Abstract
We examined the incidence of candidemia, Candida species distribution, and antifungal susceptibility patterns in a pediatric institution. We identified 301 episodes of candidemia from 2001 to 2010 inclusive. Annual incidence decreased from 0.68 to 0.12 cases/1,000 patient days between 2004 and 2010. Candida albicans was the most common species, followed by C. parapsilosis. All isolates tested were susceptible to amphotericin B and caspofungin, but 11% were resistant or dose-dependently susceptible to fluconazole.
INTRODUCTION
Among the etiologic agents responsible for bloodstream infections in children, Candida spp. raise particular concern due to the elevated mortality associated with candidemia (1, 7, 15). This heightened mortality is the consequence of numerous factors, including organism virulence (6) and the inherent vulnerability of children at risk of developing candidemia (4, 12).
In the adult literature, early initiation of effective antifungal therapy (5, 11) and timely removal of central venous catheters (8, 19) are strategies that appear to mitigate mortality in some populations. Initiation of appropriate empirical therapy is, in turn, dependent on knowledge of the distribution of Candida spp. and antifungal susceptibility trends in a given population. Our study objective was to investigate changes in the incidence of candidemia, causative Candida spp., and antifungal susceptibility patterns over a 10-year period at a tertiary pediatric institution.
(This study was presented as an oral abstract at the 111th American Society of Microbiology General Meeting in New Orleans, LA [14].)
MATERIALS AND METHODS
This was a retrospective cohort study. All cases of candidemia in patients from 0 to 18 years of age in 2001 to 2010 inclusive at The Children's Hospital of Philadelphia were examined. This study was approved by the Institutional Review Board at The Children's Hospital of Philadelphia.
All Candida sp.-positive blood cultures from 1 January 2001 to 31 December 2010 inclusive were identified using microbiology laboratory records. Postmortem blood cultures were excluded. Available antifungal susceptibility data and limited demographic information, including age at diagnosis and the responsible clinical service, were recorded from 1 January 2008 (when antifungal susceptibility testing was initiated at our institution) through 31 December 2010.
A new candidemia episode was deemed to have occurred with a first episode of candidemia, if >4 weeks had elapsed between positives, or if a new Candida species was isolated.
The annual candidemia incidence rate was calculated as the number of candidemia episodes per 1,000 patient days per fiscal year. Patient day data are provided at our institution by fiscal year (as opposed to calendar year). Because only aggregate data were available for Candida sp.-positive blood cultures in 2001, 2002, and 2003, incidence was calculated only for fiscal years 2004 through 2010 inclusive.
The BacT/Alert continuous-monitoring blood culture system and BacT/Alert PF Pediatric FAN blood culture bottles (bioMérieux, Durham, NC) were in use throughout the study period.
Yeast isolates were identified to the species level using microscopy, colonial morphology, the Vitek 2 YST ID Card (bioMérieux, Durham, NC), and where appropriate, the BBL CHROMagar Candida (BD, Sparks, MD); the rapid trehalose assimilation test was used for Candida glabrata isolates.
Yeast susceptibility testing was performed using the YeastOne susceptibility microtiter system (TREK Diagnostic Systems, Cleveland, OH) and adhering to manufacturer's instructions. All Candida sp. isolates were tested for susceptibility to amphotericin B, fluconazole, itraconazole, voriconazole, caspofungin, and 5-flucytosine. MICs were interpreted using Clinical and Laboratory Standards Institute (CLSI) breakpoints (2, 3).
RESULTS
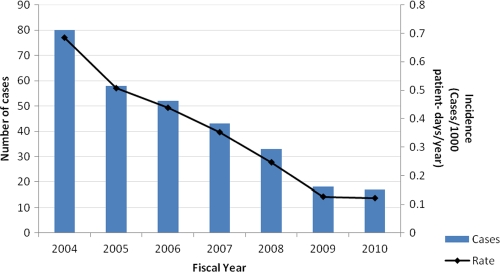
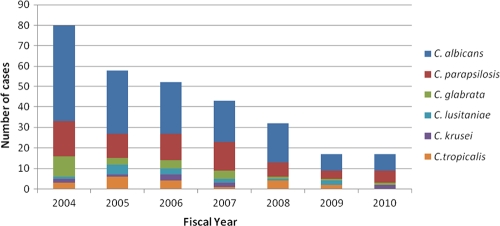
Candida spp. were identified in 1,350 blood cultures between 2001 and 2010 inclusive, and 301 unique episodes of candidemia were identified in fiscal years 2004 through 2010 inclusive. The annual candidemia incidence rate decreased from 0.68 to 0.12 episodes/1,000 patient days/year between fiscal years 2004 and 2010 (Fig. 1). C. albicans was the most common causative species (46% to 59% yearly), followed by C. parapsilosis (Fig. 2).
Fig 1.
Total candidemia cases and incidence by fiscal year.
Fig 2.
Candidemia cases by Candida species by fiscal year.
Among the 59 candidemia episodes identified between 2008 and 2010, 22% were under the care of the gastroenterology service, 19% were under oncology, 12% were under neonatal intensive care, 12% were under critical care, and the remaining 35% were under the care of various services, including general pediatrics, hematology, cardiology, and adolescent medicine.
All of the candidemia-causing isolates that underwent antifungal susceptibility testing (n = 53) were susceptible to amphotericin B and caspofungin. Of these 53 isolates, 6 (11%) had MICs that indicated resistance or dose-dependent susceptibility to fluconazole. These included a child under the care of the gastroenterology service who had two separate episodes of C. krusei candidemia (MICs, 64 and 128 μg/ml), an episode of C. glabrata (MIC = 32 μg/ml) candidemia and an episode of C. parapsilosis (MIC = 16 μg/ml) candidemia in children under the care of the neonatal intensive care service, and an episode of C. tropicalis (MIC = 64 μg/ml) candidemia and a case of C. glabrata (MIC = 16 μg/ml) candidemia in children under the care of the oncology service.
DISCUSSION
The epidemiology of pediatric candidemia is currently in evolution due to increases in at-risk patients as a result of advances in oncology and transplant and critical care medicine, the introduction of new antifungal agents, and expansion of antifungal use to include prophylactic regimens. This study examined the impact of these developments on the epidemiology of candidemia at a tertiary pediatric hospital.
With respect to our findings, our calculated incidence of 0.12 to 0.68 infections/1,000 patient days/year was generally in the same range as other published analyses from pediatric institutions that used patient days in the denominator. Reported incidence rates in the literature range from 0.06 to 0.28 infections/1,000 patient days/year (4, 10). We noted, however, a striking decline in incidence between 2004 and 2010, a trend that has not been described in the pediatric literature. Aggressive infection control initiatives at our institution substantially decreased overall central-line-associated bloodstream infections (CLABSI) during the study period and may have played a central role in the trend observed. Particularly effective initiatives included “bundling” of recommended best practices that are known to reduce the incidence of CLABSI (13) and intensified training of nursing personnel.
In our cohort, 23% of the candidemia patients were under the care of the gastroenterology service, 19% were under the care of oncology, 12% were under the care of neonatal intensive care, and 12% were under the care of critical-care services, which is consistent with other pediatric data described in the literature (4, 12).
The predominant causative Candida sp. was C. albicans throughout the period examined, consistently accounting for about half of the candidemia cases in our cohort. Despite the introduction of caspofungin into our hospital formulary in 2007, we did not see selection of and emergence of C. parapsilosis as a leading causative species, as reported by others (elevated in vitro caspofungin MICs have been noted for this Candida species) (9, 12).
This study did have two limitations. First, it was limited to a single tertiary pediatric center. As a result, the generalizability of our findings is somewhat limited. Second, it was designed to examine long-term hospital level trends related to the incidence of candidemia, causative Candida spp., and antifungal susceptibility patterns with the specific aim of providing guidance for antifungal use in our institution. We therefore did not investigate clinical outcomes or risk factors for candidemia.
Finally, the Infectious Diseases Society of America's Clinical Practice Guidelines for the Management of Candidiasis allow for the empirical use of fluconazole in clinically well nonneutropenic patients with no recent azole exposure (16). Our antifungal susceptibility data revealed cases of fluconazole-nonsusceptible candidemia outside populations that would typically receive azole prophylaxis at our institution (e.g., patients undergoing stem cell transplantation and selected oncology patients), indicating that fluconazole should be used with caution for empirical candidemia therapy. We noted proposed revisions to the CLSI Candida sp. breakpoints in the literature, but application of the lower breakpoints for fluconazole did not result in additional cases of candidemia dose-dependently susceptible or resistant to fluconazole in our cohort (17). Amphotericin B and caspofungin appeared to be appropriate first-line agents for the empirical treatment of candidemia in all of the patients in our institution. Interpretation of C. albicans MICs using proposed lowered caspofungin breakpoints revealed two cases of C. albicans candidemia with MICs of 0.5 μg/ml (intermediate), although to our knowledge, these revisions have yet to be ratified by CLSI (18).
ACKNOWLEDGMENTS
We thank Susan Coffin, the Medical Director of Infection Prevention and Control at The Children's Hospital of Philadelphia, for her assistance in relating our findings with her program's CLABSI reduction initiatives. We also thank Michelle Precourt, Tim Stewart, and Annie O'Neill for their assistance in obtaining administrative data for this study.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Blyth CC, et al. 2009. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics 123:1360–1368 [DOI] [PubMed] [Google Scholar]
- 2. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—third edition. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; third informational supplement. CLSI document M27-S3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Dutta A, Palazzi DL. 2011. Candida non-albicans versus Candida albicans fungemia in the non-neonatal pediatric population. Pediatr. Infect. Dis. J. 30:664–668 [DOI] [PubMed] [Google Scholar]
- 5. Garey KW, et al. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25–31 [DOI] [PubMed] [Google Scholar]
- 6. Haynes K. 2001. Virulence in Candida species. Trends Microbiol. 9:591–596 [DOI] [PubMed] [Google Scholar]
- 7. Horn DL, et al. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 48:1695–1703 [DOI] [PubMed] [Google Scholar]
- 8. Karlowicz MG, Hashimoto LN, Kelly RE, Jr, Buescher ES. 2000. Should central venous catheters be removed as soon as candidemia is detected in neonates? Pediatrics 106:E63. [DOI] [PubMed] [Google Scholar]
- 9. Levy I, Rubin LG, Vasishta S, Tucci V, Sood SK. 1998. Emergence of Candida parapsilosis as the predominant species causing candidemia in children. Clin. Infect. Dis. 26:1086–1088 [DOI] [PubMed] [Google Scholar]
- 10. MacDonald L, Baker C, Chenoweth C. 1998. Risk factors for candidemia in a children's hospital. Clin. Infect. Dis. 26:642–645 [DOI] [PubMed] [Google Scholar]
- 11. Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neu N, et al. 2009. Epidemiology of candidemia at a children's hospital, 2002 to 2006. Pediatr. Infect. Dis. J. 28:806–809 [DOI] [PubMed] [Google Scholar]
- 13. O'Grady NP, et al. 2011. Guidelines for the prevention of intravascular catheter-related infections, 2011. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf [Google Scholar]
- 14. Ota KV, McGowan KL. 2011. Declining incidence of candidemia in a pediatric population, abstr 176, p 62 Abstr. 111th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC [Google Scholar]
- 15. Pappas PG, et al. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634–643 [DOI] [PubMed] [Google Scholar]
- 16. Pappas PG, et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. 2011. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Diagn. Microbiol. Infect. Dis. 71:252–259 [DOI] [PubMed] [Google Scholar]
- 18. Pfaller MA, et al. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14:164–176 [DOI] [PubMed] [Google Scholar]
- 19. Rex JH, et al. 1995. Intravascular catheter exchange and duration of candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Clin. Infect. Dis. 21:994–996 [DOI] [PubMed] [Google Scholar]