Abstract
In the elderly patient population, it has become increasingly evident that immune dysregulation is a contributing factor to age-related pathologies and their associated morbidity and mortality. In particular, elderly subjects are plagued by poor responses to infectious challenge and immunization and are at heightened risk for the development of autoimmune, neuroinflammatory and tumor-associated pathologies. Rodent models of aging and age-related disorders have been utilized to better describe how innate immune cell dysfunction contributes to these clinical scenarios. As the elderly population continues to increase in size, use of these aging rodent models to study immune dysregulation may translate into increased healthy living years for these individuals.
Keywords: Inflammation, Neutrophils, Macrophages, Dendritic Cells, Natural Killer Cells, Natural Killer T Cells
In the elderly population, individuals become more susceptible to opportunistic bacterial and viral infections and have an increased incidence of autoimmune diseases and malignancy [1–3]. A predominate contributing factor to these clinical ailments is dysfunction of the immune system. In particular, dysregulation of the innate immune response contributes directly to aging pathologies, as well as indirectly by altering activation of the adaptive arm of the immune response. In aged individuals, there is a paradoxical activation of baseline inflammation referred to as “inflamm-aging” coupled with a refractory response to immune challenge known as “immunosenescence” (reviewed in [3–6]). With advanced age, the inability to balance pro- and anti-inflammatory responses generates a basal elevation in pro-inflammatory mediators that may blunt appropriate innate and adaptive cellular immune activation and function. This disrupted balance between inflammation and immune activation may contribute to a variety of co-morbidities and increased mortality following both local and systemic insults [7–11]. Use of rodent models allows examination of how advanced age impacts basic innate immune cell biology. Further, clinically relevant rodent models of trauma and tissue injury, infection, tumor immunology and autoimmunity highlight the role of innate immune cell subsets in age-related pathologies. Herein, we discuss dysregulation of neutrophil, macrophage, dendritic cell (DC), natural killer (NK) cell and natural killer T (NKT) cell function in rodent models, and how age-associated alterations in these innate immune cell subsets can negatively impact the host immune response and contribute to poor clinical outcomes.
Rodent Models of Aging: Animal Strains and Age Ranges
In the rodent aging literature, a variety of animal strains and respective ages are often utilized. In murine studies, the majority of aging research is conducted in C57/BL6, BALB/c or CBA mice, and typically young mice are 2–4 months old and aged mice are between 18–24 months [12–18]. However, other investigators have utilized aged mice less than 18 or greater than 24 months of age [19–22]. Though fewer aging and innate immune studies have been conducted in rats, rat models of aging characteristically use inbred F344 rats or outbred Wistar rats. In these studies, young rats were typically between 1–3 months of age, with a wider range between 9–24 months being considered an aged animal [23–26]. Basal differences in animal strains and designated age ranges may contribute to the inconsistent outcomes observed in studies regarding innate immune cell number or function. In turn, these baseline variations can be exacerbated in in vivo model systems of injury and trauma, infection, tumorigenesis and autoimmunity, potentially cofounding outcomes. When examining the aging literature, it is important to take these inconsistencies into account in data analysis and interpretation. As we discuss aging and innate immune studies in rodent models, we will highlight where difference in rodent models may contribute to divergent data.
Neutrophils
Neutrophils are a critical first line of defense for the innate arm of the immune system in response to infectious challenge. Following recruitment, activated neutrophils phagocytose pathogens and cellular debris, generate reactive oxygen species (ROS) and release a variety of antimicrobial peptides, pro-inflammatory cytokines and hydrolytic enzymes [27–30]. While a multitude of human studies not discussed here demonstrate cellular changes in neutrophil functions such as phagocytosis and ROS production with advanced age [6, 31–33], differences are not as robust in rodent research models. However, many rodent studies do elucidate how age-related differences in the neutrophil population contribute to the host response to trauma, infection and tissue injury [12, 14, 17, 34, 35].
Neutrophil Differentiation
With increased age, there is a shift in the hematopoietic stem cell (HSC) pool towards generation of myeloid lineage cells, including neutrophils (reviewed in [36]). To extend these findings, Miyamoto et al. examined the impact of forkhead box O3a transcription factor (FoxO3a) in regulation of HSC differentiation in aged mice [37]. The family of FoxO transcription factors mediate many cellular processes [38], and have been shown to be a player in HSC differentiation via modulation of oxidative stress [38]. Considering the contribution of oxidative stress to immune dysregulation with age (reviewed in [39]), the impact of this particular pathway in the maintenance of the circulating neutrophil pool is of interest. Data from this group demonstrated that during recovery from myelosuppressive treatments, aged FoxO3a deficient mice exhibited profound neutrophilia compared to young knockout mice [37]. This increase in neutrophils was associated with loss of inhibition of FoxO3a on Sprouty-related Ena/VASP homology 1 domain containing proteins 2 (Spred2), a negative regulator of cell proliferation, as well as activation of AKT and extracellular signal-related kinase (ERK) in the aged knockouts. This study provides novel insight into maintenance of the HSC pool in aging, and extensions of these studies to examine the affect of age on expression of the FoxO family of transcription factors may further characterize the hematopoietic disruption and altered neutrophil development and function observed in aged animals.
Trauma and Tissue Injury
In the setting of systemic insults, such as those seen following traumatic injury or sepsis, an over exuberant neutrophil response may contribute to the systemic immune response syndrome (SIRS), and the associated morbidity and mortality in aging patients [40–42]. Since the age of the patient is a major clinical predictor of outcome following trauma, several murine studies have investigated how neutrophils as early immune mediators may be contributing to poor clinical outcomes after major trauma and tissue injury [11, 12, 14, 17]. Our laboratory has previously shown that aged mice exhibit increased mortality following a 15% total body surface area (TBSA) full-thickness burn trauma as compared to young mice subjected to the same injury [11]. These aged mice have elevated levels of the neutrophil chemokine CXCL1 in lung tissue which translated into heightened neutrophil infiltration and pulmonary inflammation 24 hours following burn injury [14]. Administration of anti-CXCR2 antibody in aged mice reduced levels of neutrophil accumulation and pulmonary inflammation to levels observed in young mice [14], perhaps suggesting that modulating the neutrophil response following systemic insult may decrease pulmonary co-morbidities.
In the setting of local tissue injury, alterations in neutrophil infiltration may also play a role in resolution. Following cutaneous tissue injury, neutrophils are the first phagocyte to enter the wound bed, helping to incite an inflammatory response against invading organisms. In aged BALB/c mice, Swift et al. observed a significant delay in wound closure as reported in other studies [12, 17, 43, 44]. In this study age-related differences in neutrophil recruitment to the site of cutaneous injury were observed at early time points, they did not reach significance [17]. Conversely, Nishio et al. demonstrated that neutrophil peak infiltration was delayed in aged C57BL/6 mice following cutaneous injury [12]. Moreover, Gr-1 mediated neutrophil depletion further delayed wound closure in aged mice. Systemic administration of granulocyte-colony stimulation factor (G-CSF) or topical application of peritoneal-derived neutrophils from young mice restored rates of wound closure to those observed in young mice [12]. In the setting of cutaneous wound infection, our laboratory has observed that aged BALB/c mice exhibit increased bacterial colonization at the wound site and that this correlated with decreased neutrophil accumulation (AL Brubaker and EJ Kovacs, unpublished observations). Moreover, aged mice exhibit increased CXCL1 and CXCL2 levels in wound homogenates relative to the percentage of neutrophil recruitment to the wound bed, suggesting that aged mice may require a stronger chemotatic stimulus to mediate a similar migratory response (AL Brubaker and EJ Kovacs, unpublished observations).
Though fetal or adult wound healing studies often consider neutrophil recruitment detrimental to wound closure and scar formation [45], these studies highlight how findings in young model organisms are not always directly applicable to the aged organism. Moreover, the differing results in these studies reflect a major caveat in rodent aging studies, as different animal strains can lead to alternative outcomes. Future in vivo rodent models and injury paradigms should be evaluated to determine their relative correlation with findings in human studies to better optimize rodent aging research. Though additional studies need to be conducted to better establish the role of neutrophils in wound healing and trauma in aged animals, the impact of age on neutrophil infiltration kinetics and function may contribute to adverse clinical outcomes such as suboptimal healing and infectious complications in the elderly.
Infection and Environmental Exposures
Several other studies have examined the role of neutrophils in aged rodents following infectious challenge or environmental exposures [15, 25, 34, 35, 46]. In one such study, exacerbated lipopolysaccharide (LPS)-induced pulmonary inflammation in aged mice was correlated with increased levels of CXCL1, CXCL2, IL-1β and prolonged pulmonary neutrophilia at 72 hours [46]. In another model of pulmonary challenge, intranasal infection with Francisella tularensis lead to an altered lung inflammatory response, with a delay in production of neutrophil chemokines CXCL2 and CXCL6 and attenuated neutrophil infiltration into the lung tissue in aged mice [34]. Moreover, environmental insults, such as air pollutants or cigarette smoke, contribute to airway inflammation and chronic pulmonary co-morbidities [47, 48]. Aged mice subjected to inhaled diesel exhaust demonstrate enhanced pulmonary congestion and neutrophilia 24 hours after exposure as compared to young mice [35]. Similarly, single or chronic cigarette smoke exposure in aged mice resulted in elevated levels of CXCL1 and CXCL2, and prolonged neutrophil recruitment to the lung [49]. This age-related increase in cytokine production was associated with amplified nuclear factor (NF)-κb expression and nuclear translocation [49]. Looking at these findings, in response to pulmonary challenge, aged mice have an elevated and/or prolonged neutrophil response. Considering the delicate lung alveolar architecture and the highly hydrolytic enzymatic degranulation products of activated neutrophils, this may contribute to excessive tissue damage and reduced lung function over time.
The role of neutrophils in other models of infection has also garnered interesting data. Following oral infection with Salmonella Typhimurium, aged mice have elevated bacterial colonization in the ileum, colon, Peyer’s patches, mesenteric lymph nodes (MLN) and liver as compared to young mice [15]. Despite a baseline elevation in neutrophil numbers in the spleen and MLN of aged mice, aged mice did not increase neutrophil numbers in response to infection whereas young mice mounted a more significant neutrophil response [15]. Following challenge with the fungal pathogen Candida albicans, decreases in neutrophil recruitment to the peritoneal cavity were associated with reduced fungicidal activity in aged mice [50], again highlighting how neutrophil recruitment may be dysregulated with advanced age.
Combined, these studies offer significant evidence suggesting that neutrophil infiltration kinetics are dysregulated with aging. This dysfunction may have pleiotropic effects on the host depending on the inciting injury or clinical paradigm by promoting pathogen dissemination, compromising organ function and delaying time to recovery. While delayed neutrophil infiltration may impair the ability of the innate immune response to contain microbes to site of origin, prolonged neutrophilia can result in significant tissue damage and inflammation via potent neutrophil hydrolytic enzymes, demonstrating the importance in precise temporal regulation of this particular immune cell subset. Considering the relative abundance of this cell type in humans as compared to rodents, differences in this cell population in rodents may be further exaggerated in human studies.
Macrophages
Macrophages play pivotal roles in modulating immune function following infection, tissue injury and in tumor cytotoxity [51–53]. Following extravasation from the peripheral blood pool, monocytes undergo maturation into tissue macrophages. As professional antigen presenting cells, macrophages help shape the innate and adaptive immune responses through the release of a variety of pro-inflammatory mediators at early time points following injury or infection. These cells also serve to dampen the inflammatory response through phagocytosis of apoptotic cells and secretion of soluble factors like IL-10 [54]. Age-related alterations in macrophage number and function have been correlated with enhanced susceptibility to infection, differential responses to tissue injury and enhanced tumor progression in rodent models [50, 55–57].
Macrophage Population Dynamics
With age, there is some discrepancy regarding changes in cell numbers in rodent models [15, 57, 58]. Some studies suggest that there is an increase in the Mac1/CD11b population in aged murine bone marrow [59], though others have found there were no differences in the number or DNA content of developing macrophages between young and aged mice [58]. In other cellular compartments, aged mice have been reported to have increased resident macrophages in the spleen [15], though there is a reduction in the marginal zone macrophage population and a breakdown of the marginal zone architecture with advanced age [57]. This collapse in marginal zone structure may impair clearance of circulating pathogens and follicular responses in the elderly, increasing susceptibility to infection and prolonging recovery. Though rodent studies have yet to generate a conclusive answer regarding macrophage numbers in various compartments, alterations in macrophage environmental distribution may generate organ specific dysregulation. For example, increased infiltration of macrophages into adipose tissue of aged C57/BL6 mice fed high caloric diets was associated with increased inflammasome activation and cytokine production [60]. These data suggest that a tissue-specific shift in macrophage populations can contribute to basal differences in the inflammatory profile of aged individuals.
Cytokine production and TLR Signaling
In rodent models, advance aged is associate with increased levels of circulating cytokines, in particular IL-6, and it has been speculated that these pro-inflammatory mediators may alter immune function in aged animals [11, 13, 23, 61]. Despite this basal elevation, challenge of bone-marrow derived macrophages with Porphyromonas gingivalis (P. gingivalis) produce significantly less TNF-α and IL-6 in aged mice [62]. Similarly, in response to LPS, splenic macrophages from aged wild type mice demonstrated diminished responsiveness to LPS stimulation, producing lower levels of TNF-α, IL-1β, IL-6 and IL-12 as compared to young controls [63]. Interestingly, cytokine production was restored following in vitro culture of LPS with splenic macrophages from aged IL-6 deficient mice [63], suggesting that elevated systemic IL-6 may directly impair macrophage function in aged animals. Several studies also support a similar reduction in cytokine production, including TNF-α, IL-6, IFN-γ, nitric oxide (NO), monocyte chemoattractant protein-1 (CCL2) and macrophage inflammatory protein-1α (CCL3), following a variety of stimuli [15, 50, 62, 64]. Generation of cytokines is often examined following stimulation of a variety of Toll-like receptors (TLRs). TLRs help initiate the innate immune response to a variety pathogen-associated molecular patterns (PAMPs), and signal through either MyD88-dependent or -independent pathways to promote NF-κB pro-inflammatory cytokine production. Reduced expression of these receptors, or alterations in their respective downstream pathways, can increase susceptibility to infection [65–67], and a number of studies demonstrate alterations in the macrophage TLR profile in aged animals. In both splenic and peritoneal macrophages of aged C57/BL6 mice, decreased expression in all TLRs (TLR1-9) have been found [50, 68], though other studies report no difference in expression of some TLRs, including TLR2 and TLR4, in aged BALB/c mice [19, 56, 69]. Again, these studies highlight murine strain-dependent differences that must be taken into consideration when working with rodent models. Determining which rodent models are most relevant to findings ascertained in human studies will help guide and streamline future animal research efforts.
While divergent results regarding TLR expression may be due to purity of the isolated macrophage population or murine background strain, the pathways downstream of TLRs may also be impaired with age. In one such study, LPS or zymosan induced TNF-α and IL-6 production was attenuated in splenic macrophages from aged mice due to reduced activation of p38 mitogen-activated protein kinase (MAPK), MAPK-activated protein kinase-2 and NF-κB [69]. Others have shown that thioglycollate-elicited peritoneal macrophages from aged mice exposed to P. gingivalis have increased gene expression of the single immunoglobulin interleukin-1-related receptor (SIGIRR) which acts to inhibit TLR MyD88-dependent signaling [19, 70]. In aged rodent models, downregulation of other members of these pathways, like MyD88 and tumor necrosis factor receptor-associated factor 6 (TRAF6), lead to decreased activation of the NF-κB family of transcription factors [25, 71]. Downregulation of mediators of these pathways would dampen the immune response, and may account for the decrease in cytokine production seen in aged rodent macrophages following immunogenic challenge.
ROS/RNI Generation and Macrophage Phenotype
Another major mediator of macrophage effector function is the generation of ROS and reactive nitrogen intermediates (RNI) that aid in intracellular microbicidal activity and tumor cytotoxicity. ROS and RNI, alongside IL-1, IL-12, TNF-α, represent the pro-inflammatory M1 macrophage profile, and these cells function in driving Th1 responses, resisting tumor development and killing of intracellular foreign pathogens [54]. On the other hand, M2, or anti-inflammatory, macrophages have elevated levels of IL-10 and arginase expression. M2 macrophages predominately function in immunoregulation, neovascularization, tissue remodeling and tumor growth [54, 72]. Macrophages from aged mice have been shown to produce elevated levels of IL-10 and reduced FasL, IL-12 and TNF-α, mediators that contribute to an M2 anti-inflammatory profile [73]. Several studies have also reported decreases in ROS or RNI generation with age that may represent a skewing of M1 and M2 polarization with age [24, 55, 74]. As compared to young animals, alveolar macrophages from senescent rats demonstrated a decreased baseline generation of ROS and nitric oxide (NO), and LPS treatment did not significantly change ROS and NO production in aged animals [24]. In response to phorbol 12-myristate 13-acetate (PMA) or zymosan, peritoneal macrophages from aged mice produce 50% less hydrogen peroxide (H2O2) in contrast to cells from young animals [74]. These studies are supported by previous studies in which NO production by splenic or peritoneal macrophages was reduced in several mouse strains following LPS or peptidoglycan-polysaccharide [75]. In addition to these findings, macrophages from aged mice exhibit reduced granulocyte-monocyte colony stimulating factor (GM-CSF) mediated cell proliferation following exposure to H2O2 due to diminished signal transducer and activator of transcription (STAT) 5a oxidation and phosphorylation, suggesting that aging increases macrophage susceptibility to oxidative stress [76].
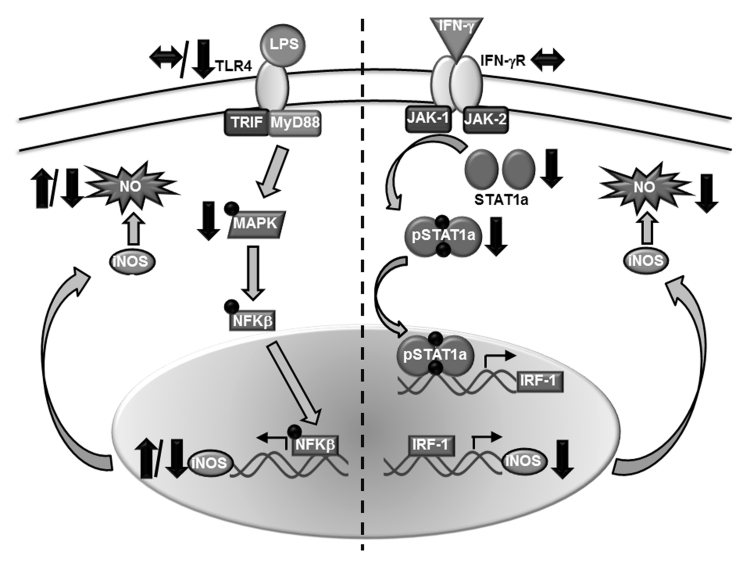
NO production is modulated by enzymatic activity of inducible nitric oxide synthase (iNOS) via activation of the interferon-gamma (IFN-γ) receptor. In aged BALB/c mice, mRNA expression of iNOS was found to be decreased after stimulation with LPS as compared to young mice [75]. Similarly, our laboratory has observed decreased iNOS expression in splenic adherent cells from aged BALB/c mice following stimulation with IFN-γ and TNF-α [77]. As macrophage signaling through the IFN-γ receptor promotes ROS and RNI generation, several studies examined if alterations in this receptor and the downstream signaling pathway may exist with advanced age [26, 74, 78]. Though there are no differences in IFN-γ receptor with age [74, 78], culture with IFN-γ reduced H2O2 and NO2− production in aged animals due to decreased phosphorylation of MAPK [74]. Others also found decreased STAT1a gene and protein expression and reduced STAT1 phosphorylation after IFN-γ stimulation in aged BALB/c mice [78]. While this reduction in iNOS and NO production may suggest suppression of M1 macrophage phenotype with age, our lab has also observed a reduction in arginase-1 expression after IL-4 stimulation in adherent splenocytes from aged mice [77], which may imply a global immunosuppression of macrophage function. Together, these studies suggest that the ROS and RNI pathways are dysregulated with age, translating into potential differences in macrophage phenotype and intracellular killing capacity and ultimately negatively impacting immune function in the elderly (Figure 1).
Figure 1. Effect of age on the macrophage iNOS pathway.
Macrophage iNOS signaling is imperative in generation of reactive nitrogen species to kill bacterial and viral pathogens, as well as a marker of macrophage polarization. Following stimulation of TLR4, activation of MAPK and NFκB modulate iNOS expression. With advanced aged, reports are still inconclusive regarding iNOS activation and NO production after stimulation with TLR4 ligands, like LPS. Activation of the IFN-γ pathway results in phosphorylation and translocation of STAT1a, initiating IRF-1 transcription and subsequent iNOS expression. In aging rodents, studies suggest that reduction in NO production and iNOS transcription may be due to decreases in STAT1a levels and phosphorylation.
Contrary to these reports, Chen et al. found enhanced NO production in both thioglycollate-derived and resident peritoneal macrophages from inbred CBA/CA aged mice following culture with LPS, zymosan or heat-killed Staphylococcus aureus [16]. In these animals, elevated NO production was correlated with a persistent increase in iNOS expression. Yet another study found no difference in iNOS induction, NO production or intracellular killing in thioglycollate-elicited macrophages from aged BALB/cByJ mice in response to in vitro stimulation with P. gingivalis [19]. These studies highlight that a variety of factors, including the murine strain, macrophage population and inciting stimuli, all play are role in determining potential age-related differences in macrophage function in rodent models.
Tissue Injury
As temporal changes in macrophage phenotype have recently been implicated in wound resolution, alteration in macrophage phenotype and function in aged animals may provide insight into altered tissue repair with advanced age [17, 44, 79]. In particular, in murine models of tissue injury, aged animals have a prolonged course of wound repair, associated with delays in re-epithelialization, neovascularization and restoration of the extracellular matrix [43]. Following excisional wound injury, macrophage accumulation was elevated 56% in aged mice as compared to young, and this correlated with increased CCL2 production [17]. Macrophages isolated from polyvinyl alcohol sponges implanted subcutaneously in aged mice demonstrated an approximate 40% decrease in phagocytosis as compared to young mice [17]. These delays in wound closure and phagocytic ability of wound macrophages may contribute to the increased susceptibility to wound infection seen in elderly patients [80–82]. Additionally, reduced macrophage phagocytosis may impair the ability to clear apoptotic keratinocytes or neutrophils from the wound bed, further delaying wound repair. Aprahamian et al. recently demonstrated that following ultraviolet B irradiation, aged mice demonstrated an elevated percentage of apoptotic keratinocytes that was later correlated with reduced phagocytic capacity and apoptotic cell clearance [83]. These findings may provide the foundation for improving our understanding of poor clinical outcomes following tissue injury with aging, and may lead to design of therapeutic interventions to enhance macrophage function and accelerate wound closure.
Tumor Immunity
Deficits in macrophage function also directly contribute to the increased incidence of neoplastic growth and metastasis in elderly patients. In tumor immunology, recruitment of tumor associated macrophages (TAMs) results in the release of growth factors, proteases and inflammatory mediators, promoting vascularization of tumor tissue and metastasis [84]. Infiltration of tumors by TAMs is associated with a poor prognosis [84], and age-related differences in TAM effector functions within tumors have been identified [85]. In a B16 intraocular tumor model, aged mice exhibit increased infiltration of M2-polarized macrophages in contrast to young mice. These macrophages had elevated expression of pro-angiogenic vascular epidermal growth factor (VEGF) and tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (TIE-2) [85]. Depletion of macrophages by subconjuctival injections of clodronate-containing liposomes reduced tumor growth in 86% of aged mice compared to only a 10% reduction in young animals. Interestingly, the M2 macrophages in tumors from aged mice shared some phenotypic characteristics of myeloid-derived suppressor cells from the monocytic lineage, namely Ly6-C [85], that were also subject to depletion by the methods used above. In addition to TAM recruitment, macrophages from aged rodents have elevated cycloxygenase activity and prostaglandin E2 (PGE2) synthesis, mediators that have been recently implicated in tumorigenesis [86, 87]. Further work to better characterize TAMs with aging and cancer subtypes may aid in development of targeted cancer treatments for patients with poor prognoses due to TAM infiltration and subsequent neovascularization and metastasis.
Age-related differences in macrophage function described above highlight how alterations in macrophage phenotype and function can facilitate development of infection, delays in tissue repair and impairment of tumor regression. Utilizing targeted rodent models to increase our understanding of how macrophages can contribute to specific pathological conditions may position us to better understand disease states at the bedside.
Dendritic Cells
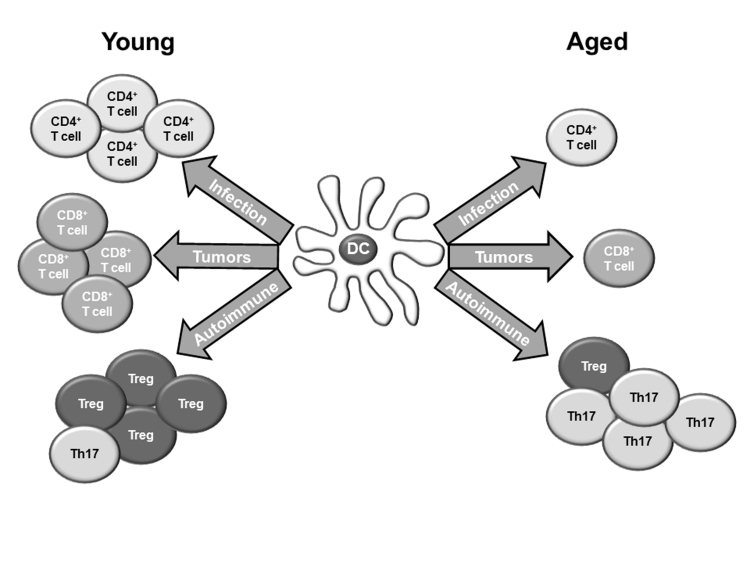
Dendritic cells (DCs) are unique mediators of the innate immune response, and their role in modulating T cell effector function in the context of aging is an expanding field of interest. Immature DCs are responsible for peripheral surveillance and phagocytosis of pathogens, and following antigenic uptake, these cell migrate to regional lymph nodes, stimulating T cell proliferation [4, 88]. DCs also play a pivotal role in establishment and maintenance of self tolerance through regulatory T cell (Treg) and Th17 induction [88–90]. Together, the dysregulation of one or more of the functions of DCs with advanced age can contribute to poor responses to infections and immunization, anti-tumor immunity and autoimmune pathology in the elderly (Figure 2).
Figure 2. Impact of age on DC-priming of the adaptive immune response.
DCs are critical in the crosstalk between the innate and adaptive immune response, helping to generate and regulate CD4+ T cell, CD8+ T cell, Treg and Th17 immune reactions. In response to infection or tumorigenesis, DCs from young animals are able to induce proliferation of CD4+ and CD8+ T cells, respectively. However, the ability to prime CD4+ and CD8+ T cells is dampened in aged mice, resulting in decreased proliferation. In the context of autoimmunity, DCs help maintain a balance between immunosuppressive Tregs and inflammatory Th17 cells. Disturbance of this equilibrium in aged mice may contribute to increase incidence of autoimmune pathologies with advanced age.
DC Differentiation and Surface Marker Expression
A multitude of murine aging studies on dendritic cell function utilize isolation of bone marrow from young and aged mice, followed by in vitro differentiation of these precursors into CD11c+ DCs [18, 91–93]. In one such study, Paula et al. demonstrated that following culture of bone marrow precursors from aged mice, the percentage and absolute number of DCs that differentiated into the mature phenotype (MCHIIhi, CD86hi) was reduced with a concomitant increase in immature DCs (MHCIIlo, CD86lo) in contrast to their young counterparts [93]. Importantly, this possible decrease in bone marrow DC maturation may contribute to age-related decreases in the DC populations in the epidermis [21], thymic tissue [94] and spleen [22], though other studies report no difference is DC subpopulations in murine bone marrow, blood or spleen [18].
Migration and Homing
The reported reduction in density of resident epidermal DCs, known as Langerhan’s cells (LC), in aged mice was also associated with functional losses [21]. Namely, the LC migratory capacity to home to regional lymph nodes in middle aged mice (6 months) was impaired in response to either oxalozone or fluorescein isothiocyanate [21]. TNF-α and IL-1β are inciting stimuli for LC mobilization from the epidermis to draining lymph nodes [95, 96] and intradermal administration of TNF-α, but not IL-1β, in middle aged mice resulted in an impaired migratory response [21]. While the authors attributed these differences to available IL-1β in the epidermal environment of middle aged mice, additional studies that evaluate the effect of these cytokines on receptor expression or cytoskeletal changes may prove beneficial. Moreover, extending these studies to examine the classical age ranges used in aging studies may provided a more definitive answer regarding the impact of age on LC migration. Another report on the homing potential of DCs found that following injection of equal numbers of fluorescently labeled mixed DCs from young and aged mice into young recipients, fewer DCs from aged mice migrated to regional lymph nodes [97]. Of particular importance in DC homing is CCR7, which mediates a chemotatic response to the migratory chemokines CCL19 and CCL21 that are concentrated in lymphoid tissues. Focusing on the CCR7 receptor, in vitro CCL21-driven chemotaxis of DCs from aged mice was diminished as compared to young mice despite adequate CCR7 expression, implying signaling downstream of CCR7 may be altered with age [97].
Activation of Cell Mediated Immunity and Infection
These defects in migration are further confounded by the ability of DCs to effectively engage T cells and promote proliferation in lymphoid organs [97, 98]. Following phagocytosis of a pathogen, DCs must present the antigenic epitope in the context of major histocompatibility complex (MHC) class II molecules along with co-stimulatory factors to induce cell-mediated immunity. Though no age-dependent differences in phagocytosis were observed in several studies [21, 93, 97], presentation of antigen in MHC class II was decreased in bone marrow DCs from aged mice [93], although no alterations were seen in MHC class I presentation [97]. Additionally, some studies report diminished surface receptor expression of MHC class II or co-stimulatory molecules CD40, CD80, CD86 and DC-SIGN correlates with reduced CD4+ T cell proliferation [20, 98–101], though others did not find differences in expression of either MHC class II or other co-stimulatory molecules [21, 102].
To examine the interplay between DCs and T cell proliferation with age, Moretto et al. utilized Encephalitozoon cuniculi (E. cuniculi), a parasite requiring T cell priming and cytotoxicity for eradication. As compared to young animals, aged mice exhibit increased mortality following E. cuniculi oral infection that was associated with reduced splenic and MLN T cell proliferation [20]. DCs isolated from MLNs of aged mice exposed to E. cuniculi in vitro failed to induce a significant proliferative response in T lymphocytes from young animals. However, E. cuniculi exposed DCs from young mice resulted in similar proliferation of T cells from young and aged mice, suggesting the defect in T cell proliferation was due to an age-related reduction in priming potential by the DC population [20]. These findings were supported by other studies in which DCs from young mice induced a four-fold increase in T cell proliferation as compared to aged mice [98] and the age-related lack of T cell proliferation was associated with decreases in MHC class II, CD40, CD80, CD86 and DC-SIGN expression [20, 97–99]. As DC-mediated T cell priming acts as a vital link between the innate and adaptive immune responses, defects in these interactions would decrease the ability of an individual to successfully combat infection and respond to vaccination, two problems common in elderly patients. In an effort to restore T cell priming by DCs, Moretto et al. added IL-15 to the co-culture systems, as IL-15 has been shown to play a role in DC-induced T cell proliferation and IL-15 mRNA was decreased in DCs from aged mice in their model. Restitution of IL-15 restored T cell proliferation and heightened DC expression of CD80 and CD86, suggesting a plausible vaccine adjuvant to generate a more robust immunization response in the elderly [20].
In addition to aiding the immune response to bacterial or parasitic antigens, DCs are major contributors to viral immunity. Endosomal recognition of common viral antigens such as single stranded RNA by TLR7 or unmethylated CpG islands by TLR9 triggers type 1 IFN production via the interferon regulatory factor 7 (IRF7) in DCs [103]. Stimulation of bone marrow isolated plasmacytoid DCs (pDCs, PDCA-1+, CD11clo/int, B220hi) from aged mice with either CpG sequences (TLR9), herpes simplex virus 2 (HSV2; TLR9), RNA40 (TLR7) or murine cytomegalovirus (MCMV; TLR7/9) resulted in decreased IFN-α production as compared to young mice [18]. These findings correlated with a failure to mount a pDC IFN-α response to in vivo CpG, HSV2 and MCMV inoculation and higher HSV2 or MCMV titers in aged mice. Reduced IFN-α levels were attributed to a failure to induce phosphatidylinostiol 3-kinase (PI3-kinase) mediated nuclear translocation of IRF7 in aged mice following TLR9 or IFN-αβ receptor activation [18]. This is in contrast to a study by Wong et al. in which total splenic DCs and splenic pDCs from aged mice demonstrated similar IFN-α production and stimulation of T cell proliferation following exposure to CpG sequences [22]. While these divergent results may be explained by the different murine background strains utilized and the tissue origin of isolated pDCs in the two studies, it may also suggest that DC function is maintained in certain immune compartments with age, such as the spleen, as seen in other studies [20]. Taken together, these studies demonstrate the inability of DCs from aged mice to adequately stimulate cell-mediated immunity in response to bacterial, parasitic or viral antigens. Future work focusing on modulation of DC and T cell interaction in the context of advanced age may lead to innovative therapeutic approaches to combat this heightened susceptibility to infection and diminished responsiveness to immunization observed in the elderly population.
Tumor Immunity
The inability to effectively generate cell-mediated immunity also extends into studies that examine the effect of aged DCs on CD8+ T cell proliferation and cytotoxicity in the setting of tumor growth and regression. In a B16-OVA melanoma tumor model, vaccination with ovalbumin (OVA) pulsed DCs from young mice resulted in enhanced percentage of OVA-specific splenic CD8+ T lymphocytes, increased cytotoxicity and elevated IFN-γ, TNF-α, IL-6 and IL-10 production compared to DCs from aged mice [104]. Other studies confirm these findings as transfer of OVA pulsed DCs from young mice into tumor-bearing recipients resulted in greater tumor reduction in vivo than in mice that received OVA pulsed DCs from aged mice [97]. The inability to reduce tumor burden was coupled with an age-dependent decrease in DC-induced proliferation of CD8+ T cells [97], implying that DC dysfunction with age may contribute to the elevated rates of tumorigenesis in the elderly.
Autoimmunity
Dysregulation of DC function may also contribute to autoimmune pathology mediated by affecting Treg or Th17 populations in aged patients. In the context of central tolerance, thymic DCs play a critical role in negative selection of highly reactive developing T lymphocytes as well as Treg induction [105]. With advanced age, three distinct thymic DC subsets demonstrate differential reductions that can be restored by sex steroid ablation [94]. Following depletion of sex hormones, the DC:thymocyte proportions were restored to levels seen in young mice and this coincided with generalized thymic growth [94]. Loss of thymic DCs would potentially increase the proportion of autoreactive cells in the periphery and contribute to the decrease in thymic Treg levels seen with age [100], leading to a potential increase in autoimmune pathology. Alternatively, elevations in Th17 cells have been documented with aging, and increases in this cell population are associated with autoimmune conditions such as inflammatory bowel disease and excessive inflammatory responses following infection [106–108]. Naïve T cells are driven toward a Th17 phenotype under the influence of DC produced IL-23 alongside transforming growth factor β and IL-6. In aged mice, TLR4 and/or TLR7/8 stimulation resulted in a pronounced 40-fold increase in IL-23 production from bone marrow derived DC in a PGE2 dependent manner [102]. This group also demonstrated that age-related elevations in IL-23 were due to heightened message of the p19 subunit of this cytokine [109]. Methylation differences between young and aged mice at H3K4 in DCs promoted c-Rel binding, elevating transcription of the p19 subunit of IL-23 and IL-23 production in aged mice [109], highlighting the role of epigenetic modifications in alterations of the aging innate immune system. Elevated production of IL-23 in DCs from aged mice is a potential mechanism by which T cell maturation is skewed towards a Th17 phenotype with age, increasing autoimmune conditions in the elderly. Interestingly, blocking of PGE2 production or use of the PGE2 receptor antagonist EP2/EP4 reduced the age associated increase in IL-23 and provides a possible target for therapeutic investigation [102].
NK cells
Natural Killer (NK) cells provide vital innate defenses against infections and malignancies using MHC-independent cytotoxicity. Once activated, NK cells exhibit direct cytolytic activity on infected cells, and also release pro-inflammatory cytokines and chemokines that contribute to the adaptive Th1 immune response [110]. While they share a common precursor with T cells that expresses FcγRIII, NK cells do not require the thymus for further development. T cell progenitors differentiate and mature in the thymus, while NK cells can continue developing in the bone marrow [111]. Differentiation in the bone marrow is not completely understood, but is driven in part by bone marrow stromal cell production of IL-2, IL-5, and IL-21 [112].
Age-related changes in both NK cell number and function in rodents have been described in the literature. Increased percentages of NK cells occur with advanced age, yet these cells exhibit decreased cytotoxicity on a per-cell basis [113]. Early studies reported that mice reached peak levels of basal NK cell activity around 5–8 weeks of age, while 25-month old animals had almost no activity [114]. The limited literature on the topic suggests that cytokines and chemokines produced by NK cells in rodents decreases with advanced age. For example, Provinciali et al. found that after IL-2 stimulation, NK cell cytotoxicity by cells from aged mice was restored to the level of cells from young, but responsiveness to IFN-γ was decreased [115]. A recent report has suggested a more direct link between NK cells and reduced adaptive immunity in aged mice. Increased numbers of NK cells were found in the developmental bone marrow Hardy Fraction A (CD19-B220+) population, which appeared to directly inhibit surrogate light chain expression in B cell precursors in aged BALB/c mice [116].
Diminished NK cell activity has also been correlated with a decrease in healthy living years in human studies [117]. Fang et al. found that an age-dependent increase in susceptibility to mousepox, the mouse equivalent of smallpox in humans, could be attributed to a decreased proportion of NK cells in circulation and an intrinsic defect in the ability of those cells to migrate to the draining lymph nodes [118]. Inability of NK cells to home to the lymph nodes resulted in poor early virus containment in older mice, increasing viral replication and systemic spread even in a mouse strain genetically resistant to the disease [118].
Though many studies investigating NK cell function in healthy aging subjects exist [119–121], less in known about the role of NK cells in pathologic conditions associated with advanced age. As the roles of NK cells in clinical settings continues to emerge, examination of this unique cell subset in a variety of animals disease models will undoubtedly aid in understanding their role in immunologic dysfunction with age.
NKT cells
Natural Killer T (NKT) cells are regulatory lymphocytes that co-express receptors found on T cells (CD3, α/β T cell receptor) and NK cells (NK1.1, CD56). NKT cells play an immunomodulatory role and this distinctive subset serves as a bridge between the innate and adaptive arms of the immune system, providing immunity against tumors and infections as well as suppression of cell-mediated autoimmunity [122, 123]. “Classical” or “invariant” NKT (iNKT) cells express a CD1d-restricted T cell receptor (Vα14/Vβ8.2 in mice, Vα24/Vβ11 in humans) which recognizes glycolipids in the context of CD1d, a MHC I-like molecule found on professional antigen presenting cells. Though they represent only 1% or less of the total lymphocyte pool, NKT cells can produce significant amounts of cytokines such as IFN-γ and IL-4 once they are activated, altering the cytokine milieu and skewing T cell polarization towards a Th1 or Th2 immune response [123–125].
In mice, NKT cells are found in greater numbers with increased age within the lymphoid compartment and the liver, with reports of altered function as compared to young animals [124, 126–128]. Systemic administration of anti-CD1d antibody to inhibit iNKT cell activation prevented the age-related decrease of both an antigen-specific delayed type hypersensitivity response in vivo and the proliferative capacity of T cells in vitro [126]. In these studies, NKT cells were found to contribute to increased amounts of the immunosuppressive cytokine IL-10 observed with increased age. iNKT cells have also been reported to release diminished levels of IFN-γ as compared to young mice, even after IL-12 stimulation [129].
NKT cells can contribute to an exaggerated state of inflammation in aged animals by changing the environmental cytokine milieu. For example, Stout-Delgado et al. found that hepatic NKT cells from HSV2 infected aged animals responded by producing markedly elevated levels of IL-17A as compared to cells from their young infected counterparts [106]. As a result, aged mice had exaggerated chemokine production and increased neutrophil influx into the liver, resulting in liver damage and mortality. Adoptive transfer of aged NKT cells into young recipients challenged with HSV2 caused hepatic damage and necrosis from overproduction of IL-17A, further demonstrating an intrinsic age-dependent defect in NKT cells [106]. The elevated and persistent inflammation observed in aged mice could help explain why elderly individuals succumb more easily to viral infections and sepsis.
As another critical link between the innate and adaptive immune response, the role of NKT cells in mediating a variety of age related diseases, in particular cancer and autoimmune conditions, need to be examined in clinically-relevant models. Like NK cells, NKT cells exhibit a profound effect on immune regulation despite their small number, implying that they would be an ideal target for therapeutic modulation.
Conclusions
As investigations of how advanced age interferes with the innate immune system continue, we will begin to better appreciate the etiology of specific age-related diseases and plausible mechanisms to improve treatments and patient outcomes. Rodent models are valuable for examining specific cellular functions and mechanisms in a controlled fashion. Use of rodent and animals models in studies focused on innate immune dysfunction as it pertains to age-related pathologies may allow for future targeted clinical research. However, as strain- and stimulus- dependent differences are observed in innate immune cell subsets in the aging rodent studies discussed, it would be beneficial for aging researchers to reach a consensus on the validity of particular rodent models with respect to the human population. Namely, use of animal models that are consistent with data generated from human studies will provide a stronger foundation for evaluation and translation of rodent aging research to clinical studies. Moreover, directing research efforts toward clinically relevant models of infection, injury and pathologic conditions will help to elucidate the intrinsic and extrinsic factors that contribute to innate immunologic dysfunction associated with human pathology. Future studies into how age impairs the innate immune response will allow for a comprehensive understanding of aging immune function and lead to improvements in clinical outcomes.
Acknowledgements
This work was supported by NIH R21 AI073987 (EJK), R01 AG018859 (EJK), T32 AG031780 (PWL) and Dr. Ralph and Marian C. Falk Medical Research Trust (EJK).
References
- 1.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- 3.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation:underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahbub S, Brubaker AL, Kovacs EJ. Aging of the Innate Immune System:An Update. Curr Immunol Rev. 2011;7:104–115. doi: 10.2174/157339511794474181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacs EJ, Palmer JL, Fortin CF, Fulop T, Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse:impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30:319–24. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence:causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–51. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 8.Nomellini V, Gomez CR, Kovacs EJ. Aging and impairment of innate immunity. Contrib Microbiol. 2008;15:188–205. doi: 10.1159/000136358. [DOI] [PubMed] [Google Scholar]
- 9.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115:278–83. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 10.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs EJ, Grabowski KA, Duffner LA, Plackett TP, Gregory MS. Survival and cell mediated immunity after burn injury in aged mice. J Amer Aging Assoc. 2002;25:3–10. doi: 10.1007/s11357-002-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishio N, Okawa Y, Sakurai H, Isobe K. Neutrophil depletion delays wound repair in aged mice. Age (Dordr) 2008;30:11–19. doi: 10.1007/s11357-007-9043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez CR, Nomellini V, Baila H, Oshima K, Kovacs EJ. Comparison of the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury:scald injury versus i.p. LPS administration. Shock. 2009;31:78–84. doi: 10.1097/SHK.0b013e318180feb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomellini V, Faunce DE, Gomez CR, Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol. 2008;83:1493–501. doi: 10.1189/jlb.1007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren Z, Gay R, Thomas A, Pae M, Wu D, Logsdon L, Mecsas J, Meydani SN. Effect of age on susceptibility to Salmonella Typhimurium infection in C57BL/6 mice. J Med Microbiol. 2009;58:1559–67. doi: 10.1099/jmm.0.013250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen LC, Pace JL, Russell SW, Morrison DC. Altered regulation of inducible nitric oxide synthase expression in macrophages from senescent mice. Infect Immun. 1996;64:4288–98. doi: 10.1128/iai.64.10.4288-4298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–35. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 18.Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181:6747–56. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang S, Domon H, Hosur KB, Wang M, Hajishengallis G. Age-related alterations in innate immune receptor expression and ability of macrophages to respond to pathogen challenge in vitro. Mech Ageing Dev. 2009;130:538–46. doi: 10.1016/j.mad.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol. 2008;181:7977–84. doi: 10.4049/jimmunol.181.11.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cumberbatch M, Dearman RJ, Kimber I. Influence of ageing on Langerhans cell migration in mice:identification of a putative deficiency of epidermal interleukin-1beta. Immunol. 2002;105:466–77. doi: 10.1046/j.1365-2567.2002.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong CP, Magnusson KR, Ho E. Aging is associated with altered dendritic cells subset distribution and impaired proinflammatory cytokine production. Exp Gerontol. 2010;45:163–9. doi: 10.1016/j.exger.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Gomez CR, Acuna-Castillo C, Nishimura S, Perez V, Escobar A, Salazar-Onfray F, Sabaj V, Torres C, Walter R, Sierra F. Serum from aged F344 rats conditions the activation of young macrophages. Mech Ageing Dev. 2006;127:257–63. doi: 10.1016/j.mad.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Tasat DR, Mancuso R, O'Connor S, Molinari B. Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging Cell. 2003;2:159–64. doi: 10.1046/j.1474-9728.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 25.Sunil VR, Laumbach RJ, Patel KJ, Turpin BJ, Lim HJ, Kipen HM, Laskin JD, Laskin DL. Pulmonary effects of inhaled limonene ozone reaction products in elderly rats. Toxicol Appl Pharmacol. 2007;222:211–20. doi: 10.1016/j.taap.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davila DR, Edwards CK, 3rd, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. Faseb J. 1990;4:2906–11. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- 27.Jann NJ, Schmaler M, Kristian SA, Radek KA, Gallo RL, Nizet V, Peschel A, Landmann R. Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J Leukoc Biol. 2009;86:1159–69. doi: 10.1189/jlb.0209053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson KE, Chessa TA, Davidson K, Henderson RB, Walker S, Tolmachova T, Grys K, Rausch O, Seabra M, Tybulewicz VL, Stephens LR, Hawkins PT. PtdIns3P and Rac direct the assembly of the NADPH oxidase on a novel, pre-phagosomal compartment during FcR-mediated phagocytosis in primary mouse neutrophils. Blood. 2010;116:4978–89. doi: 10.1182/blood-2010-03-275602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Appelberg R. Neutrophils and intracellular pathogens:beyond phagocytosis and killing. Trends Microbiol. 2007;15:87–92. doi: 10.1016/j.tim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules:a library of innate immunity proteins. Trends Immunol. 2007;28:340–5. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Fortin CF, Larbi A, Dupuis G, Lesur OTF., Jr GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology. 2007;8:173–87. doi: 10.1007/s10522-006-9067-1. [DOI] [PubMed] [Google Scholar]
- 32.Fulop T, Larbi A, Douziech N, Fortin C, Guerard KP, Lesur O, Khalil A, Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–26. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 33.Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, O'Mahony D, Lord JM. Senescence in innate immune responses:reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70:881–6. [PubMed] [Google Scholar]
- 34.Mares CA, Ojeda SS, Li Q, Morris EG, Coalson JJ, Teale JM. Aged mice display an altered pulmonary host response to Francisella tularensis live vaccine strain (LVS) infections. Exp Gerontol. 2010;45:91–96. doi: 10.1016/j.exger.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunil VR, Patel KJ, Mainelis G, Turpin BJ, Ridgely S, Laumbach RJ, Kipen HM, Nazarenko Y, Veleeparambil M, Gow AJ, Laskin JD, Laskin DL. Pulmonary effects of inhaled diesel exhaust in aged mice. Toxicol Appl Pharmacol. 2009;241:283–293. doi: 10.1016/j.taap.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geiger H, Rudolph KL. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;30:360–5. doi: 10.1016/j.it.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto K, Miyamoto T, Kato R, Yoshimura A, Motoyama N, Suda T. FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood. 2008;112:4485–93. doi: 10.1182/blood-2008-05-159848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monsalve M, Olmos Y. The Complex biology of FOXO. Curr Drug Targets. 2011;12:1322–50. doi: 10.2174/138945011796150307. [DOI] [PubMed] [Google Scholar]
- 39.Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 2011 2011 Jun 21; doi: 10.1016/j.jprot.2011.06.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–74. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 41.Sikora JP, Chlebna-Sokol D, Andrzejewska E, Chrul S, Polakowska E, Wysocka A, Sikora A. Clinical evaluation of proinflammatory cytokine inhibitors (sTNFR I, sTNFR II, IL-1 ra), anti-inflammatory cytokines (IL-10, IL-13) and activation of neutrophils after burn-induced inflammation. Scand J Immunol. 2008;68:145–52. doi: 10.1111/j.1365-3083.2008.02126.x. [DOI] [PubMed] [Google Scholar]
- 42.Ono S, Aosasa S, Tsujimoto H, Ueno C, Mochizuki H. Increased monocyte activation in elderly patients after surgical stress. Eur Surg Res. 2001;33:33–8. doi: 10.1159/000049690. [DOI] [PubMed] [Google Scholar]
- 43.Swift ME, Kleinman HK, DiPietro LA. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 1999;79:1479–87. [PubMed] [Google Scholar]
- 44.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–6. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 45.Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound:adding insult to injury? Thromb Haemost. 2004;92:275–80. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- 46.Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccaride-induced neutrophil inflammation in the lungs differs with age. Exp Lung Res. 2007;33:375–84. doi: 10.1080/01902140701634843. [DOI] [PubMed] [Google Scholar]
- 47.Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–9. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 48.Sydbom A, Blomberg A, Parnia S, Stenfors N, Sandstrom T, Dahlen SE. Health effects of diesel exhaust emissions. Eur Respir J. 2001;17:733–46. doi: 10.1183/09031936.01.17407330. [DOI] [PubMed] [Google Scholar]
- 49.Moriyama C, Betsuyaku T, Ito Y, Hamamura I, Hata J, Takahashi H, Nasuhara Y, Nishimura M. Aging enhances susceptibility to cigarette smoke-induced inflammation through bronchiolar chemokines. Am J Respir Cell Mol Biol. 2010;42:304–11. doi: 10.1165/rcmb.2009-0025OC. [DOI] [PubMed] [Google Scholar]
- 50.Murciano C, Yanez A, O'Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol Med Microbiol. 2008;53:214–21. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 51.Gordon S. The macrophage:past, present and future. Eur J Immunol. 2007;37:S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 52.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Adamson R. Role of macrophages in normal wound healing:an overview. J Wound Care. 2009;18:349–51. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- 54.Mantovani A. Macrophage diversity and polarization:in vivo veritas. Blood. 2006;108:408–9. [Google Scholar]
- 55.Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11:177–85. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- 56.Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–13. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Birjandi SZ, Ippolito JA, Ramadorai AK, Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol. 2011;186:3441–51. doi: 10.4049/jimmunol.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrero C, Sebastian C, Marques L, Comalada M, Xaus J, Valledor AF, Lloberas J, Celada A. Immunosenescence of macrophages:reduced MHC class II gene expression. Exp Gerontol. 2002;37:389–94. doi: 10.1016/s0531-5565(01)00205-4. [DOI] [PubMed] [Google Scholar]
- 59.Wang CQ, Udupa KB, Xiao H, Lipschitz DA. Effect of age on marrow macrophage number and function. Aging (Milano) 1995;7:379–84. doi: 10.1007/BF03324349. [DOI] [PubMed] [Google Scholar]
- 60.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. J Leukoc Biol. 2004;76:36–41. doi: 10.1189/jlb.1103538. [DOI] [PubMed] [Google Scholar]
- 62.Shaik-Dasthagirisaheb YB, Kantarci A, Gibson FC., 3rd Immune response of macrophages from young and aged mice to the oral pathogenic bacterium Porphyromonas gingivalis. Immun Ageing. 2010;7:15. doi: 10.1186/1742-4933-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25:581–5. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- 64.Murciano C, Villamon E, Yanez A, O'Connor JE, Gozalbo D, Gil ML. Impaired immune response to Candida albicans in aged mice. J Med Microbiol. 2006;55:1649–56. doi: 10.1099/jmm.0.46740-0. [DOI] [PubMed] [Google Scholar]
- 65.Takeuchi O, Hoshino K, Akira S. Cutting edge:TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–6. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 66.Kong KF, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, Fikrig E, Montgomery RR. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–23. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 68.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge:impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 69.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–9. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 70.Gong J, Wei T, Stark RW, Jamitzky F, Heckl WM, Anders HJ, Lech M, Rössle SC. Inhibition of Toll-like receptors TLR4 and 7 signaling pathways by SIGIRR:A computational approach. J Struct Biol. 2010;169:323–30. doi: 10.1016/j.jsb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–27. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- 72.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization:tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 73.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–6. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153:2146–52. [PubMed] [Google Scholar]
- 75.Kissin E, Tomasi M, McCartney-Francis N, Gibbs CL, Smith PD. Age-related decline in murine macrophage production of nitric oxide. J Infect Dis. 1997;175:1004–7. doi: 10.1086/513959. [DOI] [PubMed] [Google Scholar]
- 76.Sebastian C, Herrero C, Serra M, Lloberas J, Blasco MA, Celada A. Telomere Shortening and Oxidative Stress in Aged Macrophages Results in Impaired STAT5a Phosphorylation. J Immunol. 2009;183:2356–64. doi: 10.4049/jimmunol.0901131. [DOI] [PubMed] [Google Scholar]
- 77.Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2011 doi: 10.1089/jir.2011.0058. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev. 2004;125:137–43. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 79.Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. J Tissue Viability. 2009;18:13–9. doi: 10.1016/j.jtv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 81.Butcher SK, Killampalli V, Chahal H, Kaya Alpar E, Lord JM. Effect of age on susceptibility to post-traumatic infection in the elderly. Biochem Soc Trans. 2003;31:449–51. doi: 10.1042/bst0310449. [DOI] [PubMed] [Google Scholar]
- 82.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–77. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 83.Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. 2008;152:448–55. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vasievich EA, Huang L. The Suppressive Tumor Microenvironment:A Challenge in Cancer Immunotherapy. Mol Pharm. 2011;8:635–41. doi: 10.1021/mp1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ly LV, Baghat A, Versluis M, Jordanova ES, Luyten GP, van Rooijen N, van Hall T, van der Velden PA, Jager MJ. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J Immunol. 2010;185:3481–8. doi: 10.4049/jimmunol.0903479. [DOI] [PubMed] [Google Scholar]
- 86.Hayek MG, Mura C, Wu D, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol. 1997;159:2445–51. [PubMed] [Google Scholar]
- 87.Wu D, Meydani SN. Mechanism of age-associated up-regulation in macrophage PGE2 synthesis. Brain Behav Immun. 2004;18:487–94. doi: 10.1016/j.bbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Steinman RM. Dendritic Cells In Vivo:A Key Target for a New Vaccine Science. Immunity. 2008;29:319–24. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–5. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shurin MR, Shurin GV, Chatta GS. Aging and the dendritic cell system:implications for cancer. Crit Rev Oncol Hematol. 2007;64:90–105. doi: 10.1016/j.critrevonc.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Espia M, Sebastian C, Mulero M, Giralt M, Mallol J, Celada A, Lloberas J. Granulocyte macrophage--colony-stimulating factor-dependent proliferation is impaired in macrophages from senescence-accelerated mice. J Gerontol. 2008;63:1161–7. doi: 10.1093/gerona/63.11.1161. [DOI] [PubMed] [Google Scholar]
- 92.Kim HJ, Barajas B, Wang M, Nel AE. Nrf2 activation by sulforaphane restores the age-related decrease of T(H)1 immunity:role of dendritic cells. J Allergy Clin Immunol. 2008;121:1255–1261. e7. doi: 10.1016/j.jaci.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paula C, Motta A, Schmitz C, Nunes CP, Souza AP, Bonorino C. Alterations in dendritic cell function in aged mice:potential implications for immunotherapy design. Biogerontology. 2009;10:13–25. doi: 10.1007/s10522-008-9150-x. [DOI] [PubMed] [Google Scholar]
- 94.van Dommelen SL, Rizzitelli A, Chidgey A, Boyd R, Shortman K, Wu L. Regeneration of dendritic cells in aged mice. Cell Mol Immunol. 2010;7:108–15. doi: 10.1038/cmi.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cumberbatch M, Fielding I, Kimber I. Modulation of epidermal Langerhans' cell frequency by tumour necrosis factor-alpha. Immunology. 1994;81:395–401. [PMC free article] [PubMed] [Google Scholar]
- 96.Cumberbatch M, Kimber I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31–5. [PMC free article] [PubMed] [Google Scholar]
- 97.Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008;68:6341–9. doi: 10.1158/0008-5472.CAN-07-5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grolleau-Julius A, Garg MR, Mo R, Stoolman LL, Yung RL. Effect of aging on bone marrow-derived murine CD11c+CD4-CD8alpha- dendritic cell function. J Gerontol. 2006;61:1039–47. doi: 10.1093/gerona/61.10.1039. [DOI] [PubMed] [Google Scholar]
- 99.Pereira LF, Duarte de Souza AP, Borges TJ, Bonorino C. Impaired in vivo CD4+ T cell expansion and differentiation in aged mice is not solely due to T cell defects:Decreased stimulation by aged dendritic cells. Mech Ageing Dev. 2011;132:187–94. doi: 10.1016/j.mad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 100.Chiu BC, Stolberg VR, Zhang H, Chensue SW. Increased Foxp3(+) Treg cell activity reduces dendritic cell co-stimulatory molecule expression in aged mice. Mech Ageing Dev. 2007;128:618–27. doi: 10.1016/j.mad.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Toapanta FR, Ross TM. Impaired immune responses in the lungs of aged mice following influenza infection. Respir Res. 2009;10:112. doi: 10.1186/1465-9921-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myer RG, El Mezayen R, High KP. Prostaglandin E2-dependent IL-23 production in aged murine dendritic cells. Exp Gerontol. 2010;45:834–41. doi: 10.1016/j.exger.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saitoh T, Satoh T, Yamamoto N, Uematsu S, Takeuchi O, Kawai T, Akira S. Antiviral protein Viperin promotes Toll-like receptor 7- and Toll-like receptor 9-mediated type I interferon production in plasmacytoid dendritic cells. Immunity. 2011;34:352–63. doi: 10.1016/j.immuni.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 104.Plowden J, Renshaw-Hoelscher M, Gangappa S, Engleman C, Katz JM, Sambhara S. Impaired antigen-induced CD8+ T cell clonal expansion in aging is due to defects in antigen presenting cell function. Cell Immunol. 2004;229:86–92. doi: 10.1016/j.cellimm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–76. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe. 2009;6:446–56. doi: 10.1016/j.chom.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ouyang X, Yang Z, Zhang R, Arnaboldi P, Lu G, Li Q, Wang W, Zhang B, Cui M, Zhang H, Liang-Chen J, Qin L, Zheng F, Huang B, Xiong H. Potentiation of Th17 cytokines in aging process contributes to the development of colitis. Cell Immunol. 2011;266:208–17. doi: 10.1016/j.cellimm.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murdaca G, Colombo BM, Puppo F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern Emerg Med. 2011 Jul 30; doi: 10.1007/s11739-011-0517-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 109.El Mezayen R, El Gazzar M, Myer R, High KP. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell. 2009;8:553–65. doi: 10.1111/j.1474-9726.2009.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18:1613–20. doi: 10.1016/s0264-410x(99)00495-8. [DOI] [PubMed] [Google Scholar]
- 111.Budzynski W, Radzikowski C. Cytotoxic cells in immunodeficient athymic mice. Immuno-pharmacol Immunotoxicol. 1994;16:319–46. doi: 10.3109/08923979409007097. [DOI] [PubMed] [Google Scholar]
- 112.Toomey JA, Gays F, Foster D, Brooks CG. Cytokine requirements for the growth and development of mouse NK cells in vitro. J Leukoc Biol. 2003;74:233–42. doi: 10.1189/jlb.0303097. [DOI] [PubMed] [Google Scholar]
- 113.Mocchegiani E, Giacconi R, Cipriano C, Malavolta M. NK and NKT cells in aging and longevity:role of zinc and metallothioneins. J Clin Immunol. 2009;29:416–25. doi: 10.1007/s10875-009-9298-4. [DOI] [PubMed] [Google Scholar]
- 114.Albright JW, Albright JF. Age-associated impairment of murine natural killer activity. Proc Natl Acad Sci U S A. 1983;80:6371–5. doi: 10.1073/pnas.80.20.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Provinciali M, Muzzioli M, Fabris N. Timing of appearance and disappearance of IFN and IL-2 induced natural immunity during ontogenetic development and aging. Exp Gerontol. 1989;24:227–36. doi: 10.1016/0531-5565(89)90014-4. [DOI] [PubMed] [Google Scholar]
- 116.King AM, Keating P, Prabhu A, Blomberg BB, Riley RL. NK cells in the CD19− B220+ bone marrow fraction are increased in senescence and reduce E2A and surrogate light chain proteins in B cell precursors. Mech Ageing Dev. 2009;130:384–92. doi: 10.1016/j.mad.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levy SM, Fernstrom J, Herberman RB, Whiteside T, Lee J, Ward M, Massoudi M. Persistently low natural killer cell activity and circulating levels of plasma beta endorphin:risk factors for infectious disease. Life Sci. 1991;48:107–16. doi: 10.1016/0024-3205(91)90403-x. [DOI] [PubMed] [Google Scholar]
- 118.Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J Exp Med. 2010;207:2369–81. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mariani E, Neri S, Cattini L, Mocchegiani E, Malavolta M, Dedoussis GV, Kanoni S, Rink L, Jajte J, Facchini A. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly:interactive influence of +647 MT1a and -174 IL-6 polymorphic alleles. Exp Gerontol. 2008;43:462–71. doi: 10.1016/j.exger.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 120.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 121.Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Pena J, Solana R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 122.Taniguchi M, Seino K, Nakayama T. The NKT cell system:bridging innate and acquired immunity. Nat Immunol. 2003;4:1164–5. doi: 10.1038/ni1203-1164. [DOI] [PubMed] [Google Scholar]
- 123.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–36. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 124.Inui T, Nakagawa R, Ohkura S, Habu Y, Koike Y, Motoki K, Kuranaga N, Fukasawa M, Shinomiya N, Seki S. Age-associated augmentation of the synthetic ligand- mediated function of mouse NK1.1 ag(+) T cells:their cytokine production and hepatotoxicity in vivo and in vitro. J Immunol. 2002;169:6127–32. doi: 10.4049/jimmunol.169.11.6127. [DOI] [PubMed] [Google Scholar]
- 125.Joyce S. CD1d and natural T cells:how their properties jump-start the immune system. Cell Molec Life Sci. 2001;58:442–69. doi: 10.1007/PL00000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Faunce DE, Palmer JL, Paskowicz KK, Witte PL, Kovacs EJ. CD1d-restricted NKT cells contribute to the age-associated decline of T cell immunity. J Immunol. 2005;175:3102–9. doi: 10.4049/jimmunol.175.5.3102. [DOI] [PubMed] [Google Scholar]
- 127.Kawabata T, Kinoshita M, Inatsu A, Habu Y, Nakashima H, Shinomiya N, Seki S. Functional alterations of liver innate immunity of mice with aging in response to CpG-oligodeoxynucleotide. Hepatology. 2008;48:1586–97. doi: 10.1002/hep.22489. [DOI] [PubMed] [Google Scholar]
- 128.Tsukahara A, Seki S, Iiai T, Moroda T, Watanabe H, Suzuki S, Tada T, Hiraide H, Hatakeyama K, Abo T. Mouse liver T cells:their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301–9. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 129.Mocchegiani E, Giacconi R, Cipriano C, Gasparini N, Bernardini G, Malavolta M, Menegazzi M, Cavalieri E, Muzzioli M, Ciampa AR, Suzuki H. The variations during the circadian cycle of liver CD1d-unrestricted NK1.1+TCR gamma/delta+ cells lead to successful ageing. Role of metallothionein/IL-6/gp130/PARP-1 interplay in very old mice. Exp Gerontol. 2004;39:775–88. doi: 10.1016/j.exger.2004.01.014. [DOI] [PubMed] [Google Scholar]