Abstract
Infectious diseases contribute to significant morbidity and mortality in elderly populations. One of the major contributing factors to this is age-related declines in the immune system that diminish the response o both infections and vaccinations. In order to understand how specific changes in the immune system influence the generation of immunity in older individuals, immunologists have developed aging mouse models that allow for experimental manipulation of immune system components. These models have shown that there are dramatic age-related changes in naive CD4 T cell function that have the potential to impact a myriad of immune responses. In this review, we will summarize these findings on the intrinsic changes in CD4 T cell function and discuss how these changes influence immunity.
Keywords: Aging, CD4, T cell, Mouse, Model
With aging, the immune system declines leading to an increased susceptibility to infectious diseases in older individuals. Because of this, the elderly are often targeted for vaccinations to prevent serious illness. The drawback of this approach is that because of age-related changes in the immune system, vaccines exhibit significantly reduced efficacy in the elderly. Reduced immune responses have been observed in elderly individuals vaccinated against tetanus and Streptococcus pneumoniae (1, 2) and this is especially evident when elderly individuals are vaccinated with a new vaccine such as that for Hepatitis B (3). In this case, 96% of young adults exhibited seroconversion following vaccination, while only 44% of adults aged 71–80 showed similar seroconversion. With a substantial percentage of the global population approaching an advanced age, coupled with the threat of emerging diseases that can severely impact the aged, such as pandemic influenza, West Nile virus and severe acute respiratory syndrome (SARS), it is critical that we devise means to improve the vaccination of the aged and therapies to increase immunity in this susceptible population. However, in order to achieve this, a thorough understanding of the mechanisms responsible for age-related declines in immune function is required.
While both innate and adaptive immune responses can be influenced by aging, the adaptive immune response is most important for generation of protection following vaccination. The immunological memory generated following vaccination can be incredibly potent and can also persist for an extended period of time (4, 5). Most of the vaccines currently in use aim to generate protective antibodies, which can neutralize invading pathogens and prevent development of serious illness. In order to generate highly protective antibodies both CD4 T cells and B cells need to function properly. CD4 T cells deliver cognate help to B cells, which results in the generation of high affinity antibodies and the development of both CD4 T cell and B cell memory. Importantly, as individuals age, the ability to generate these high affinity antibodies that can protect from infection wanes. Not only do older individuals produce lower titers of antibodies, they produce antibodies that exhibit reduced function, such as neutralizing and opsonizing activities, compared to younger individuals (6, 7). In this review, we focus on how aging influences CD4 T cell function and cognate helper activity and the impact that this has on the response to vaccination and development of a robust humoral response.
In vitro model demonstrating aging CD4 T cell defects
The introduction of T cell receptor transgenic (TCR Tg) mouse models to examine how aging influences CD4 T cell function allowed for the examination of antigen-specific responses from both naive and memory CD4 T cell populations. Previously, this sort of study had been extremely difficult since there are few naive CD4 T cells present in older animals (8) and precise examination of antigen specific responses is quite difficult in wild type mice. In vitro studies with TCR Tg CD4 T cells definitively demonstrated that phenotypically naive (CD44loCD45RBhi) CD4 T cells from aged (over 16 months) mice exhibited reduced proliferation to specific peptide antigen along with reduced IL-2 and IL-3 production (9). Thus, this was the initial determination of intrinsic defects in antigen-specific naive CD4 T cells derived from aged mice.
Subsequent studies revealed that the main defect of naive CD4 T cells from aged mice was reduced IL-2 production following TCR stimulation with peptide antigen (10). This observation was important since the in vitro responses of antigen-stimulated CD4 T cells, including the tempo, magnitude and duration of clonal expansion, is regulated by the availability of IL-2 (11). The presence of adequate amounts of IL-2 also prevents apoptosis of expanding T cell populations, leading to accumulation of significantly larger numbers of highly activated effectors (12, 13). By comparing in vitro IL-2 production from the same number of young and aged antigen-specific CD4 T cells, it was discovered that young T cells produce up to 3 times as much IL-2 compared to aged T cells. This reduced availability of IL-2 resulted in the aged cells undergoing fewer rounds of cell division, exhibiting less clonal expansion and expressing a less differentiated phenotype (10), as summarized in Table 1.
Table 1.
Summary of age-related changes in CD4 T cell function and how they can be overcome
| In vitro changes in aged CD4 T cell function |
|
| In vivo changes in aged CD4 T cell function |
|
| Enhancement of aged CD4 T cell function |
|
Major defects in the intracellular signaling in T cells develop with aging and likely contribute to these functional impairments observed in naive CD4 T cells, including reduced IL-2 production [recently reviewed in (14)]. Upon antigenic stimulation, defective immunological synapse formation, an essential event of naive CD4 T cell activation and cognate helper function, was observed in aged CD4 T cell (15, 16). Furthermore, TCR signaling was also diminished in aged CD4 T cells. This includes reduced ZAP-70 activation resulting in decreased tyrosine phosphorylation of the zeta-chains (17). Downstream events of ZAP-70 activation are vital for cytoskeleton remodeling, which is an important aspect of several essential functions of T cells including cell migration, cytokine secretion, receptor endocytosis and many more. Thus, impairments in signaling pathways associated with the cytoskeleton arise with age and, therefore, compromise important cellular functions [reviewed in (18)]. Another downstream event of TCR signaling, Raf-1 activation, is reduced in aged naive CD4 T cells compared to young CD4 T cells (19). This decrease likely contributes to the reduced production of IL-2 by CD4 T cells from old mice as Raf-1 activates the ERK/MAPK pathway that leads to IL-2 transcription. Additionally, the activation of the transcription factors NF-AT and AP-1, which are implicated in IL-2 transcription, were also found to be decreased with age in T cells (20, 21).
Importantly, the age-related defect in aged naive T cell responses could be overcome by the addition of exogenous IL-2 to aged cell cultures (10). When IL-2 was added, the aged cells proliferated much like the young cells and also exhibited enhanced subsequent IL-2 production. In addition, aged T cells stimulated in the presence of exogenous IL-2 also exhibited a more activated cell surface phenotype, including enhanced expression of CD25 and down regulation of CD62L when compared to aged cells stimulated without exogenous IL-2. Finally, the addition of exogenous IL-2 to aged CD4 T cells could enhance their differentiation to both Th1 and Th2 subsets, such that their cytokine production was similar to young T cells (10). These results demonstrated that the main defect of aged naive CD4 T cells was their inability to produce enough IL-2, and did not involve defects in response to exogenously provided IL-2.
Reduced in vivo function of aged CD4 T cells
While the in vitro studies definitively showed that there were intrinsic defects in the antigen-specific responses of naive CD4 T cells from aged mice, the in vivo relevance of these studies was not apparent. Further studies employed an adoptive transfer model in which naive CD4 T cells harvested from young or aged TCR Tg mice were transferred into young hosts which were then immunized with specific antigen with alum as the adjuvant. Thus, by using young hosts for these studies, the influence of the aged environment could be eliminated and the intrinsic defect in the transferred CD4 T cells could be specifically examined. Interestingly, aged CD4 T cells primed in young hosts exhibit similar defects as those stimulated with antigen and APC in vitro (22). This includes reduced IL-2 production and reduced clonal expansion. This age-related defect in clonal expansion was observed over the first 7 days following vaccination but was no longer evident by day 14 even though the Carboxyfluorescein succinimidyl ester (CFSE) profiles of the aged population showed fewer rounds of cell division at this time point (23).
In addition to reduced in vivo expansion and IL-2 production, aged naive CD4 T cells transferred into young hosts also exhibited reduced cognate helper function for B cell responses. CD4 T cells are critical for the generation of a high affinity antibody response following vaccination. The CD4 T cell subset identified as follicular helper T cells (Tfh) is specialized for this function and the interaction of Tfh with responding B cells induces the generation of germinal centers, long-lived plasma cells and memory B cells (24). In order to examine the impact of aging on cognate helper activity, hosts lacking endogenous CD4 T cells were employed in order to specifically examine the in vivo activity of the young and aged donor T cell populations. For these studies, aged T cells were transferred into young hosts, which were immunized with a haptenated protein (NP conjugated to pigeon cytochrome c) and the hapten-specific B cell responses were examined. In the hosts receiving aged donor T cells there was significantly less hapten-specific B cell expansion and differentiation to a germinal center phenotype, even though these B cells were young (23). This difference was apparent at all time points examined and with transfer of a wide range of cell numbers. Thus, the aged CD4 T cell function was not just slower but was quantitatively reduced on a per cell basis.
This reduced cognate helper activity of aged CD4 T cells also resulted in reduced hapten-specific serum IgG titers at all time points examined (23). Furthermore, the antibodies generated in the presence of the aged donor cells also exhibited a significantly lower frequency of somatic hypermutation, which is necessary for proper affinity maturation. The end result of this was that the antibody response generated when aged CD4 T cells provide help was reduced in both magnitude and quality. Since most current human vaccines aim to generate an antibody response, this could account for the reduced efficacy of vaccinations observed in the elderly.
Enhancement of aged CD4 T cell in vivo responses
Initial studies to determine if the in vivo responses of aged CD4 T cells could be enhanced examined whether the use of an adjuvant other than aluminum hydroxide (alum) could boost function. The rationale for this approach was that by boosting the innate inflammatory immune response with a potent adjuvant, the response of aged CD4 T cells could be improved. In fact, it has been appreciated for some time that inflammatory cytokines can provide a “third” signal that enhances the costimulation of naive CD4 T cells in vivo (25). Thus, studies with aged TCR Tg CD4 T cells specific for pigeon cytochrome c (PCC) demonstrated that vaccination with PCC in Complete Freund’s Adjuvant (CFA) could enhance both the clonal expansion and IL-2 production of aged naive T cells adoptively transferred into young hosts so that their response was equivalent to that seen with young donor T cells (22). The mechanism of this enhancement was found to be due to the fact that CFA induced the pro-inflammatory (PI) cytokines TNFα, IL-1 and IL-6 to much higher levels when compared to alum (22). These studies went on to determine that these three cytokines administered with antigen could act as an adjuvant for vaccination and enhance the in vivo clonal expansion of aged CD4 T cells in young hosts. In vitro, these PI cytokines were also shown to enhance the clonal expansion and IL-2 production by antigen (PCC peptide) stimulated aged CD4 T cells via a mechanism involving the enhanced activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) (22). This is quite important since NFκB activation is important for T cell activation, cytokine production and migration [reviewed in (26)].
More recently, the use of Toll Like Receptor (TLR) agonists to enhance aged CD4 T cell responses in vitro has also been examined (27). TLRs are a type of pattern recognition receptor that is expressed by numerous cell types in the mammalian immune system (28). TLRs bind molecules that are associated with perceived threats such as pathogen products and signal for responses such as cellular activation and cytokine production. The rationale for these studies was that a TLR agonist binding to a TLR on antigen presenting cells (APC), such as dendritic cells, induces the production of pro-inflammatory cytokines by the APC, which in turn can act to deliver additional costimulatory signals to responding T cells. In fact, this study went on to show that APC treated with TLR ligands produced enhanced levels of IL-6 and supported increased in vitro clonal expansion and survival of responding aged naive TCR Tg CD4 T cells (27). This increased survival was also shown to be associated with higher levels of the anti-apoptotic molecule Bcl-2. Furthermore, since CFA contains microbial products, namely mycobacteria, we have hypothesized that cytokine production in response to a vaccine containing CFA is the result of these microbial products activating innate immune cells via TLRs to produce pro-inflammatory cytokines. This then results in enhanced responses seen with aging CD4 T cell populations following vaccination with a TLR containing adjuvant (22).
In addition to the above in vitro studies, PI cytokines were also used as an adjuvant in vivo. Vaccination with antigen plus PI cytokines was shown to dramatically enhance the in vivo cognate helper activity of adoptively transferred aged CD4 T cells (29). This resulted in enhanced expansion of antigen-specific B cell populations as well as enhanced germinal center formation and IgG production. Not only was the quantity of IgG enhanced, the frequency of somatic hypermutations was also enhanced in the presence of PI cytokines, leading to a more high affinity antibody response. The mechanism of how these PI cytokines act to enhance the function of aged CD4 T cells is not totally clear but likely involves the enhanced production of IL-17 and IL-21, both of which can influence cognate helper activity (29, 30).
Influence of aging on memory CD4 T cells
Memory CD4 T cells are critical for long-lived protection from infection following vaccination. The advantage of memory T cells is that they respond faster and with a lower activation threshold when compared to naive T cells and, thus, are poised for a rapid and vigorous response to infection. To examine the influence of aging on memory CD4 T cells, a model that was developed by Swain and colleagues was employed (31). In this model, in vitro generated CD4 T cell effectors from young or aged TCR Tg donors were transferred into young hosts and allowed to return to rest. The advantage of this model is that equivalent primary responses can be ensured by generating in vitro effectors in the presence of IL-2 and type 1 (IL-12) or type 2 (IL-4) polarizing cytokines resulting in Th1 or Th2 effector populations, respectively, with no age-related differences in phenotype or cytokine production at the time of transfer (32). These in vitro generated effectors exhibited a typical effector memory (Tem) phenotype with high levels of CD44 and CD25 and reduced levels of CD62L. Four weeks after transfer of equal numbers of antigen specific effectors into hosts, the effectors had returned to a resting central memory (Tcm) phenotype (CD44hiCD25negCD62Lhi) and there was no difference in the recovery of young and aged memory CD4 T cell populations.
The recall response of these memory cells was examined by ex vivo stimulation with peptide antigen (32). Upon initial stimulation, both young and aged Th1 and Th2 memory cells upregulated the activation markers CD25 and CD69. With regards to Th1 memory cells, those generated from young T cells produced both IL-2 and IFNγ, while those generated from aged T cells only produced IFNγ. For Th2 memory cells, those generated from young T cells produced both IL-4 and IL-5, while those generated from aged T cells produced very low levels of these cytokines. In addition, only those memory cells generated from young T cells exhibited proliferation in response to antigenic stimulation; those generated from aged T cells did not proliferate at all. These responses correspond with the concept of two pathways of memory CD4 T cell responses that has been put forth by Farber and colleagues (33). This model proposes that there is an early pathway of memory responses that is CD28 independent and results in memory cell activation and IFNγ production. This pathway is intact in memory cell populations derived from both young and aged naive CD4 T cells. The later pathway, which leads to proliferation and additional cytokine production, including IL-2, is CD28 dependent. This pathway is only functional in memory cells generated from young naive CD4 T cells. These results suggest that memory cells generated from aged naive T cells have a defect in CD28 signaling that results in their inability to respond properly to antigenic stimulation.
Importantly, this defect in responses seen in memory cells generated from aged naive CD4 T cells has dramatic in vivo consequences. Memory CD4 T cells were generated from Th1 and Th2 effectors as described above using TCR Tg CD4 T cell effectors. Four weeks following transfer of young and aged effectors, hosts were vaccinated with haptenated protein (NP-PCC) in alum and the humoral response to the hapten NP was assessed. Both Th1 and Th2 memory cells generated from aged naive CD4 T cells exhibit significantly reduced cognate helper activity following vaccination. This resulted in reduced NP-specific B cell expansion, germinal center formation and IgG production (32). These results demonstrate that one of the major consequences of aging is that the response to novel vaccinations in older individuals is dramatically reduced.
Finally, the responses of aged memory cells was also examined using the same adoptive transfer model (34). In this case, Th2 effectors were generated in vitro from young T cells and then transferred into young hosts. After either 1 month or 12 months post-transfer, the ex vivo and in vivo response of these memory cells was examined. There was no difference in ex vivo proliferation or cytokine production by these two memory cell populations. Both exhibited robust clonal expansion and Th2 cytokine production. In addition, both populations exhibited strong in vivo cognate helper function in response to vaccination. These results are very intriguing since they demonstrate that aging has minimal impact on the function of memory CD4 T cells, while it has a dramatic impact on the responses of naive CD4 T cells.
Origin of the age-related defect in CD4 T cell function
As individuals age, the production of new naive T cells declines as a result of thymic involution. In mice, the production of new T cells declines from 2×106 per day in 1 month old mice to 105 per day in 6 month old mice (35). The consequence of this is that there are fewer new naive T cells entering the circulation as individuals age. This issue is compounded by the fact that it has been recently demonstrated that in a lymphoreplete individual, recent thymic emigrants are at a competitive disadvantage compared to mature naive T cells already in the periphery (36). Thus, not only are there fewer new T cells produced in older individuals, they survive less well in the periphery. We have developed the hypothesis that in order to compensate for this significant decline in new T cell production, a change in homeostasis occurs with aging. Since the total number of T cells does not dramatically change with aging and there is less new T cell input, we propose that there is reduced replacement of existing naive T cells in the periphery which is achieved by extending the lifespan of naive CD4 T cells already in the periphery. While this change does maintain peripheral homeostasis within the T cell compartment, it comes at a price, namely the function of those naive CD4 T cells. In addition to changes in function that have been delineated above, there is evidence that this also results in a highly restricted oligoclonal TCR repertoire in aging humans (37).
In order to examine this further, the lifespan of naive CD4 T cells from young and aged animals was carefully examined. The increased lifespan of naive T cells from aged mice was demonstrated following thymectomy and in an adoptive transfer model (38). In both instances, naive CD4 T cells from aged mice exhibited significantly longer lifespans than those from young mice. Furthermore these studies correlated this increased lifespan of naive T cells from aged mice to the loss of function of these T cells. Importantly, this was not due to the fact that the aged naive cells underwent a greater degree of homeostatic turnover. Furthermore, this was not dependent upon self peptide/MHC interaction or IL-7, both of which were thought to be vital for naive CD4 T cell homeostasis [reviewed in (39)]. Instead, it was found that there was an inverse correlation between the expression of the Bcl-2 protein family member Bim and the intrinsic lifespan of naive CD4 T cells. Bim, which shares only a short BH3 domain with the Bcl-2 family, is a key trigger of apoptosis and a critical regulator of homeostasis (40). Thus, it is thought that Bim may be a major physiological antagonist of prosurvival proteins. Since naive CD4 T cells from young mice express high levels of Bim, they would be more susceptible to Bim mediated apoptosis compared to aged cells, which express lower levels. These studies went on to show that reduced Bim expression induced a cell intrinsic accelerated development of age-related immune defects in naive CD4 T cells. This was also correlated with increased expression of the senescence-related genes INK4a and p19ARF, both of which may be considered biomarkers for cellular senescence (41, 42).
Enhancing CD4 T cell function in aged individuals
It is very clear that new T cell production declines with age due to thymic involution and that naive CD4 T cells loose function as they age in the periphery. In order to determine if new functional naive CD4 T cells could be generated in aged mice, young and aged TCR Tg mice were treated with a CD4-depleting antibody and the newly generated T cells were examined (43). In both young and aged mice, the newly generated T cells exhibited a prototypical naive phenotype (CD44loCD62LhiCD25neg) and, importantly, no new T cells were generated in previously thymectomized animals. Ex vivo stimulation of newly generated CD4 T cells demonstrated that aged mice could generate new T cells that were highly functional and similar to young in both clonal expansion and IL-2 production. In addition, in an adoptive transfer assay, newly generated CD4 T cells from both young and aged animals exhibited robust cognate helper function. These results demonstrated two important points. First, the aged environment, including an aging involuted thymus, can support the generation of new naive CD4 T cells. Second, these new naive CD4 T cells generated in the aged environment can function as well as those generated in a young environment.
Other studies went on to examine the ability of bone marrow (BM) from young or aged TCR Tg donors to generate new naive CD4 T cells (34). Young and aged BM was harvested and transferred into lethally irradiated young recipients. After 3 months, the newly generated TCR Tg CD4 T cells were harvested and Th2 effectors were generated in vitro. These effectors were then transferred into hosts to generate memory CD4 T cells as described above. After 4 weeks, memory cells were harvested and assayed in vitro or the host mice were immunized with a haptenated protein. In both cases, memory cells derived from young or aged BM were highly functional and could help a robust humoral response. These results indicate that there are no apparent age-related intrinsic defects in BM stem cells with regards to the generation of functional CD4 T cells. This also supports our hypothesis that new naive CD4 T cells leave the thymus highly functional, even in older animals, and loss of function is something that happens over time in the periphery.
Conclusions
Naive CD4 T cells are an important component of the adaptive immune response and are vital for a robust response to vaccination. In older individuals, there are significant defects in naive CD4 T cell function that can influence both primary and memory responses. These defects seem to develop post-thymically and are associated with reduced expression of Bim. Importantly, the in vivo function of aged naive CD4 T cells can be enhanced by the use of adjuvants that induce the production of proinflammatory cytokines. This strategy could significantly enhance the efficacy of vaccinations in elderly populations.
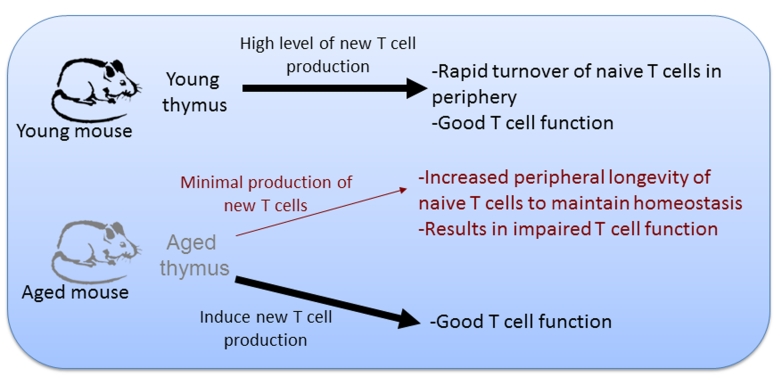
Figure 1. Model for basis of age-related changes in CD4 T cells.
In young animals thymic output is high, resulting in a rapid replacement of naive T cell populations in the periphery. As animals age and thymic involution occurs, thymic output of new naive T cells declines. To maintain the homeostasis of peripheral T cell numbers, the lifespan of naive T cells in the periphery is increased. This increase in longevity is accompanied by a decline the naive T cell function. Induction of new naive CD4 T cell production in the aged environment can generate T cells with robust function.
References
- 1.Burns EA, Lum LG, L’Hommedieu G, Goodwin JS. Specific humoral immunity in the elderly: in vivo and in vitro response to vaccination. J Gerontol. 1993;48:B231–236. doi: 10.1093/geronj/48.6.b231. [DOI] [PubMed] [Google Scholar]
- 2.Musher DM, Chapman AJ, Goree A, Jonsson S, Briles D, Baughn RE. Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 3.Denis F, Mounier M, Hessel L, Michel JP, Gualde N, Dubois F, Barin F, Goudeau A. Hepatitis-B vaccination in the elderly. J Infect Dis. 1984;149:1019. doi: 10.1093/infdis/149.6.1019. [DOI] [PubMed] [Google Scholar]
- 4.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 5.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 6.Nicoletti C, Yang X, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150:543–549. [PubMed] [Google Scholar]
- 7.Miller C, Kelsoe G. Ig VH hypermutation is absent in the germinal centers of aged mice. J Immunol. 1995;155:3377–3384. [PubMed] [Google Scholar]
- 8.Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, Hayakawa K, Hardy RR, Weigle WO. Differences in the expression profiles of CD45RB, Pgp-1 and 3G11 membrane antigens and the pattern of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295. [PubMed] [Google Scholar]
- 9.Linton P-J, Haynes L, Klinman NR, Swain SL. Antigen independent changes in CD4 T cells with aging. J Exp Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynes L, Linton P-J, Eaton SM, Tonkonogy SL, Swain SL. IL-2, but not other common g chain (gc)-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1023. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 12.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 14.Larbi A, Pawelec G, Wong SC, Goldeck D, Tai JJ, Fulop T. Impact of age on T cell signaling: a general defect or specific alterations? Ageing Res Rev. 2011;10:370–378. doi: 10.1016/j.arr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 16.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 17.Whisler RL, Chen M, Liu B, Newhouse YG. Age-related impairments in TCR/CD3 activation of ZAP-70 are associated with reduced tyrosine phosphorylations of zeta-chains and p59fyn/p56lck in human T cells. Mech Ageing Dev. 1999;111:49–66. doi: 10.1016/s0047-6374(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 18.Garcia GG, Miller RA. Age-related defects in the cytoskeleton signaling pathways of CD4 T cells. Ageing Res Rev. 2011;10:26–34. doi: 10.1016/j.arr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirk CJ, Miller RA. Analysis of Raf-1 activation in response to TCR activation and costimulation in murine T-lymphocytes: effect of age. Cell Immunol. 1998;190:33–42. doi: 10.1006/cimm.1998.1382. [DOI] [PubMed] [Google Scholar]
- 20.Whisler RL, Beiqing L, Chen M. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell Immunol. 1996;169:185–195. doi: 10.1006/cimm.1996.0109. [DOI] [PubMed] [Google Scholar]
- 21.Whisler RL, Chen M, Beiqing L, Carle KW. Impaired induction of c-fos/c-jun genes and of transcriptional regulatory proteins binding distinct c-fos/c-jun promoter elements in activated human T cells during aging. Cell Immunol. 1997;175:41–50. doi: 10.1006/cimm.1996.1048. [DOI] [PubMed] [Google Scholar]
- 22.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 25.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 26.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 27.Jones SC, Brahmakshatriya V, Huston G, Dibble J, Swain SL. TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6-dependent mechanism. J Immunol. 2010;185:6783–6794. doi: 10.4049/jimmunol.0901296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 29.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. 2009;182:6129–6135. doi: 10.4049/jimmunol.0804226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swain SL. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1994;1:543–552. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 32.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farber DL. Biochemical signaling pathways for memory T cell recall. Semin Immunol. 2009;21:84–91. doi: 10.1016/j.smim.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eaton SM, Maue AC, Swain SL, Haynes L. Bone Marrow Precursor Cells from Aged Mice Generate CD4 T Cells That Function Well in Primary and Memory Responses. J Immunol. 2008;181:4825–4831. doi: 10.4049/jimmunol.181.7.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 36.Houston EG, Jr, Higdon LE, Fink PJ. Recent thymic emigrants are preferentially incorporated only into the depleted T-cell pool. Proc Natl Acad Sci U S A. 2011;108:5366–5371. doi: 10.1073/pnas.1015286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Mowes B, Julke K, Romagnani C, Thiel A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35:1987–1994. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- 38.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, Haynes L, Swain SL. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 40.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 41.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, Thomas NE, Sharpless NE. Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]