Abstract
Aging is associated with a variety of changes to immune responsiveness. Reduced protection against infection, reduced responses to vaccination and increased risk of autoimmunity are all hallmarks of advanced age. Here we consider how changes in the expression of regulatory receptors on the T cell surface contribute to altered immunity during aging.
Keywords: Aging, T cell costimulation, inhibitory receptors, T cell regulation, NK receptors
T cells sense their environment through receptors that complement or regulate TCR-induced signals and determine the outcome of antigen recognition. In addition to the classical stimulation of the CD28 pathway to prevent anergy, a number of costimulatory and coinhibitory receptors have been identified. It is now clear that T cells from elderly people display different patterns of these regulatory receptors and experience differences in downstream signaling compared with T cells isolated from young people. The extent to which these changes are responsible for the immune defects observed in the elderly is still contested. Aging is associated with hyperresponsiveness to stimulation in some situations (autoimmunity) but hyporesponsiveness in others (infection and vaccination). Elderly people are at greater risk of infectious disease both from novel pathogens and from the reactivation of latent infections owing to a decrease in immune responses to these stimuli. In addition, elderly people are less responsive to vaccination than younger recipients and have reduced delayed-type hypersensitivity (DTH) responses [1]. Conversely, age represents the major risk factor for selected autoimmune diseases. This review will summarize what is currently known about the age-related changes to costimulatory and coinhibitory molecules, the potential causes of these changes, their downstream consequences in terms of effector function, and their involvement in age-dependent diseases.
Age-related changes to regulatory molecules in T cells
During aging, the expression patterns of T cell regulatory receptors change and these changes have been observed at the gene and protein level (Table 1). Regulatory receptors on the surface of T cells can be grouped into stimulatory and inhibitory molecules. These proteins include members of the TNF receptor superfamily (TNFRSF), the CD28 family, killer Ig-like receptors (KIRs), killer cell lectin-like receptors (KLRs) and the immunoglobulin-like transcript (ILT/LILR/CD85) receptors. Broadly speaking, aging is associated with the acquisition of inhibitory surface receptors, such as KIRs, KLRs and ILT receptors, accompanied by a decrease in the expression of costimulatory CD28 family and TNFRSF members.
Table 1.
Changes to surface expression of regulatory receptors on T cells with age
| Regulatory receptors | T cell affected | Young | Old | Reference |
|---|---|---|---|---|
| CD28 | CD4, CD8 | +++ | +/− | [2], [137] |
| KLRG1 | CD4, CD8 | + | +++ | [22], [56], [52–54] |
| CD85j | CD4, CD8 | + | +++ | [23], [33], [34] |
| KIRs | CD4, CD8 | + | +++ | [9], [19–25], [28] |
| CD56 | CD4, CD8 | +/− | ++ | [20], [21], [38] |
| CD16 | CD8 | + | ++ | [20–22] |
| NKG2D | CD8 | + | ++ | [22] |
| CD94 | CD8 | + | ++ | [8], [21], [22] |
| CD27 | CD4, CD8 | +++ | + | [8] |
One of the first observed differences in regulatory receptor expression in the elderly was the progressive loss of CD28 from the T cell surface [2]. This constitutively expressed costimulatory molecule provides signals that are vital for efficient T cell activation and its loss during aging may be partly responsible for reduced T cell responses. However, memory T cells are less reliant on CD28 signaling for activation, particularly when provided with stimulatory signals through inducible TNFRSF members [3–6]. OX40 is a TNFRSF member, which provides survival signals to T cells and increases the production of pro-inflammatory cytokines. While OX40 expression is maintained on tumor-specific T cells isolated from aged mice, these cells are less responsive to OX40 stimulation partly owing to extrinsic decreases in IL-12 [7] and the expression patterns of TNFRSF members during aging in humans have not been fully described.
Advances in systems biology and high-throughput analyses have aided studies which aim to monitor changes in gene expression in T cells from healthy young and elderly individuals. Examination of the total CD8+ T cell pool by Cao and colleagues showed changes in 754 genes of known function with aging [8]. Two thirds of these genes are down-regulated with advancing age and one third up-regulated. The changes observed include genes involved in transcription, translation, signal transduction and protein transport. Observed increases in expression include genes involved in oxidative stress, apoptosis and inflammatory processes, which could explain the increases in inflammation observed during aging. With reference to costimulation, Cao and colleagues observed that Cd8 and Cd28 were down-regulated, along with the naïve cell markers ccr7 and Cd27. Up-regulated genes also include Cd244, Cd96, Klrf1 and CD94, (described below) which encode NK cell-related receptors that are associated with a reduction in T cell activity.
Lazuardi and colleagues took a different approach, isolating CD28+ and CD28− CD8+ cells from young and elderly individuals and comparing gene expression between these four groups [9]. Analysis showed three clusters based on gene expression profiles: young CD28+ cells, old CD28+ cells and CD28− cells from both young and old donors, which clustered together. CD28+ cells isolated from elderly people showed gene expression profiles intermediate between young CD28+ cells and CD28− cells from all donors suggesting that major differences in gene expression reflect changes in the proportion of T cell subsets in combination with chronological aging. This study also showed that CD28+ cells from young people express more genes associated with cell proliferation, adhesion and differentiation, supporting previous data that CD28− cells are less able to proliferate and suggesting that T cells may traffic differently in elderly people. This study documented an increase in NK cell-associated KIR and KLR gene expression in CD8+ CD28− T cells from both young and elderly individuals compared with CD8+ CD28+ T cells, so the overall increase described by Cao et al. may be a reflection of the accumulation of CD28− T cells in elderly individuals rather than a change in expression at the single cell level.
In addition to surface-expressed regulatory molecules, these studies identified many changes in downstream signaling pathways. Large increases were observed in the expression of the dual-specificity phosphatase genes Dusp2, 4, 5, 6 and 10, which are associated with proliferation and differentiation. Of particular interest, DUSP4 is a key mediator of replicative senescence. Its increased expression in elderly people has been confirmed by PCR and at the protein level and silencing of this gene can improve T cell responses to TCR ligation in the elderly [10]. Though many cellular processes are subject to decreased expression in most of the genes involved, both studies found that immune response pathways contain genes that are up- and down-regulated in roughly equal number. Further studies on these gene candidates may provide insight into the dichotomy of infection- and autoimmune-related changes to immunity during aging.
Age-related expression patterns of regulatory receptors - a switch in focus to environmental signals
Regulatory receptors provide information on the environment in which antigen recognition occurs. Changes in their expression patterns therefore indicate a fundamental shift in the information that is integrated in the T cell response. As well as sharing structural features, the different families of regulatory receptors tend to have family-specific expression patterns and signaling outcomes. CD28 provides the strongest and earliest costimulatory signal following TCR ligation owing to its constitutive expression on the cell surface and close association with the TCR complex. Its loss is associated with the induction of anergy and loss of T cell effector function [11–13]. Its ligands are limited to antigen presenting cells (APCs) and are inducible upon activation [14, 15]. Unlike CD28, the majority of costimulatory and inhibitory molecules are not constitutively expressed on the T cell surface. Following TCR signaling TNFRSF members, including OX40 (CD134) and 4-1BB (CD137), are up-regulated. These proteins provide archetypal costimulatory signals that augment the TCR signal following antigen recognition. Signaling through these receptors induces proliferation and pro-inflammatory cytokine production, and promotes T cell survival. The ligands for these receptors are also inducible rather than constitutive and tend to have a limited distribution, again being expressed primarily by APCs [16]. In addition to costimulatory molecules, activated T cells up-regulate surface receptors that inhibit activation and reduce inflammatory signals. CTLA-4, for example, is structurally related to CD28 but competitively inhibits CD28 signaling by binding its ligands, CD80 and CD86 [17]. PD-1 is another CD28−related molecule that provides inhibitory signals to T cells but which has distinct ligands (PD-L1 and PD-L2) [18]. Because the expression of these receptors is dependent on prior encounter with antigen, and signaling relies on the availability of the ligand, T cell activation, proliferation and survival can be controlled both temporally and spatially. This ensures that antigen-specific T cells receive pro-inflammatory signals to increase their effector function; proliferative and survival signals to establish a pathogen-specific memory T cell pool; and inhibitory signals to reduce inflammation and resolve the immune response once the pathogen is cleared.
Distinct from the classical costimulatory and inhibitory receptors up-regulated on the surface of T cells following activation are NK cell-associated receptors including KIRs, CD16, CD94, CD56, CD85j and NKG2D. These molecules, typically associated with NK cells, have very different expression patterns on T cells. Genetic and protein analyses show that the expression of multiple activating and inhibitory KIRs is increased with age [19–24]. Like in NK cells, the expression of different isoforms appears to be stochastic. Analysis of clonal progenies of T cells sharing the same T cell receptor suggest the model that acquisition of different isoforms on each cell is successive and cumulative generating increasingly complex, clone family-specific expression patterns [25]. While all T cells including naïve and memory T cells express the transcriptional activators required for KIR transcription and express KIRs upon DNA demethylation [26], only end-differentiated T cells have accumulated the extent of CpG demethylation in the KIR promotors that is necessary for expression [27, 28].
NK receptors are divided into two groups based on their structure: the immunoglobulin superfamily receptors and C-type lectin superfamily receptors. Among the NK receptors that are up-regulated in aged T cells, KIRs, CD16, CD56 and CD85j belong to the Ig-like NK receptors, while CD94/NKG2A, NKG2D, CD161 and KLRG1 belong to the C-type lectin NK receptors [29–32]. Although varying in structure, KIRs, CD85/ILTs/LILRs and CD94/NKG2A all recognize MHC class I molecules. Specifically, human KIRs recognize HLA-A, HLA-B and HLA-C. CD85 receptors bind to a broader range of HLA molecules, including the nonclassical MHC molecules HLA-G and HLA-F. In contrast, KLRG1 binds to cadherins but not MHC class I molecules. CD85j (LILR1/ILT2) is broadly expressed on hematopoietic cells, but is mostly up-regulated on CD8+ T cells during aging with little expression on CD4+ T cells [33]. Changes in gene expression of CD85j were not observed in the gene array studies described above. However, CD85j on CD8+ T cells is associated with aging and is expressed on a substantial proportion of CD8+ T cells in the elderly, including CMV-specific T cells [23,34], implying that posttranscriptional modifications may induce changes in the expression pattern of this receptor with age.
Unlike the classical T cell regulatory receptors induced after peptide recognition and TCR signaling, NK cell associated receptors are stably maintained once expressed on CD8 T cells [35–39]. The expression of their ligands is likewise constitutive and not restricted to APCs. As a result, signaling through these receptors is not subject to the same level of control, implying that their function is fundamentally different from the traditional costimulatory or inhibitory receptors that fine-tune antigen-induced T cell activation.
Age-related expression patterns of regulatory receptors – a reflection of defective T cell subset homeostasis or a consequence of genetic differentiation or senescence programming?
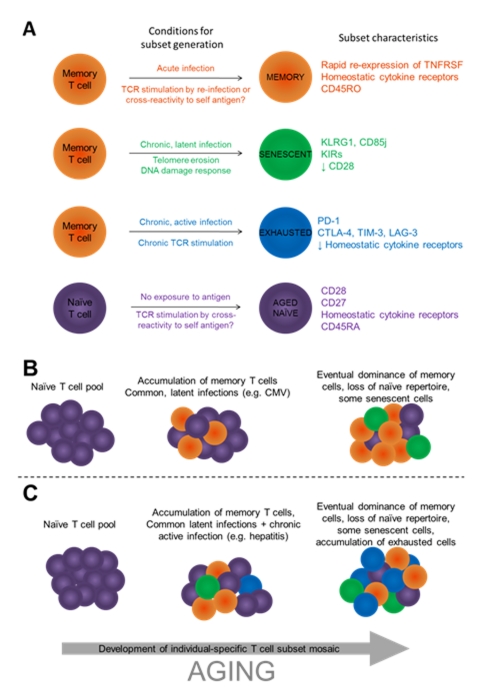
The above studies have shown a variety of changes that take place in the lymphocyte population during aging. However, crucial to the understanding of age-related changes in immune function is the distinction between intrinsic changes associated with chronological aging of immune cells and the accumulation of highly differentiated cells, which can occur independently of aging, for example during chronic antigen exposure (Figure 1). It has been well documented that the T cell pool undergoes substantial changes in subset proportions with aging [40, 41]. The accumulation of antigen-experienced, memory T cells can be tracked by the expression of the short isoform of CD45, CD45RO, which increases with increasing age. There is a concurrent decrease in the number of T cells expressing the naïve T cell marker CD45RA. However, CD45RA can be regained by memory T cells later in life and other markers must be used to identify memory and naïve T cells with certainty [42]. CD27, for example, is not regained on CD45RA+ memory cells but is present on CD45RA+ naïve T cells [43]. The loss of CD28 is associated with the gain of CD45RA and the acquisition of negative cell surface receptors, so it is often difficult to distinguish whether observed changes are the result of changes to individual T cells or merely reflections of changes in the proportion of T cell subsets. In addition to the steady accumulation of memory T cells, other highly-differentiated T cell subsets increase with age as a result of chronic antigen exposure. These include exhausted and senescent cells, the presence of which is likely to further complicate the analysis of changes to T cell function during aging.
Figure 1.
Changes to the T cell pool during aging reflect differences in subset proportions as well as age-related changes to individual T cells. A) The proportional representation of T cell subsets depends on regulatory molecule expression, the infection history of the individual, current infections and stochastic events. T cell survival may be enhanced by TCR cross-reactivity, which would increase TCR stimulation and thus increase positive signaling. T cell location may also influence survival based on access to cytokines, contact with APCs, CD4+ T cell help or inflammatory signals. Finally, access to positive and negative signals both in terms of ligand accessibility and the expression of regulatory receptors on the T cell surface is likely to shape the development of the memory pool and select for certain T cell clones. B) During the course of aging, individuals are exposed to infection, and antigen-specific T cells form a memory population (orange) which comes to dominate the T cell pool. Common, chronic latent infections such as CMV induce the development of senescent cells (green). C) Individual-specific exposure to infection, for example to chronic active infections, can alter the proportion of T cell subsets in the total T cell pool. The resultant memory pool is very heterogeneous, involving the accumulation of memory cells, senescent cells, exhausted cells (blue) and aged naïve cells (purple) following acute, latent chronic and active chronic infection, and homeostatic proliferation.
APC: antigen-presenting cell; TCR: T cell receptor
Some studies have begun to shed light on this issue by directly comparing highly differentiated, memory T cells with exhausted cells, senescent cells and with naïve cells from young and elderly people. Most consider CD8+ T cells, but CD4+ cells are subject to similar changes in terms of memory progression or loss of function, although much less frequently. CD4+ CD28− T cells do appear in elderly people [27] and share some phenotypic characteristics with CD8+ CD28− T cells, including an increase in the expression of KIR2DL2, KIR2DL3, and KIR2DL4 [19, 44, 45].
Most studies to date have considered rather broad T cell populations. For example comparing total CD8+ T cells from young and elderly people [8] or total CD8+ CD28+ with CD8+ CD28− populations [9]. It is now becoming clear that, especially when considering the changes that occur with age, T cell populations must be considered separately. Of particular interest is the growing consensus that late-stage, highly differentiated T cells can differ greatly depending on the reasons underlying their differentiation. Late-stage T cells can be highly differentiated, with full effector function and the ability to transition into the memory pool. However, they can also follow pathways into senescence and exhaustion. The main differences between these fates and their relation to aging and immune function are discussed below. Anergy is distinct from all these processes because it arises following TCR stimulation in the absence of efficient costimulation. In contrast, differentiation, senescence and exhaustion typically occur following full activation of the T cell and robust TCR signaling. Processes leading to T cell anergy are important for peripheral tolerance and have been discussed elsewhere [46, 47]. Whilst exhausted, senescent and memory T cells do share certain characteristics, the signaling pathways involved in their fate determination are distinct and this suggests that they have at least some independent mechanisms of development. Memory cells are produced following TCR engagement, efficient costimulation and cytokine provision. Senescent T cell development typically follows the detection of DNA damage after the erosion of telomeres or as a result of telomere-independent stresses. Finally, exhaustion is a state established following chronic exposure to antigen and involves the active ligation of surface inhibitory receptors by their cognate ligands as well as the provision of negative signals by extrinsic, immunoregulatory cytokines.
Memory cells can be highly functional on re-exposure to antigen, even decades after their formation. However, aging and homeostatic proliferation are accompanied by telomere shortening and can increase damage to the cell. DNA damage, telomere shortening and limited access to growth factors can all induce senescence [48–51]. Senescent cells are typified by a loss of CD28 from the cell surface, reduced telomere lengths and telomerase activity, and the up-regulation of surface inhibitory markers such as the NK cell receptor KLRG1 [52]. These cells are unable to proliferate but maintain effector functions such as cytokine production and cytotoxicity, even at the extreme end of the differentiation process. Repetitive antigen stimulation of naïve CD8+ T cells in vitro induces KLRG1 expression, suggesting that the expression of KLRG1 is associated with senescent CD8+ T cells [53]. The expression of KLRG1 is increased with age in both CD4+ and CD8+ T cells from mice and humans [53–55]. In humans, the frequency of KLRG1+ T cells increases with T cell differentiation, being lowest in the naïve population and highest in terminally differentiated effector T cells [56]. Similar to data from mice, CD4+ and CD8+ T cells expressing KLRG1 in humans do not proliferate well in vitro [53, 54, 56]. However, senescent cells can maintain effector function and are likely to provide at least some protection against chronic infection.
In summary T cell subsets change both in terms of relative frequency and in terms of subset-specific phenotypic characteristics. The extent to which these processes contribute to the aging immune phenotype is still unclear but the accumulation of regulatory receptors on the surface of individual T cells, along with subset-specific changes in gene expression, has shown that age-related changes are not solely the result of changes in relative subset frequencies.
Age-related expression patterns of regulatory receptors – not a classical case of T cell exhaustion
Recent review articles have provided comparisons between exhausted and senescent cells [52, 57] and current studies suggest that the development of these cell subsets is at least partly the result of independent programs. Both subsets require active changes in gene transcription and are not passively dependent on other age-related events such as DNA hypomethylation. Indeed, one study comparing effector and memory T cell subsets with exhausted T cells showed that, in terms of gene transcription, exhausted cells differ from effector and memory subsets as much as these latter two subsets differ from each other [58].
In contrast to acute infection, persistent antigen in the setting of chronic active infection or cancer leads to persistent T cell stimulation. Cells that are chronically exposed to antigen gradually lose the ability to respond to stimulation and reach a stage of exhaustion. Unlike senescent cells, exhausted cells are defined by their progressive loss of function, though this population also proliferates less. This process may reduce autoimmunity but could also decrease immunity to tumors and chronic infections. Exhausted cells lose functions progressively, starting with a reduction in IL-2 production, followed by a loss of TNF-α production, cytotoxic activity, and eventually IFN-γ production [59]. If the antigen persists, exhausted cells are deleted and the level of exhaustion correlates with the duration of infection and the strength of the signal provided. However, there is evidence to suggest that antigen is also needed to maintain this population. In chronic infection models in mice, it has been shown that exhausted, CD8+ virus-specific T cells cannot survive without constant exposure to antigen and are deleted when adoptively-transferred to naïve mice, despite the provision of the homeostatic cytokines which usually allow survival in the absence of TCR stimulation [60]. CD8+ cells from HIV-infected individuals have lower expression of the IL-7 receptor [61] and virus-specific cells can be lost after anti-retroviral therapy [62], suggesting that antigen exposure, rather than provision of homeostatic cytokines, plays a role in the survival and maintenance of this population. Dual costimulation through the TNFRSF members OX40 and 4-1BB induces the up-regulation of the IL-7 receptor alpha chain on the surface of T cells [63] and could therefore be involved in the maintenance of memory cells. OX40 and 4-1BB expression is typically limited to T cells receiving strong stimulation and so this mechanism could act to enhance the survival of antigen-specific T cells following infection [63]. The loss of OX40 could be indicative of exhaustion, leading to reduced homeostatic cytokine receptor expression and an increased reliance on antigen for survival.
The continuing production of viral escape mutants during chronic viral infection supports the notion that exhausted T cells, despite reduced effector function, do provide protection and can drive mutation in pathogens. As described above, senescence usually follows DNA damage or telomere shortening and does not result in significant loss of effector function. Exhaustion appears to be the result of the gradual acquisition of inhibitory receptors on the cell surface, signaling through which results in the progressive loss of cytokine production and killing activity. While senescence could provide a mechanism for reduced proliferation of damaged, potentially precancerous cells, exhaustion could be a means of preventing excessive inflammation in the face of chronic infection. This may be particularly important if viruses are latent, with bouts of replication, in delicate tissues, which need to avoid prolonged or repeated periods of inflammation in order to perform their bodily function.
Following stimulation through the TCR, activated T cells do express inhibitory receptors, but this expression is transient, and prolonged expression is limited to exhausted cells [64]. As such, increased inhibitory receptor expression during aging could reflect an increase in the number of exhausted T cells. Memory T cells, however, maintain the ability to proliferate and express few inhibitory receptors, and are the T cell population that expands the most in the absence of chronic, active infection. Exhausted T cells gradually acquire inhibitory receptors on their cell surface and their expression increases in terms of density and diversity as long as the antigen persists [64]. Exhaustion, therefore, is more reliant on extrinsic factors than senescence – signaling through the inhibitory receptor PD-1 appears to be important for exhaustion, and other inhibitory receptors such as 2B4, LAG-3, TIM-3 and CTLA-4 all require ligation by their cognate ligands to suppress the T cell. Changes in the external cytokine milieu, including increases in IL-10 and TGF-β concentrations, also appear to play a role in exhaustion [65–67] and are provided for the most part by APCs and CD4+ T cells. The provision of cytokines that provide positive signals to T cells can increase effector function so there may be a concurrent decrease in the concentrations of, or signaling to T cells by, cytokines such as IL-2 and IL-21 during chronic infection. The key role of cell surface inhibitory receptors in T cell exhaustion was first identified in chronic LCMV-infected mice [68]. PD-1 is dramatically increased and maintained to a high level in exhausted CD8+ T cells in this model. More importantly, the in vivo blockade of PD1/PD-L1 signaling rescues IFN-γ production, proliferation and cytotoxic activity of virus specific CD8+ T cells, and reduces viral load. The role of PD-1 in T cell exhaustion has been extended to several human chronic viral infections, such as HIV [69–72], HBV [73, 74] and HCV [75–79]. High levels of PD-1 are seen on HIV-specific CD8+ T cells, and its expression is associated with the dysfunction of HIV-specific exhausted CD8+ T cells and with viral load [69–71]. Up-regulated expression of PD-1 has also been detected in HIV-specific CD4+ T cells [72]. Blocking the PD-1 pathway ex vivo increases the proliferation, cytokine production, and cytotoxicity of HIV specific CD8+ and CD4+ T cells upon cognate antigen stimulation [69, 70, 72]. In addition, blockade of PD-1 in vivo during Simian Immunodeficiency Virus (SIV) restores the proliferation of SIV-specific CD4+ and CD8+ T cells and memory B cells, leading to the improvement of SIV-specific immunity and prolonged survival of SIV-infected macaques, suggesting the potential of blocking the PD-1 pathway to control HIV infection [80]. CTLA-4, another member of CD28 family, may also be involved in HIV-specific CD4+ T cell exhaustion, as blocking this molecule in vitro restores HIV-specific CD4+ T cell proliferation and cytokine production [81]. Studies following the downstream signaling of inhibitory receptors suggest that ligation of PD-1 inhibits TCR-induced activation of PI3-Kinase through recruitment of either SHP-1 or SHP-2 to the cell membrane [82]. Quigley et al. recently demonstrated that a panel of genes is up-regulated with PD-1 ligation of HIV-specific exhausted T cells [83]. Their results further suggest that inhibitory receptors regulate T cell function not only by attenuating TCR signaling, but also by inducing the expression of proteins that actively impair T cell function.
It is now clear that exhausted, senescent and aged T cells have different expression patterns of inhibitory receptors. Whether the inhibitory receptors that are important in T cell exhaustion also play role in T cell senescence is still not well characterized. The expression of PD-1, CTLA-4, LAG-3 and TIM-3 all increase with age, which at least partly reflects the increased frequency of memory T cells with age [84–86]. However, the expression of PD-1 is inversely correlated with the expression of age-related inhibitory receptors [87], and exhausted T cells do not express KLRG1 or age-associated KIRs [58]. In addition, blockade of PD-1 in vitro does not increase the proliferation or survival of T cells from aged mice [84], suggesting that although PD-1 plays an important role in T cell exhaustion, its role in T cell dysfunction during aging is minor and T cell exhaustion itself should not be considered a hallmark of aging in the absence of active chronic infections such as HIV or Hepatitis.
Age-related expression patterns of regulatory receptors - one common genetic program or one for each family?
Shifts in the expression of regulatory receptors with aging are correlated. For example, KIRs, KLRs and CD85 receptors are all preferentially found on T cells lacking CD27 and CD28 [88–90]. Although strict correlation studies on single cells have not been done, this preferential subset distribution suggests that the genetic programs controlling these expressions may be linked or even shared. CD28 down-regulation and loss is seen with CD28 engagement, exposure to TNF-α and replication, in particular for CD8+ T cells [91–93]. All three mechanisms induce the same genetic program, the progressive loss of DNA-binding proteins, which leads to loss of binding to the CD28 initiator complex and thus reduced transcription of CD28 [92, 93]. This process is partly reversible through an IL-12 dependent mechanism and CD8+ cells are more prone to the loss of DNA binding proteins than CD4+ which may explain the comparative preservation of CD28 on CD4+ T cells [94].
CD85j expression is uniquely regulated by T cells, which use a different promoter than monocytes [33]. T cells use an upstream promoter that includes an additional distal exon in the 5′-promoter region, while monocytes use a downstream promoter. The exon inhibits the translation of CD85j, leading to a lower expression level of CD85j in T cells compared with that in monocytes. Studies from our lab show that the transcriptional regulation of KIR expression in T cells is also distinct from that in NK cells supporting the idea that KIRs may have important functions in T cells [26]. We further demonstrated that KIR expression in T cells is regulated by CpG methylation of promoter regions. DNA demethylation up-regulates KIR expression [28]. With advanced age the recruitment of DNA methyltransferase to, and the methylation level of, the KIR2DL3 promoter is decreased in CD8+ T cells, contributing to the increased expression of KIRs in these cells.
It is currently unclear at what stage the expression of inhibitory receptors is determined both in terms of chronological age and differentiation status of the cell. While expression increases with age [23, 95], and T cells expressing inhibitory receptors have short telomeres indicative of antigenic experience [54, 96], there is evidence to suggest that T cells begin to express inhibitory receptors relatively early on in differentiation. Several groups have observed diverse KIR expression patterning on the surface of clonally-derived T cells [25, 97], and recent advances in TCR sequencing could help to determine the extent to which clonal selection determines the mosaic of stimulatory and inhibitory receptor expression in T cells during aging.
The mechanism behind the increased expression of inhibitory receptors on exhausted T cells is likely to be an active process involving the transcription factor BLIMP-1, which is very highly expressed in exhausted T cells [98]. Silencing of BLIMP-1 reduces the expression of surface inhibitory receptors, and functional memory T cells express low levels of BLIMP-1. Low levels of Tbet and reduced translocation of NFATc1 to the nucleus have also been linked to increased exhaustion [58, 99]. Progressive demethylation of DNA occurs during aging and is considered to be one mechanism by which previously silent genes become expressed [100]. However, in addition to this, the above changes in transcription factor activity suggest active alterations in gene expression during exhaustion.
In summary, the genetic programs driving the expression of inhibitory receptors in senescent and aged T cells are not related and are different from those in exhausted T cells, further hinting that the gain and loss of regulatory receptors is a complex, regulated process rather than a stochastic by-product of senescence or exhaustion. In addition, whilst several processes can lead to the loss or gain of one receptor through common signaling pathways, each regulatory receptor or family of receptors responds to distinct pathways induced by a variety of age-related stimuli.
Do inhibitory receptors account for decreased immune function in the elderly?
To understand the role of inhibitory NK receptors in T cells, it is useful to examine their role in NK cells. Unlike T cells, NK cells do not express antigen receptors. Instead, they express a complicated array of activating and inhibitory receptors on the cell surface, which work in synergy to regulate their function and cytolytic activity. Inhibitory receptors of the KIR, KLR and ILT families all contain cytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIMs), which initiate inhibitory signals following their phosphorylation by providing a docking site for tyrosine phosphatases. How ITIMs are phosphorylated is still unclear. Studies have shown that when NK cells contact susceptible target cells, both activating and inhibitory receptors are recruited to the cSMAC [101–103]. The clustering of activating receptors recruits tyrosine kinases, such as Syk, Zap-70 and Lck to the synapse and data suggest that Lck, recruited by activating receptors, phosphorylates ITIMs in the cytoplasmic tail of inhibitory receptors thus initiating the inhibitory signal [104, 105]. In addition, the inhibition of activating receptors even before their phosphorylation suggests that a Lck-independent mechanism may exist to regulate the phosphorylation of ITIMs in NK inhibitory receptors. Whether NK cells execute cytolytic function on target cells depends on the ratio of activating and inhibitory signaling in the immune synapse [106–109]. If inhibitory signals dominate, they abrogate actin cytoskeleton rearrangement, reduce granule exocytosis, and prevent the cytolytic activity of NK cells [110, 111]. Rather than spreading over the cell membrane, inhibitory signaling is confined to the immune synapse in contact with a susceptible target cell [105]. This allows NK cells simultaneously in contact with resistant and susceptible target cells, to direct their cytolytic function towards the susceptible target cell only [105, 112]. The location of these inhibitory receptors on the T cell membrane has not been identified and the mechanisms behind the phosphorylation of their ITIMs are still unknown.
Most published studies suggest that these receptors have a similar coinhibitory function in T cells as in NK cells. In vitro cross-linking of CD85j and CD3 has been shown to reduce proliferation and IFN-γ production in both CD4+ and CD8+ T cell clones [113–115]. Blockade of CD85j up-regulates IFN-γ production and proliferation of T cells in response to CD3 stimulation [114]. The function of CD85j in CD4+ T cell clones is surprising, because the frequency of CD85j+ CD4+ T cells is very low. CD85j has also been demonstrated to inhibit actin cytoskeleton rearrangement upon both superantigen and TCR stimulation [115]. In addition, CD85j binds with high affinity to a CMV MHC class I homolog UL18, suggesting that it may be involved in T cell function in response to CMV infection [116, 117]. Blockade of CD85j induces IFN-γ production but not cytotoxic activity in CD8+ T cells in response to CMV peptide stimulation [88]. Blockade of KIRs in vitro up-regulates cytokine production by KIR+ T cell clones upon stimulation, suggesting that KIRs regulate cytokine production by T cells [118]. Transfection of KIR3DL1 to a tumor specific CTL cell line blocks its ability to lyse the target cells, and adding KIR3DL1 blocking antibody to the transfected CTL restores their cytolytic function [119]. KIRs have also been shown to inhibit the activation-induced cell death of T cells upon antigen-specific stimulation [24, 120].
As discussed above most published studies have shown that inhibitory receptor signaling impairs effector function and that blockade of these receptors can enhance antigen-specific T cell responses in vitro. While these observations are consistent with the paradigm derived from their function in NK cells, they raise the question of why CD8 T cells are programmed to eventually stably express these receptors. There is, less obvious from the published literature, evidence that the inhibitory action is not as global as assumed and that the application of the NK model to T cells is an oversimplification and may be even misleading. Many CMV- specific CD8 T cells express CD85j but are completely competent to respond to antigen stimulation with cytokine production. Work from our lab showed that cross-linking of TCR and KIR2DL2 inhibits IFN-γ production and proliferation, but not the cytotoxicity of KIR2DL2+ CD4+ T cell clones [121]. Further analysis showed that the late recruitment of KIR2DL2 to cSMACs means that KIR2DL2 only affects late TCR-mediated signaling events but not early events. In this way, KIRs selectively inhibit cytokine production and proliferation, but not cytotoxic function [121]. In chronic virus infections such as CMV, the effect of KIRs on CMV-specific CD8+ T cells seems to be limited, since cytolytic function and cytokine production of KIR+ CMV-specific T cells are normal upon antigen-specific stimulation. The inhibitory function of KIRs on cytokine production and proliferation of CD8+ T cells upon antigen-stimulation is dependent on TCR signaling strength [122]. Many of the in vitro model systems used are rather artificial, and the inhibition of effector function is frequently modest, selective or for a large number of T cells not demonstrable at all suggesting that the function of the inhibitory receptors are to modify responses, but not to paralyze effector function [118, 121, 123, 124]. This selectivity may be beneficial, restricting the expansion of oligoclonal T cells without inhibiting their function.
Is the expression of inhibitory receptors an attempt to prevent unopposed clonal expansion and maintain T cell homeostasis?
The controlled acquisition of inhibitory receptors on the surface of T cells during aging has been well documented. However, the possible benefits of such negative regulation are rarely discussed. Several aspects of immune aging are a reflection of the accumulation of end-differentiated T cells. These, along with exhausted and senescent cells, have proliferation defects that can be partly restored by blocking inhibitory receptors.
In vitro blockade of KLRG1 signaling (by blocking its ligand E-cadherin) recovers the defective proliferation in terminally differentiated CD8+ T cells by up-regulating the expression of cyclins and down-regulating cyclin inhibitor [54]. In contrast, decreased telomerase activity in terminally differentiated effector T cells is not recovered by KLRG1 blockade. The development of senescent cells during chronic infection may be dependent on continual presentation of antigen. In the context of chronic infection there is also likely to be continual activation and division of cells, which would increase the risk of DNA damage, and increase the requirement for growth factors, which may be limited when inflammation is low or absent. Senescence could, therefore, be viewed as a safety mechanism to prevent the proliferation of T cells that have a high risk of genetic mutation owing to substantial DNA damage or that lead to memory inflation. In the case of highly replicating viruses, clonal exhaustion occurs, which, however, also impairs function and not only proliferation. In the case of latent infections such as CMV, EBV and VZV, cells stay functional but express inhibitory receptors that limit clonal expansion [124]. This model, however, does not explain why memory inflation is frequently found with CMV infection although many of the CMV-specific clones express KLRs, KIRs and CD85 receptors.
While there is some indication that exhaustion and senescence could act as a barrier to uncontrolled proliferation, much more evidence is needed. Studies investigating the blockade of senescence-associated and exhaustion-associated receptors during chronic infection and examination of the resultant changes to disease progression and T cell diversity will be useful in this regard.
Age-related expression patterns of regulatory receptors – a risk factor for autoimmunity in the elderly?
A consistent feature of aging is a reduction in responses to infection and vaccination, but a concurrent increase in the incidence of autoimmunity. Some clues to the mechanisms behind this discrepancy can be found in genetic studies. A decrease in the expression of the complement regulators Cd55 and Cd46 was observed in T cells isolated from elderly individuals [8]. CD55-deficient mice show increased autoimmune disease development [125] and the loss of CD46 is associated with a switch from a regulatory phenotype to a Th1 phenotype in T cells [126]. A reduction in the expression of these genes could, therefore, contribute to the increased incidence of autoimmune diseases in the elderly. This could be exacerbated by changes in the expression of T cell regulatory molecules despite the trend for increased inhibitory receptor expression. In addition to inhibitory effects, KIRs can have stimulatory effects on the surface of NK cells and it is thought that these effects could apply equally to T cells. The KIR receptor KIR2DL4, whilst containing an ITIM motif in its cytoplasmic tail, can provide stimulatory as well as inhibitory signals to NK cells, in which population it is ubiquitously expressed [127]. Unlike other KIRs, KIR2DL4 transcripts have been found in all human NK cells rather than just a subset. Gene expression of KIR2DL4 has also been observed in T cells. KIR2DL4 appears to be different from other KIR members in that it signals at the level of the endosome, activating DNA-PKcs and downstream PI3K, [128]. When KIR2DL4 binds HLA-G, one of its ligands, the complex is internalized. The presence of HLA-G in an endosome then triggers inflammation and TNF-α and IFN-γ production [129]. KIR2DL4 is increased on T cells during aging and the amount of IFN-γ produced following ligation is proportional to disease severity in SLE patients [130]. This pro-inflammatory molecule may, therefore, play a larger role in T cell activation with increasing age, further suggesting that the mechanisms controlling the T cell response in elderly people are distinct.
Clearly, costimulation plays a large role in the survival and activation of T cells. Indeed, provision of costimulation in mouse models overcomes the reduction in anti-tumor immunity seen with advancing age [131]. However, increasing costimulatory signals to T cells could exacerbate autoimmunity. An increase in costimulatory molecule expression has been seen in the site of inflammation in many autoimmune diseases, including rheumatoid arthritis [132, 133]. Some autoimmune diseases are much more common in the elderly despite an overall reduction in T cell activation as described above. Though T cells isolated from peripheral blood have reduced costimulatory molecule expression, those isolated from the site of inflammation, e.g. the inflamed synovial membrane, have increased expression of OX40 [132]. This may reflect a decreased activation threshold of T cells at the inflamed site, which could exacerbate autoimmunity. Distinct from classical costimulatory molecules, aging-related increases in other activating receptors have been observed. These stimulatory molecules provide positive signals to T cells, possibly even in the absence of antigen and, again, their ligands are not restricted to APCs. As such, they can provide inflammatory signals to T cells without the temporal and spatial control afforded by traditional costimulators. One such potentially activating receptor is the NK cell-related molecule NKG2D. When expressed in cells that also express DAP10, NKG2D provides a stimulatory signal in the absence of antigen [134, 135]. KIR2DS2 behaves in a similar fashion in cells expressing DAP12 [136], and KIR2DL4, as discussed above, can provide a stimulatory signal after binding its ligand, HLA-G.
Increased costimulatory activity may explain the increased incidence of autoimmunity in the elderly which at first sight seems contradictory to expectations considering the almost universal increase in negative regulators on the surface of immune cells. The potential for some of the NK associated receptors, such as NKG2D, KIR2DL4 and KIR2DS2 to provide positive signals, the tissue-specific expression of costimulatory receptors and the loss of certain inhibitory receptors may imbalance immunity following the initiation of inflammation. Further studies investigating the full range of costimulatory and inhibitory molecules expressed on the surface of individual T cells, as well as the expression patterns and tissue distribution of their ligands, will be needed to provide clues to the mechanisms behind T cell dysfunction during autoimmune disease and aging.
Conclusions
In considering the changes to T cells and T cell regulation during aging, it appears that immune aging is at least partly an active process and may, therefore, provide some benefits in the clearance of pathogens and tumors and/or the suppression of autoimmunity. Rather than observing changes to the T cell pool as a whole, we must now consider immune aging in terms of chronological aging of individual cells in combination with the accumulation of specific cell subsets. As immune therapies develop, it will be important to consider how aging affects their efficacy. Targeting specific populations may be more effective than targeting receptors that affect the T cell population as a whole, and dissection of the expression patterns of costimulatory and inhibitory molecules within each subset will help to identify specific receptors for targeting. The accumulation of, for example, exhausted T cells in a patient with a chronic, active infection such as HCV may contraindicate certain immune therapies which may not be the case in a patient with a higher proportion of senescent, CMV-specific cells. Targeting exhausted T cell-specific receptors may provide more effective therapy against chronic infections, whilst senescence-specific receptor targeting may lead to the proliferation of damaged or at least unwanted T cell clones. Figure 1 shows the main mechanisms leading to the formation of each T cell subset and the regulatory receptors currently known to be associated with that subset. Future studies should address the extent to which memory formation, senescence and exhaustion are independent processes; the roles played by costimulatory and inhibitory receptors during aging and autoimmunity; the effects of T cell location (periphery vs. lymphoid organs vs. inflamed sites); and the potential for therapeutic interventions based on reversing exhaustion and/or promoting effective memory.
References
- 1.Goodwin JS, Searles RP, Tung KS. Immunological responses of healthy elderly population. Clin Exp Immunol. 1982;48:403–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schachter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29:601–9. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 3.Debenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28− T lymphocytes by 4–1BB ligand. J Immunol. 1997;158:551–9. [PubMed] [Google Scholar]
- 4.Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, Mackall CL. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179:4910–8. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidric M, Suh WK, Dianzani U, Mak TW, Watts TH. Cooperation between 4-1BB and ICOS in the immune response to influenza virus revealed by studies of CD28/ICOS-deficient mice. J Immunol. 2005;175:7288–96. doi: 10.4049/jimmunol.175.11.7288. [DOI] [PubMed] [Google Scholar]
- 6.Bansal-Pakala P, Croft M. Defective T cell priming associated with aging can be rescued by signaling through 4-1BB (CD137) J Immunol. 2002;169:5005–9. doi: 10.4049/jimmunol.169.9.5005. [DOI] [PubMed] [Google Scholar]
- 7.Ruby CE, Weinberg AD. OX40-enhanced tumor rejection and effector T cell differentiation decreases with age. J Immunol. 2009;182:1481–9. doi: 10.4049/jimmunol.182.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao JN, Gollapudi S, Sharman EH, Jia Z, Gupta S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell. 2010;9:19–31. doi: 10.1111/j.1474-9726.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- 9.Lazuardi L, Herndler-Brandstetter D, Brunner S, Laschober GT, Lepperdinger G, Grubeck-Loebenstein B. Microarray analysis reveals similarity between CD8+CD28− T cells from young and elderly persons, but not of CD8+CD28+ T cells. Biogerontology. 2009;10:191–202. doi: 10.1007/s10522-008-9167-1. [DOI] [PubMed] [Google Scholar]
- 10.Yu M, Li G, Lee W-W, Yuan M, Weyand CM, Goronzy JJ. Mechanisms of defective T cell-dependent B cell responses with age. J Immunol. 2011;186:104.17. [Google Scholar]
- 11.Koulova L, Clark EA, Shu G, Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991;173:759–62. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding FA, Mcarthur JG, Gross JA, Raulet DH, Allison JP. CD28− mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 13.Gimmi CD, Freeman GJ, Gribben JG, Sugita K, Freedman AS, Morimoto C, Nadler LM. B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci U S A. 1991;88:6575–9. doi: 10.1073/pnas.88.15.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young JW, Koulova L, Soergel SA, Clark EA, Steinman RM, Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992;90:229–37. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman AS, Freeman GJ, Rhynhart K, Nadler LM. Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991;137:429–37. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 16.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney ER, Walunas TL, Karr RW, Morton PA, Loh DY, Bluestone JA, Jenkins MK. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–6. [PubMed] [Google Scholar]
- 18.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 19.Van Bergen J, Thompson A, Van Der Slik A, Ottenhoff TH, Gussekloo J, Koning F. Phenotypic and functional characterization of CD4 T cells expressing killer Ig-like receptors. J Immunol. 2004;173:6719–26. doi: 10.4049/jimmunol.173.11.6719. [DOI] [PubMed] [Google Scholar]
- 20.Abedin S, Michel JJ, Lemster B, Vallejo AN. Diversity of NKR expression in aging T cells and in T cells of the aged: the new frontier into the exploration of protective immunity in the elderly. Exp Gerontol. 2005;40:537–48. doi: 10.1016/j.exger.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Tarazona R, Delarosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 22.Fann M, Chiu WK, Wood WH, 3rd, Levine BL, Becker KG, Weng NP. Gene expression characteristics of CD28null memory phenotype CD8+ T cells and its implication in T-cell aging. Immunol Rev. 2005;205:190–206. doi: 10.1111/j.0105-2896.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 23.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–18. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arlettaz L, Degermann S, De Rham C, Roosnek E, Huard B. Expression of inhibitory KIR is confined to CD8+ effector T cells and limits their proliferative capacity. Eur J Immunol. 2004;34:3413–22. doi: 10.1002/eji.200324756. [DOI] [PubMed] [Google Scholar]
- 25.Snyder MR, Muegge LO, Offord C, O’fallon WM, Bajzer Z, Weyand CM, Goronzy JJ. Formation of the killer Ig-like receptor repertoire on CD4+CD28null T cells. J Immunol. 2002;168:3839–46. doi: 10.4049/jimmunol.168.8.3839. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Vallejo AN, Jiang Y, Weyand CM, Goronzy JJ. Distinct transcriptional control mechanisms of killer immunoglobulin-like receptors in natural killer (NK) and in T cells. J Biol Chem. 2005;280:24277–85. doi: 10.1074/jbc.M500727200. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Weyand CM, Goronzy JJ. Epigenetic mechanisms of age-dependent KIR2DL4 expression in T cells. J Leukoc Biol. 2008;84:824–34. doi: 10.1189/jlb.0807583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Yu M, Weyand CM, Goronzy JJ. Epigenetic regulation of killer immunoglobulin-like receptor expression in T cells. Blood. 2009;114:3422–30. doi: 10.1182/blood-2009-01-200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang C, Rodriguez A, Carretero M, Lopez-Botet M, Phillips JH, Lanier LL. Molecular characterization of human CD94: a type II membrane glycoprotein related to the C-type lectin superfamily. Eur J Immunol. 1995;25:2433–7. doi: 10.1002/eji.1830250904. [DOI] [PubMed] [Google Scholar]
- 30.Giorda R, Rudert WA, Vavassori C, Chambers WH, Hiserodt JC, Trucco M. NKR-P1, a signal transduction molecule on natural killer cells. Science. 1990;249:1298–300. doi: 10.1126/science.2399464. [DOI] [PubMed] [Google Scholar]
- 31.Guthmann MD, Tal M, Pecht I. A new member of the C-type lectin family is a modulator of the mast cell secretory response. Int Arch Allergy Immunol. 1995;107:82–6. doi: 10.1159/000236938. [DOI] [PubMed] [Google Scholar]
- 32.Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203:289–95. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamar DL, Weyand CM, Goronzy JJ. Promoter choice and translational repression determine cell type-specific cell surface density of the inhibitory receptor CD85j expressed on different hematopoietic lineages. Blood. 2010;115:3278–86. doi: 10.1182/blood-2009-09-243493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northfield J, Lucas M, Jones H, Young NT, Klenerman P. Does memory improve with age? CD85j (ILT-2/LIR-1) expression on CD8 T cells correlates with ‘memory inflation’ in human cytomegalovirus infection. Immunol Cell Biol. 2005;83:182–8. doi: 10.1111/j.1440-1711.2005.01321.x. [DOI] [PubMed] [Google Scholar]
- 35.Anfossi N, Doisne JM, Peyrat MA, Ugolini S, Bonnaud O, Bossy D, Pitard V, Merville P, Moreau JF, Delfraissy JF, Dechanet-Merville J, Bonneville M, Venet A, Vivier E. Coordinated expression of Ig-like inhibitory MHC class I receptors and acquisition of cytotoxic function in human CD8+ T cells. J Immunol. 2004;173:7223–9. doi: 10.4049/jimmunol.173.12.7223. [DOI] [PubMed] [Google Scholar]
- 36.Morice WG, Kurtin PJ, Leibson PJ, Tefferi A, Hanson CA. Demonstration of aberrant T-cell and natural killer-cell antigen expression in all cases of granular lymphocytic leukaemia. Br J Haematol. 2003;120:1026–36. doi: 10.1046/j.1365-2141.2003.04201.x. [DOI] [PubMed] [Google Scholar]
- 37.De Maria A, Ferraris A, Guastella M, Pilia S, Cantoni C, Polero L, Mingari MC, Bassetti D, Fauci AS, Moretta L. Expression of HLA class I-specific inhibitory natural killer cell receptors in HIV-specific cytolytic T lymphocytes: impairment of specific cytolytic functions. Proc Natl Acad Sci U S A. 1997;94:10285–8. doi: 10.1073/pnas.94.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemster BH, Michel JJ, Montag DT, Paat JJ, Studenski SA, Newman AB, Vallejo AN. Induction of CD56 and TCR-independent activation of T cells with aging. J Immunol. 2008;180:1979–90. doi: 10.4049/jimmunol.180.3.1979. [DOI] [PubMed] [Google Scholar]
- 39.Jamieson AM, Diefenbach A, Mcmahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 40.Hong MS, Dan JM, Choi JY, Kang I. Age-associated changes in the frequency of naive, memory and effector CD8+ T cells. Mech Ageing Dev. 2004;125:615–8. doi: 10.1016/j.mad.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Hsu HC, Scott DK, Zhang P, Zhou J, Yang P, Wu Q, Schroeder HW, Jr, Gerald LB, Ravussin E, Jazwinski SM, Mountz JD. CD8 T-cell immune phenotype of successful aging. Mech Ageing Dev. 2006;127:231–9. doi: 10.1016/j.mad.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Arlettaz L, Barbey C, Dumont-Girard F, Helg C, Chapuis B, Roux E, Roosnek E. CD45 isoform phenotypes of human T cells: CD4(+)CD45RA(-)RO(+) memory T cells re-acquire CD45RA without losing CD45RO. Eur J Immunol. 1999;29:3987–94. doi: 10.1002/(SICI)1521-4141(199912)29:12<3987::AID-IMMU3987>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–40. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- 44.Saez-Borderias A, Guma M, Angulo A, Bellosillo B, Pende D, Lopez-Botet M. Expression and function of NKG2D in CD4+ T cells specific for human cytomegalovirus. Eur J Immunol. 2006;36:3198–206. doi: 10.1002/eji.200636682. [DOI] [PubMed] [Google Scholar]
- 45.Namekawa T, Snyder MR, Yen JH, Goehring BE, Leibson PJ, Weyand CM, Goronzy JJ. Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J Immunol. 2000;165:1138–45. doi: 10.4049/jimmunol.165.2.1138. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 47.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010;22:552–9. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’adda Di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–8. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 49.Campisi J, D’adda Di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 50.D’adda Di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–22. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 51.Passos JF, Von Zglinicki T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res. 2006;40:1277–83. doi: 10.1080/10715760600917151. [DOI] [PubMed] [Google Scholar]
- 52.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–95. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 53.Voehringer D, Blaser C, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–43. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 54.Henson SM, Franzese O, Macaulay R, Libri V, Azevedo RI, Kiani-Alikhan S, Plunkett FJ, Masters JE, Jackson S, Griffiths SJ, Pircher HP, Soares MV, Akbar AN. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113:6619–28. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 55.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Muller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003;38:911–20. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 56.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 57.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;131:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 58.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Wherry EJ, Blattman JN, Murali-Krishna K, Van Der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–9. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macpherson PA, Fex C, Sanchez-Dardon J, Hawley-Foss N, Angel JB. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:454–7. doi: 10.1097/00042560-200112150-00008. [DOI] [PubMed] [Google Scholar]
- 62.Ogg GS, Jin X, Bonhoeffer S, Moss P, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Hurley A, Markowitz M, Ho DD, Mcmichael AJ, Nixon DF. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee SJ, Rossi RJ, Lee SK, Croft M, Kwon BS, Mittler RS, Vella AT. CD134 costimulation couples the CD137 pathway to induce production of supereffector CD8 T cells that become IL-7 dependent. J Immunol. 2007;179:2203–14. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 64.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–57. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, Mcgavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, Von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–72. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 69.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, Depierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 70.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 71.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–87. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 73.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–70. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 75.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 76.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–58. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 82.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, Russell K, Toth I, Piechocka-Trocha A, Dolfi D, Angelosanto J, Crawford A, Shin H, Kwon DS, Zupkosky J, Francisco L, Freeman GJ, Wherry EJ, Kaufmann DE, Walker BD, Ebert B, Haining WN. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–51. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lages CS, Lewkowich I, Sproles A, Wills-Karp M, Chougnet C. Partial restoration of T-cell function in aged mice by in vitro blockade of the PD-1/PD-L1 pathway. Aging Cell. 2010;9:785–98. doi: 10.1111/j.1474-9726.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M. Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp Gerontol. 2009;44:517–22. doi: 10.1016/j.exger.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev. 2009;130:709–12. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 87.Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, Tata P, Gupta S, Zilliox MJ, Nakaya HI, Pulendran B, Haining WN, Freeman GJ, Ahmed R. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186:4200–12. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ince MN, Harnisch B, Xu Z, Lee SK, Lange C, Moretta L, Lederman M, Lieberman J. Increased expression of the natural killer cell inhibitory receptor CD85j/ILT2 on antigen-specific effector CD8 T cells and its impact on CD8 T-cell function. Immunology. 2004;112:531–42. doi: 10.1046/j.1365-2567.2004.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933–41. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]
- 90.Alter G, Rihn S, Streeck H, Teigen N, Piechocka-Trocha A, Moss K, Cohen K, Meier A, Pereyra F, Walker B, Altfeld M. Ligand-independent exhaustion of killer immunoglobulin-like receptor-positive CD8+ T cells in human immunodeficiency virus type 1 infection. J Virol. 2008;82:9668–77. doi: 10.1128/JVI.00341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eck SC, Chang D, Wells AD, Turka LA. Differential down-regulation of CD28 by B7-1 and B7-2 engagement. Transplantation. 1997;64:1497–9. doi: 10.1097/00007890-199711270-00025. [DOI] [PubMed] [Google Scholar]
- 92.Vallejo AN, Brandes JC, Weyand CM, Goronzy JJ. Modulation of CD28 expression: distinct regulatory pathways during activation and replicative senescence. J Immunol. 1999;162:6572–9. [PubMed] [Google Scholar]
- 93.Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-alpha. J Immunol. 2001;167:3231–8. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- 94.Warrington KJ, Vallejo AN, Weyand CM, Goronzy JJ. CD28 loss in senescent CD4+ T cells: reversal by interleukin-12 stimulation. Blood. 2003;101:3543–9. doi: 10.1182/blood-2002-08-2574. [DOI] [PubMed] [Google Scholar]
- 95.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–9. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 96.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, Akbar AN. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–25. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 97.Uhrberg M, Valiante NM, Young NT, Lanier LL, Phillips JH, Parham P. The repertoire of killer cell Ig-like receptor and CD94:NKG2A receptors in T cells: clones sharing identical alpha beta TCR rearrangement express highly diverse killer cell Ig-like receptor patterns. J Immunol. 2001;166:3923–32. doi: 10.4049/jimmunol.166.6.3923. [DOI] [PubMed] [Google Scholar]
- 98.Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–20. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8(+) T cell responses during chronic infection. Nat Immunol. 2011;12:663–71. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y, Chen Y, Richardson B. Decreased DNA methyltransferase levels contribute to abnormal gene expression in “senescent” CD4(+)CD28(-) T cells. Clin Immunol. 2009;132:257–65. doi: 10.1016/j.clim.2009.03.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vyas YM, Mehta KM, Morgan M, Maniar H, Butros L, Jung S, Burkhardt JK, Dupont B. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol. 2001;167:4358–67. doi: 10.4049/jimmunol.167.8.4358. [DOI] [PubMed] [Google Scholar]
- 102.Vyas YM, Maniar H, Dupont B. Cutting edge: differential segregation of the SRC homology 2-containing protein tyrosine phosphatase-1 within the early NK cell immune synapse distinguishes noncytolytic from cytolytic interactions. J Immunol. 2002;168:3150–4. doi: 10.4049/jimmunol.168.7.3150. [DOI] [PubMed] [Google Scholar]
- 103.Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev. 2010;235:24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Binstadt BA, Brumbaugh KM, Dick CJ, Scharenberg AM, Williams BL, Colonna M, Lanier LL, Kinet JP, Abraham RT, Leibson PJ. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–38. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 105.Treanor B, Lanigan PM, Kumar S, Dunsby C, Munro I, Auksorius E, Culley FJ, Purbhoo MA, Phillips D, Neil MA, Burshtyn DN, French PM, Davis DM. Microclusters of inhibitory killer immunoglobulin-like receptor signaling at natural killer cell immunological synapses. J Cell Biol. 2006;174:153–61. doi: 10.1083/jcb.200601108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wulfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci U S A. 2003;100:7767–72. doi: 10.1073/pnas.1336920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci U S A. 2003;100:14151–6. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lou Z, Jevremovic D, Billadeau DD, Leibson PJ. A balance between positive and negative signals in cytotoxic lymphocytes regulates the polarization of lipid rafts during the development of cell-mediated killing. J Exp Med. 2000;191:347–54. doi: 10.1084/jem.191.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J Immunol. 2006;177:3590–6. doi: 10.4049/jimmunol.177.6.3590. [DOI] [PubMed] [Google Scholar]
- 110.Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–9. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Billadeau DD, Brumbaugh KM, Dick CJ, Schoon RA, Bustelo XR, Leibson PJ. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J Exp Med. 1998;188:549–59. doi: 10.1084/jem.188.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eriksson M, Leitz G, Fallman E, Axner O, Ryan JC, Nakamura MC, Sentman CL. Inhibitory receptors alter natural killer cell interactions with target cells yet allow simultaneous killing of susceptible targets. J Exp Med. 1999;190:1005–12. doi: 10.1084/jem.190.7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saverino D, Fabbi M, Ghiotto F, Merlo A, Bruno S, Zarcone D, Tenca C, Tiso M, Santoro G, Anastasi G, Cosman D, Grossi CE, Ciccone E. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J Immunol. 2000;165:3742–55. doi: 10.4049/jimmunol.165.7.3742. [DOI] [PubMed] [Google Scholar]
- 114.Saverino D, Merlo A, Bruno S, Pistoia V, Grossi CE, Ciccone E. Dual effect of CD85/leukocyte Ig-like receptor-1/Ig-like transcript 2 and CD152 (CTLA-4) on cytokine production by antigen-stimulated human T cells. J Immunol. 2002;168:207–15. doi: 10.4049/jimmunol.168.1.207. [DOI] [PubMed] [Google Scholar]
- 115.Dietrich J, Cella M, Colonna M. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J Immunol. 2001;166:2514–21. doi: 10.4049/jimmunol.166.4.2514. [DOI] [PubMed] [Google Scholar]
- 116.Chapman TL, Heikeman AP, Bjorkman PJ. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity. 1999;11:603–13. doi: 10.1016/s1074-7613(00)80135-1. [DOI] [PubMed] [Google Scholar]
- 117.Yang Z, Bjorkman PJ. Structure of UL18, a peptide-binding viral MHC mimic, bound to a host inhibitory receptor. Proc Natl Acad Sci U S A. 2008;105:10095–100. doi: 10.1073/pnas.0804551105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.D’andrea A, Chang C, Phillips JH, Lanier LL. Regulation of T cell lymphokine production by killer cell inhibitory receptor recognition of self HLA class I alleles. J Exp Med. 1996;184:789–94. doi: 10.1084/jem.184.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bakker AB, Phillips JH, Figdor CG, Lanier LL. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, gamma delta T cells, and antigen-specific CTL. J Immunol. 1998;160:5239–45. [PubMed] [Google Scholar]
- 120.Gati A, Guerra N, Gaudin C, Da Rocha S, Escudier B, Lecluse Y, Bettaieb A, Chouaib S, Caignard A. CD158 receptor controls cytotoxic T-lymphocyte susceptibility to tumor-mediated activation-induced cell death by interfering with Fas signaling. Cancer Res. 2003;63:7475–82. [PubMed] [Google Scholar]
- 121.Henel G, Singh K, Cui D, Pryshchep S, Lee WW, Weyand CM, Goronzy JJ. Uncoupling of T-cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood. 2006;107:4449–57. doi: 10.1182/blood-2005-06-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van Der Veken LT, Campelo MD, Van Der Hoorn MA, Hagedoorn RS, Van Egmond HM, Van Bergen J, Willemze R, Falkenburg JH, Heemskerk MH. Functional analysis of killer Ig-like receptor-expressing cytomegalovirus-specific CD8+ T cells. J Immunol. 2009;182:92–101. doi: 10.4049/jimmunol.182.1.92. [DOI] [PubMed] [Google Scholar]
- 123.Phillips JH, Gumperz JE, Parham P, Lanier LL. Superantigen-dependent, cell-mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science. 1995;268:403–5. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- 124.Van Bergen J, Kooy-Winkelaar EM, Van Dongen H, Van Gaalen FA, Thompson A, Huizinga TW, Feltkamp MC, Toes RE, Koning F. Functional killer Ig-like receptors on human memory CD4+ T cells specific for cytomegalovirus. J Immunol. 2009;182:4175–82. doi: 10.4049/jimmunol.0800455. [DOI] [PubMed] [Google Scholar]
- 125.Miwa T, Maldonado MA, Zhou L, Sun X, Luo HY, Cai D, Werth VP, Madaio MP, Eisenberg RA, Song WC. Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/lpr mice. Am J Pathol. 2002;161:1077–86. doi: 10.1016/S0002-9440(10)64268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, Hayday A, Kemper C. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–71. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kikuchi-Maki A, Yusa S, Catina TL, Campbell KS. KIR2DL4 is an IL-2-regulated NK cell receptor that exhibits limited expression in humans but triggers strong IFN-gamma production. J Immunol. 2003;171:3415–25. doi: 10.4049/jimmunol.171.7.3415. [DOI] [PubMed] [Google Scholar]
- 128.Rajagopalan S, Moyle MW, Joosten I, Long EO. DNA-PKcs controls an endosomal signaling pathway for a proinflammatory response by natural killer cells. Sci Signal. 2010;3:ra14. doi: 10.1126/scisignal.2000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rajagopalan S. Endosomal signaling and a novel pathway defined by the natural killer receptor KIR2DL4 (CD158d) Traffic. 2010;11:1381–90. doi: 10.1111/j.1600-0854.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 130.Basu D, Liu Y, Wu A, Yarlagadda S, Gorelik GJ, Kaplan MJ, Hewagama A, Hinderer RC, Strickland FM, Richardson BC. Stimulatory and inhibitory killer Ig-like receptor molecules are expressed and functional on lupus T cells. J Immunol. 2009;183:3481–7. doi: 10.4049/jimmunol.0900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jensen SM, Maston LD, Gough MJ, Ruby CE, Redmond WL, Crittenden M, Li Y, Puri S, Poehlein CH, Morris N, Kovacsovics-Bankowski M, Moudgil T, Twitty C, Walker EB, Hu HM, Urba WJ, Weinberg AD, Curti B, Fox BA. Signaling through OX40 enhances antitumor immunity. Semin Oncol. 2010;37:524–32. doi: 10.1053/j.seminoncol.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshioka T, Nakajima A, Akiba H, Ishiwata T, Asano G, Yoshino S, Yagita H, Okumura K. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–23. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 133.Brugnoni D, Bettinardi A, Malacarne F, Airo P, Cattaneo R. CD134/OX40 expression by synovial fluid CD4+ T lymphocytes in chronic synovitis. Br J Rheumatol. 1998;37:584–5. doi: 10.1093/rheumatology/37.5.584. [DOI] [PubMed] [Google Scholar]
- 134.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 135.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100:9452–7. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Snyder MR, Nakajima T, Leibson PJ, Weyand CM, Goronzy JJ. Stimulatory killer Ig-like receptors modulate T cell activation through DAP12-dependent and DAP12-independent mechanisms. J Immunol. 2004;173:3725–31. doi: 10.4049/jimmunol.173.6.3725. [DOI] [PubMed] [Google Scholar]
- 137.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–18. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]