Abstract
Confirmative diagnosis of visceral leishmaniasis (VL) is still a challenge at the primary health care facilities in most of the rural areas of endemicity in the Indian subcontinent. Conventional methods for parasitological confirmation are risky and require skilled personnel, and hence they are unavailable to the poor people in the regions of endemicity. Buffy coat smear microscopy, as a minimally invasive, simple alternative for the parasitological diagnosis of VL, was evaluated in this prospective study. One hundred twelve VL patients were enrolled in this study. The buffy coat was separated from peripheral blood of all enrolled subjects using Histopaque-1119 solution. Leishman-stained buffy coat smears were examined for Leishmania donovani bodies, and buffy coat was also utilized for detection of parasite DNA by Leishmania nested PCR (LnPCR) for all cases. Concomitant splenic smears could be examined for L. donovani bodies in 66 cases, and the parasite load was graded on a scale of 1+ to 6+ for L. donovani-positive smears. All splenic smear-positive cases were also found to be positive by LnPCR. Of 112 enrolled VL cases, 103 (92%) were found to be positive for L. donovani bodies in buffy coat smear microscopy, which is promising as a confirmative diagnosis tool. We have also found a significant association of the buffy coat smear positivity with parasitic burden in the spleen smear. In this preliminary observation in Bangladesh, buffy coat smear microscopy has been found to be very simple, minimally invasive, and risk-free method of parasitological diagnosis of VL with a good diagnostic accuracy and potential for field use.
INTRODUCTION
Visceral leishmaniasis (VL), or Indian kala-azar, is a vector-borne parasitic disease caused by an obligate intracellular hemoflagellate of the genus Leishmania (11). Not all leishmanial infections lead to overt clinical disease, but in those infected persons who do develop the disease, multiplication of the parasite in the reticuloendothelial system causes prolonged fever, anemia, hepatosplenomegaly, and weight loss (3). VL is fatal if it is not adequately treated. The current prevalence is estimated to be 45,000 cases, with more than 40.6 million people at risk of developing the disease in Bangladesh. Of 64 districts, at least 34 districts, including 105 upazilas (subdistricts), have been reportedly affected by kala-azar (17).
Diagnostic tests for VL need to be highly sensitive and specific because of the fatal evolution of the disease without adequate treatment and the serious toxicity of antimonials (25), the most commonly used first-line therapy. Moreover, tests must be inexpensive and easy to perform since VL occurs in poor and remote rural communities with limited access to referral hospitals. The development of diagnostic tests for improved case management of VL has been rated as one of the most needed among the infectious diseases prevalent in the developing world (13). Currently, diagnostic options for VL include parasitological, serological, and molecular methods, but each has its own advantages and disadvantages (23, 24). Although the need for accurate VL diagnostics is obvious, innovation in this field has been slow. There is always a search for new diagnostic tool particularly suitable for field use, with high sensitivity and minimal invasiveness. Isolation of the parasite in culture or demonstration in relevant tissues such as spleen or bone marrow by light microscopic examination of the stained specimen remains the “gold standard” and leads to the definitive diagnosis of leishmaniasis. However, for parasitological diagnosis to be made from these tissues, painful and sometimes fatal invasive procedures are necessary, which limits their scope in routine clinical practice and makes them not feasible in the field. Serodiagnosis is a valid and attractive choice for kala-azar, like for many other infectious diseases, and the detection in serum and urine specimens of antileishmanial antibody against recombinant antigens such as rK39 by rapid immunochromatographic tests has been carried out in different regions where the disease is endemic, including India, Bangladesh, and Nepal (9, 20, 26). Although this rapid test has been found to be highly sensitive in the Indian subcontinent, its specificity is yet to be established (26). Further, its use to discriminate disease and asymptomatic infection is limited because antibody titers may vary with the infecting species, tissue tropism, and the immunocompetence of the host (1). The development of PCR has provided a powerful approach to the application of molecular biology techniques to the diagnosis of leishmaniasis for more than a decade (2). PCR assay with buffy coat preparations to detect Leishmania DNA has been found to be 10 times more sensitive than that with whole-blood preparation (10, 19). However, for PCR, the need for sophisticated machines and trained personnel, as well as cost, are limiting factors.
Detection of Leishmania donovani bodies in peripheral blood buffy coat smears is an alternative and minimally invasive procedure for the parasitological diagnosis of VL. In a few studies its sensitivity ranged from 50 to 99% (16). Buffy coat is the portion of blood that contains concentrates of white blood cells, including monocytes and platelets, and for obvious reasons its smear can be examined for detection of L. donovani bodies in suspected VL patients. There are clear advantages of buffy coat over conventional smears for parasitological diagnosis in terms of minimal invasiveness, simplicity, and cost-effectiveness. As an alternative but definitive diagnostic tool, buffy coat smear has the potential to be carried out for point-of-care VL case management. We report, for the first time, this preliminary observation on the usefulness of buffy coat smear microscopy as a simple and effective method for parasitological diagnosis of VL in Bangladeshi patients.
MATERIALS AND METHODS
Patients.
The subjects were 112 patients with VL as defined by the Bangladesh national kala-azar elimination guideline (8). One of the 112 cases was diagnosed as congenital VL and had been reported earlier (7). All of them were from an area of Bangladesh where VL is endemic. On admission to the Rajshahi Medical College Hospital (RMCH) during June 2009 to June 2010, all patients had fever (perceived by patient or guardian) for more than 2 weeks, splenomegaly, and a positive rK39 immunochromatographic test on a finger prick blood specimen, which was later confirmed by doing splenic smear microscopy and Leishmania nested PCR (LnPCR).
Blood collection.
With all aseptic precautions, 3.0 ml of blood from each patient was collected into a vacuette (K3 EDTA tube).
Splenic aspiration.
After relevant laboratory evaluations and following standard techniques as described by Bryceson (4), splenic aspiration was able to be carried out for 66 patients by an experienced physician. Two good-quality smears were prepared at bedside for microscopic examination.
Buffy coat preparation.
Buffy coat was separated following the principle of concentration gradient separation by using Histopaque solution (Histopaque-1119; Sigma-Aldrich). Three milliliters of collected blood was layered onto 3 ml of the Histopaque-1119 solution in a sterile 15-ml centrifuge tube. The tube was capped and then centrifuged in a tabletop centrifuge at 4,000 × g for 10 min at ambient temperature. A diffuse gray band of leukocytes (buffy coat) in between the Histopaque solution and plasma above the erythrocyte pellet was aseptically removed with a pipette and transferred to a sterile 1.5-ml microcentrifuge tube to be utilized for smear preparation and as a sample for LnPCR.
DNA extraction.
Buffy coat DNA was extracted for PCR using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The DNA was eluted in 0.2 ml of AE buffer (supplied with the Qiagen kit). The purity of the DNA was satisfactory, since the A260/A280 ratio was within 1.7 to 1.9 for all DNA samples. We used molecular-grade water instead of blood as an extraction control to check for carryover contamination in every run of DNA extraction and PCR amplification.
LnPCR.
We used a previously reported LnPCR with primers targeting the parasite's small-subunit rRNA region (6). An advantage of this LnPCR is its high sensitivity and specificity due to the use of a second set of Leishmania-specific primers (R223 [5′-TCCCATCGCAACCTCGGTT-3′] and R333 [5′-AAAGCGGGCGCGGTGCTG-3′]) designed to an internal sequence of the first PCR product. For the first PCR run, we used Kinetoplastida-specific primers (R221 [5′-GGTTCCTTTCCTGATTTACG-3′] and R332 [5′-GGCCGGTAAAGGCCGAATAG-3′]). In the first PCR, 2 μl of extracted DNA was amplified in a final volume of 25 μl containing 12.5 μl of Bio-Rad iQ Supermix (catalog number 170-8862), which contains 100 mM KCl, 40 mM Tris-HCl (pH 8.4), 1.6 mM deoxynucleoside triphosphates, 50 U/ml iTaq DNA polymerase, and 6.0 mM MgCl2. A 0.3-μmol/liter concentration of each Kinetoplastida-specific primer (R221 and R332) and an additional 3.0 mM MgCl2 were also added. Amplification was performed on a Bio-Rad MyCycler. The PCR program was run for 40 cycles that consisted of denaturation at 94°C for 30 s, annealing at 65°C for 30 s, and extension at 72°C for 30 s.
Prior to the second amplification, or nested PCR, the amplified products from the first run were diluted at 1:50 with molecular-grade water, and 1 μl was added to a 25-μl reaction volume, as described above, containing 0.15 μmol/liter of the Leishmania-specific primers R223 and R333. For the second round of amplification, 35 cycles were used, consisting of denaturation at 94°C for 30 s, 65°C for 30 s, and 72°C for 30 s. In both amplifications, initial Taq DNA polymerase activation was performed at 95°C for 3 min, and a final extension at 72°C for 5 min was included. Amplification products were separated by electrophoresis on a 2% agarose gel with a 50-bp DNA ladder (Invitrogen, catalog no. 15628-019) as molecular size marker and stained with ethidium bromide (0.1 mg/ml). Stained gels were visualized and photographed under UV light emission with a UV transilluminator (75S/03589; Bio-Rad, Milan, Italy). Amplification products were visualized, and positive samples yielded a PCR product of 350 kb. In every run, molecular-grade water and healthy human DNA were used as negative controls, and DNA from cultured promastigotes served as a positive control.
Buffy coat smear microscopy.
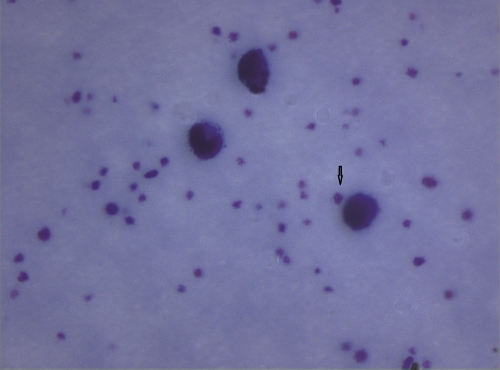
Two good-quality smears prepared from buffy coat and stained with Leishman stain were examined under an oil immersion light microscope (CH-20; Olympus, Japan). L. donovani bodies in the smear (Fig. 1) were confirmed independently by two experienced microscopists by detection of the standard parasite morphology using the following guidelines. (i) Amastigotes are usually seen extracellularly in the buffy coat smear under the oil immersion objective of a light microscope. (ii) The hallmark of identification of structures as amastigotes in the buffy coat smear is the typical conjugation of a nucleus and kinetoplast of unequal sizes. (iii) The typical morphology of amastigotes (oval or elliptical cells 2 to 4 μm in size, bounded by a cytoplasmic membrane containing the nucleus and kinetoplast, which are bound together at a right angle to each other and where the nucleus is larger than the kinetoplast) as demonstrated in splenic or bone marrow smears may not be well preserved in all amastigotes seen in the buffy coat smear. However, the distinct conjugation with variable size of nucleus and kinetoplast, which are covered by a complete or partially complete cytoplasmic rim, is characteristic of the presence of amastigotes and makes them sufficiently different from the platelets, which are the only structures that may be confused with them. Platelets usually remain as a cluster, and they are smaller than amastigotes. Moreover, there certainly is no separate nucleus and structure such as a kinetoplast inside the platelets. (iv) The number of amastigotes is low in a buffy coat smear in comparison to a splenic smear, which is logical. Therefore, careful and patient searching is always required for clear demonstration of amastigotes.
Fig 1.
L. donovani amastigote in buffy coat smear.
Splenic smear microscopy.
Two experienced microscopists independently examined spleen smears taken from the 66 of 112 patients in whom contraindications for splenic aspiration were absent. Splenic smears were stained with Leishman stain and read in a standard way under magnification of ×1,000 for the presence of L. donovani amastigotes. The presence of L. donovani bodies was graded on a scale from 1+ to 6+. If the number of amastigotes counted per field was >100, 10 to 100, or 1 to 10, the grade was 6+, 5+, and 4+, respectively. Similarly, the presence of 1 to 10 amastigotes in 10, 100, or 1,000 fields was graded as 3+, 2+, and 1+, respectively (5).
Serological test.
The rK39 immunochromatographic test had been done by using the Kala-azar Detect rapid test (InBios International, Seattle, WA) as per the manufacturer's instructions (20).
Case definition.
A VL case was defined as per the Bangladesh national kala-azar elimination guideline (8).
Data analysis.
Descriptive statistical analysis, the chi-square test, the McNemar paired test, and analysis of variance (ANOVA) were used for data analysis, using SPSS 11.5 and R software.
Ethical considerations.
Ethical issues relating to this research protocol were reviewed by the Institutional Review Board of Rajshahi Medical College, Bangladesh, which gave approval. Informed written consent was obtained from each patient or from the legal guardian before splenic aspiration and venipuncture for the collection of blood samples.
RESULTS
Study population characteristics.
Of 112 VL patients, 75 (67%) and 37 (33%) were male and female, respectively. The median age was 276 months (quartiles, 120 months and 384 months). All patients had splenomegaly ranging from just palpable to 12 cm from the costal margin along midclavicular line. All had positive rK39 tests and underwent buffy coat smear microscopy for L. donovani bodies and buffy coat LnPCR analysis for Leishmania DNA. Sixty-six of 112 VL patients consented to spleen aspiration diagnosis. Patients were treated with sodium antimony gluconate (SAG) at a dose of 10 to 20 mg/kg of body weight, with a maximum dose not exceeding 10 ml (800 mg), given intravenously (i.v.) daily without any interruption. All patients were discharged from hospital after 30 days of treatment with clinical improvements.
Laboratory results.
Ninety-two percent (103/112), 95.5% (107/112), and 100% (66/66) of VL patients were positive by buffy coat microscopy, buffy coat PCR, and spleen smear microscopy, respectively. The buffy coat microscopy results did not show any relation to the sex and age of the patients. The mean ages ± standard errors (SE) of patients with and without positive buffy coat microscopy test results were 243 ± 72 and 276 ± 16, respectively (P = 0.58). Ninety percent (68/75) of male patients and 94.6% (35/37) of female patients were positive by buffy coat microscopy (P = 0.47). However, buffy coat PCR positivity was more common among female patients (93% [70/75] versus 100% [37/37]; P = 0.16).
Leishmania amastigotes were found by buffy coat microscopy in 93.5% (100/107) of those positive by buffy coat PCR and in 92.4% (61/66) of those positive for Leishmania amastigotes by spleen smear microscopy. Compared to spleen smear microscopy, the positivity rates of buffy coat PCR and buffy coat microscopy was comparable (66/66 for PCR versus 61/66; P = 0.06 by the McNemar paired test).
Of 66 spleen smears positive for amastigotes, most of them (54/66) had a parasite burden of grade ≥2+. Rates of positivity of buffy coat smears correlated significantly with the spleen smear parasite burden. Buffy coat smears were 100% positive among cases having higher (grade 2+ to 5+) parasite burdens (P = 0.003 by the Fisher exact test) (Table 1).
Table 1.
Association of buffy coat smear positivity with spleen parasite burden
| Parasite load grade | No. (%) positive by: |
|
|---|---|---|
| Spleen smear | Buffy coat smear | |
| 1+ | 12 (18.18) | 8 (66.67) |
| 2+ | 26 (39.39) | 25 (96.15) |
| 3+ | 16 (24.24) | 16 (100) |
| 4+ | 7 (10.61) | 7 (100) |
| 5+ | 5 (07.58) | 5 (100) |
DISCUSSION
The most important finding of the study is that buffy coat smear microscopy correlated excellently with clinical diagnosis of VL as per the national kala-azar elimination guideline, buffy coat PCR for Leishmania donovani DNA, and confirmatory diagnosis of VL by spleen aspirate smear. Another important finding is that the recommendation for diagnosis and treatment of VL based on clinical criteria in the national kala-azar elimination program is correct, since clinical diagnosis in 66 VL patients was justified by spleen aspirate smear, which is the gold standard for diagnosis of VL. So far this is the first report of a comparison of the Bangladesh national kala-azar elimination guideline criteria for VL diagnosis with spleen aspirate microscopy.
The development of an accurate, practical, and affordable diagnostic test is essential for any attempt to control VL in areas of endemicity. The conventional methods of parasite demonstration not only are associated with painful invasive procedures but may be fatal. Moreover, prior laboratory evaluation of the patient is a prerequisite for conventional invasive procedures, which are not feasible to be performed at the point of care in regions where VL is endemic. Therefore, an alternative method for the parasitological diagnosis of VL with minimum invasiveness and risk for the patients needs to be explored. The buffy coat positivity rate among clinically defined VL patients was 92%. The positivity rate was also high among confirmed VL patients (92.4%). Further, buffy coat smear was comparable with buffy coat PCR for detection of Leishmania donovani DNA. Thus, buffy coat smear is a promising less invasive, affordable, virtually risk-free diagnostic tool for VL which can be used for point-of-care diagnosis in the subdistrict hospitals of Bangladesh. Currently most VL patients are clinically diagnosed and treated in the subdistrict hospitals of Bangladesh, where facilities for the use of confirmatory diagnostic tools are not available. Buffy coat smear can be used as a confirmatory diagnostic tool in these health facilities. However, its sensitivity and specificity have to be validated by further study following the standards for field evaluation of VL diagnostics for its recommendation as a confirmatory test (3).
The idea for diagnosis of VL using peripheral blood buffy coat smears originated from studies in the early 1990s (12, 14, 15). These studies showed that the Leishmania parasite could be demonstrated by microscopy of peripheral blood smears of HIV-infected patients with visceral leishmaniasis. Leishmania amastigotes in peripheral blood specimens from Indian kala-azar patients were also demonstrated later, with a rate of 46% to 66% depending on the time of blood sampling (21). Leishmania-stained blood smears revealed 1.3% parasitemia among 450 healthy individuals in another study conducted by Sharma et al. in areas of Bihar, India, where VL is endemic (22). We also examined conventionally prepared buffy coat smears from 200 asymptomatic VL patients (defined as a person from a household or nearest to a household with a kala-azar patient[s] in the past who was positive by the rK39 test and clinically completely healthy) and found that none were positive by buffy coat smear microscopy (our unpublished data from an ongoing cohort study of asymptomatic VL patients in Trishal, Mymensingh, Bangladesh). The study by Sharma et al. and our unpublished data indicated that the buffy coat smear should be a good diagnostic method for active VL, since parasitemia among asymptomatic VL patients and healthy controls from areas of endemicity was very low (0% to 1.3%).
So far, only one study conducted in Bangladesh has tried to investigate the diagnostic sensitivity and specificity of buffy coat smear for diagnosis of VL (18). The study found a positivity rate of 31% by buffy coat smear among 67 clinically suspected VL patients. Unfortunately the results of present study cannot be compared with the results of the study by Roy et al. (18), because they did not report the buffy coat smear positivity rates among their confirmed VL cases (44/67) and also among those who had been positive by the rK39 rapid test (57/67). Nevertheless, the low positivity rate for buffy coat smear found by Roy et al. might be due to the conventional method for preparation of buffy coat smears which they used.
The positivity rate for buffy coat smear correlated well with the spleen parasite burden, and most of the cases had spleen parasite burdens of grade 2+ and above during admission. The buffy coat smear was positive in 98% of VL patients with spleen parasite burdens of grade 2+ and above. This finding is very encouraging for recommendation of buffy coat smear as a routine confirmatory test for diagnosis of VL.
In conclusion, we found buffy coat smear to be a promising confirmatory diagnostic tool for VL which can be used for point-of-care diagnosis in resource-limited health facilities. However, a well-designed study following the recommended standards for evaluation of VL diagnostic tools is highly desired for recommendation of buffy coat smear as a confirmatory diagnostic tool for VL.
ACKNOWLEDGMENTS
We are thankful to all the study participants and staff of Rajshahi Medical College and the International Centre for Diarrhoeal Disease Research, Bangladesh, who were involved in this study for their assistance and cooperation.
We declare that we have no competing interests. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Footnotes
Published ahead of print 28 December 2011
REFERENCES
- 1. Alvar JC, et al. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andresen K, et al. 1997. Diagnosis of visceral leishmaniasis by polymerase chain reaction using blood, bone marrow and lymph node samples from patients from the Sudan. Trop. Med. Int. Health 2:440–444 [PubMed] [Google Scholar]
- 3. Boelaert M, et al. 2007. Evaluation of rapid diagnostic tests: visceral leishmaniasis. Nat. Rev. Microbiol. 5:S30–S39 [Google Scholar]
- 4. Bryceson A. 1987. Splenic aspiration procedure as performed at the Clinical Research Center, Nairobi, 1982, p 728–729 In Peter W, Killick-Kendrick R. (ed), The leishmaniasis, vol 2 Academic Press, London, United Kingdom [Google Scholar]
- 5. Chulay JD, Bryceson AD. 1983. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am. J. Trop. Med. Hyg. 32:475–479 [DOI] [PubMed] [Google Scholar]
- 6. Curz I, et al. 2002. A nested polymerase chain reaction (Ln-PCR) for diagnosing and monitoring Leishmania infantum infection in patients co-infected with human immunodeficiency virus. Trans. R. Soc. Trop. Med. Hyg. 96:185–189 [DOI] [PubMed] [Google Scholar]
- 7. Haque A, Ekram ARMS, Sharmin LS, Belaluddin M, Salam MA. 2010. Congenital visceral leishmaniasis. Pak. J. Med. Sci. 26:485–487 [Google Scholar]
- 8. Hossain M. (ed). 2008. National guideline and training module for kala-azar elimination in Bangladesh. Directorate General of Health Services, Ministry of Health and Family Welfare, Dhaka, Bangladesh [Google Scholar]
- 9. Khan MGM, et al. 2010. Evaluation of rK-39 strip test using urine for diagnosis of visceral leishmaniasis in an endemic area in Bangladesh. Parasit. Vectors 26:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lachaud L, et al. 2001. Comparison of various sample preparation methods of PCR diagnosis of visceral leishmaniasis using peripheral blood. J. Clin. Microbiol. 38:613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leishman WB. 1903. On the possibility of occurrence of trypanosomiasis in India. Br. Med. J. 1:1252–1254 [PubMed] [Google Scholar]
- 12. López-Vélez R, et al. 1995. Parasitic culture of buffy coat for diagnosis of visceral leishmaniasis in human immunodeficiency virus-infected patients. J. Clin. Microbiol. 33:937–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mabey D, Peelin RW, Ustianowsk A, Perkin MD. 2004. Tropical infectious diseases: diagnostics for the developing world. Nat. Rev. Microbiol. 2:231–240 [DOI] [PubMed] [Google Scholar]
- 14. Martínez P, et al. 1993. Diagnosis of visceral leishmaniasis in HIV-infected individuals using peripheral blood smears. AIDS 7:227–230 [DOI] [PubMed] [Google Scholar]
- 15. Medrano FJ, Jiménez-Mejías E, Calderón E, Regordan C, Leal M. 1993. An easy and quick method for the diagnosis of visceral leishmaniasis in HIV-1-infected individuals. AIDS 7:1399. [DOI] [PubMed] [Google Scholar]
- 16. Monica C. 1998. District laboratory practice in tropical countries, part I, p 275–280 Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 17. Rahman R, Bangali M, Kabir H, Naher FB, Mahboob S. 2008. Kala-azar situation in Bangladesh, p 11–12 In Hossain M. (ed), National guideline and training module for kala-azar elimination in Bangladesh. Directorate General of Health Services, Ministry of Health and Family Welfare, Dhaka, Bangladesh [Google Scholar]
- 18. Roy CK, Safiullah SA, Saleh AA, Miah MRA. 2009. Detection of LD body in peripheral blood buffy-coat from suspected kala-azar cases of Bangladesh. Bangladesh J. Med. Microbiol. 3:27–32 [Google Scholar]
- 19. Salam MA, Mondal D, Kabir M, Ekram ARMS, Haque R. 2010. PCR for diagnosis and assessment of cure in kala-azar patients in Bangladesh. Acta Trop. 113:52–55 [DOI] [PubMed] [Google Scholar]
- 20. Salam MA. 2008. Immunochromatographic strip test detection of anti-rK39 antibody for the diagnosis of kala-azar in an endemic zone of Bangladesh. Pak. J. Med. Sci. 24:497–501 [Google Scholar]
- 21. Saran R, Sharma MC, Gupta AK, Sinha SP, Kar SK. 1997. Diurnal periodicity of Leishmania amastigotes in peripheral blood of Indian kala-azar patients. Acta Trop. 68:357–360 [DOI] [PubMed] [Google Scholar]
- 22. Sharma MC, et al. 2000. Leishmania donovani in blood smears of asymptomatic persons. Acta Trop. 76:195–196 [DOI] [PubMed] [Google Scholar]
- 23. Singh S, Sivakumar R. 2003. Recent advances in the diagnosis of leishmaniasis. J. Postgrad. Med. 49:55–60 [DOI] [PubMed] [Google Scholar]
- 24. Sundar S, Rai M. 2002. Laboratory diagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 9:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sundar S, et al. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31:1104–1107 [DOI] [PubMed] [Google Scholar]
- 26. Sundar S, Pai K, Sahu M, Kumar V, Murray HW. 2002. Immunochromatographic strip test detection of anti k39 antibody in Indian visceral leishmaniasis. Ann. Trop. Med. Parasitol. 96:19–23 [DOI] [PubMed] [Google Scholar]