratios are below normal in obese subjects and are not normalized by substantial weight reduction. Am J Clin Nutr. 2003;77:1112–1118. doi: 10.1093/ajcn/77.5.1112. [DOI] [PubMed] [Google Scholar]
- 94.Hasegawa-Ishii S, Takei S, Inaba M, Umegaki H, Chiba Y, Furukawa A, Kawamura N, Hosokawa M, Shimada A. Defects in cytokine-mediated neuroprotective glial responses to excitotoxic hippocampal injury in senescence-accelerated mouse. Brain Behav Immun. 2011;25:83–100. doi: 10.1016/j.bbi.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 95.Gramsbergen JB, Schmidt W, Turski WA, Schwarcz R. Age-related changes in kynurenic acid production in rat brain. Brain Res. 1992;588:1–5. doi: 10.1016/0006-8993(92)91337-e. [DOI] [PubMed] [Google Scholar]
- 96.Moroni F, Russi P, Carlá V, Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci Lett. 1998;94:145–150. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- 97.Urbańska EM, Luchowski P, Luchowska E, Pniewski J, WoŸniak R, Chodakowska-Zebrowska M, Lazarewicz J. Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharmacol Rep. 2006;58:507–511. [PubMed] [Google Scholar]
- 98.Frick B, Schroecksnadel K, Neurauter G. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 99.Niinisalo P, Raitala A, Pertovaara M, Oja SS, Lehtimäki T, Kähönen M, Reunanen A, Jula A, Moilanen L, Kesäniemi YA, Nieminen MS, Hurme M. Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 study. Scand J Clin Lab Invest. 2008;68:767–770. doi: 10.1080/00365510802245685. [DOI] [PubMed] [Google Scholar]
- 100.Pertovaara M, Raitala A, Lehtimäki T, Karhunen PJ, Oja SS, Jylhä M, Hervonen A, Hurme M. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 101.Fahey JL, Schnelle JF, Boscardin J, Thomas JK, Gorre ME, Aziz N, Sadeghi H, Nishanian P. Distinct categories of immunologic changes in frail elderly. Mech Ageing Dev. 2000;15:1–20. doi: 10.1016/s0047-6374(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 102.Spencer ME, Jain A, Matteini A, Beamer BA, Wang NY, Leng SX, Punjabi NM, Walston JD, Fedarko NS. Serum levels of the immune activation marker neopterin change with age and gender and are modified by race, BMI, and percentage of body fat. J Gerontol A Biol Sci Med Sci. 2010;65:858–865. doi: 10.1093/gerona/glq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grammer TB, Fuchs D, Boehm BO, Winkelmann BR, Maerz W. Neopterin as a predictor of total and cardiovascular mortality in individuals undergoing angiography in the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2009;55:1135–1146. doi: 10.1373/clinchem.2008.118844. [DOI] [PubMed] [Google Scholar]
- 104.Ledochowski M, Murr C, Widner B, Fuchs D. Association between insulin resistance, body mass and neopterin concentrations. Clin Chim Acta. 1999;282:115–123. doi: 10.1016/s0009-8981(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 105.Oxenkrug G, Tucker K, Requintina P, Summergrad P. Neopterin, a marker of interferon-gamma-inducible inflammation, correlates with pyridoxal-5′-phosphate, waist circumference, HDL-cholesterol, insulin resistance and mortality risk in adult Boston community dwellers of Puerto Rican origin. American Journal of Neuroprotection and Neuroregeneration. 2011;3:48–52. doi: 10.1166/ajnn.2011.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140:103–110. doi: 10.3945/jn.109.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen J, Lai CQ, Mattei J, Ordovas JM, Tucker KL. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: the Boston Puerto Rican Health Study. Am J Clin Nutr. 2010;91:337–342. doi: 10.3945/ajcn.2009.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rudzite V, Jurica E, Fuchs D, Kalnins U, Erglis A, Trusinskis K. Inflammation, homocysteine, pyridoxal-5-phosphate and lipids in patients with coronary artery disease before and six months after coronary angioplasty followed by stent implantation. Pteridines. 2005;16:190–194. [Google Scholar]
- 109.Campayo A, de Jonge P, Roy JF, Saz P, de la Cámara C, Quintanilla MA, Marcos G, Santabárbara J, Lobo A ZARADEMP Project. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167:580–588. doi: 10.1176/appi.ajp.2009.09010038. [DOI] [PubMed] [Google Scholar]
- 110.White RE, Gerrity R, Barman SA, Han G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids. 2010;75:788–793. doi: 10.1016/j.steroids.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]