Abstract
The Senescence-Accelerated Mouse (SAM) represents a group of inbred mouse strains developed as a model for the study of human aging and age-related diseases. Senescence-prone (SAMP) strains exhibit an early onset of age-related decline in the peripheral immunity such as thymic involution, loss of CD4+ T cells, impaired helper T cell function, decreased antibody-forming capacity, dysfunction of antigen-presenting cells, decreased natural killer activity, increased auto-antibodies, and susceptibility to virus infection. Senescence-prone SAMP10 mice undergo age-related changes in the brain such as brain atrophy, shrinkage and loss of cortical neurons, retraction of cortical neuronal dendrites, loss of dendritic spines, loss of synapses, impaired learning and memory, depressive behavior, accumulation of neuronal DNA damage, neuronal ubiquitinated inclusions, reduced hippocampal cholinergic receptors, decreased neurotrophic factors, decreased hippocampal zinc and zinc transporters, increased sphyngomyelinase, and elevated oxidative-nitrative stress. Recent data indicating increased pro-inflammatory cytokines in the brain of SAMP10 mice are directing investigators toward an integration of immune and neural abnormalities to enhance understanding of the principles of brain aging. We highlight how mouse brain cells adopt cytokine-mediated responses and how SAMP10 mice are defective in these responses. SAMP10 model would be useful to study how age-related disturbances in peripheral immunity have an impact on dysregulation of brain tissue homeostasis, resulting in age-related neurodegeneration.
Keywords: Brain Aging, Senescence-accelerated Mice, Immunosenescence, Model, CD4, T cells
The Senescence-Accelerated Mouse (SAM) represents a group of inbred mouse strains developed by Toshio Takeda and his colleagues at Kyoto University as a model for the study of human aging and age-related diseases [1]. Progenitors of SAM strains were generated as a result of an accidental outcrossing of AKR/J mice and another unknown albino mouse strain [2]. Beginning in 1975, five litters of mice showing an accelerated senescence phenotype and short life span were selected to become the progenitors of senescence-prone strains (SAMPs), while three litters of mice resistant to accelerated aging were selected as progenitors of senescence-resistant strains (SAMRs). Thereafter, selective sibling mating has been continued for more than 20 generations. Since 2002, four inbred SAMPs (SAMP1/Ka, SAMP6/Ta, SAMP8/Ta, and SAMP10/Ta) and one SAMR (SAMR1/Ta) have been available to investigators worldwide through the Council for SAM Research (http://samrc.md.shinshu-u.ac.jp/firste.html). These strains are interrelated recombinant inbred mice. SAMP strains presumably share a combination of gene mutations responsible for the common senescence-prone phenotype. In addition, each strain is thought to carry its own gene mutation(s) causing age-related pathological phenotypes that are unique to each strain (e.g., brain atrophy and neurodegeneration in SAMP10, learning and memory deficits in SAMP8, osteoporosis in SAMP6). SAMR1 mice represent a normal aging control. Genealogy for the SAM strains has been one of the major subjects for investigators involved in the development of SAM mice [1]. Genetic profiles of individual SAM strains have been studied using microsatellite DNA markers [3]. Japanese researchers recently launched a collaborative exome analysis, using next-generation sequencing technology, of SAM strains and the AKR/J strain, in comparison with a reference C57BL/6J strain, to identify gene mutations responsible for a general senescence-prone phenotype and for strain-unique age-related pathologies.
Although investigators studying SAM mice understand the importance of interdisciplinary collaboration, as aging is a complex process that simultaneously affects both mind and body, it has not been easy to examine global questions about the relationships between mind and body in aging and disease. However, recent data obtained from SAM mice, including our own, are directing us toward an integration of immunology and neuroscience. In the earliest part of this article, we briefly review recent advances in the neuroinflammatory aspects of the brain aging. We then describe immunosenescence and brain aging of SAM mice. Thereafter, we highlight data on cytokines in the brains of SAM mice and the adoption of cytokine-mediated responses by brain cells, which could be integral to interconnecting the two disciplines.
1. Brain aging and neuroinflammation
Changes in the structural complexity of the aged brain have been well documented in humans, non-human primates, dogs, and rodents. It has been established that there is no overt loss of neurons during normal aging. Rather, more subtle changes occur in individual neurons, including shrinkage in cell body size, loss or regression of dendrites and dendritic spines, alterations in neurotransmitter receptors, and changes in electrophysiological properties [4–9]. Aging is a major risk factor for neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease.
Complex biological systems are under constant homeostatic regulation, involving maintenance and repair mechanisms. Such mechanisms include various stress responses, antioxidative mechanisms, and removal and turnover of defective cellular components and molecules [10]. Abundant evidence suggests that the effects of aging result from the accumulation of many forms of cellular and tissue damage. Therefore, understanding the cellular and molecular basis of aging requires exploration of both the mechanisms causing the accumulation of damage and the systems controlling such damage [11]. Similar to cells in other organ systems, it has been documented that cells in the brain experience increased levels of oxidative stress, DNA damage, and accumulation of non-degraded molecules as a result of normal quiescent aging [10].
Perturbed immune responses may be associated with neural dysfunction. Under quiescent conditions, where immune mechanisms exert physiologically beneficial effects, the immune system is activated by environmental and/or psychological stimuli and positively regulates neuroplasticity and neurogenesis, promoting learning, memory, and hippocampal long-term potentiation [12]. On the other hand, detrimental effects of inflammatory conditions induced by infections and injury, as well as severe or chronic stress, are associated with the production of inflammatory mediators such as interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, and prostaglandins. These mediators disrupt the delicate physiological balance between immune and neural processes, resulting in neuronal hyperexcitability, hormonal aberrations, reduced neurotrophic factor production and suppressed neurogenesis, and leading to impairments in learning, memory and neuroplasticity [12].
It has been well recognized that the intact aging brain is characterized by a shift from a homeostatic balance of inflammatory mediators to a proinflammatory state [13–14]. Neuroinflammation provides a common basis linking many factors associated with age-related cognitive decline [15–18]. The level of IL-6 in whole brain, as well as in the hippocampus, cerebral cortex, and cerebellum, is increased in aged mice [19]. In contrast, anti-inflammatory cytokines, such as IL-10, are decreased [20]. NFκB binding to the IL-6 gene promoter is increased in the brain of aged mice [21]. This increase in neuroinflammation is reported to be associated with increased numbers of microglia with an activated phenotype [22–23], which may actually be “primed” rather than “activated” microglia [17]. Microglia are cells of bone marrow myeloid origin that populate the CNS during early development and form the brain’s major innate immune cell type [24]. Under unstimulated conditions, these cells adopt a ramified appearance and their main role is constant surveillance of the brain tissue microenvironment, enabling quick responses to even the slightest perturbations using highly motile cytoplasmic processes [25]. When stimulated, microglia undergo a morphological and functional transformation. “Primed” microglia represent a subset of microglia that appear to reside in an intermediate state characterized by shortened processes and expression of cell surface markers, similar to “activated” microglia, but without appreciable secretion of cytokines [13]. “Primed” microglia are thought to sensitize the aged brain to produce an exaggerated response, including proinflammatory cytokine overproduction, to immune stimulation in the periphery or following exposure to a stressor, resulting in severe detriments in cognitive function [13].
Paradoxically, microglia in the human brain have been reported to undergo “dystrophic” morphological changes that are indicative of cellular senescence and are presumably accompanied by the loss of neuroprotective functions [26–28]. We may have reached a turning point in terms of our understanding of the neuroimmunology of Alzheimer’s disease by realizing that neurodegeneration may not be the result of an overactive immune response, but rather may occur because of a decline in immunological functions [14, 29]. Because CNS microglia are both immunological defenders and neuroprotective glia, the consequences of microglial senescence include both increased susceptibility to brain infections as well as an increased susceptibility to neurodegeneration. However, the factors or conditions that exacerbate microglial aging remain to be identified.
The major issues and opinions on the involvement of the brain immune system in aging and age-related diseases have been presented in a special edition of the journal Aging and Disease Vol.1, Number 3, December 2010 [29–33].
2. Immunosenescence in SAM mice
In SAMP1 mice, involution of the thymus occurs earlier than in SAMR1 mice [34]. The proportion of CD4+ T cells and the CD4/CD8 ratio in the peripheral blood declines as a function of age earlier in SAMP1 mice than in SAMR1 mice. Serological studies have indicated that SAMP1 mice produce a number of auto-antibodies from 5 months of age, including a natural thymocytotoxic auto-antibody, anti-nuclear antibodies, anti-collagen type II antibodies, and IgG anti-single-stranded and anti-double-stranded DNA antibodies [35].
In vitro studies, using cultured spleen cells, have revealed that in SAMP1 mice at 2 months of age, the antibody-forming capacity to T-independent antigens, such as DNP-Ficoll, and the activity of natural killer (NK) cells undergo an early onset of regression with a sharp decline from the level of age-matched control SAMR1 mice [36]. SAMP1 mice also exhibit profound defects in the antibody responses to T-dependent antigens, such as sheep red blood cells (SRBC) and ovalbumin (OVA). There are only feeble antibody responses to SRBC and OVA at the age of 2 months and a negligible response at later ages. In contrast, other in vitro cellular immune responses, such as mixed leukocyte reaction (MLR), allo-specific cytotoxic T lymphocyte (CTL) response, and delayed-type hypersensitivity (DTH) reaction, of SAMP1 mice are equivalent to SAMR1 mice at 2 months of age and decline little with advancing age [37]. Nishimura et al. found that splenic CD4+ T cells from young SAMP1 mice exhibited abnormally short-lasting production of IL-2 in response to stimulation with concanavalin A, leading to impaired proliferation and survival of these cells [38]. The short-lasting IL-2 production and resulting insufficient production of IL-2 may limit the propagation of antigen-specific T cells during immune responses, reducing their magnitude.
The production of interferon (IFN)-γ, a TH1 cytokine, and interleukin (IL)-4, a TH2 cytokine, by spleen cells prepared from SAMP1 mice has been studied in comparison to responses in C3H/He mice. Here, Hosokawa and his colleagues immunized mice with a single i.p. injection of OVA with alum adjuvant, a potent inducer of TH2-type immune responses [39]. When cultured, these OVA-primed C3H/He spleen cells produced large amounts of IL-4 and relatively small amounts of IFN-γ, consistent with a TH2-type immune response, in the presence, but not in the absence, of OVA. In contrast, spleen cells prepared from SAMP1 mice produced a substantial amount of both IFN-γ and IL-4 regardless of the presence or absence of OVA in the culture. These observations indicate that TH2 cell activity is impaired in SAMP1 mice [39].
Noradrenaline (NA) is known to modulate antibody responses [40]. Hosokawa and his colleagues found that NA exhibited significant dose-dependent modulatory effects on the production of IL-4, but not IFN-γ, in C3H/He spleen cells [39]. NA augmented IL-4 production from OVA-specific TH2 cells in C3H/He spleen cell culture via enhancing the release of TH2-promoting cytokines by antigen presenting cells (APCs). The IL-4 production by TH2 cells was augmented by the addition of NA at a concentration of 3.0 × 10−5 M and suppressed at a concentration of 3.0 × 10−4 M. In contrast, NA did not augment but suppressed IL-4 production by spleen cells from SAMP1 mice in a dose-dependent manner at concentrations higher than 3.0 × 10−5 M. These observations indicate that the NA-mediated regulatory mechanisms of TH2 cell function are impaired in SAMP1 mice [39].
Hosono et al. immunized mice with suboptimal doses of xenogenic red blood cells and found that in vivo antibody responses by SAMP1 mice was low [41]. They performed several in vivo studies and have reported that SAMP1 mice exhibit an early onset of age-related decline in antibody and DTH responses [42]. Injection of thymic T cells from young mice before sensitization completely restores antibody responses in aged SAMP1 mice; suggesting that the age-related decline in antibody responses is due to the early age-related loss of helper T cells. Toichi et al. transferred spleen cells, prepared from sensitized aged donors, into the footpads of naive syngenic recipients and found that the procedure evoked strong DTH responses, demonstrating the existence of DTH-mediating T cells in the spleens of aged SAMP1 mice. They injected naive spleen cells from young donors into the footpads, together with the antigen, and found that the procedure caused DTH responses in sensitized aged recipients. These findings indicate that DTH-mediating T cells are actually induced and migrate to function as effector cells in aged SAMP1 mice. When they injected naive spleen cells from aged SAMP1 donor mice into the footpad, the procedure restored DTH reactions in aged SAMP1 mice; however, this was ineffective when they injected spleen cells intravenously. Intravenous injection of naive spleen cells from young SAMP1 donors restored the DTH response in aged mice. These results suggest that lowered DTH responses in aged SAMP1 mice are not due to the loss of DTH-mediating T cells but the impaired migration of inflammatory cells into the local site [42].
Age-related changes in the function of APCs have been examined using SAMP1 mice. In primary MLR, dendritic cells (DCs) from aged SAMP1 mice show less stimulatory activity than those of age-matched BALB/c or young SAMP1 mice. In secondary MLR, the stimulatory activity of B cells is lower in aged SAMP1 mice, but not in age-matched BALB/c or young SAMP1 mice. These age-related decreases in the stimulatory activity of APCs are related to reductions in the surface density of major histocompatibility complex (MHC) class II and intercellular adhesion molecule (ICAM)-1 on APCs (DCs and B cells), which are essential for helper T-APC interaction and co-stimulation. [43].
Studies of intranasal inoculation with influenza A virus [44] as well as with respiratory syncytial virus [45] have indicated that SAMP1 mice are more susceptible to viral infection and exhibit a higher rate of mortality and prolonged virus growth in the lungs. The increased susceptibility to these viruses is associated with diminished cellular immunity by local virus-specific CTLs and NK cells. The deficiency in cellular immune responses is due to a lack of clonal expansion of CD4+ and CD8+ T lymphocytes. The production of IFN-γ and IL-12 is significantly restrained, which suggests a partial deficiency in TH1 cells. In contrast, the immunologic activity of TH2 cells appears to be functionally normal, judging from the release of large amounts of IL-4 followed by the production of appropriate amounts of influenza virus-specific antibodies. These observations suggest that SAMP1 mice have alterations in the storage and function of CD4+ T cells and a TH2-biased immune response, which results in increased susceptibility to influenza virus infection. Although there is apparent discrepancy between data from in vitro studies, indicating TH2 deficiency, and those from in vivo infection studies, indicating TH1 deficiency, both findings suggest that SAMP1 mice are defective in making appropriate immune responses to individual inflammatory stimuli. Further investigation would unravel the mechanisms for dysregulated immunity of SAMP1 mice.
The SAMP1, P8, and P10 strains have the same allele of the H-2K gene, H-2Kk (SAMR1 has H-2Ks), and probably share similar immune system deficiencies, such as accelerated age-associated decline in T cell-dependent immune functions [39]. For example, it has been reported that SAMP8 mice exhibit similar impairments in antibody responses. Abe et al. studied the immune profiles of SAMP8 mice in cultured spleen cells by assessing NK cell activity, anti-SRBC antibody responses, cell proliferation, and IL-2-producing activity in response to concanavalin A [46]. At 2 months of age, SAMP8 mice showed markedly decreased activity for all these parameters. Endogenous NK cell activity of SAMP8 mice was low or at the background level for a few months. Flow cytometry analyses revealed that the size of the lymphocyte fraction of spleen cells was comparable between SAMP8 and SAMR1 mice; however, the T cell fraction, especially that of CD4+ T cells, was smaller in SAMP8 mice than in SAMR1 mice. These results indicate that there are certain defects in the function of CD4+ T cells in SAMP8 mice that could be due to low endogenous NK cell activity, which leads to the impairment of helper T cell activity in antibody responses [46].
3. Fundamental age-related changes in the brain morphology of SAMP10 mice
The SAMP10 strain of mouse was established by Shimada and colleagues [1, 47–48]. In SAMP10 mice, age-related retraction of dendritic arbors, decreases in the density of dendritic spines, loss of synapses, and impairments in learning and memory, together with relatively smaller changes in the number of cortical neurons, are more consistent with observations in normal human aging than in Alzheimer’s disease. In the first paper on the SAMP10 strain, we reported age-related brain atrophy in SAMP10 mice, which is most evident in the olfactory bulbs, and frontal and temporal portions of the cerebrum [47]. The average brain weight of SAMP10 mice increases postnatally as rapidly as that of SAMR1 mice up to 4 months of age. After 4 months of age, and throughout the lifespan, a 9% reduction in average brain weight occurs in SAMP10 mice relative to the average brain weight of 4-month-old mice. Reduction in brain weight is not evident throughout the lifespan of SAMR1 mice. Age-related brain atrophy is not common in other strains of mice or rats, and appears to be unique to SAMP10 mice [49].
The neocortex of SAMP10 mice is more prone to atrophy than that of SAMR1 mice with advancing age. Cortical atrophy is most evident in the frontal cortex, although affected cortical areas range widely from the frontal to the occipital regions in SAMP10 mice [47]. Neocortical thickness in all cerebral areas is decreased with advancing age, without obvious alterations in cytoarchitecture. Neuronal packing density in the cortex is similar across all ages. Other characteristic features include age-related atrophy in the amygdala and entorhinal cortex. There is a mild to moderate increase in the density of hypertrophic astrocytes in the neocortex, entorhinal cortex, amygdala, and subcortical white matter in aged SAMP10 mice. Mean neuronal cell body size gradually shrinks with aging in layers II/III, V, and VI of the cerebral cortex and in the cingulate and insular cortices. There is a 36% decrease in the number of large neurons in the atrophied cortex, which is due to both perikaryal shrinkage of large neurons and possible neuronal loss. In contrast, in SAMR1 mice, the mean neuronal cell body size in the cerebral cortex does not change and the number of large neurons remains constant throughout life, indicating that age-related neuronal degeneration is unique to SAMP10 mice [48].
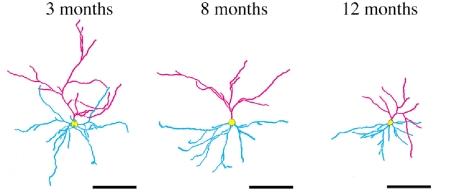
Based on quantitative evaluations of neuronal morphology using the Golgi method, we reported that dendrites of prefrontal cortical neurons in SAMP10 mice gradually retracted towards the somata with age, with a relative preservation of overall complexity (Fig. 1) [50]. Apical dendrites were much more severely affected than basal dendrites. The combined length of the apical dendrites and spine density decreased by 45% and 55%, respectively, in mice at 12 months of age compared to mice at 3 months of age. Immunohistochemical and immunoblot analyses indicated that the expression of microtubule-associated protein 2, a marker of dendrites, decreased with age not only in the frontal cortex, but also in the posterior cortex and olfactory-related structures. In contrast, in SAMR1 mice, neuronal dendrites and spines were well preserved and underwent no age-related decline in any measure of cell morphology. A reduction in the complexity of dendritic arbors, reduced dendritic length, and a decrease in spine numbers are well-documented characteristics of age-related changes in the morphology of cortical and hippocampal neurons occurring in humans and nonhuman primates [4]. Given that age-related changes in neuronal morphology are subtle in normal mouse strains, the SAMP10 strain represents a useful rodent model in which neuronal changes characteristic of human brain aging occur spontaneously as a function of age. Associated with degenerative changes in neuronal dendrites and spines, synapses are lost from the frontal cortex of SAMP10 mice. We reported that levels of expression of synaptophysin, a presynaptic marker, and postsynaptic density protein 95, a postsynaptic marker, decreased in the anterior cerebral cortex by 52% and 56%, respectively [51].
Fig. 1. Age-related retraction of neuronal dendrites in SAMP10 mice.
Dendritic arbors of prefrontal pyramidal cells of SAMP10 mice are represented by skeletonized camra lucida drawings. Apical and basal dendrites and somata are indicated in pink, blue, and yellow, respectively. Entire arbors, particularly apical dendrites retract towards the cell bodies. Scale bars, 100 μm. Reproduction of copyrighted material permitted by John Wiley and Sons [50], License Number 2507530701423.
4. Age-related behavioral changes in SAMP10 mice
The learning and memory ability of SAMP10 mice declines as a function of age. Deficits in the single-trial passive avoidance task occur earlier in a more marked manner in SAMP10 mice than in SAMR1 mice [52–53]. In our previous study on active avoidance task using a T-maze, SAMP10 mice exhibited significant impairments at 10–12 months of age, compared to performance at 4 months of age. In SAMR1 mice, significant impairment occurred at 23–26 months of age [52]. Miyamoto et al. reported that SAMP10 mice exhibited poorer performance in a two-way active avoidance task using a shuttle box [53]. Okuma et al. examined SAM mice in a spatial learning task using the Morris water maze and found that 9-month-old SAMP10 mice failed to shorten the escape latency or path length during space discrimination training [54].
Deficits in the learning and memory task performance reported in SAMP10 mice may be due, at least in part, to emotional changes such as lack of motivation and depressive mood [53, 55–56]. Emotional behaviors have been measured in SAMP10 mice using the tail suspension test, forced swimming test, and the elevated plus-maze task. Miyamoto et al. reported that SAMP10 mice exhibited depressive behavior but did not exhibit abnormalities in anxiety-like behavior [53]. The effects of chronic social isolation stress on learning and memory have been examined in SAMP10 mice. Chida et al. chronically exposed SAMP10 mice to social isolation stress from the age of 5 weeks [57]. At 12 weeks of age, conditioning memory and spatial memory were evaluated by the single-trial passive avoidance and Y-maze tests, respectively. Chronic social isolation stress significantly reduced conditioning memory but did not affect spatial memory. Isolation stress elevated serum corticosterone levels and inhibited increases in c-Fos expression in the central amygdaloid nucleus, which is required for conditioning memory during passive avoidance learning. These results suggest that chronic social isolation stress exacerbates conditioning memory in SAMP10 mice, probably via a glucocorticoid-mediated decrease in neural activation in the central amygdala [57].
5. Age-related neuropathological changes in SAMP10 mice
It has been reported that the DNA in cerebral cortical neurons in patients with Alzheimer’s disease is extensively damaged, although the morphological features of apoptosis are absent [58–59]. In our previous study, quantitative terminal deoxynucleotidyl transferase-mediated digoxigenin-labeled dUTP nick end labeling (TUNEL) on paraffin sections of various brain regions revealed that TUNEL-positive cells increased in number with aging in the cerebral neurons of SAMP10 mice [60]. TUNEL-positive cells were widely distributed in mice at 13–14 months of age: they appeared chiefly in the olfactory tubercle, anterior cingulate cortex, insular cortex, and amygdala. These TUNEL positive cells did not exhibit the morphological features of apoptosis. Thus, DNA in brain tissues of SAMP10 mice becomes damaged with advancing age through a non-apoptotic mechanism, presumably mimicking the progressive neuronal DNA damage associated with human neurodegenerative diseases.
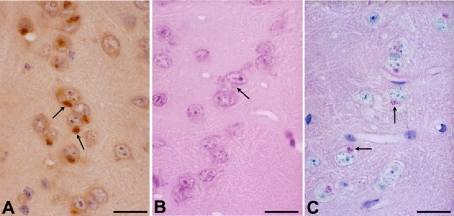
The ubiquitin proteasomal system provides both a precise and a general means for cells to dispose of misfolded proteins or biologically non-useful proteins. Abnormal enrichment of ubiquitin in neuropathological lesions is a feature of age-related neurodegenerative disorders including Alzheimer’s disease (neurofibrillary tangles) and Parkinson’s disease (Lewy bodies). We first reported that inbred strains of mice also develop ubiquitin-positive neuronal cytoplasmic inclusions as a function of age (Fig. 2) [61]. We found that inclusions were formed in association with lipofuscin in neuronal perikarya and occurred most frequently in the limbic-related forebrain structures, such as the entorhinal cortex, subiculum, hippocampus, amygdala, piriform cortex, insular cortex, medial prefrontal cortex, hypothalamus, and medial part of the thalamus [61]. Inclusions were relatively sparse in non-limbic structures. SAMP10 mice developed these ubiquitinated inclusions much more frequently and from much earlier ages than SAMR1 and C57BL mice. There was a significant increase in the percentage of inclusion-bearing neurons in SAMP10 mice at 12 months of age, compared with that at 3 months of age. In SAMR1 and C57BL mice, a significant increase in the percentage of inclusion-bearing neurons was evident in mice at 20 months of age and older.
Fig. 2. Neuronal cytoplasmic ubiquitinated inclusions in aged SAMP10 mice.
Neuronal cytoplasmic inclusions (arrows) in the entorhinal cortex of 17-month-old SAMP10 mice are detected by immunohistochemistry for ubiquitin (A). Corresponding structures are indicated on sections stained with hematoxylin & eosin (B) and periodic acid-Schiff (C). Scale bars, 20 μm. Reproduction of copyrighted material permitted by John Wiley and Sons [61], License Number 2507531234682.
We reported that the proteasome activity in the limbic-related forebrain decreased much more rapidly and remarkably upon aging in SAMP10 than in SAMR1 mice [61]. The tissue proteasome activity was significantly lower in SAMP10 mice at 7 months than in mice at 3 months of age. Only 26% activity was detected in 17-month-old SAMP10 mice as compared with 3-month-old mice. It has been reported that a suboptimally functioning ubiquitin-proteasome system plays a role in pathogenetic mechanisms underlying neurodegenerative disorders [62–65]. It is also known that there is a hierarchical distribution of ubiquitin-positive inclusions, particularly of neurofibrillary tangles and neuritic plaques [66–70]. SAMP10 mice may be useful to study how impairments in the ubiquitin-proteasome system are causally related to neuronal degeneration and to investigate the mechanisms underlying regional selectivity of neuronal degeneration.
Another issue regarding intracellular inclusion bodies is whether inclusion formation is beneficial or detrimental to cells. To solve this issue, we prepared primary cultures of neurons from the cerebral cortices of embryonic SAMP10 and SAMR1 mice, and inhibited proteasome activity with MG115 [61]. Proteasomal inhibition was found to enhance the formation of ubiquitinated inclusions in neurons in vitro. Neurons bearing inclusions exhibited retracted neurites, indicative of neuronal degeneration. Thus, inclusions do not protect neurons perfectly. Rather, inclusions may be harmful to cells or just an indication of cellular damage in that inclusion-bearing neurons fail to maintain cytoplasmic processes. The regional selectivity of proteasomal impairments may be causally related to the selectivity of inclusion formation and associated dendritic degeneration in neurons of aging SAMP10 mice [61].
6. Changes in neurotransmitters, growth factors, and electrophysiological properties in SAMP10 brains
The ligand binding activities of muscarinic acetylcholine (mACh) receptors and protein kinase C (PKC) in the hippocampus of 9-month-old SAMP10 mice are lower than those of age-matched SAMR1 mice [54]. There is no significant difference in the ligand binding activity of α1 and α2 adrenoreceptors between SAMP10 and SAMR1 mice. Thus, a reduction in mACh receptors and PKC in the hippocampus appears to contribute to impairments in the capacity for learning and memory in SAMP10 mice.
In association with depression-like behavior in SAMP10 mice, dopamine content is decreased in the cerebral cortex, hippocampus, and midbrain, and the content of 5-hydroxyindoleacetic acid, a 5-hydroxytryptamine (5-HT) metabolite, is decreased in the hippocampus and midbrain (unpublished data, as mentioned in [55]). [3H]Quinpirole binding to D2/D3 dopamine receptors in the cerebral cortex is increased in SAMP10 mice at 8 months of age, compared with age-matched SAMR1 mice [55]. In the hippocampus, [3H]8-hydroxy DPAT binding to 5-HT1A receptors is increased. In the midbrain, binding of [3H]quinpirole and [3H]8-hydroxy DPAT are increased. There is no difference between SAMP10 and SAMR1 mice in the binding of [3H]SCH23390 to D1/D5 receptors, nor is there any difference between the two strains in the binding of [3H]ketanserin to 5-HT2 receptors.
The serine/threonine kinase Akt is critical for cell signaling and neuronal survival. The effect of aging on Akt signaling in hippocampal tissue has been studied using SAM mice. The expression of Akt mRNA and protein remains stable during aging, but the constructive phosphorylation of AktSer473 exhibits a continuous decrease in SAMP10 mice from 6 to 10 months of age [56]. There is a significant reduction in the phosphorylation of AktSer473 in SAMP10 mice compared with that in SAMR1 mice at ages 8 and 10 months.
Nerve growth factor (NGF) is one of the neurotrophins involved in the survival and maintenance of certain populations of neurons, such as sensory and sympathetic neurons in the peripheral nervous system [71–72]. In the brain, basal forebrain cholinergic neurons are highly dependent on the supply of NGF for the maintenance of their cholinergic phenotype as well as their synaptic integrity [73]. In aged rats, NGF protein and its mRNA decrease in parallel with learning impairments [74]. A dysregulation in the maturation or degradation of mature NGF might contribute to the vulnerability of the cholinergic system in Alzheimer’s disease [75]. Age-related changes in the distribution of brain NGF have been studied in SAMP10 mice. There is a decline in NGF immunoreactivity in the substantia innominata in SAMP10 mice at 10 months of age compared with that in age-matched SAMR1 mice [76]. Along with neuronal degeneration in cortical regions, decreased NGF levels in the basal forebrain may contribute to the pathogenesis of age-related learning and memory impairments in SAMP10 mice.
Glial cell line-derived neurotrophic factor (GDNF) is widely expressed in the brain, and promotes the survival and maturation of central dopaminergic and noradrenergic neurons and motorneurons, as well as various subpopulations of peripheral sensory and sympathetic neurons [77–78]. GDNF improves the function of dopaminergic neurons, which have been shown to augment locomotor behavior in 24-month-old Fischer 344 rats [79–80]. GDNF also protects nigrostriatal dopaminergic neurons against the toxicity of 6-hydroxy-dopamine in aged Fischer 344 rats [81]. Age-related changes in GDNF expression in SAM mice have been investigated. The expression of mRNA for GDNF in the hippocampus decreases in an age-dependent manner in SAMR1 mice [82]. The GDNF mRNA levels in 10-month-old SAMR1 mice are lower than those of 2-month-old SAMR1 mice. The hippocampal GDNF mRNA levels in SAMP10 mice at 2 months of age are significantly lower than those of age-matched SAMR1 mice and are comparable to those of 10-month-old SAMR1 mice, suggesting an early-onset of hippocampal GDNF insufficiency in SAMP10 mice. These abnormalities in the GDNF pathway may contribute to hippocampal dysfunction, including deficits in learning and memory.
Nakanishi et al. examined amygdala slice preparations obtained from young and aged SAMP10 and SAMR1 mice in an electrophysiological study of spontaneous and evoked bursts in regard to glutamate receptor subtypes [83]. Spontaneous bursts were recorded in the medial, central and basolateral nuclei. The mean frequency of the spontaneous bursts was significantly increased in both young and aged SAMP10 mice compared with SAMR1 mice. Burst responses were evoked in the medial, central, and basolateral amygdala following stimulation of the stria terminalis. Evoked and spontaneous bursts were completely suppressed by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 4 μM), an alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)/kainate receptor antagonist, but not by (+)-5-methyl-10,11-dihydro-5H-dibenzo-[a,d]-cyclohepten-5,10-imine hydrogen maleate (MK-801, 30 μM), an N-methyl-d-aspartate (NMDA) receptor antagonist. The hyperexcitability of amygdala neurons was studied using optical recordings. Following stimulation of the stria terminalis, the optical signals reflecting postsynaptic responses spread into the medial and central amygdaloid areas at 2–5 ms and faded out at 20–30 ms after stimulation. The intensity of the optical signal recorded in slices from young SAMP10 mice was significantly higher than those from SAMR1 mice. These observations indicate that bursts mediated by AMPA/kainate receptors are only transiently generated in the amygdala of SAMR1 mice at young ages, while bursts with higher frequency are continuously generated in the amygdala of SAMP10 mice for an extended period of time. Such chronic neuronal hyperexcitability in the amygdala may be involved in age-related impairments in learning and memory in SAMP10 mice.
7. Molecular abnormalities and genetic defects in SAMP10 mice
To study molecular senescence of the hippocampus, Carter performed gene-expression analysis and polymorphism screening using tissues from SAMP10, SAMP8, SAMR1, and C57BL/6 mice [84]. The analyses led to the identification of mutations that could have an impact on neurodegenerative processes in SAMP10 mice, including a mutation in a fibroblast growth factor gene, Fgf1. We sequenced Fgf1 and found a 6-bp insertion into the 3′ region of the third intron of Fgf1, which shifts the splicing site 15-bp upstream of the usual 5′ end of exon 4 (unpublished data). The predicted amino-acid sequence of FGF1 protein from SAMP10 mice indicates an altered amino-acid sequence with a truncation at the carboxy-terminus resulting in the absence of the entire fourth exon. Western blots (and our unpublished immunohistochemistry) with an antibody specific for the carboxy-terminus of FGF1 confirmed the absence of normal FGF1 protein in SAMP10 mouse brain. It has been reported that reduced expression of FGF1 in layer 2 entorhinal cortical neurons in Alzheimer’s disease patients leads to lower levels of calbindin, resulting in a decreased neuroprotective capacity [85]. Given the link between FGF1 and neuroprotection, the mutation in this gene may play an important role in the pathogenesis of age-related neurodegeneration in SAMP10 mice.
Zinc (Zn) has been shown to play a variety of roles in the brain [86]. Zn is highly concentrated in the synaptic boutons of the hippocampal mossy fiber pathway [87], which forms a dense plexus of terminals on apical dendrites of CA3 pyramidal neurons and is involved in learning and memory. Zn is released from presynaptic vesicles, along with glutamate [88–89], and subsequently attenuates the excessive presynaptic glutamate release [90] and the activity of glutamate receptors [91–92], thereby protecting neurons from glutamate excitotoxicity. Hippocampal Zn content decreases with aging, and this may be involved in age-related impairments in learning and memory [93]. Zn transporters 3 (ZnT3) and 4 (ZnT4) have been identified in mammalian brains and are thought to transport Zn into synaptic vesicles and to regulate Zn efflux in the brain [94]. Age-related decreases in the amount of Zn in the hippocampal mossy fiber pathway are more marked in SAMP10 mice than in SAMR1 mice [95]. The expression of ZnT3 is markedly reduced in SAMP10 mice even at young ages (2 months). Additionally, excessive presynaptic release of glutamate and glycine occurs in the hippocampus of 12-month-old SAMP10 mice compared to age-matched SAMR1 mice [95]. These findings suggest that an age-related decline in the amount of Zn in synaptic vesicles of the mossy fiber pathway, induced by low levels of the hippocampal ZnT3 expression, may cause excessive release of glutamate and glycine, resulting in chronic excitotoxic damage in hippocampal synapses and neurons. Such impairments in the regulatory system exerted by Zn may contribute to age-related deficits in learning and memory in SAMP10 mice.
Sphingomyelinase is an important effector enzyme in signal transduction pathways. Ceramide, a relatively recently identified lipid second messenger, is produced by sphingomyelinase and plays a key role in various antiproliferative responses including apoptosis, cell cycle arrest, and aging [96–97]. Sphingomyelinase and ceramide may act as mediators of cellular senescence. It has been reported that the levels of endogenous ceramide and sphingomyelinase activity are markedly elevated in senescent human diploid fibroblasts, and that exogenous ceramide induces a senescent phenotype in young human fibroblasts [98]. The sphingomyelinase activity in membrane fractions prepared from the cerebral cortex is increased in SAMP10 mice at ages 10 and 17 months compared to that in mice at age 2 months. In contrast, there is no age-related change in the enzyme activity in SAMR1 mice. These findings suggest that elevation of membrane-bound sphingomyelinase activity, and the resultant increase in ceramide production, may be involved in neuronal degeneration in the cerebral cortex in SAMP10 mice.
The perineuronal net (PNN) is a reticular structure that covers cell bodies and proximal dendrites of certain neurons, in which a variety of proteoglycans are constituents. PNNs may play a role in stabilizing synaptic structures [99]. Saitoh reported that Immunohistochemistry with proteoglycan-related antibody 6B4 (MAb6B4, which has been reported to recognize phosphacan/PTPζ in immature brains) stained PNNs in the cerebral cortex of SAMR1 mice [100]. In contrast, immunoreactivity with MAb6B4 was markedly reduced in the cortex of SAMP10 mice. Since the aggrecan core glycoprotein is preserved in SAMP10 mouse brain, the reduced expression of MAb6B4 epitope suggests a deficit in posttranslational modification, which could lead to PNN functional impairment in SAMP10 mice. The structure and function of the MAb6B4 epitope has not been well documented. SAMP10 mice may provide novel information about the MAb6B4 epitope, particularly in close association with age-related neurodegeneration [100–101].
One of the most striking aspects of the pathology of neurodegenerative diseases is the selective vulnerability of neuronal populations to damage in various diseases. The neurofibrillary tangles in Alzheimer’s disease occur preferentially in the limbic forebrain, including the entorhinal cortex, perirhinal cortex, hippocampal CA1, subiculum and amygdala, as well as the temporal and frontal association cortex [66, 68]. Tangles that appear in normal aging also preferentially affect limbic structures [67, 69–70]. SAMP10 mice develop numerous intraneuronal ubiquitinated inclusions, particularly in the limbic and related forebrain structures, as we previously described [61]. Recently, we performed a proteomic analysis to identify proteins that exhibit changes in expression in association with aging in the limbic and related forebrain areas of SAMP10 mice [102]. Age-related changes in protein expression were compared between limbic-related and non-limbic forebrain, as well as between SAMP10 and SAMR1 mice by two-dimensional difference gel electrophoresis coupled with peptide mass fingerprinting and peptide sequencing. Among protein spots in which aging-related expression patterns in the limbic forebrain differed between SAMP10 and SAMR1 mice, we identified three proteins: pyridoxal phosphate phosphatase (PLPP), collapsin response mediator protein 2 (CRMP-2) and α-internexin. PLPP expression was increased in the limbic forebrain of 3-month-old SAMP10 mice, while CRMP-2 and phosphorylated α-internexin levels were increased in the limbic forebrain of SAMP10 mice at age 8 months and remained high until 14 months. Western blot analysis revealed an elevation in the level of phosphorylated CRMP-2 and the phosphorylation ratio of α-internexin. Immunohistochemistry revealed that α-internexin was chiefly distributed in axons. Aging in SAMP10 mice is associated with abnormalities in PLPP, CRMP-2 and α-internexin, all of which are known to be involved in brain cytoskeleton formation and are associated with acute and chronic neurodegenerative conditions [103–105].
8. Elevated oxidative-nitrative stress in the brains of SAMP10 mice
Since the ‘free-radical theory of aging’ was proposed [106], oxidative stress has been linked to biological aging at molecular, cellular, and organismal levels [107–108]. There have been many studies indicating higher oxidative-nitrative status in various tissues of SAMP1, SAMP8, and SAMP10 mice in comparison with SAMR1 mice [109]. The following are examples of studies reporting higher oxidative-nitrative status in SAMP10 mouse brains. Based on real-time bioradiography, a photonic imaging method using Lucigenin as a chemilumigenic probe for detecting superoxide anion radical production, Sasaki et al. in collaboration with our group examined age-related changes in superoxide-dependent chemiluminescence in ex vivo brain tissue slices prepared from SAM mice [110]. An age-related increase was observed in the levels of superoxide generated from brain slices before and after hypoxia-reoxygenation treatment in both SAMP10 and SAMR1 mice. The rate of age-related increases in brain superoxide levels in SAMP10 mice was significantly greater than that in SAMR1 mice throughout the lifespan. These findings suggest that the excessive production of superoxide in SAMP10 mouse brain may be associated with accelerated brain aging.
Unno et al. examined cellular damage due to oxidative stress by measuring the amount of 8-oxo-deoxyguanosine (8-oxodG) contained in the hippocampus and olfactory-related structures using an electron capture detector (ECD) [111]. SAMP10 mice showed elevated levels of 8-oxodG in these brain regions compared to SAMR1 mice. The same group also examined carbonylation, a marker for oxidative damage of proteins. Carbonyl protein levels in the cerebral cortex were higher in SAMP10 mice at 2, 6, and 12 months of age compared with levels in SAMR1 mice at 12 months of age [112]. An age-related decline in glutathione peroxidase activity may contribute to the accumulation of oxidatively damaged proteins in the brains of SAMP10 mice. Ubiquitinated neuronal inclusions, as previously described, may also be a manifestation of elevated oxidative stress status, since oxidative damage is implicated in lipofuscin formation [113].
The expression of neuronal nitric oxide synthase (nNOS) increases with age in the cerebral cortex in SAMP10 mice. Levels of nNOS are higher in both 3- and 12-month-old SAMP10 mice compared to those in age-matched SAMR1 mice [114]. Such elevated nitrative stress may cause DNA damage in neuronal cells.
9. Age-related increases in pro-inflammatory cytokines in SAMP10 and SAMP8 mice
Elevated oxidative-nitrative stress may induce a pro-inflammatory status in the brains of SAM mice. In the hippocampus of 10-month-old SAMP8 mice, the levels of IL-1 mRNA are significantly elevated in comparison with that of age-matched SAMR1 mice. In both SAM strains, the IL-1β protein levels in the brain are increased in mice at 10 months of age compared with mice at ages 2 and 5 months. The protein levels of IL-1β in the hippocampus and hypothalamus, as well as TNF-α and IL-6 in the hippocampus and cerebral cortex, are significantly elevated in SAMP8 mice as compared to SAMR1 mice [115].
In SAMP10 mice, we evaluated cytokine expression in brain tissue prepared from the frontal part of the cerebrum that underwent the most marked atrophy in SAMP10 mice [116]. IL-1β and IFN-γ mRNA levels in SAMP10 mice were about 2-fold higher than those in SAMR1 mice throughout life. The levels of IL-6 mRNA in SAMP10 mice were 2-fold higher than in SAMR1 mice at 14 months of age, although the levels were similar between these mice at 3 months of age. The levels of monocyte chemotactic protein-1 mRNA were greater in SAMP10 mice than in SAMR1 mice, and tended to increase with advancing age. Mice at 14 months of age exhibited a 2.1-fold elevation in TNF-α mRNA levels as compared to mice at 3 months of age in both SAMP10 and SAMR1 strains. These findings suggest the presence of elevated pro-inflammatory status in the atrophy-prone brain region of SAMP10 mice. Therefore, neuroinflammation may be a likely mechanism underlying age-related neurodegeneration in SAMP10 mice.
10. Alterations in microglial cells in SAMP10 mice
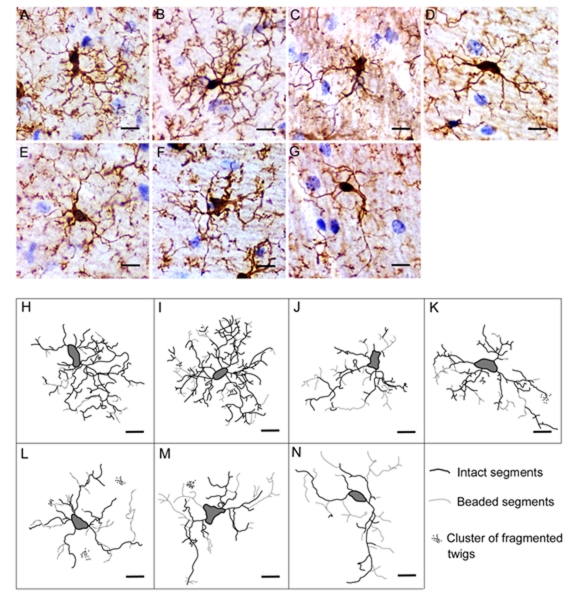
Different types of alterations in microglial cells have been reported in various brain regions of SAMP10 mice. Although neuronal dendrites are known to retract with aging, as described, age-related changes in the morphology of microglia have not been well documented. In our recent study [117], hippocampal sections prepared from SAMP10 and SAMR1 mice at various ages were stained with immunohistochemistry for ionized calcium-binding adapter molecule 1 (Iba-1), a microglial marker. Camera lucida drawings of individual microglia were used to characterize the exact length and complexity of cytoplasmic processes. Parameters representing morphological features of microglia were quantified using an image analyzer, including: area of convex closure, cell body area, number of primary processes, maximal branch order, combined projection length, number of segments and number of tips. Pathological changes in processes, such as beading and clusters of fragmented twigs, were counted. In microglia of 3- and 8-month-old SAMP10 mice, the combined projection length was shorter, and the numbers of segments and tips were fewer than those in age-matched SAMR1 mice. Similar changes were detected in SAMR1 mice at age 14 months and older. Microglia of SAMP10 mice at all ages were characterized by having frequent pathological changes in processes, which were not apparent in SAMR1 mice at any age (Fig. 3). These morphological abnormalities in microglia of SAMP10 mice preceded the onset of neuronal degeneration [117]. Given that ramified cytoplasmic processes are essential for microglia to exert surveillance activity [25], morphological abnormalities in microglial processes in SAMP10 mice may make the tissue microenvironment of the hippocampus less favorable for neurons. These microglial abnormalities may contribute to the vulnerability to age-related neuronal degeneration in SAMP10 mice.
Fig. 3. Age-related alterations in microglial morphology in SAMR1 and SAMP10 mice.
(A-G) Iba-1 immunostaining with hematoxylin counterstain reveals representative microglia in the hippocampal CA1 regions of 3-, 8-, 14- and 24-month-old SAMR1 (A, B, C and D, respectively) and 3-, 8- and 14-month-old SAMP10 (E, F and G, respectively) mice. (H-N) Skeletonized camera lucida drawings of microglia. Drawings in H-K and L-N correspond to cells in A-D and E-G, respectively. Solid lines represent intact segments. Dotted lines and groups of small dots represent beaded segments and clusters of fragmented twigs of microglia, respectively. Scale bars, 10 μm. Reproduction of copyrighted material permitted by John Wiley and Sons [117], License Number 2507540929136.
Cathepsin E is a non-lysosomal aspartic proteinase in mammalian cells with a limited distribution in certain cell types of lymphoid, gastrointestinal, and urinary organs [118]. Cathepsin E accumulates markedly in the brain stem of SAMP10 mice by 2 months of age, although it is barely detectable in the brains of SAMP10 mice at 1 week of age [23]. The main cellular source of cathepsin E is activated microglia that forms clusters in response to vacuolar degeneration in the brain stem. Cathepsin E expression is not detectable in SAMR1 mice at any age. Therefore, microglia in the brain stem of SAMP10 mice are activated with the expression of cathepsin E and are likely to respond to spongiform neurodegeneration that occurs spontaneously in SAMP10 mice from 2 months of age as in SAMP8 mice [47, 119].
One of the possible factors that may potentially perturb brain immune function is the endogenous retrovirus that has been reported to be integrated into the genome of SAMP strains, including SAMP10 [120]. Given the fact that AKR mice have high levels of endogenous ecotropic murine leukemia virus (MuLV), it is not surprising that most SAMP strains contain the Emv11 provirus that encodes the predominant MuLV found in AKR mice [120–121]. It has not yet been elucidated whether or how endogenous MuLV is involved in the acceleration of aging processes in various organs in SAMP mice. Reportedly, the capsid antigen CAgag is expressed in neurons, astrocytes, oligodendrocytes, and vascular endothelial cells in affected brain regions of SAMP8 mice where spongy degeneration is noted [122]. Spongiform neurodegeneration in the brain stem and spinal cord in SAMP10 mice is likely to be induced by MuLV provirus expression as in SAMP8 mice. Certain neurovirulent MuLV retroviruses, when exogenously inoculated, cause noninflammatory spongiform neurodegeneration in the brain stem and spinal cord in susceptible mice and rats, which manifests clinically by progressive paralysis [123–125]. However, neither SAMP10 nor SAMP8 mice develop progressive paralysis throughout the lifespan. Therefore, MuLV-associated neurodegeneration may be relatively mild in SAMP strains, but still may potentially perturb immune responses exerted by microglia and other glial cells.
11. Upregulation of cytokines following KA-induced hippocampal injury in SAMR1 mice
In the hippocampus of mice injected with kainic acid (KA), a glutamate analogue excitotoxin, pyramidal cells undergo degeneration and cell death in the CA3 region with relative preservation in the CA1 region [126]. Although the number of glial cells increases in both the CA3 and CA1 regions following KA injection, most microglia located in the CA1 region undergo morphological transformation to bushy cells [126] and are considered to be non-phagocytic activated microglia [127]. In our recent study, DNA microarray analysis, followed by real-time RT-PCR confirmation, of samples prepared from the mouse hippocampus revealed significant increases in the expression levels of the Ifng, Spp1, Ccl3, Cxcl10, Cd44, Socs3, Csf2, Ccl4, and Osmr genes [encoding IFN-γ, osteopontin (OPN), macrophage inflammatory protein (MIP)-1α, chemokine (C-X-C motif) ligand 10 (CXCL10), CD44, suppressor of cytokine signaling 3 (SOCS3), colony-stimulating factor (GM-CSF), MIP-1β, and oncostatin M-specific receptor (OSMR)] in KA-injected SAMR1 mice over saline-injected SAMR1 mice 3 days post-treatment [127].
Our immunohistological analyses using SAMR1 mouse brain sections revealed that the expressions of IFN-γ and GM-CSF were increased in non-phagocytic activated microglial cells in SAMR1 mice at 3 days after KA injection [127]. The expressions of IFNγ receptor, CXCL10, and MIP-1α were increased in astrocytes. The expression of OPN was markedly increased throughout the neuropil and more intensively in perikarya and dendrites of scattered neurons in the pyramidal layer and dentate hilus. Non-phagocytic activated microglia expressing OPN often appeared in close association with nearby neurons and contact neuronal dendrites with microglial processes or with cell bodies. Reactive astrocytes were devoid of OPN. The expression of CD44, an OPN receptor, was dramatically increased throughout the hippocampal formation. Some astrocytes expressed CD44 on the surface of cytoplasmic endfeet but non-phagocytic activated microglia were devoid of CD44 expression [127].
12. Biological significance of the cytokine-mediated glia-neuronal network following excitotoxic hippocampal injury
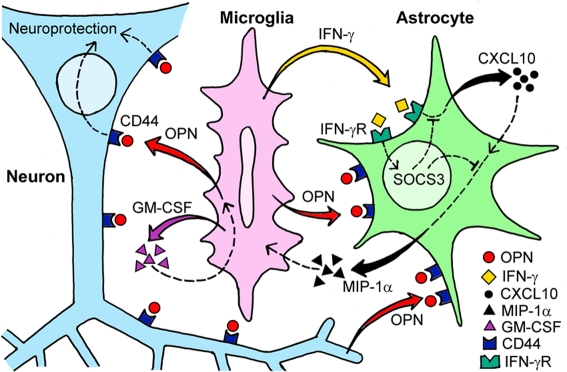
Although IFN-γ was previously thought to be produced exclusively by T and NK cells, cells of the monocyte/macrophage lineage have been shown to produce IFN-γ in the early phase of the host response to infectious agents [128]. There is emerging evidence that microglia have the capacity to produce IFN-γ [129–130] and reactive astrocytes can express the IFN-γ receptor [131]. CXCL10 is induced by IFN-γ and induction of CXCL10 in astrocytes has been reported in various types of brain pathology [132–134]. The ability of astrocytes to release MIP-1α and MIP-1β has also been reported [135]. CXCL10 induces MIP-1α expression when CXCL10 initiates inflammatory responses in the eye [136], and MIP-1α plays a critical role in the recruitment of microglia in the brain [137–138]. GM-CSF is one of the many cytokines that have been reported to be secreted by microglia [139]. GM-CSF is an important inducer of microglial proliferation [140], and GM-CSF-primed neutrophils are targets for migration induced by MIP-1α [141]. Therefore, in the injured hippocampus of SAMR1 mice, microglia-derived IFN-γ activates astrocytes via the IFN-γ receptor, which induces astrocytic CXCL10 production leading to the upregulation of MIP-1α secretion by astrocytes and results in the recruitment of GM-CSF-primed microglia (Fig. 4).
Fig. 4. Construction of cytokine-mediated glia-neuronal intercellular communication network in the hippocampus of SAMR1 mice following excitotoxic injury.
Cytokine-mediated intercellular communication among microglia, astrocytes, and neurons following KA-induced hippocampal injury in SAMR1 mice is represented by schematic illustration. Solid curved arrows indicate interactions confirmed in our previous study. Broken lines indicate hypothetical interactions. (reproduction of copyrighted material permitted by Elsevier [127], License Number 2673921041383)
OPN is not expressed in intact brain but rather is upregulated to serve as a potent neuroprotectant in the injured brain [142]. OPN mRNA upregulation is most marked 3 days after KA injection and occurs in a subset of activated microglial cells. Similarly, in models of ischemia, OPN mRNA reaches peak levels 3–5 days after the ischemic insult, and is expressed in microglia and macrophages [143–144]. OPN reduces infarct size [145] and attenuates delayed secondary neurodegeneration [146]. OPN accumulates in the matrix of ischemic brain and plays a functional role in cell-matrix interactions and tissue remodeling [147]. OPN is produced in response to GM-CSF to exert anti-apoptotic activity through binding to CD44 [148]. OPN-deficient mice exhibit exacerbated secondary neurodegeneration in the thalamus compared to wild-type mice following excitotoxin-induced cortical lesion [149]. CD44 has been reported to be upregulated in association with mossy fiber sprouting in the dentate gyrus following KA treatment [150] and status epilepticus [151]. CD44 interacts with OPN to promote axon generation [152]. These findings suggest a role for the OPN/CD44 system in neural repair. Therefore, our findings of marked elevation of OPN/CD44 expression over almost the entire neuropil of the hippocampus indicate that the hippocampal tissue of SAMR1 mice 3 days after KA injection is in the process of remodeling toward re-establishment of the hippocampal neural network that has been damaged due to acute excitotoxicity.
13. Lack of cytokine-mediated glia-neuronal communication in SAMP10 mice
Using C57BL/6 mice, we previously investigated the characteristic bushy transformation of non-phagocytic activated hippocampal microglia following KA-induced injury [153]. These non-phagocytic activated microglia exhibited increases in both the number and area of cell-to-cell contacts between microglial processes and neuronal dendrites. Direct contact between neurons and microglia is required for manifestation of neuroprotective effects [154]. One advantage of this intimate physical arrangement is the facilitation of reciprocal, targeted exchange of beneficial molecules between neurons and microglia.
In contrast to remarkable cytokine-mediated responses evident in the hippocampus of SAMR1 mice following KA administration, hippocampal tissue reactions of SAMP10 mice in response to KA-induced injury are surprisingly weak. In our recent study, the bushy transformation of non-phagocytic activated hippocampal microglia following KA-induced injury was insufficient in SAMP10 mice, in terms of branch morphology and cell body size, compared to that in SAMR1 mice [127]. In the same study, we reported that KA-induced hippocampal upregulation of cytokine-related genes was strikingly reduced in SAMP10 mice compared to SAMR1 mice. There were no significant increases in the expression of any of Ifng, Spp1, Ccl3, Cxcl10, Cd44, Socs3, Csf2, Ccl4, and Osmr genes in KA-injected SAMP10 mice compared to saline-injected SAMP10 mice. Impaired induction of a pleiotropic cytokine such as IFN-γ may lead to failure of other cytokine-mediated tissue responses. Neither CXCL10 nor MIP-1α mRNA were significantly upregulated in the injured hippocampus of SAMP10 mice. Correspondingly, reactive astrocytes expressed nearly basal levels of CXCL10 and MIP-1α. Microglial expression of GM-CSF was not obvious in the injured hippocampus of SAMP10 mice. The magnitude of upregulation of OPN/CD44 was much less in the injured hippocampus of SAMP10 mice than in SAMR1 mice. Injured hippocampus in SAMP10 mice did not exhibit a significant upregulation of OSMR mRNA, suggesting that OsM-OSMR signaling is not utilized by hippocampal neurons for protection following excitotoxic injury in these mice. Consistent with the markedly reduced OSMR upregulation, SOCS3 expression was not induced significantly in the hippocampus of SAMP10 mice after KA injection.
Orchestration of intercellular communication via cytokines thus fails to occur in the injured hippocampus of SAMP10 mice. The lack of activation of the OPN-CD44 system, together with the failure of OsM-OSMR pathway utilization, suggests that the hippocampal tissue of SAMP10 mice at 3 days after KA injection is not oriented toward neuroprotection and remodeling, despite tissue damage. This dysfunction of intercellular communication via a series of cytokines is probably related to the resultant atrophy of hippocampal layers in SAMP10 mice observed as a remote after-effect 30 days after KA injection [127].
The findings described above appear to indicate that microglia of SAMP10 mice undergo cellular senescence-like changes with a loss of neuroprotective functions at an early age. An early onset of neurodegeneration in SAMP10 mice may be due to deficits in cytokine-mediated neuroprotective glia-neuron interactions, rather than the result of an overactive immune response exerted by microglia. Although it remains to be determined whether the changes in microglia in SAMP10 mice correspond to the “dystrophic” microglia in the human brain [27], the identification of factors and/or microenvironmental conditions that cause microglial changes in SAMP10 mice would make a great contribution to understanding how microglial senescence potentially increases susceptibility to neurodegeneration in humans.
14. Interface between the CNS and the peripheral immune system
To identify potential factors and/or microenvironmental conditions that could cause microglial changes in SAM mice, it is important to determine the route by which brain parenchyma generates pro-inflammatory cytokines in response to peripheral inflammatory signals. How peripheral inflammatory cytokines instigate communication between the immune system and brain during infection has been studied [155]. Inflammatory stimuli in the periphery (e.g., LPS and inflammatory cytokines) induce IL-1β, IL-6, and TNF-α expression in discrete brain areas [156–157]. There have been studies to locate the interface between the peripheral immune system and the brain. For example, the number of IL-1β-expressing cells (macrophages and microglial cells) following peripheral inflammatory challenge with LPS administration increases in the meninges, choroid plexus, circumventricular organs, cerebral cortex and hypothalamus, and reaches a maximum 8 h after administration of LPS [158–159]. In particular, the perivascular space, meninges, and choroid plexus are inter-related compartments that are thought to constitute the brain-immune interface.
CNS perivascular cells (also called as perivascular macrophages, perivascular microglia, fluorescent granular perithelial cells, or Mato cells) are known to express cytokines in response to inflammatory stimuli in the periphery [158–159]. Perivascular cells also function as APCs in CNS inflammation, sensors of neural injury and death, phagocytic and pinocytotic cells taking up CNS antigens in the perivascular space, and as sensory cells sensitive to peripheral nerve injury, cytokine levels, and endotoxin outside the CNS [160]. Perivascular cells exert dual roles in immune-to-brain signaling by providing prostaglandin-mediated drive to initiate hypothalamo-pituitary-adrenal responses in response to peripheral IL-1 challenge, as well as providing an anti-inflammatory action that constrains endothelial responses to inflammatory challenge [161].
The mouse meninges and choroid plexus contain resident macrophages and dendritic cells (DCs), and the choroid plexus stroma represents a niche for myeloid progenitors and may serve as a reservoir for brain macrophages [162]. Meningeal/choroid plexus macrophages and DCs participate in immune surveillance, phagocytosis of cellular debris, uptake of antigens from the surrounding cerebrospinal fluid, and immune regulation in many pathologic processes. In a recent study using bone marrow chimeric mice, prepared by bone marrow transplantation following lethal irradiation, Chinnery et al. reported that there was a rapid replenishment of bone marrow-derived macrophages and DCs in the meninges (at 4 weeks) and the choroid plexus was fully reconstituted by 8 weeks [163]. Prodinger et al. reported that the periventricular area, adjacent fiber tracts, and optical nerve were preferentially populated by CD11c+ DC cells [164]. Most CD11c+ cells were located within the juxtavascular parenchyma rather than the perivascular spaces. Virtually all CD11c+ cells co-expressed Iba-1 and CD11b, while detectable levels of MHC-II was restricted to CD11c+ cells in the choroid plexus. Cellular processes project into the glia limitans, which may allow transport and/or presentation of intraparenchymal antigens to extravasated T cells in perivascular spaces [164].
The importance of meningeal immunity has been highlighted in association with the T cell effects on cognitive function [165–166]. Derecki et al. introduced mice to a cognitive task and found substantial immunological changes, including the accumulation of T cells, in the areas immediately adjacent to the brain parenchyma in task-trained mice, such as the subarachnoid meningeal spaces and choroid plexus, although no T cells were detectable in the parenchyma [167].
How age-related changes in the peripheral immune system could be transferred to the brain parenchyma via perivascular cells and resident myeloid cells in the meninges/choroid plexus awaits future studies. Given the defective peripheral immune functions in SAMP mice, we presume that the immunity constructed by bone marrow-derived cells of the meninges and choroid plexus, and perivascular macrophages might be perturbed relatively early in life in these mice. It would be useful to study what kind of cell-to-cell interactions, factors, and pathways might be involved in transferring abnormalities from the peripheral immunity to the brain microenvironment. It is challenging to determine the mechanism underlying the impact of age-related disturbances in peripheral immunity on the dysregulation of brain tissue homeostasis resulting in age-related neurodegeneration.
15. Coda
Owing to a 30-year history of data accumulation by immunologists and neuroscientists using SAM mice, SAMP strains are known to exhibit changes in various aspects of brain morphology and function that mimic age-related changes occurring in humans. It is important that SAMP mice achieve good health conditions during postnatal development and during their reproductive age (young adult SAMP10 mice exhibit good learning and memory performance comparable to age-matched SAMR1 and C57BL mice). Abnormalities in brain functions begin to appear after SAMP mice reach reproductive age; however, early signs of perturbation in the peripheral immune status become detectable before the appearance of brain dysfunction. These features of SAM mice are beneficial for investigation of the causal relationship of immune-brain dysregulation with aging.
Our hope is that SAM mice will serve the needs of scientists worldwide who are interested in linking certain aspects of immunology to aging and age-related diseases. We would be grateful if future data obtained from SAM mice, through powerful collaborations between immunologists and neuroscientists, would make substantial contributions to the rapidly growing field of psychoneuroimmunology, the study of behaviorally associated immunological changes and immunologically associated behavioral changes that result from reciprocal interactions among the nervous, endocrine, and immune systems [168].
Acknowledgements
Our laboratory is supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Contract grant numbers: 21590458 to AS and 22790392 to SHI).
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res. 2009;34:639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- 2.Sprott RL, Austad SN. Historical Development of Animal Models of Aging. In: Conn PM, editor. Handbook of Models for Human Aging. Elsevior Academic Press; 2006. pp. 1–8. [Google Scholar]
- 3.Mori M, Higuchi K. Genetic monitoring system for SAM strains utilizing DNA markers. In: Nomura Y, Takeda T, Okuma Y, editors. The Senescence-Accerelated Mouse (SAM): An Animal Model of Senescence. Amsterdam: Elsevier; 2004. pp. 187–190. [Google Scholar]
- 4.Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- 7.Hof PR, Duan H, Page TL, Einstein M, Wicinski B, He Y, Erwin JM, Morrison JH. Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Res. 2002;928:175–186. doi: 10.1016/s0006-8993(01)03345-5. [DOI] [PubMed] [Google Scholar]
- 8.Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- 9.Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex. 2005;15:409–418. doi: 10.1093/cercor/bhh144. [DOI] [PubMed] [Google Scholar]
- 10.Ron-Harel N, Schwartz M. Immune senescence and brain aging: can rejuvenation of immunity reverse memory loss? Trends Neurosci. 2009;32:367–375. doi: 10.1016/j.tins.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Bernhardi R, Tichauer JE, Eugenin J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- 15.Gemma C. Neuroimmunomodulation and Aging. Aging Dis. 2010;1:169–172. [PMC free article] [PubMed] [Google Scholar]
- 16.Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RW, Godbout JP. Aging, Neuroinflammation, and Behavior. In: Ader R, editor. Psychoneuroimmunology. 4th edn. Amsterdam: Elsevier Acdemic Press; 2007. pp. 379–391. [Google Scholar]
- 18.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 20.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 21.Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappaB. J Neuroimmunol. 2001;117:87–96. doi: 10.1016/s0165-5728(01)00316-2. [DOI] [PubMed] [Google Scholar]
- 22.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amano T, Nakanishi H, Oka M, Yamamoto K. Increased expression of cathepsins E and D in reactive microglial cells associated with spongiform degeneration in the brain stem of senescence-accelerated mouse. Exp Neurol. 1995;136:171–182. doi: 10.1006/exnr.1995.1094. [DOI] [PubMed] [Google Scholar]
- 24.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 26.Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- 27.Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- 29.Streit WJ, Xue Q-S. The brain’s aging immune system. Aging Dis. 2010;1:254–261. [PMC free article] [PubMed] [Google Scholar]
- 30.Bachstetter AD, Van Eldik LJ. The p38 MAP kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010;1:199–211. [PMC free article] [PubMed] [Google Scholar]
- 31.Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging Dis. 2010;1:212–231. [PMC free article] [PubMed] [Google Scholar]
- 32.Gemma C, Bachstetter AD, Bickford PC. Neuron-microglia dialogue and hippocampal neurogenesis in the aged brain. Aging Dis. 2010;1:232–244. [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch AM, Murphy KJ, Deighan BF, O’Reilly J-A, Gun’ko YK, Cowley TR, Gonzalez-Reyes RE, Lynch MA. The impact of glial activation in the aging brain. Aging Dis. 2010;1:262–278. [PMC free article] [PubMed] [Google Scholar]
- 34.Toichi E, Hanada K, Hosono M, Hosokawa T, Hosokawa M, Baba M, Imamura S, Takeda T. Early decline of T cell function in humoral immunity and long-lasting inflammatory T cell activity in aging SAM mice. In: Takeda T, editor. The SAM model of senescence. Amsterdam: Excerpta Medica; 1994. pp. 175–178. [Google Scholar]
- 35.Yoshioka H, Yoshida H, Doi T, Muso E, Ohshio G, Higuchi K, Inada M, Miyake T, Kita T, Hamashima Y, et al. Autoimmune abnormalities in a murine model of accelerated senescence. Clin Exp Immunol. 1989;75:129–135. [PMC free article] [PubMed] [Google Scholar]
- 36.Hosokawa T, Hosono M, Higuchi K, Aoike A, Kawai K, Takeda T. Immune responses in newly developed short-lived SAM mice. I. Age-associated early decline in immune activities of cultured spleen cells. Immunology. 1987;62:419–423. [PMC free article] [PubMed] [Google Scholar]
- 37.Hosokawa T, Hosono M, Hanada K, Aoike A, Kawai K, Takeda T. Immune responses in newly developed short-lived SAM mice. Selectively impaired T-helper cell activity in in vitro antibody response. Immunology. 1987;62:425–429. [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura Y, Hosokawa T, Hosono M, Baba M, Hosokawa M. Insufficient interleukin-2 production from splenic CD4+ T cells causes impaired cell proliferation and early apoptosis in SAMP1, a strain of senescence-accelerated mouse. Immunology. 2002;107:190–198. doi: 10.1046/j.1365-2567.2002.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosokawa T. Immune system deficiencies in SAM. In: Nomura Y, Takeda T, Okuma Y, editors. The Senescence-Accerelated Mouse (SAM): An Animal Model of Senescence. Amsterdam: Elsevier; 2004. pp. 41–46. [Google Scholar]
- 40.Sanders VM, Munson AE. Beta adrenoceptor mediation of the enhancing effect of norepinephrine on the murine primary antibody response in vitro. J Pharmacol Exp Ther. 1984;230:183–192. [PubMed] [Google Scholar]
- 41.Hosono M, Hanada K, Toichi E, Naiki H, Higuchi K, Hosokawa T. Immune abnormality in relation to nonimmune diseases in SAM mice. Exp Gerontol. 1997;32:181–195. doi: 10.1016/s0531-5565(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 42.Toichi E, Hanada K, Hosokawa T, Higuchi K, Hosokawa M, Imamura S, Hosono M. Age-related decline in humoral immunity caused by the selective loss of TH cells and decline in cellular immunity caused by the impaired migration of inflammatory cells without a loss of TDTH cells in SAMP1 mice. Mech Ageing Dev. 1997;99:199–217. doi: 10.1016/s0047-6374(97)00100-0. [DOI] [PubMed] [Google Scholar]
- 43.Haruna H, Inaba M, Inaba K, Taketani S, Sugiura K, Fukuba Y, Doi H, Toki J, Tokunaga R, Ikehara S. Abnormalities of B cells and dendritic cells in SAMP1 mice. Eur J Immunol. 1995;25:1319–1325. doi: 10.1002/eji.1830250528. [DOI] [PubMed] [Google Scholar]
- 44.Dong L, Mori I, Hossain MJ, Kimura Y. The senescence-accelerated mouse shows aging-related defects in cellular but not humoral immunity against influenza virus infection. J Infect Dis. 2000;182:391–396. doi: 10.1086/315727. [DOI] [PubMed] [Google Scholar]
- 45.Liu B, Kimura Y. Local immune response to respiratory syncytial virus infection is diminished in senescence-accelerated mice. J Gen Virol. 2007;88:2552–2558. doi: 10.1099/vir.0.83089-0. [DOI] [PubMed] [Google Scholar]
- 46.Abe Y, Yuasa M, Kajiwara Y, Hosono M. Defects of immune cells in the senescence-accelerated mouse: a model for learning and memory deficits in the aged. Cell Immunol. 1994;157:59–69. doi: 10.1006/cimm.1994.1205. [DOI] [PubMed] [Google Scholar]
- 47.Shimada A, Ohta A, Akiguchi I, Takeda T. Inbred SAM-P/10 as a mouse model of spontaneous, inherited brain atrophy. J Neuropathol Exp Neurol. 1992;51:440–450. doi: 10.1097/00005072-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Shimada A. Age-dependent cerebral atrophy and cognitive dysfunction in SAMP10 mice. Neurobiol Aging. 1999;20:125–136. doi: 10.1016/s0197-4580(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 49.Shimada A, Hosokawa M, Ohta A, Akiguchi I, Takeda T. Localization of atrophy-prone areas in the aging mouse brain: Comparison between the brain atrophy model SAM-P/10 and the normal control SAM-R/1. Neuroscience. 1994;59:859–869. doi: 10.1016/0306-4522(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 50.Shimada A, Tsuzuki M, Keino H, Satoh M, Chiba Y, Saitoh Y, Hosokawa M. Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: a model of cerebral degeneration. Neuropathol and Appl Neurobiol. 2006;32:1–14. doi: 10.1111/j.1365-2990.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 51.Shimada A, Keino H, Satoh M, Kishikawa M, Hosokawa M. Age-related loss of synapses in the frontal cortex of SAMP10 mouse: A model of cerebral degeneration. Synapse. 2003;48:198–204. doi: 10.1002/syn.10209. [DOI] [PubMed] [Google Scholar]
- 52.Shimada A, Ohta A, Akiguchi I, Takeda T. Age-related deterioration in conditional avoidance task in the SAM-P/10 mouse, an animal model of spontaneous brain atrophy. Brain Res. 1993;608:266–272. doi: 10.1016/0006-8993(93)91467-7. [DOI] [PubMed] [Google Scholar]
- 53.Miyamoto M. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp Gerontol. 1997;32:139–148. doi: 10.1016/s0531-5565(96)00061-7. [DOI] [PubMed] [Google Scholar]
- 54.Okuma Y, Murayama T, Tha KK, Yamada C, Hosokawa M, Ishikawa A, Watanabe R, Maekawa M, Nomura Y. Learning deficiency and alterations in acetylcholine receptors and protein kinase C in the brain of senescence-accelerated mouse (SAM)-P10. Mech Ageing Dev. 2000;114:191–199. doi: 10.1016/s0047-6374(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 55.Onodera T, Watanabe R, Tha KK, Hayashi Y, Murayama T, Okuma Y, Ono C, Oketani Y, Hosokawa M, Nomura Y. Depressive behavior and alterations in receptors for dopamine and 5-hydroxytryptamine in the brain of the senescence accelerated mouse (SAM)-P10. Jpn J Pharmacol. 2000;83:312–318. doi: 10.1254/jjp.83.312. [DOI] [PubMed] [Google Scholar]
- 56.Nie K, Yu JC, Fu Y, Cheng HY, Chen FY, Qu Y, Han JX. Age-related decrease in constructive activation of Akt/PKB in SAMP10 hippocampus. Biochem Biophys Res Commun. 2009;378:103–107. doi: 10.1016/j.bbrc.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Chida Y, Sudo N, Mori J, Kubo C. Social isolation stress impairs passive avoidance learning in senescence-accelerated mouse (SAM) Brain Res. 2006;1067:201–208. doi: 10.1016/j.brainres.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 58.Lucassen PJ, Chung WC, Kamphorst W, Swaab DF. DNA damage distribution in the human brain as shown by in situ end labeling; area-specific differences in aging and Alzheimer disease in the absence of apoptotic morphology. J Neuropathol Exp Neurol. 1997;56:887–900. doi: 10.1097/00005072-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Stadelmann C, Bruck W, Bancher C, Jellinger K, Lassmann H. Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol. 1998;57:456–464. doi: 10.1097/00005072-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Shimada A, Keino H, Satoh M, Kishikawa M, Seriu N, Hosokawa M. Age-related progressive neuronal DNA damage associated with cerebral degeneration in a mouse model of accelerated senescence. J Gerontol A Biol Sci Med Sci. 2002;57:B415–421. doi: 10.1093/gerona/57.12.b415. [DOI] [PubMed] [Google Scholar]
- 61.Shimada A, Keino H, Kawamura N, Chiba Y, Hosokawa M. Limbic structures are prone to age-related impairments in proteasome activity and neuronal ubiquitinated inclusions in SAMP10 mouse: a model of cerebral degeneration. Neuropathol Appl Neurobiol. 2008;34:33–51. doi: 10.1111/j.1365-2990.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 62.de Vrij FMS, Sluijs JA, Gregori L, Fischer D, Hermens WT, Goldgaber D, Verhaagen J, van Leeuwen FW, Hol EM. Mutant ubiquitin expressed in Alzheimer’s disease causes neuronal death. FASEB J. 2001;15:2680–2688. doi: 10.1096/fj.01-0438com. [DOI] [PubMed] [Google Scholar]
- 63.Hernandez F, Diaz-Hernandez M, Avila J, Lucas JJ. Testing the ubiquitin-proteasome hypothesis of neurodegeneration in vivo. Trends Neurosci. 2004;27:66–69. doi: 10.1016/j.tins.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Petrucelli L, Dawson TM. Mechanism of neurodegenerative disease: role of the ubiquitin proteasome system. Ann Med. 2004;36:315–320. doi: 10.1080/07853890410031948. [DOI] [PubMed] [Google Scholar]
- 65.Vigouroux S, Briand M, Briand Y. Linkage between the proteasome pathway and neurodegenerative diseases and aging. Mol Neurobiol. 2004;30:201–221. doi: 10.1385/MN:30:2:201. [DOI] [PubMed] [Google Scholar]
- 66.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 67.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- 68.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 69.Hof PR, Giannakopoulos P, Vickers JC, Bouras C, Morrison JH. The morphologic and neurochemical basis of dementia: aging, hierarchical patterns of lesion distribution and vulnerable neuronal phenotype. Rev Neurosci. 1995;6:97–124. doi: 10.1515/revneuro.1995.6.2.97. [DOI] [PubMed] [Google Scholar]
- 70.Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 71.Hamburger V, Brunso-Bechtold JK, Yip JW. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci. 1981;1:60–71. doi: 10.1523/JNEUROSCI.01-01-00060.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987;6:1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cuello AC, Bruno MA, Allard S, Leon W, Iulita MF. Cholinergic involvement in Alzheimer’s disease. A link with NGF maturation and degradation. J Mol Neurosci. 2010;40:230–235. doi: 10.1007/s12031-009-9238-z. [DOI] [PubMed] [Google Scholar]
- 74.Larkfors L, Ebendal T, Whittemore SR, Persson H, Hoffer B, Olson L. Decreased level of nerve growth factor (NGF) and its messenger RNA in the aged rat brain. Brain Res. 1987;427:55–60. doi: 10.1016/0169-328x(87)90044-1. [DOI] [PubMed] [Google Scholar]
- 75.Cuello AC, Bruno MA, Bell KF. NGF-cholinergic dependency in brain aging, MCI and Alzheimer’s disease. Curr Alzheimer Res. 2007;4:351–358. doi: 10.2174/156720507781788774. [DOI] [PubMed] [Google Scholar]
- 76.Ohnishi K, Tomimoto H, Akiguchi I, Seriu N, Kawamata T, Nakamura S, Kimura J, Nishio T, Higuchi K, Hosokawa M. Age-related decrease of nerve growth factor-like immunoreactivity in the basal forebrain of senescence-accelerated mice. Acta Neuropathol. 1995;90:11–16. doi: 10.1007/BF00294454. [DOI] [PubMed] [Google Scholar]
- 77.Arenas E, Trupp M, Akerud P, Ibanez CF. GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron. 1995;15:1465–1473. doi: 10.1016/0896-6273(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 78.Buj-Bello A, Buchman VL, Horton A, Rosenthal A, Davies AM. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;15:821–828. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 79.Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- 80.Bowenkamp KE, Ujhelyi L, Cline EJ, Bickford PC. Effects of intra-striatal GDNF on motor coordination and striatal electrophysiology in aged F344 rats. Neurobiol Aging. 2000;21:117–124. doi: 10.1016/s0197-4580(99)00112-8. [DOI] [PubMed] [Google Scholar]
- 81.Fox CM, Gash DM, Smoot MK, Cass WA. Neuroprotective effects of GDNF against 6-OHDA in young and aged rats. Brain Res. 2001;896:56–63. doi: 10.1016/s0006-8993(00)03270-4. [DOI] [PubMed] [Google Scholar]
- 82.Miyazaki H, Okuma Y, Nomura J, Nagashima K, Nomura Y. Age-related alterations in the expression of glial cell line-derived neurotrophic factor in the senescence-accelerated mouse brain. J Pharmacol Sci. 2003;92:28–34. doi: 10.1254/jphs.92.28. [DOI] [PubMed] [Google Scholar]
- 83.Nakanishi H, Miyazaki M, Takai N, Wang HD, Yamamoto T, Watanabe S, Yamamoto K. Hyperexcitability of amygdala neurons of senescence-accelerated mouse revealed by electrical and optical recordings in an in vitro slice preparation. Brain Res. 1998;812:142–149. doi: 10.1016/s0006-8993(98)00968-8. [DOI] [PubMed] [Google Scholar]
- 84.Carter TA, Greenhall JA, Yoshida S, Fuchs S, Helton R, Swaroop A, Lockhart DJ, Barlow C. Mechanisms of aging in senescence-accelerated mice. Genome Biol. 2005;6:R48. doi: 10.1186/gb-2005-6-6-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thorns V, Licastro F, Masliah E. Locally reduced levels of acidic FGF lead to decreased expression of 28-kda calbindin and contribute to the selective vulnerability of the neurons in the entorhinal cortex in Alzheimer’s disease. Neuropathology. 2001;21:203–211. doi: 10.1046/j.1440-1789.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 86.Cuajungco MP, Lees GJ. Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol Dis. 1997;4:137–169. doi: 10.1006/nbdi.1997.0163. [DOI] [PubMed] [Google Scholar]
- 87.Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 88.Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 89.Charton G, Rovira C, Ben-Ari Y, Leviel V. Spontaneous and evoked release of endogenous Zn2+ in the hippocampal mossy fiber zone of the rat in situ. Exp Brain Res. 1985;58:202–205. doi: 10.1007/BF00238969. [DOI] [PubMed] [Google Scholar]
- 90.Westergaard N, Banke T, Wahl P, Sonnewald U, Schousboe A. Citrate modulates the regulation by Zn2+ of N-methyl-D-aspartate receptor-mediated channel current and neurotransmitter release. Proc Natl Acad Sci U S A. 1995;92:3367–3370. doi: 10.1073/pnas.92.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 92.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 93.Barili P, Fringuelli C, Ricci A, Rossodivita I, Sabbatini M. Age-related changes of sulphide-silver staining in the rat hippocampus. Mech Ageing Dev. 1997;99:83–94. doi: 10.1016/s0047-6374(97)00095-x. [DOI] [PubMed] [Google Scholar]
- 94.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saito T, Takahashi K, Nakagawa N, Hosokawa T, Kurasaki M, Yamanoshita O, Yamamoto Y, Sasaki H, Nagashima K, Fujita H. Deficiencies of hippocampal Zn and ZnT3 accelerate brain aging of Rat. Biochem Biophys Res Commun. 2000;279:505–511. doi: 10.1006/bbrc.2000.3946. [DOI] [PubMed] [Google Scholar]
- 96.Testi R. Sphingomyelin breakdown and cell fate. Trends Biochem Sci. 1996;21:468–471. doi: 10.1016/s0968-0004(96)10056-6. [DOI] [PubMed] [Google Scholar]
- 97.Sallusto F, Nicolo C, De Maria R, Corinti S, Testi R. Ceramide inhibits antigen uptake and presentation by dendritic cells. J Exp Med. 1996;184:2411–2416. doi: 10.1084/jem.184.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 99.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 100.Saitoh Y, Matsui F, Chiba Y, Kawamura N, Keino H, Satoh M, Kumagai N, Ishii S, Yoshikawa K, Shimada A, Maeda N, Oohira A, Hosokawa M. Reduced expression of MAb6B4 epitopes on chondroitin sulfate proteoglycan aggrecan in perineuronal nets from cerebral cortices of SAMP10 mice: a model for age-dependent neurodegeneration. J Neurosci Res. 2008;86:1316–1323. doi: 10.1002/jnr.21582. [DOI] [PubMed] [Google Scholar]
- 101.Saitoh Y, Chiba Y, Shimada A. Aging and proteoglycans. In: Maeda N, editor. Neural proteoglycans. Kerala, India: Research Signpost; 2007. pp. 183–199. [Google Scholar]
- 102.Furukawa A, Oikawa S, Hasegawa-Ishii S, Chiba Y, Kawamura N, Takei S, Yoshikawa K, Hosokawa M, Kawanishi S, Shimada A. Proteomic analysis of aging brain in SAMP10 mouse: A model of age-related cerebral degeneration. Mech Ageing Dev. 2010;131:379–388. doi: 10.1016/j.mad.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Hwang IK, Yoo KY, Kim do H, Lee BH, Kwon YG, Won MH. Time course of changes in pyridoxal 5′-phosphate (vitamin B6 active form) and its neuroprotection in experimental ischemic damage. Exp Neurol. 2007;206:114–125. doi: 10.1016/j.expneurol.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 104.Soutar MP, Thornhill P, Cole AR, Sutherland C. Increased CRMP2 phosphorylation is observed in Alzheimer’s disease; does this tell us anything about disease development? Curr Alzheimer Res. 2009;6:269–278. doi: 10.2174/156720509788486572. [DOI] [PubMed] [Google Scholar]
- 105.Cairns NJ, Zhukareva V, Uryu K, Zhang B, Bigio E, Mackenzie IR, Gearing M, Duyckaerts C, Yokoo H, Nakazato Y, Jaros E, Perry RH, Lee VM, Trojanowski JQ. alpha-Internexin is present in the pathological inclusions of neuronal intermediate filament inclusion disease. Am J Pathol. 2004;164:2153–2161. doi: 10.1016/s0002-9440(10)63773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 107.Harman D. Aging: overview. Ann N Y Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 108.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 109.Chiba Y, Shimada A, Kumagai N, Yoshikawa K, Ishii S, Furukawa A, Takei S, Sakura M, Kawamura N, Hosokawa M. The senescence-accelerated mouse (SAM): a higher oxidative stress and age-dependent degenerative diseases model. Neurochem Res. 2009;34:679–687. doi: 10.1007/s11064-008-9812-8. [DOI] [PubMed] [Google Scholar]
- 110.Sasaki T, Unno K, Tahara S, Shimada A, Chiba Y, Hoshino M, Kaneko T. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell. 2008;7:459–469. doi: 10.1111/j.1474-9726.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 111.Unno K, Takabayashi F, Kishido T, Oku N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10) Exp Gerontol. 2004;39:1027–1034. doi: 10.1016/j.exger.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 112.Kishido T, Unno K, Yoshida H, Choba D, Fukutomi R, Asahina S, Iguchi K, Oku N, Hoshino M. Decline in glutathione peroxidase activity is a reason for brain senescence: consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology. 2007;8:423–430. doi: 10.1007/s10522-007-9085-7. [DOI] [PubMed] [Google Scholar]
- 113.Brunk UT, Jones CB, Sohal RS. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res. 1992;275:395–403. doi: 10.1016/0921-8734(92)90042-n. [DOI] [PubMed] [Google Scholar]
- 114.Numata T, Saito T, Maekawa K, Takahashi Y, Saitoh H, Hosokawa T, Fujita H, Kurasaki M. Bcl-2-linked apoptosis due to increase in NO synthase in brain of SAMP10. Biochem Biophys Res Commun. 2002;297:517–522. doi: 10.1016/s0006-291x(02)02155-1. [DOI] [PubMed] [Google Scholar]
- 115.Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 2000;885:25–31. doi: 10.1016/s0006-8993(00)02883-3. [DOI] [PubMed] [Google Scholar]
- 116.Kumagai N, Chiba Y, Hosono M, Fujii M, Kawamura N, Keino H, Yoshikawa K, Ishii S, Saitoh Y, Satoh M, Shimada A, Hosokawa M. Involvement of pro-inflammatory cytokines and microglia in an age-associated neurodegeneration model, the SAMP10 mouse. Brain Res. 2007;1185:75–85. doi: 10.1016/j.brainres.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 117.Hasegawa-Ishii S, Takei S, Chiba Y, Furukawa A, Umegaki H, Iguchi A, Kawamura N, Yoshikawa K, Hosokawa M, Shimada A. Morphological impairments in microglia precede age-related neuronal degeneration in senescence-accelerated mice. Neuropathology. 2011;31:20–28. doi: 10.1111/j.1440-1789.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- 118.Sakai H, Saku T, Kato Y, Yamamoto K. Quantitation and immunohistochemical localization of cathepsins E and D in rat tissues and blood cells. Biochim Biophys Acta. 1989;991:367–375. doi: 10.1016/0304-4165(89)90130-x. [DOI] [PubMed] [Google Scholar]
- 119.Yagi H, Irino M, Matsushita T, Katoh S, Umezawa M, Tsuboyama T, Hosokawa M, Akiguchi I, Tokunaga R, Takeda T. Spontaneous spongy degeneration of the brain stem in SAM-P/8 mice, a newly developed memory-deficient strain. J Neuropathol Exp Neurol. 1989;48:577–590. doi: 10.1097/00005072-198909000-00008. [DOI] [PubMed] [Google Scholar]
- 120.Carp RI, Meeker HC, Chung R, Kozak CA, Hosokawa M, Fujisawa H. Murine leukemia virus in organs of senescence-prone and -resistant mouse strains. Mech Ageing Dev. 2002;123:575–584. doi: 10.1016/s0047-6374(01)00377-3. [DOI] [PubMed] [Google Scholar]
- 121.Kim BH, Meeker HC, Shin HY, Kim JI, Jeong BH, Choi EK, Carp RI, Kim YS. Physiological properties of astroglial cell lines derived from mice with high (SAMP8) and low (SAMR1, ICR) levels of endogenous retrovirus. Retrovirology. 2008;5:104. doi: 10.1186/1742-4690-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jeong BH, Jin JK, Choi EK, Lee EY, Meeker HC, Kozak CA, Carp RI, Kim YS. Analysis of the expression of endogenous murine leukemia viruses in the brains of senescence-accelerated mice (SAMP8) and the relationship between expression and brain histopathology. J Neuropathol Exp Neurol. 2002;61:1001–1012. doi: 10.1093/jnen/61.11.1001. [DOI] [PubMed] [Google Scholar]
- 123.Kay DG, Gravel C, Robitaille Y, Jolicoeur P. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc Natl Acad Sci U S A. 1991;88:1281–1285. doi: 10.1073/pnas.88.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gravel C, Kay DG, Jolicoeur P. Identification of the infected target cell type in spongiform myeloencephalopathy induced by the neurotropic Cas-Br-E murine leukemia virus. J Virol. 1993;67:6648–6658. doi: 10.1128/jvi.67.11.6648-6658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clase AC, Dimcheff DE, Favara C, Dorward D, McAtee FJ, Parrie LE, Ron D, Portis JL. Oligodendrocytes are a major target of the toxicity of spongiogenic murine retroviruses. Am J Pathol. 2006;169:1026–1038. doi: 10.2353/ajpath.2006.051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jorgensen MB, Finsen BR, Jensen MB, Castellano B, Diemer NH, Zimmer J. Microglial and astroglial reactions to ischemic and kainic acid-induced lesions of the adult rat hippocampus. Exp Neurol. 1993;120:70–88. doi: 10.1006/exnr.1993.1041. [DOI] [PubMed] [Google Scholar]
- 127.Hasegawa-Ishii S, Takei S, Inaba M, Umegaki H, Chiba Y, Furukawa A, Kawamura N, Hosokawa M, Shimada A. Defects in cytokine-mediated neuroprotective glial responses to excitotoxic hippocampal injury in senescence-accelerated mouse. Brain Behav Immun. 2011;25:83–100. doi: 10.1016/j.bbi.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 128.Gessani S, Belardelli F. IFN-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998;9:117–123. doi: 10.1016/s1359-6101(98)00007-0. [DOI] [PubMed] [Google Scholar]
- 129.Kawanokuchi J, Mizuno T, Takeuchi H, Kato H, Wang J, Mitsuma N, Suzumura A. Production of interferon-gamma by microglia. Mult Scler. 2006;12:558–564. doi: 10.1177/1352458506070763. [DOI] [PubMed] [Google Scholar]
- 130.Wang X, Suzuki Y. Microglia produce IFN-gamma independently from T cells during acute toxoplasmosis in the brain. J Interferon Cytokine Res. 2007;27:599–605. doi: 10.1089/jir.2006.0157. [DOI] [PubMed] [Google Scholar]
- 131.Wang Y, Zhou CF. Involvement of interferon-gamma and its receptor in the activation of astrocytes in the mouse hippocampus following entorhinal deafferentation. Glia. 2005;50:56–65. doi: 10.1002/glia.20152. [DOI] [PubMed] [Google Scholar]
- 132.Amin DN, Rottenberg ME, Thomsen AR, Mumba D, Fenger C, Kristensson K, Buscher P, Finsen B, Masocha W. Expression and role of CXCL10 during the encephalitic stage of experimental and clinical African trypanosomiasis. J Infect Dis. 2009;200:1556–1565. doi: 10.1086/644597. [DOI] [PubMed] [Google Scholar]
- 133.Bhowmick S, Duseja R, Das S, Appaiahgiri MB, Vrati S, Basu A. Induction of IP-10 (CXCL10) in astrocytes following Japanese encephalitis. Neurosci Lett. 2007;414:45–50. doi: 10.1016/j.neulet.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 134.Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 135.Bakhiet M, Mousa A, Seiger A, Andersson J. Constitutive and inflammatory induction of alpha and beta chemokines in human first trimester forebrain astrocytes and neurons. Mol Immunol. 2002;38:921–929. doi: 10.1016/s0161-5890(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 136.Carr DJ, Chodosh J, Ash J, Lane TE. Effect of anti-CXCL10 monoclonal antibody on herpes simplex virus type 1 keratitis and retinal infection. J Virol. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang HK, Park UJ, Kim SY, Lee JH, Kim SU, Gwag BJ, Lee YB. Free radical production in CA1 neurons induces MIP-1alpha expression, microglia recruitment, and delayed neuronal death after transient forebrain ischemia. J Neurosci. 2008;28:1721–1727. doi: 10.1523/JNEUROSCI.4973-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, Kovac AD, Gerard C, Boddeke HW, Nitsch R, Kettenmann H. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci. 2004;24:8500–8509. doi: 10.1523/JNEUROSCI.2451-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85:352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- 140.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 141.Ottonello L, Montecucco F, Bertolotto M, Arduino N, Mancini M, Corcione A, Pistoia V, Dallegri F. CCL3 (MIP-1alpha) induces in vitro migration of GM-CSF-primed human neutrophils via CCR5-dependent activation of ERK 1/2. Cell Signal. 2005;17:355–363. doi: 10.1016/j.cellsig.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 142.Kim SY, Choi YS, Choi JS, Cha JH, Kim ON, Lee SB, Chung JW, Chun MH, Lee MY. Osteopontin in kainic acid-induced microglial reactions in the rat brain. Mol Cells. 2002;13:429–435. [PubMed] [Google Scholar]
- 143.Choi JS, Kim HY, Cha JH, Choi JY, Lee MY. Transient microglial and prolonged astroglial upregulation of osteopontin following transient forebrain ischemia in rats. Brain Res. 2007;1151:195–202. doi: 10.1016/j.brainres.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 144.Wang X, Louden C, Yue TL, Ellison JA, Barone FC, Solleveld HA, Feuerstein GZ. Delayed expression of osteopontin after focal stroke in the rat. J Neurosci. 1998;18:2075–2083. doi: 10.1523/JNEUROSCI.18-06-02075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meller R, Stevens SL, Minami M, Cameron JA, King S, Rosenzweig H, Doyle K, Lessov NS, Simon RP, Stenzel-Poore MP. Neuroprotection by osteopontin in stroke. J Cereb Blood Flow Metab. 2005;25:217–225. doi: 10.1038/sj.jcbfm.9600022. [DOI] [PubMed] [Google Scholar]
- 146.Schroeter M, Zickler P, Denhardt DT, Hartung HP, Jander S. Increased thalamic neurodegeneration following ischaemic cortical stroke in osteopontin-deficient mice. Brain. 2006;129:1426–1437. doi: 10.1093/brain/awl094. [DOI] [PubMed] [Google Scholar]
- 147.Ellison JA, Velier JJ, Spera P, Jonak ZL, Wang X, Barone FC, Feuerstein GZ. Osteopontin and its integrin receptor alpha(v)beta3 are upregulated during formation of the glial scar after focal stroke. Stroke. 1998;29:1698–1706. doi: 10.1161/01.str.29.8.1698. discussion 1707. [DOI] [PubMed] [Google Scholar]
- 148.Lin YH, Huang CJ, Chao JR, Chen ST, Lee SF, Yen JJ, Yang-Yen HF. Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin-3 or granulocyte-macrophage colony-stimulating factor. Mol Cell Biol. 2000;20:2734–2742. doi: 10.1128/mcb.20.8.2734-2742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maetzler W, Berg D, Funke C, Sandmann F, Stunitz H, Maetzler C, Nitsch C. Progressive secondary neurodegeneration and microcalcification co-occur in osteopontin-deficient mice. Am J Pathol. 2010;177:829–839. doi: 10.2353/ajpath.2010.090798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bausch SB. Potential roles for hyaluronan and CD44 in kainic acid-induced mossy fiber sprouting in organotypic hippocampal slice cultures. Neuroscience. 2006;143:339–350. doi: 10.1016/j.neuroscience.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 151.Borges K, McDermott DL, Dingledine R. Reciprocal changes of CD44 and GAP-43 expression in the dentate gyrus inner molecular layer after status epilepticus in mice. Exp Neurol. 2004;188:1–10. doi: 10.1016/j.expneurol.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 152.Ries A, Goldberg JL, Grimpe B. A novel biological function for CD44 in axon growth of retinal ganglion cells identified by a bioinformatics approach. J Neurochem. 2007;103:1491–1505. doi: 10.1111/j.1471-4159.2007.04858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hasegawa S, Yamaguchi M, Nagao H, Mishina M, Mori K. Enhanced cell-to-cell contacts between activated microglia and pyramidal cell dendrites following kainic acid-induced neurotoxicity in the hippocampus. J Neuroimmunol. 2007;186:75–85. doi: 10.1016/j.jneuroim.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 154.Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K. Microglia provide neuroprotection after ischemia. FASEB J. 2006;20:714–716. doi: 10.1096/fj.05-4882fje. [DOI] [PubMed] [Google Scholar]
- 155.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ban E, Haour F, Lenstra R. Brain interleukin 1 gene expression induced by peripheral lipopolysaccharide administration. Cytokine. 1992;4:48–54. doi: 10.1016/1043-4666(92)90036-q. [DOI] [PubMed] [Google Scholar]
- 157.Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 158.Van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- 159.van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- 160.Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- 161.Serrats J, Schiltz JC, Garcia-Bueno B, van Rooijen N, Reyes TM, Sawchenko PE. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nataf S, Strazielle N, Hatterer E, Mouchiroud G, Belin MF, Ghersi-Egea JF. Rat choroid plexuses contain myeloid progenitors capable of differentiation toward macrophage or dendritic cell phenotypes. Glia. 2006;54:160–171. doi: 10.1002/glia.20373. [DOI] [PubMed] [Google Scholar]
- 163.Chinnery HR, Ruitenberg MJ, McMenamin PG. Novel characterization of monocyte-derived cell populations in the meninges and choroid plexus and their rates of replenishment in bone marrow chimeric mice. J Neuropathol Exp Neurol. 2010;69:896–909. doi: 10.1097/NEN.0b013e3181edbc1a. [DOI] [PubMed] [Google Scholar]
- 164.Prodinger C, Bunse J, Kruger M, Schiefenhovel F, Brandt C, Laman JD, Greter M, Immig K, Heppner F, Becher B, Bechmann I. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445–458. doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 165.Kipnis J, Derecki NC, Yang C, Scrable H. Immunity and cognition: what do age-related dementia, HIV-dementia and ‘chemo-brain’ have in common? Trends Immunol. 2008;29:455–463. doi: 10.1016/j.it.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 166.Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 167.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cohen N, Kinney KS. Exploring the phylogenetic history of neural-immune system interactions: an update. In: Ader R, editor. Psychoneuroimmunology. 4th edn. Amsterdam: Elsevier; Academic Press; 2007. pp. 1–38. [Google Scholar]