Abstract
Dried blood spots (DBS) may be a promising alternative specimen type to plasma for measuring the viral load (VL) in HIV-infected individuals in resource-limited settings. However, characterization of assay performance using DBS is incomplete. In this prospective study, the VL was measured in parallel using plasma and DBS specimens collected at the same time from 157 HIV-1-infected individuals. DBS were prepared by dispensing 50 μl of blood onto filter paper cards and were stored desiccated at −20°C. Nucleic acid extraction from plasma and DBS was performed automatically using the Abbott m2000sp instrument, and the VL was measured using the RealTime HIV-1 VL assay, which has a lower limit of detection of 40 HIV RNA copies/ml. The correlation between plasma and DBS results was good (R = 0.91; P < 0.001). The mean difference in the VL (DBS minus plasma) was 0.35 log copies (standard deviation [SD], 0.47 log copies). A total of 40 (26%) paired specimens had a difference of >0.5 log copy, and in 12 (7.8%) it was >1 log copy. the VL from DBS was measurable in 95.7% of specimens with a plasma VL of >2.74 log copies (550 HIV RNA copies/ml). In summary, the VL can reliably be measured using DBS with the Abbott RealTime HIV-1 assay. The estimated lower limit of detection of this automated methodology on DBS is 550 copies/ml, a threshold that may be acceptable for periodic VL monitoring in patients on antiretroviral therapy in resource-limited settings, where early detection of virologic treatment failure is often problematic.
INTRODUCTION
Periodic measurement of the viral load (VL) in plasma remains a key parameter in the follow-up of HIV-infected individuals, especially those on antiretroviral therapy (ART) (21). However, reliable VL testing in plasma requires specialized facilities that often are not available in resource-poor settings. Dried blood spots (DBS) may be an interesting alternative to plasma for periodic VL testing (1, 5, 7, 9, 12, 23). DBS can be easily collected and stored without being frozen or refrigerated (2, 17, 22). Several studies have demonstrated the feasibility of DBS as a specimen type for VL testing using different methodologies, although limitations in terms of sensitivity and stability have been noted (3, 5, 9, 10, 12, 16). In addition, the recognition of distinct subtypes is an important problem in countries where HIV variability is high, and some VL assays occasionally underquantify some variants (19, 24).
In a previous study, we reported the results of VL testing on DBS using the Abbott mSample preparation system, a manual RNA isolation method. Although the correlation with plasma values was acceptable, the sensitivity was significantly lower on DBS (∼3.5 log copies) (11). Improvements in the detection limit (similar to plasma values of ∼400 copies/ml) are important for early recognition of virological failure in patients on antiretroviral treatment. Here, we evaluate the feasibility of using an automated RNA isolation method, m2000sp, and the Abbott RealTime HIV-1 VL assay to quantify HIV-1 RNA on DBS and compare the results to those obtained by testing plasma samples in parallel.
MATERIALS AND METHODS
Study population.
A total of 157 HIV-1 (subtype B)-infected adult individuals, 70% of whom were antiretroviral therapy naïve, were identified at Hospital Carlos III, a reference HIV/AIDS clinic located in Madrid, Spain. In addition, a group of 15 specimens from HIV-negative individuals were used as controls for the specificity analyses. Specimens were obtained from residual blood collected for routine analysis and were anonymously tested.
Specimen preparation.
DBS were prepared by dispensing 50 μl of blood per spot (5 spots per card) onto filter paper cards (S&S903 filter paper; Schleicher & Schuell, BioScience GmbH, Barcelona, Spain) and dried at room temperature for 4 to 6 h. The DBS were stored in zip-lock plastic bags with a silica gel desiccant at −20°C. Plasma was prepared by centrifugation of the whole blood, aliquoted, and stored at −20°C until use.
RNA isolation and viral load determination.
RNA isolation from DBS was performed by cutting a 12-mm-diameter piece of each spot using a hole punch. Two spots were transferred into a 50-ml conical tube containing 2 ml of lysis buffer (Abbott Molecular, Madrid, Spain). The tubes were incubated at room temperature for 120 min with constant mixing. After the incubation, the spots were removed, and the supernatant was clarified by centrifugation and transferred to the tubes of the Abbott mSample preparation system. RNA isolation was done following the manufacturer's recommendations on the m2000sp platform, which uses a method based on iron particles, in both plasma and DBS (20). The VL testing was carried out following the manufacturer's instructions. The linear dynamic range when processing 0.6 ml of plasma is 40 to 107 HIV RNA copies/ml (20).
Statistical analysis.
All RNA values are reported as log10-transformed copy numbers of HIV RNA per ml of blood (DBS) or plasma. Results reported as not detected or below the detection limit (<40 copies/ml) were all grouped as <40 copies/ml and analyzed using a value of 1.59 log copies of HIV RNA per ml. The results were expressed as means and standard deviations. Linear regression analyses were performed in order to compare the results obtained for each sample type; correlations were expressed as the R value. Concordance between plasma and DBS was assessed using the Bland and Altman test (6). All analyses were performed using the statistical software SPSS v15.0 (SPSS Inc., Chicago, IL). Differences were considered significant only when P values were <0.05.
RESULTS
Paired DBS and plasma VL results were obtained for 154 of 157 (98%) specimens. Three DBS reported invalid results, whereas corresponding plasma values were 2.2, 3.48, and 4.14 log HIV RNA copies/ml. Specimens were classified into strata defined by the plasma VL. The results from plasma showed that 57 specimens had VL values of <40 copies/ml. The VL results for the other 97 specimens were stratified as follows: 26 specimens (26.8%), between 40 and 1,000 copies/ml; 33 specimens (34%), between 1,000 and 10,000 copies/ml; 33 specimens (34%), between 10,000 and 100,000 copies/ml; and 5 specimens (5.2%), over 100,000 copies/ml. All 15 plasma specimens collected from HIV-negative controls gave VL values of <40 copies/ml.
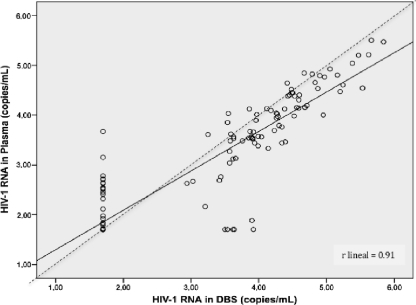
The correlation between the DBS and plasma VL results was high (R = 0.91; P < 0.001) (Fig. 1). The mean difference for quantitative data between measurements (DBS minus plasma) was 0.35 log copy (standard deviation, 0.47 log copy). Overall, 40 pairs (26%) had a difference of more than 0.5 log copy, and in 12 (7.8%), it was greater than 1 log copy. The mean difference and standard deviation in samples with a VL between 40 and 1,000 copies/ml was 0.65 (0.44); they were 0.51 (0.43) for those between 1,000 and 5,000, 0.38 (0.18) for those between 5,000 and 10,000, 0.29 (0.26) for those between 10,000 and 100,000, and 0.31 (0.096) for those with a VL of >100,000 copies/ml. Rank correlation was performed with similar results (data not shown).
Fig 1.
Linear regression comparing VL values obtained by testing DBS versus plasma using the Abbott RealTime HIV-1 assay. The dashed line represents perfect agreement between plasma and DBS, with a slope of 1.0 and intercept in 0.
Classification of the VL as above or below the limit of detection was concordant in plasma and DBS for 82.4% of specimens. The VL was above the limit of detection in 95.7% of DBS, with corresponding plasma VL values of >2.74 log copies (550 copies/ml), rising to 100% in specimens with more than 1,400 (3.1 log) copies/ml in plasma. Five (9%) out of 57 samples with plasma VL of <40 copies/ml yielded DBS VL results above the limit of detection (mean, 3.6 log copies; standard deviation, 0.16 log copy) (Table 1). Lack of further DBS material precluded retesting. However, a specific reverse transcription (RT)-PCR in the protease region was performed on the RNA material originally extracted from DBS, following previously described optimized conditions (10), and the VL was then considered undetectable on DBS, suggesting that nonspecific amplification had initially occurred. All DBS from HIV-negative individuals tested gave negative results, confirming good specificity of the assay.
Table 1.
Qualitative agreement comparing VL results in plasma and DBS in 154 samples with paired results
| Plasmaa | DBSa |
||
|---|---|---|---|
| No. positive | No. negative | Total | |
| No. positive | 75 | 22b | 97 |
| No. negative | 5b | 52 | 57 |
| Total | 80 | 74 | 154 |
Positive, VL values of >40 HIV RNA copies/ml; negative, VL values of <40 HIV RNA copies/ml (1.59 log HIV-RNA copies/ml).
For the 22 samples with VL results positive in plasma and negative in DBS, the mean plasma VL was 2.29 log copies (range, 1.72 to 3.15). For samples with negative results in plasma and positive in DBS, the values were 3.92, 3.62, 3.5, 3.54, and 3.64 HIV-RNA copies/ml.
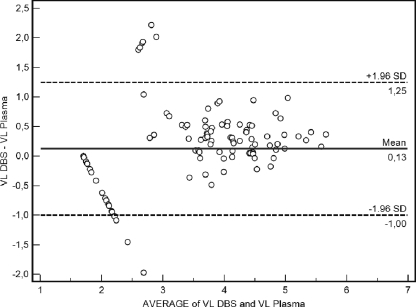
A Bland and Altman plot showed that the mean difference between DBS and plasma values was 0.13, and the standard deviations multiplied by 1.96 ranged from −1 to 1.25 (Fig. 2).
Fig 2.
Bland and Altman plots of average VL values comparing DBS versus plasma using the Abbott RealTime HIV-1 assay.
DISCUSSION
The ability to preserve viral nucleic acids on DBS has resulted in growing interest in the material as a source for HIV molecular testing, including measurement of the VL (10), drug resistance testing (4, 5, 13, 15), subtyping (10), and molecular diagnosis (8). This is particularly interesting for resource-limited countries, where access to standard testing procedures using blood samples is often difficult and maintenance of cold chain conditions before shipment to reference laboratories is challenging.
In the present study, we evaluated the feasibility of an automated RNA extraction and commercial real-time amplification assay to measure HIV-1 RNA on DBS using plasma determinations as a reference. This study demonstrated good correlation between the VL results obtained on DBS and plasma using the Abbott RealTime HIV assay. In addition, we demonstrated good sensitivity for the assay, with a corresponding lower limit of detection of 550 copies/ml in plasma, which may be considered enough for early detection of virological treatment failure in most instances during longitudinal follow-up of patients on ART. Marconi and colleagues have recently published similar results, although the sensitivity of their assay was slightly lower (14). For early detection of virological failure with low-level viremia, it is clear that the VL sensitivity on DBS must improve (18).
A previous study showed that manual RNA nucleic acid isolation using the Abbott mSample preparation system resulted in good correlation compared with automatic procedures (11). However, automation of the procedure may improve agreement between the results obtained testing DBS and plasma due to the reduced human intervention, lower risk of cross contamination, and more rapid turnaround time. In our current study, the correlation between the VL measurements in plasma and DBS was satisfactory and was superior to the results obtained previously using manual RNA nucleic acid isolation (11).
Overall, 26% of specimens had >0.5 log copy difference between DBS and plasma, and 8% had >1 log copy difference. The main difference between the VL measurements on DBS and plasma was the recognition of a trend toward greater values with DBS than with plasma, especially when the plasma VL values were below 1,000 HIV RNA copies/ml. In terms of specificity, 5 out of 57 specimens (9%) with plasma VL of <40 copies/ml gave low-level VL values on DBS. As intracellular HIV-1 DNA and RNA may be more easily recognized on DBS than in plasma, hypothetically this may be the source of apparently false-positive results when testing DBS collected from infected subjects with undetectable plasma VL. It also may account for the greater quantitative values in DBS than in plasma, especially in subjects harboring low-level plasma viremia. However, it should be noted that repeated PCR amplifications using nucleic acids extracted from these DBS specimens were all negative. Thus, discordant results should always be considered carefully and, when possible, confirmed by retesting.
Although the samples examined in our study all belonged to persons infected with HIV-1 clade B variants, the Abbott RealTime HIV assay has been designed to accurately quantify the VL in subjects infected with diverse HIV-1 group M and group O subtypes (20), as well as group P strains (19). The high diversity of HIV-1 in many developing countries must not be neglected and may represent a major challenge for molecular tests. Direct comparisons between the latest approved real-time VL assays for HIV monitoring are warranted in order to provide reliable information about their respective sensitivities and specificities when testing the VL on DBS. Thereafter, the performance of drug resistance tests using DBS must be similarly evaluated to maximally improve therapeutic HIV monitoring (4, 13, 15). The combination of early detection of virological treatment failure on DBS with the opportunity for drug resistance testing in the material should provide adequate time for medication switches and appropriate selection of antiretroviral drugs for subsequent regimens in patients on ART in resource-limited regions, preserving the clinical benefits of therapy and avoiding unwanted accumulation of drug resistance (5, 18). Moreover, we report the feasibility of an automated VL assay under ideal conditions. However, storage conditions for DBS need further validation, especially under unfavorable conditions, such as high temperature and high humidity.
In summary, our study showed that the Abbott RealTime platform provided good sensitivity and reliability for VL monitoring using DBS collected from individuals infected with HIV-1 clade B viruses using an automated extraction method. This information is relevant when considering how to improve access to VL testing in resource-limited settings.
ACKNOWLEDGMENTS
This work was supported in part by grants from the World Health Organization (project HQHIV0801828), Fundación Investigación y Educación en SIDA (F-IES), Red de Investigación en SIDA (RIS) (project RD06/006), and Fondo de Investigación Sanitaria (FIS) (projects CP06/0284 and PI06/1826).
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Alvarez-Muñoz MT, et al. 2005. High correlation of human immunodeficiency virus type-1 viral load measured in dried-blood spot samples and in plasma under different storage conditions. Arch. Med. Res. 36:382–386 [DOI] [PubMed] [Google Scholar]
- 2. Amellal B, Katlama C, Calvez V. 2007. Evaluation of the use of dried spots and of different storage conditions of plasma for HIV-1 RNA quantification. HIV Med. 8:396–400 [DOI] [PubMed] [Google Scholar]
- 3. Andreotti M, et al. 2010. Correlation between HIV-1 viral load quantification in plasma, dried blood spots, and dried plasma spots using the Roche COBAS Taqman assay. J. Clin. Virol. 47:4–7 [DOI] [PubMed] [Google Scholar]
- 4. Bertagnolio S, et al. 2007. HIV-1 drug resistance surveillance using dried whole blood spots. Antivir. Ther. 12:107–113 [PubMed] [Google Scholar]
- 5. Bertagnolio S, et al. 2010. Dried blood spots for HIV-1 drug resistance and viral load testing: a review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 12:195–208 [PubMed] [Google Scholar]
- 6. Bland JM, Altman DG. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307–310 [PubMed] [Google Scholar]
- 7. Brambilla D, et al. 2003. Multicenter evaluation of use of dried blood and plasma spot specimens in quantitative assays for human immunodeficiency virus RNA: measurement, precision, and RNA stability. J. Clin. Microbiol. 41:1888–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cassol S, et al. 1992. Diagnosis of vertical HIV-1 transmission using the polymerase chain reaction and dried blood spot specimens. J. Acquir. Immune Defic. Syndr. 5:113–119 [PubMed] [Google Scholar]
- 9. Cassol S, et al. 1997. Quantification of human immunodeficiency virus type 1 RNA from dried plasma spots collected on filter paper. J. Clin. Microbiol. 35:2795–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garrido C, et al. 2008. Subtype variability, virological response and drug resistance assessed on dried blood spots collected from HIV patients on antiretroviral therapy in Angola. J. Antimicrob. Chemother. 61:694–698 [DOI] [PubMed] [Google Scholar]
- 11. Garrido C, et al. 2009. Correlation between HIV-1 RNA measurements obtained with dried blood spots and those obtained with plasma by use of Nuclisens EasyQ HIV-1 and Abbott RealTime HIV load tests. J. Clin. Microbiol. 47:1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johannessen A, et al. 2009. Dried blood spots perform well in viral load monitoring of patients who receive antiretroviral treatment in rural Tanzania. Clin. Infect. Dis. 49:976–981 [DOI] [PubMed] [Google Scholar]
- 13. Hallack R, Doherty L, Wethers J, Parker M. 2008. Evaluation of dried blood spots specimens for HIV-1 drug resistance testing using the Trugene HIV-1 genotyping assay. J. Clin. Virol. 41:283–287 [DOI] [PubMed] [Google Scholar]
- 14. Marconi A, et al. 2009. Evaluation of the Abbott Real-Time HIV-1 quantitative assay with dried blood spot specimens. Clin. Microbiol. Infect. 15:93–97 [DOI] [PubMed] [Google Scholar]
- 15. Masciotra S, et al. 2007. High concordance between HIV-1 drug resistance genotypes generated from plasma and dried blood spots in antiretroviral-experienced patients. AIDS 21:2503–2511 [DOI] [PubMed] [Google Scholar]
- 16. Mbida A, et al. 2009. Meaure of viral load by using the Abbott Real Time HIV-1 assay on dried blood spots and plasma spot specimens collected in 2 rural dispensaries in Cameroon. J. Acquir. Immune Defic. Syndr. 52:9–16 [DOI] [PubMed] [Google Scholar]
- 17. Mwaba P, et al. 2003. Whole blood versus plasma spots for measurement of HIV-1 viral load in HIV-infected African patients. Lancet 362:2067–2068 [DOI] [PubMed] [Google Scholar]
- 18. Ndembi N, et al. 2010. Viral rebound and emergence of drug resistance in the absence of viral load testing: a randomized comparison between zidovudine-lamivudine plus nevirapine and zidovudine-lamivudine plus abacavir. J. Infect. Dis. 201:106–113 [DOI] [PubMed] [Google Scholar]
- 19. Plantier JC, et al. 2009. A new human immunodeficiency virus derived from gorillas. Nat. Med. 15:871–872 [DOI] [PubMed] [Google Scholar]
- 20. Swanson P, et al. 2006. Performance of the automated Abbott RealTime HIV-1 assay on a genetically diverse panel of specimens from London: comparison to VERSANT HIV-1 RNA 3.0, AMPLICOR HIV-1 MONITOR v1.5, and LCx HIV RNA quantitative assays. J. Virol. Methods 137:184–192 [DOI] [PubMed] [Google Scholar]
- 21. Thompson M, et al. 2010. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-U. S. A. panel. JAMA 304:32133. [DOI] [PubMed] [Google Scholar]
- 22. Toure-Kane C, et al. 2008. Quantitation of HIV-1 RNA in dried blood spots by the real-time Nuclisens EasyQ HIV-1 assay in Senegal. J. Virol. Methods 148:291–295 [DOI] [PubMed] [Google Scholar]
- 23. Uttayamakul S, et al. 2005. Usage of dried blood spots for molecular diagnosis and monitoring HIV-1 infection. J. Virol. Methods 128:128–134 [DOI] [PubMed] [Google Scholar]
- 24. Vessiere A, et al. 2010. First evidence of a HIV-1 M/O recombinant form circulating outside Cameroon. AIDS 24:1079–1082 [DOI] [PubMed] [Google Scholar]