Abstract
We assessed the performance of galactomannan and (1→3)-β-d-glucan in 29 serum samples from patients with multiple myeloma and Waldenstrom's macroglobulinemia without invasive fungal disease to address issues of false positivity and uninterpretable results previously reported among patients with these conditions. Galactomannan and (1→3)-β-d-glucan assays were not falsely elevated in any patient. (1→3)-β-d-glucan assay results were uninterpretable in 24% of patients. Patients with IgG levels of >2,000 mg/dl had higher odds of uninterpretable (1→3)-β-d-glucan results.
TEXT
Invasive fungal disease (IFD) is a significant cause of morbidity and mortality in patients with hematologic malignancy (9, 14, 15, 19). Multiple myeloma (MM) and Waldenstrom's macroglobulinemia (WM) are plasma cell diseases associated with increased monoclonal serum immunoglobulin levels. Serum galactomannan (GM) and (1→3)-β-d-glucan (BG) antigen assays are useful for the diagnosis of invasive aspergillosis and other IFD in patients with appropriate risk factors and a compatible clinical syndrome (2–7, 10–13, 16–18). MM and WM patients may have uninterpretable GM or BG assay results, presumably due to optical interference from high levels of paraprotein (20), potentially making these assays less useful in this population. Other known causes of optical artifacts when using the BG assay include excessive hemolysis, hyperbilirubinemia, and lipemia. In one study, GM was reported to be falsely elevated in up to 50% of patients with immunoglobulin G (IgG)-subtype MM (8). We conducted this study to assess the performance of GM and BG assays in MM and WM patients without IFD, evaluate the rate of false-positive results, and identify potential factors associated with uninterpretable results.
Serum samples were obtained from MM and WM patients without clinical or radiologic signs of IFD who presented to Dana-Farber Cancer Institute in Boston, MA, between November 2010 and January 2011. Serum samples were tested using commercially available GM (Platelia; Bio-Rad Laboratories, Hercules, CA) and BG (Fungitell; Associates of Cape Cod, East Falmouth, MA) assays by technicians blinded to patient characteristics and sample immunoglobulin type and levels. Pertinent clinical data, including patient demographics, MM type, and Ig levels, were recorded. All analyses were performed using STATA 11 (College Station, TX). Logistic regression was used to assess factors increasing the odds of an uninterpretable BG value.
To investigate the effect of BG assay buffer pH in the generation of potential optical artifacts, serum samples from patients with high IgG levels (>2,000 mg/dl) were incubated at different pH levels (6.5 to 8.0) and assessed for paraprotein precipitation and development of optical artifacts. Samples were processed using the following protocol: 5 μl of serum was preincubated with 20 μl of an alkaline pretreatment reagent (0.125 M KOH, 0.6 M KCl) at 37°C, and then 100 μl of a 0.1 M Bis-Tris propane buffer at a range of pH values (6.5 to 8.0) or water was added to this mixture. Optical density was read at A405 minus A490 using a Molecular Devices ThermoMax microplate reader (SoftMax Pro v3.1.1). Each experiment was conducted in quadruplicate. This study was approved by the Office for Human Research Studies at Dana-Farber/Harvard Cancer Center.
Serum samples were obtained from 29 MM and WM patients. Baseline characteristics, immunoglobulin class, and levels are presented in Table 1. All serum samples tested negative for GM, with a median GM index of 0.17 (range, 0.12 to 0.30).
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| No. of patients | 29 |
| Median age, years (range) | 63 (41–88) |
| Sex, no. (%) | |
| Male | 14 (48%) |
| Female | 15 (52%) |
| Race/ethnicity, no. (%) | |
| White | 27 (93%) |
| Nonwhite | 2 (7%) |
| Underlying disease, no. (%) | |
| Multiple myeloma | 17 (59%) |
| Waldenstrom's macroglobulinemia | 11 (38%) |
| Solitary plasmacytoma | 1 (3%) |
| Immunoglobulin type | |
| IgG | 11 |
| IgM | 10 |
| IgA | 1 |
| Light chain | 5 |
| Nonsecretory | 2 |
| Immunoglobulin levels, mg/dl; median (range) | |
| Overall | |
| IgG (n = 29) | 896 (109–4,050) |
| IgM (n = 29) | 45 (2–5,080) |
| Multiple myeloma (n = 17) | |
| IgG | 1990 (896–4,050) |
| IgG > 2,000 mg/dl (n = 4) | 2,840 (2,560–4,050) |
| Waldenstrom's macroglobulinemia (n = 12) | |
| IgM | 1,700 (57–5,080) |
A total of 22 (76%) BG assays were negative, with BG values of <31 pg/ml. A total of 7 samples (24%) had uninterpretable BG assay results due to optical artifacts, including four IgG-type MM, one IgM-type WM, and two light-chain MM. Of 4 samples with IgG levels of >2,000 mg/dl, 3 had an uninterpretable BG assay result. On univariable logistic regression, an IgG level of >2,000 mg/dl, was the only factor predictive of an uninterpretable BG result (odds ratio, 7.5; 95% confidence interval [CI], 0.9 to 60.4; P = 0.05). Sample turbidity, total protein, bilirubin, and IgM levels were not predictive of uninterpretable BG results.
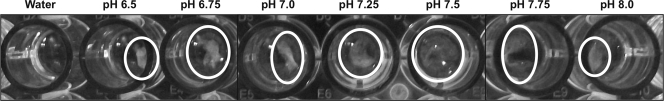
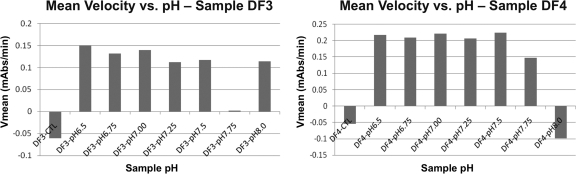
Precipitation was observed when BG assay buffer was added to serum from 3 patients with IgG levels of >2,000 mg/dl across a wide pH range (6.5 to 8.0). In contrast, precipitation was not observed when water was used as a diluent (Fig. 1). There was no correlation between pH and change in the mean velocity (Vm) of optical density of the BG assay (Fig. 2).
Fig 1.
Incubation of a representative serum sample with IgG levels of >2,000 mg/dl at different pH levels (6.5 to 8.0). White precipitate is visible in all but the first well, in which water was used as the diluent.
Fig 2.
Correlation between pH and Vm of optical density in two representative samples with IgG levels of >2,000 mg/dl. CTL, negative control; V mean, mean velocity; mAbs, milliabsorbance units.
In contrast to a previous report in which 50% (11 out of 22) of patients with IgG-type MM had false-positive GM assay results (8), we did not find any falsely elevated GM in our cohort of patients with MM and WM. Although we found no false-positive BG results, BG results were not interpretable due to optical artifacts in 24% of samples, likely due to paraprotein precipitation.
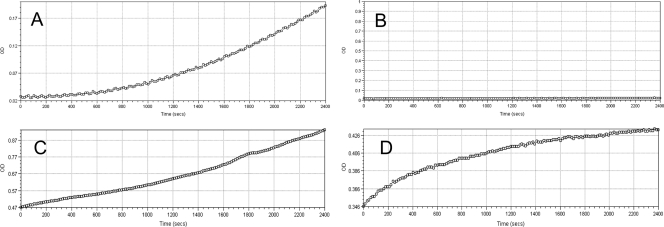
The Fungitell BG assay relies on the activation of the BG-sensitive zymogen proteases of the reagent, with hydrolysis of a chromogenic substrate (leucine–glycine–arginine–para-nitroaniline) and an increase in the A405 optical density. The final assay result is based upon the mean velocity (Vm) of the rise in optical density over the 40-min test period (Fungitell test procedure outline: http://www.acciusa.com/clinical/fungitell/FungitellInfoDownloads.html). The pattern of optical density development over the incubation period of the assay offers an opportunity to observe potential optical artifacts. In cases with optical artifacts, the optical density usually rises immediately, without the initial lag period associated with zymogen protease activation (Fig. 3).
Fig 3.
(A) BG assay kinetic trace of a positive sample without optical artifacts; (B) kinetic trace of a negative control; (C and D) kinetic trace showing optical artifacts. OD, optical density. In panels C and D note initial high OD readings and the absence of initial lag periods consistent with optical artifacts.
MM patients with IgG levels of >2,000 mg/dl had higher odds of uninterpretable BG results secondary to optical artifacts. While IgA and IgM have isoelectric points in the acidic range (pH 4.5 to 6.5), IgG has an isoelectric point in the basic range (pH 6.5 to 9.5) (1). As the BG assay buffer has a pH of 7.4, it is possible that samples with high IgG levels precipitate, since they experience a pH level close to their isoelectric point. However, changing the BG assay buffer pH (range 6.5 to 8.0) had no effect on paraprotein precipitation, and only samples with a water diluent had no observed precipitation. There was also no correlation between pH and Vm of optical density (Fig. 2). The observed variation in Vm was most likely due to paraprotein precipitation randomly interfering with the optical density reading. Whether optical interference effect is limited to monoclonal Ig needs to be further studied.
In summary, GM and BG were not falsely elevated in patients with MM or WM and can be used in patients with suspected invasive fungal disease. Patients with plasma cell disorders and IgG levels of >2,000 mg/dl have higher odds of uninterpretable BG results.
ACKNOWLEDGMENTS
M.A.F. is an employee of Associates of Cape Cod; all other authors report no conflict of interest.
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Chiodi F, Sidén Å, Ösby E. 1985. Isoelectric focusing of monoclonal immunoglobulin G, A and M followed by detection with the avidin-biotin system. Electrophoresis 6:124–128 [Google Scholar]
- 2. Hachem RY, et al. 2009. Utility of galactomannan enzyme immunoassay and (1,3) β-d-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J. Clin. Microbiol. 47:129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kami M, et al. 2000. Computed tomographic scan of the chest, latex agglutination test and plasma (1AE3)-beta-d-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica 85:745–752 [PubMed] [Google Scholar]
- 4. Kawazu M., et al. 2004. Prospective comparison of the diagnostic potential of real-time PCR, double-sandwich enzyme-linked immunosorbent assay for galactomannan, and a (1→3)-β-d-glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. J. Clin. Microbiol. 42:2733–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koo S, Bryar JM, Page JH, Baden LR, Marty FM. 2009. Diagnostic performance of the (1→3)-beta-d-glucan assay for invasive fungal disease. Clin. Infect. Dis. 49:1650–1659 [DOI] [PubMed] [Google Scholar]
- 6. Marty FM, Koo S. 2009. Role of (1→3)-beta-d-glucan in the diagnosis of invasive aspergillosis. Med. Mycol. 47(Suppl 1):S233–S240 [DOI] [PubMed] [Google Scholar]
- 7. Mennink-Kersten MA, Verweij PE. 2006. Non-culture-based diagnostics for opportunistic fungi. Infect. Dis. Clin. North Am. 20:711–727, viii [DOI] [PubMed] [Google Scholar]
- 8. Mori Y., et al. 2010. High incidence of false-positive Aspergillus galactomannan test in multiple myeloma. Am. J. Hematol. 85:449–451 [DOI] [PubMed] [Google Scholar]
- 9. Neofytos D, et al. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 48:265–273 [DOI] [PubMed] [Google Scholar]
- 10. Obayashi T, Negishi K, Suzuki T, Funata N. 2008. Reappraisal of the serum (1→3)-beta-d-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin. Infect. Dis. 46:1864–1870 [DOI] [PubMed] [Google Scholar]
- 11. Obayashi T, et al. 1995. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17–20 [DOI] [PubMed] [Google Scholar]
- 12. Odabasi Z, et al. 2004. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199–205 [DOI] [PubMed] [Google Scholar]
- 13. Ostrosky-Zeichner L, et al. 2005. Multicenter clinical evaluation of the (1→3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654–659 [DOI] [PubMed] [Google Scholar]
- 14. Pagano L, et al. 2006. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91:1068–1075 [PubMed] [Google Scholar]
- 15. Pagano L, et al. 2007. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study—Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin. Infect. Dis. 45:1161–1170 [DOI] [PubMed] [Google Scholar]
- 16. Pazos C, Ponton J, Del Palacio A. 2005. Contribution of (1→3)-β-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J. Clin. Microbiol. 43:299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Persat F, et al. 2008. Contribution of the (1→3)-β-d-glucan assay for diagnosis of invasive fungal infections. J. Clin. Microbiol. 46:1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Senn L, et al. 2008. 1,3-Beta-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin. Infect. Dis. 46:878–885 [DOI] [PubMed] [Google Scholar]
- 19. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. 2007. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin. Infect. Dis. 44:531–540 [DOI] [PubMed] [Google Scholar]
- 20. Yoshida K. 2006. Recent advances of serodiagnosis for systemic fungal infections. Nihon Ishinkin Gakkai Zasshi 47:135–142 (In Japanese.) [DOI] [PubMed] [Google Scholar]