Abstract
Older age has long been associated with altered inflammation and hemostasis regulation. Emerging evidence suggests that age-related differences in inflammation and hemostasis abnormalities may play a role in the development of and long-term outcomes after critical illness. A better understanding of underlying mechanisms may provide new possibilities for therapeutic interventions. In this review, we will examine how age-related differences in inflammatory and coagulation responses are affected through the continuum of healthy state, before infection occurs, to severe sepsis and recovery.
Keywords: Aging, Infection, Sepsis, Inflammation, Coagulation
Older age has long been associated with altered inflammatory and hemostasis regulation [1, 2]. For example, chronic elevation of inflammatory and hemostasis markers has been implicated in a number of age-related chronic conditions, such as frailty syndrome, Alzheimer’s disease, and atherosclerosis [3, 4]. The effects of age-related inflammatory and hemostasis dysregulation on critical illnesses, such as sepsis, remains incompletely understood.
Classically, sepsis has been characterized by an exuberant immune response, reflected in many-fold higher levels of inflammatory and hemostasis markers. Given the well known higher mortality seen in older adults, it has been suggested that sepsis in the elderly is driven by an age-related increase in inflammation. Recently it has been hypothesized that sepsis may also be characterized by an exuberant anti-inflammatory or immunosuppressive phase that occurs after the initial inflammatory burst [5, 6]. Unfortunately, clinical studies have failed to confirm these hypotheses. The complexity of the immune response in sepsis has befuddled clinicians and scientists alike and underscores the many failed therapeutic trails to date.
Understanding the potential effects of aging on the inflammatory and hemostasis response before, during, and after sepsis is important to design appropriate prevention and treatment strategies for the elderly. In this article, we will examine how age-related differences in inflammatory and coagulation responses are affected through the continuum of healthy state, before infection occurs, to severe sepsis and recovery.
Inflammation and hemostasis as markers of immune response
Clinical and experimental models of sepsis often measure circulating inflammatory and hemostasis cytokines/chemokines as markers of immune activity. There exist other biologic processes of systemic inflammation, however, such the stress triggered inflammatory system mediated by the hypothalamic-pituitary-adrenal axis, and are reviewed elsewhere [7]. The immune system is divided into the innate response and the adaptive response. The innate response comprises the initial recognition and destruction of pathogens and is characterized by its rapid action and lack of immunologic memory. It is triggered by the recognition of a pathogenic antigen by Toll-like receptors on circulating immune cells. This results in the activation of transcription factor NF-kB, a “nodal point” linking pathogen recognition to immune response [2]. The NF-kB system is considered the master regulator of the innate immune system and leads to the production of pro-inflammatory cytokines such as TNF and IL-6 that are crucial to activating downstream cytokines, recruiting immune effector cells, activating coagulation networks, and stimulating the adaptive immune response. Adaptive immunity differs from innate immunity in its ability to form an immunologic memory and thereby adapt its response to previously encountered pathogens. Upon elimination of the pathogen, mechanisms to resolve the immune response are activated to prevent unintended harm to the host. Such mechanisms include the prostaglandin network and the activated glucocorticoid receptor, the latter of which stimulates production of glucocorticoids that counteract the effects of activated NF-kB [8–10]. Both the initiation and resolution of the immune response are highly coordinated, active processes.
Sepsis in the elderly
Sepsis, the 10th leading cause of death in the elderly, refers to the presence of at least two systemic inflammation response syndrome criteria (fever or hypothermia, tachycardia, increased respiratory rate, and elevated leukocyte count) in the presence of infection. Most adult patients with an infection serious enough to require hospitalization are septic. Respiratory tract infections, such as community-acquired-pneumonia (CAP), are the leading cause of sepsis in developed countries [11]. When sepsis is complicated by organ failure, it is called severe sepsis and is a grave prognostic indicator. For instance, 90-day mortality after CAP is 6%, whereas mortality approaches 26% for patients who develop severe sepsis, and the mortality approaches 40% for the subset that develop septic shock [12].
Severe sepsis is a disease of the elderly. In a large epidemiologic study, Angus, et al. showed that approximately 750,000 people per year develop severe sepsis in the U.S., with a mean age of 63.8 years [11]. The incidence increases exponentially with age, and hospitalization rates for severe sepsis in those ≥ 80 years were approximately 2-fold, 6-fold, and 15–19 fold higher than those 65–79yr, 50–64yr, and 35–49 years, respectively [13, 14]. The higher incidence of severe sepsis in the elderly is likely due to a higher risk of infection and a higher risk of organ dysfunction once infection occurs.
The incidence rates of severe sepsis have increased over the past 2 decades, likely due to the aging of the population and an increased number of older adults with chronic diseases, prosthetic devices, and immunosuppressive states. Martin et al. found that, from 1979–2000, the average incidence of sepsis increased 11.5% per year in those ≥ 65 years compared to 9.5% per year in those < 65 years [14]. The true incidence rate may be higher, since sepsis is a clinical diagnosis and the elderly typically exhibit blunted signs of infection.
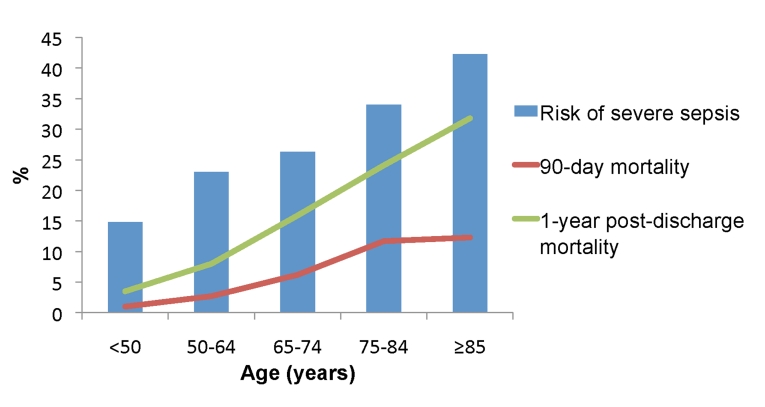
Older age is also an independent risk factor for mortality among adults hospitalized with sepsis. Between 1979–2002, case fatality increased linearly across age deciles and averaged 27.7% for those ≥ 65 years compared to 17.7% for those < 65 years [14]. In a recent prospective cohort study of patients hospitalized with pneumonia-induced sepsis, we found an age-related increase in in-hospital, 90-day, and 1-year post-discharge mortality (Figure 1) [6].
Figure 1. Age-stratified risk of severe sepsis and mortality rates from a prospective cohort study of adults hospitalized with community-acquired pneumonia.
90-day mortality measured from day of hospital admission. 1-year post-discharge mortality measured from day of hospital discharge. All rates increased significantly across age groups (P<0.001).
The reason for the higher susceptibility to infection, and once infection occurs, the higher risk of severe sepsis in older adults remains unclear. Epidemiologic studies suggest that age-related differences in clinical risk factors, such as chronic disease burden and higher rates of institutionalization in the elderly, help to explain some, but not all, of these differences [15]. Another factor may be immunosenescence, or the age-related subclinical changes in the innate and adaptive immune response, which may increase the risk of severe sepsis and subsequent mortality. To this end, we will focus on how age-related inflammation and hemostasis, two interrelated and well-studied components of the immune response, act as markers of overall immune activity and examine its role in the incidence, severity, and long-term outcomes of sepsis.
Role of inflammation and hemostasis in susceptibility to infection
Older age is associated with chronically elevated circulating levels of inflammatory markers such as interleukin (IL)-6, tumor necrosis factor (TNF), IL-1 receptor antagonist, and C-reactive protein (CRP) [2, 16]. Although chronic inflammation is associated with several chronic diseases, plasma levels of IL-6 have been shown to be associated with increased age, independent of chronic disease states [17]. Increased inflammatory markers in older adults are on average 2–4 fold higher than in younger adults. In contrast, inflammatory markers are several log-fold higher during sepsis compared to levels observed in the absence of infection, regardless of age.
Aging is also associated with a pro-thrombotic state, in part due to activation of hemostasis networks by inflammatory cytokines. Older adults exhibit increased levels of fibrinogen, F-VII, F-VIII and other clotting factors [18, 19]. In a study of 1729 subjects ≥ 70 years, high D-dimer levels were found in 7% of those 70–79 years, 13% of those 80–89 years, and 23% of those 90–99 years [20]. Clinically, this pro-thrombotic state may explain the age-related increased risk of venous thrombosis, atherosclerosis, and pulmonary emboli [19, 21].
The reasons for increased inflammation and coagulation in older adults are unclear and likely multifactorial [22]. It has been proposed that latent infections, such as cytomegalovirus (CMV), which are prevalent in up to 80% of older adults, may act as chronic stimuli of the inflammatory system [23, 24]. There is also growing research into the role oxidative stress in creating pro-inflammatory states [25]. Aging is associated with increased pro-inflammatory prostaglandins such as cycloxygenase and lipooxygenase. During aging, anti-oxidant systems decline, leading to an imbalance in redox status and activation of redox-sensitive transcription factor NF-kB [2]. Activation of NF-kB leads to the expression of pro-inflammatory mediators, including TNF and IL-6, as well as upregulation of adhesion molecules [25, 26].
Regardless of its causes, a pro-inflammatory milieu can serve as a risk factor for infection in at least two ways. First, persistent activation of B and T lymphocytes by inflammatory cytokines is thought to extinguish the replicative potential of immune cells [27]. For example, T cells of patients with inflammatory syndromes such as rheumatoid arthritis and chronic infections, including CMV, have shortened telomeres, indicative of extensive replication, and decreased T cell receptor repertoire. These changes are also characteristic of T cell aging and limit the ability of the adaptive immune system to respond to novel antigens [28].
Persistent inflammation has been shown to increase risk of bacterial invasion in rodent models. Systemic administration of TNF in rats before inoculation of bacteria into the lungs worsens pneumonia by reducing alveolar neutrophil recruitment and bacterial clearance [29, 30]. A critical step during the process of bacterial invasion is the adherence of bacteria to host cell surfaces. Recent studies show that two receptors common on many cell types, polymeric immunoglobulin receptor (pIgR) and platelet activating factor receptor (PAFr), are vulnerable to bacterial attachment [31, 32]. Mice lacking PAFr as well as those treated with PAFr antagonists are resistant to invasive pneumococcus [33, 34]. Both pIgR and PAFr are upregulated by transcription factor NF-kB. Cells in acute and chronic states of inflammation have been found to express increased pIgR and PAFr and are bound by S. pneumoniae more often than resting cells. Using a mouse model, Hinojosa and colleagues recently sought to determine if chronic, low-grade inflammation in mice would cause increased levels of pIgR and PAFr in the lung and increased susceptibility to pneumonia [35]. Young mice, when infused systemically with low levels of TNF for up to 6 days, had increased lung pIgR and PAFr protein levels and a susceptibility to pneumococcal pneumonia that was higher than young mice controls and on par with sham old mice.
Epidemiologic studies also suggest that increases in inflammation may increase the susceptibility to infection. For instance, we have previously shown that chronic inflammation is a risk factor for hospitalization with pneumonia in otherwise healthy older adults. In a cohort of well-functioning older adults 70–79 years, the highest tertiles of circulating TNF and IL-6 were associated with a higher risk of pneumonia independent of coexisting medical conditions, smoking status, or use of steroid medication [36]. Whether the small differences in circulating cytokine levels and the increased risk of serious infection observed in clinical studies constitutes a cause-effect relationship or is simply a marker for other immune defects are unclear.
Role of inflammation and hemostasis response during sepsis
A dysregulated immune response has been suggested to play an important role in the progression of sepsis to severe sepsis, and clinical trials have focused on modulating the immune response by targeting different inflammatory molecules. Epidemiologic and animal studies have shown that higher circulating inflammatory cytokines, such as IL-6, are associated with increased risk of severe sepsis and mortality [37–39]. The inflammatory response also activates, and is in turn activated by, the coagulation response and diminished fibrinolysis [40]. This promotes vascular leakage, vasodilation, microvascular dysfunction, and increases the risk of organ hypoperfusion and downstream ischemia.
While higher systemic levels of inflammation and coagulation are associated with multiple organ failure and death, several lines of evidence suggest that severe sepsis is a heterogeneous condition. For example, trials using limited duration anti-TNF strategies have failed to improve outcomes [41]. Recent evidence from well-designed epidemiologic studies have shown that, while higher pro-inflammatory and hemostasis levels are associated with organ failure and mortality, these abnormalities are present in many patients who do not develop organ dysfunction. In a large, multicenter inception cohort of 2320 subjects enrolled upon presentation to the emergency department with CAP, a broad panel of inflammatory (TNF, IL-6, IL-10) and coagulation markers (D-dimer, antithrombin, F-IX, thrombin-antithrombin complex, plasminogen-activator inhibitor-1) were measured daily for the first week and weekly thereafter [42]. Although increasing levels of inflammatory markers on day 1 were associated with increased risk of severe sepsis and mortality, the differences in inflammatory profile between survivors and non-survivors were modest. Furthermore, the onset of organ dysfunction was not associated with an increase in cytokine levels. Similarly, although day 1 coagulation abnormalities increased with illness severity and mortality, abnormalities also occurred in those subjects who never developed organ dysfunction and differences between the groups were modest.. Finally, the majority of inflammatory and coagulation markers peaked on day 1, indicating that these changes were already underway prior to presentation.
Numerous animal and human studies have been conducted to examine the effect of aging on the inflammatory and hemostasis response in sepsis. Older mice subjected to experimental sepsis, either by lipopolysaccharide (LPS) inoculation or by cecal ligation and puncture (CLP), have higher circulating inflammatory and hemostasis response and have higher mortality compared to younger mice [43–45]. While older mice tend to have an exaggerated inflammatory and hemostasis response to sepsis, the relationship between high systemic cytokine levels and mortality appears independent of age [46]. For example, at 24 hours following CLP, old mice have an approximately 7-fold increase in circulating TNF and IL-6 and a 2.5-fold increase in mortality compared to younger mice with equivalent CLP inoculum. However, when young mice receive an increased inoculum of CLP such that mortality between septic young and old mice are matched, they expressed an increased inflammatory profile similar to old mice [46].
Until recently, few studies examined whether the age-related differences seen in mouse models of sepsis were reflected in adults hospitalized with sepsis (Table 1). These studies were limited by either small sample sizes or by data collection at only one time point, thus limiting the ability to examine the trajectory of inflammation and coagulation response during the course of hospitalization. We recently sought to determine whether age related differences in inflammation and hemostasis response existed in a large cohort of adults hospitalized with pneumonia-induced sepsis [6]. Surprisingly, despite an age-related increase in risk of severe sepsis and mortality, there were no age related differences in inflammatory markers, including IL-6, TNF, and IL-10, during the first day of hospitalization or over the first week. While hemostasis markers revealed a pro-coagulant profile in older adults, the differences were modest and did not explain differences in outcome. Our findings of no age-related change in inflammatory profile are similar to results from two smaller studies [47, 48].
Table 1.
Prospective observational cohort studies examining age-related differences in inflammation and/or hemostasis response in adults hospitalized with infection.
| Author | Study design | Biomarkers | Sample Characteristics | Pertinent results | Interpretation |
|---|---|---|---|---|---|
| Kale, S., et al. (2010) |
|
Pro-inflammatory: IL-6 and TNF Anti-inflammatory: IL-10 Hemostasis: D-dimer, TAT, AT-III, PAI-1, F-IX. |
N=2183 <50yr, n=495 50–64yr, n=444 65–74yr, n=403 75–84yr, n=583 ≥85yr, n=258 |
No age-related difference in inflammatory markers at presentation and minimal pro-coagulant response in older adults. At discharge, there is a significant, but modest, age-related increase in IL-6 and a modest pro-coagulant response. | Minimal to modest age-related differences occur in inflammation and hemostatsis upon hospitalization for CAP. However, resolution of inflammation may be delayed in older adults at discharge. |
| Kelly, E., et al. (2009) |
|
Pro-inflammatory: IL-6 Anti-inflammatory: IL-10 |
N=80 <65yr, n=21 >85, n=59 |
Levels of IL-6 and IL-10 were similar between age groups. | Older age not associated with blunting of the inflammatory response. |
| Marik, P., et al. (2001) |
|
Pro-inflammatory: IL-6, TNF-α, sTNFR-75 | N=930 <50yr, n=280 50–64yr, n=242 65–74yr, n=210 75–84yr, n=150 ≥85yr, n=48 |
TNF in oldest group significantly higher than in those 50–64yr and 75–84yr groups. Other markers were similar across age groups. | Older age not associated with diminished pro-inflammatory cytokines in subjects presenting with septic shock. |
| Bruunsgaard, H., et al. (1999) |
|
Pro-inflammatory: TNF-α, IL-β, IL-6 Anti-inflammatory: IL-10, sTNFR-1, IL-1RA Chemokine: MIP-1β |
N=22 37–55yr, n=10 68–91yr, n=12 |
No age-related difference in cytokines on Day 1, but TNF-α and sTNFR-1 levels higher on Day 7 in elderly vs. young. | Older age associated with prolonged inflammatory activity following infection. |
NF= tumor necrosis factor. sTNFR= soluble TNF receptor. IL=interleukin. MIP= macrophage inflammatory protein. TAT= thrombin-antithrombin complex. AT= antithrombin. PAI= plasminogen activator inhibitor.
Subjects analyzed were placebo arm of North American Sepsis Trial (NORASEPT II) study
Our results suggest that, at least by the time of hospitalization, the dysregulated inflammatory and hemostasis host response that characterizes severe sepsis is quite similar across age groups. Furthermore, our results suggest that absolute levels of systemic inflammation and hemostasis do not alone explain worse outcomes. Although severe sepsis and mortality were associated with the highest levels of inflammation, older adults had higher levels of organ dysfunction even at less pronounced levels of inflammation.
How do we resolve the discrepant results of animal and human studies? First, animal studies measured cytokines during early sepsis, within 24–48 hours after exposure to infection, whereas human studies measure cytokines several days later after presentation to the emergency department. Whether early immune response differs among younger and older individuals is unknown. It is not practical to measure the immune response immediately after exposure to infection in clinical studies. Experimental models of sepsis, such as intravenous LPS, can measure cytokines over time under controlled conditions, but these studies are rarely performed in older adults due to safety reasons. Second, older adults have multiple chronic diseases and may have organ dysfunction prior to exposure to infection. Thus, the same inflammatory load may increase risk of organ dysfunction among older adults. Whether older animals have reduced organ reserve, similar to human studies, is not known.
Although clinical studies did not show large differences in inflammatory and coagulation markers between older and younger adults, these studies did not measure inflammation and hemostasis response at the tissue level, which could be age-dependent. Only selected markers were measured, and several important sepsis mediators, such as macrophage migration inhibitory factor (MIF), gamma interferon, and high mobility group box protein-1 (HMGB1), which have shown promising results in animal studies, were not measured [49–51].
Persistent inflammation and recovery
The traditional focus of care in patients with critical illness has been to reduce short-term and ICU mortality. However, the improved care of critically ill patients has improved short-term outcomes of sepsis. In recent years, interest in understanding the impact of infection on longer-term outcomes has increased [52, 53]. Studies examining long-term outcomes in older adults with serious infection suggest an increased risk of adverse events that persists well beyond hospital stay. In a study of more than 150,000 elderly Medicare recipients hospitalized with CAP, 1 in 3 patients who survived hospitalization for CAP died within the following year [54]. In a prospective cohort study of adults hospitalized with pneumonia, adults ≥ 85 years had a 1.3-fold and 2-fold increased risk of 1-year post-discharge mortality than those 75–84 years and 65–74 years, respectively [6]. Long-term mortality after sepsis, using data from the National Death Index, is most often due to cardiovascular disease, exacerbation of chronic obstructive lung disease, cancer, or repeat infection [55].
Adverse long-term outcomes are not limited to increased mortality risk. Several studies have also reported that survivors of critical illness develop physical and cognitive disabilities in the months and years after discharge [56, 57]. Recently, Iwashyna and colleagues analyzed data from an observational cohort of 1194 older adults to determine risk of cognitive impairment after all-cause sepsis [58]. During the 1–8-year follow-up, the risk of moderate-to-severe cognitive impairment increased 3-fold, from 6.1% before severe sepsis to 16.7% afterward. Extrapolating from national data, this would add approximately 20,000 new cases of moderate-to-severe cognitive impairment in the United States each year. Furthermore, severe sepsis was independently associated with the onset of 1.5 new physical limitations in patients who did not have a history of severe physical limitations.
Although observational studies cannot prove causation, these studies suggest that pathophysiological processes initiated during infection may lead to long-term consequences. In particular, recent studies suggest that the immune response activated during an acute infection may remain activated during recovery and is associated with higher long-term morbidity and mortality. We have recently shown that survivors of CAP have age-related increased circulating concentrations of IL-6 and hemostasis makers at hospital discharge, despite exhibiting normal vital signs and thus apparent clinical recovery [6]. Circulating markers were at least 4-fold higher than have been reported at baseline in community-dwelling individuals with similar ages, suggesting that dysregulated inflammation may persist at discharge. The higher concentrations of IL-6 at discharge were associated with increased risk of death over 3 months [59]. These studies suggest some survivors of critical illness may experience persistent inflammation. Similarly, we showed that the elevated levels of hemostasis observed during pneumonia hospitalization may persist at hospital discharge [60]. Higher levels of D-dimer at discharge were associated with higher risk of death over 1 year. Persistent inflammation and hemostasis response may interact with and worsen chronic and subclinical conditions, such as cardiovascular disease. An important limitation of our work is that inflammatory and hemostasis markers were measured up to hospital discharge. The exact duration for which these markers remain upregulated after discharge remains unclear, and whether specific trajectories of inflammatory markers over time would be associated with long-term outcomes remains unknown.
There are several explanations for a persistent pro-inflammatory state following sepsis. First, resolution of inflammation is an active and highly regulated process that may be age-dependent [8, 61]. It is now appreciated that anti-inflammatory mechanisms are activated within the first few hours of acute inflammation [61]. Prostaglandin-derived lipoxins and resolvin D2 induce apoptosis in neutrophils and help stem the inflammatory response from sepsis [62]. These systems may be impaired with age. For example, aging is associated with diminished lipoxin levels [63]. If so, the profound inflammation and coagulation that occurs during sepsis may in fact persist at a lower level for months after apparent recovery. Additionally, patients with severe sepsis and acute respiratory distress syndrome have been found to have deficient glucocorticoid mediated down-regulation of inflammation, despite elevated levels of circulating cortisol [9, 10].
Second, sepsis may accelerate cellular senescence in older adults. Cellular senescence is triggered by a wide range of stimuli, including DNA damage and oxidative stress, both of which are hallmarks of sepsis. Although thought to be primarily an adaptive mechanism that stops cancer progression in vivo though irreversible cellular arrest, cellular senescence from fibroblasts and epithelial cells are associated with secretion of several pro-inflammatory molecules, such as IL-6, IL-8, and IL-1 [22]. This unintentional deleterious effect is proposed to be a contributor to the chronic inflammatory state of aging, and may be further exacerbated after critical illness. Further studies will be needed to examine the persistence of elevated inflammatory markers in the months following hospitalization.
Conceptual model and interventions
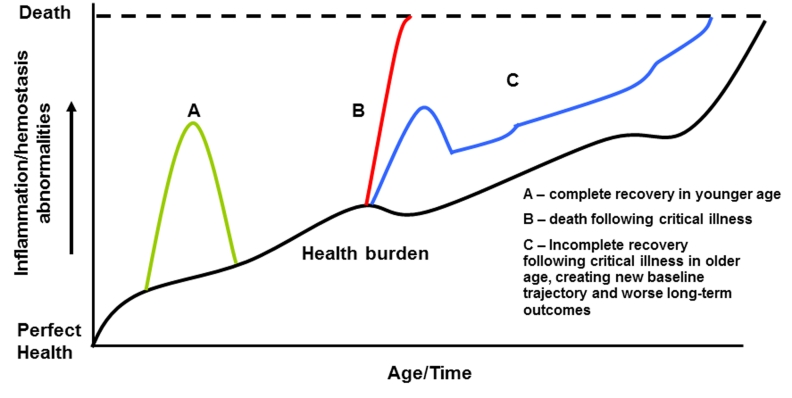
Based on our interpretation of the current literature, we have developed a conceptual model of age-related inflammatory and hemostasis abnormalities prior to, during, and after sepsis (Figure 2). As our model demonstrates, the aging process is associated with a baseline increase in inflammation and hemostasis abnormalities (dark black line). In our model, three potential outcomes are possible during hospitalization for severe sepsis and critical illness. In younger age, critical illness is associated with a sharp increase in inflammation and hemostasis response and a quick resolution (Curve A). In older age, severe sepsis results nearly 25% short-term mortality (Curve B). Even in older survivors of critical illness, however, the inflammation/hemostasis abnormalities associated with critical illness fail to fully resolve, placing them on a new trajectory (Curve C). This new trajectory is associated with a higher risk of repeat critical illness and increases the risk of subsequent death. At each stage of the continuum, specific interventions in the elderly to decrease inflammation and hemostasis abnormalities may decrease risk of sepsis and mortality.
Figure 2. Conceptual model showing the relationship between inflammation/hemostasis abnormalities throughout the continuum of critical illness and aging.
Aging is associated with a baseline increase in inflammation and hemostasis abnormalities (dark black line). Three potential outcomes are shown following sepsis. Younger adults have a lower incidence and mortality with and are expected to return to baseline inflammatory and hemostasis levels after infection (A). In older adults, a higher baseline inflammation/hemostasis burden increases the risk of sepsis and higher mortality (B). Even among older survivors of sepsis, processes that down-regulate inflammation after infection may be dysregulated, leading to a higher inflammation/hemostasis burden post-infection and worse long-term outcomes, including repeat infection and exacerbation of chronic conditions (C).
A review of current treatments for sepsis is beyond the scope of this manuscript; however recent studies attempting to modulate the early inflammation and hemostasis response during sepsis have shown some success. Recombinant activated protein C (rhAPC), known to alter the coagulation cascade, was approved by the US Food and Drug Administration for use in patients with sepsis-induced organ dysfunction. The PROWESS (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis) trial demonstrated a 61% absolute decrease in mortality (30.8% vs. 24.7%, p=0.005) in patients with severe sepsis [64]. Results of randomized clinical trials to assess efficacy of low-moderate dose glucocorticoids have shown conflicting results [65, 66]. Additionally, the efficacy of statins have been examined in observational studies and small clinical trials due to their pleiotropic effects on inflammation, endothelial dysfunction, and oxidative stress pathways [67–69]. A meta-analysis of existing observational studies showed that statins were associated with a reduction in the development of infection in high-risk patients as well as improved outcomes in patients admitted with infections [69].
Studies to date that test immunomodulatory therapies have focused on modulating the early immune response and these therapies have been used for short duration, often limited to ICU stay or during the first week of hospitalization. Whether extending these treatment strategies up to or beyond hospital discharge can improve long-term outcomes remains unknown. Trials are underway to test interventions for longer duration, such as glucocorticoid therapy for 20 days in patients with severe community-acquired pneumonia [70]. In particular, therapies employed for a longer duration may be beneficial in older adults who have higher burden of chronic disease prior to onset of severe sepsis and may be at higher risk for a dysregulated immune response.
Conclusion
Older age has long been associated with altered inflammation and hemostasis regulation, which has been linked to the development of chronic diseases. Emerging evidence suggests that age-related differences in inflammation and hemostasis abnormalities may play a role in the development of and long-term outcomes after critical illness. A better understanding of underlying mechanisms may provide new possibilities for therapeutic interventions.
References
- 1.Girard TD, Ely EW. Bacteremia and sepsis in older adults. Clin Geriatr Med. 2007;23:633–647. viii. doi: 10.1016/j.cger.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 6.Kale S, Yende S, Kong L, Perkins A, Kellum JA, Newman AB, Vallejo AN, Angus DC. The effects of age on inflammatory and coagulation-fibrinolysis response in patients hospitalized for pneumonia. PLoS One. 5:e13852. doi: 10.1371/journal.pone.0013852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuro-immunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136:1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 10.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 11.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 14.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 15.Sogaard M, Schonheyder HC, Riis A, Sorensen HT, Norgaard M. Short-term mortality in relation to age and comorbidity in older adults with community-acquired bacteremia: a population-based cohort study. J Am Geriatr Soc. 2008;56:1593–1600. doi: 10.1111/j.1532-5415.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 16.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 18.Mari D, Mannucci PM, Coppola R, Bottasso B, Bauer KA, Rosenberg RD. Hypercoagulability in centenarians: the paradox of successful aging. Blood. 1995;85:3144–3149. [PubMed] [Google Scholar]
- 19.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 20.Pieper CF, Rao KM, Currie MS, Harris TB, Chen HJ. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55:M649–657. doi: 10.1093/gerona/55.11.m649. [DOI] [PubMed] [Google Scholar]
- 21.McDermott MM, Greenland P, Green D, Guralnik JM, Criqui MH, Liu K, Chan C, Pearce WH, Taylor L, Ridker PM, Schneider JR, Martin G, Rifai N, Quann M, Fornage M. D-dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation. 2003;107:3191–3198. doi: 10.1161/01.CIR.0000074227.53616.CC. [DOI] [PubMed] [Google Scholar]
- 22.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 25.Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, Jung JH, Yu BP. Molecular Inflammation as an Underlying Mechanism of the Aging Process and Age-related Diseases. J Dent Res. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123:1589–1595. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 27.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Vallejo AN. Age-dependent alterations of the T cell repertoire and functional diversity of T cells of the aged. Immunol Res. 2006;36:221–228. doi: 10.1385/IR:36:1:221. [DOI] [PubMed] [Google Scholar]
- 29.White JC, Nelson S, Winkelstein JA, Booth FV, Jakab GJ. Impairment of antibacterial defense mechanisms of the lung by extrapulmonary infection. J Infect Dis. 1986;153:202–208. doi: 10.1093/infdis/153.2.202. [DOI] [PubMed] [Google Scholar]
- 30.Mason CM, Dobard E, Summer WR, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infect Dis. 1997;176:1293–1302. doi: 10.1086/514125. [DOI] [PubMed] [Google Scholar]
- 31.Thornton JA, Durick-Eder K, Tuomanen EI. Pneumococcal pathogenesis: “innate invasion” yet organ-specific damage. J Mol Med. 88:103–107. doi: 10.1007/s00109-009-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fillon S, Soulis K, Rajasekaran S, Benedict-Hamilton H, Radin JN, Orihuela CJ, El Kasmi KC, Murti G, Kaushal D, Gaber MW, Weber JR, Murray PJ, Tuomanen EI. Platelet-activating factor receptor and innate immunity: uptake of gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol. 2006;177:6182–6191. doi: 10.4049/jimmunol.177.9.6182. [DOI] [PubMed] [Google Scholar]
- 33.Radin JN, Orihuela CJ, Murti G, Guglielmo C, Murray PJ, Tuomanen EI. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun. 2005;73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rijneveld AW, Weijer S, Florquin S, Speelman P, Shimizu T, Ishii S, van der Poll T. Improved host defense against pneumococcal pneumonia in platelet-activating factor receptor-deficient mice. J Infect Dis. 2004;189:711–716. doi: 10.1086/381392. [DOI] [PubMed] [Google Scholar]
- 35.Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yende S, Tuomanen EI, Wunderink R, Kanaya A, Newman AB, Harris T, de Rekeneire N, Kritchevsky SB. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med. 2005;172:1440–1446. doi: 10.1164/rccm.200506-888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 41.Bone RC. Why sepsis trials fail. JAMA. 1996;276:565–566. [PubMed] [Google Scholar]
- 42.Milbrandt EB, Reade MC, Lee M, Shook SL, Angus DC, Kong L, Carter M, Yealy DM, Kellum JA. Prevalence and significance of coagulation abnormalities in community-acquired pneumonia. Mol Med. 2009;15:438–445. doi: 10.2119/molmed.2009.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Crit Care Med. 2009;37:1018–1023. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar AT, Sudhir U, Punith K, Kumar R, Ravi Kumar VN, Rao MY. Cytokine profile in elderly patients with sepsis. Indian J Crit Care Med. 2009;13:74–78. doi: 10.4103/0972-5229.56052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly E, MacRedmond RE, Cullen G, Greene CM, McElvaney NG, O’Neill SJ. Community-acquired pneumonia in older patients: does age influence systemic cytokine levels in community-acquired pneumonia? Respirology. 2009;14:210–216. doi: 10.1111/j.1440-1843.2008.01423.x. [DOI] [PubMed] [Google Scholar]
- 49.Grieb G, Merk M, Bernhagen J, Bucala R. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 23:257–264. doi: 10.1358/dnp.2010.23.4.1453629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersson U, Tracey KJ. HMGB1 in sepsis. Scand J Infect Dis. 2003;35:577–584. doi: 10.1080/00365540310016286. [DOI] [PubMed] [Google Scholar]
- 51.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 52.Yende S, Angus DC. Long-term outcomes from sepsis. Curr Infect Dis Rep. 2007;9:382–386. doi: 10.1007/s11908-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 53.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 304:1833–1834. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 54.Kaplan V, Clermont G, Griffin MF, Kasal J, Watson RS, Linde-Zwirble WT, Angus DC. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163:317–323. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 55.Yende S, Angus DC, Ali IS, Somes G, Newman AB, Bauer D, Garcia M, Harris TB, Kritchevsky SB. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc. 2007;55:518–525. doi: 10.1111/j.1532-5415.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 56.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan V, Angus DC. Surviving intensive care. Crit Care Med. 2002;30:703–705. doi: 10.1097/00003246-200203000-00037. [DOI] [PubMed] [Google Scholar]
- 58.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yende S, D’Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, Kong L, Carter M, Angus DC. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yende S, D’Angelo G, Mayr F, Kellum JA, Weissfeld L, Kaynar AM, Young T, Irani K, Angus DC. Elevated Hemostasis Markers after Pneumonia Increases One-Year Risk of All-Cause and Cardiovascular Deaths. PLoS One. 6:e22847. doi: 10.1371/journal.pone.0022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 62.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gangemi S, Pescara L, D’Urbano E, Basile G, Nicita-Mauro V, Davi G, Romano M. Aging is characterized by a profound reduction in anti-inflammatory lipoxin A4 levels. Exp Gerontol. 2005;40:612–614. doi: 10.1016/j.exger.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 65.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaud P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 66.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 67.Yende S, Milbrandt EB, Kellum JA, Kong L, Delude RL, Weissfeld LA, Angus DC. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med. 39:1871–1878. doi: 10.1097/CCM.0b013e31821b8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woerndle RH, Maxwell DL. Statins and sepsis: good bullet, disappearing target. J Am Osteopath Assoc. 2008;108:486–490. [PubMed] [Google Scholar]
- 69.Tleyjeh IM, Kashour T, Hakim FA, Zimmerman VA, Erwin PJ, Sutton AJ, Ibrahim T. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169:1658–1667. doi: 10.1001/archinternmed.2009.286. [DOI] [PubMed] [Google Scholar]
- 70.Meduri GU. Extended Steroid in CAP(e) (ESCAPe) 2011;2011 ClinicalTrials.gov. [Google Scholar]