Abstract
Outbreaks of sexually transmitted hepatitis C virus (HCV) infections have been recently reported in HIV-infected men who have sex with men (MSM) in Europe, Australia, and North America. Little is known concerning whether this also occurs in other Asia-Pacific countries. Between 1994 and 2010, a prospective observational cohort study was performed to assess the incidence of recent HCV seroconversion in 892 HIV-infected patients (731 MSM and 161 heterosexuals) who were not injecting drug users. A nested case-control study was conducted to identify associated factors with recent HCV seroconversion, and phylogenetic analysis was performed using NS5B sequences amplified from seroconverters. During a total followup duration of 4,270 person-years (PY), 30 patients (3.36%) had HCV seroconversion, with an overall incidence rate of 7.03 per 1,000 PY. The rate increased from 0 in 1994 to 2000 and 2.29 in 2001 to 2005 to 10.13 per 1,000 PY in 2006 to 2010 (P < 0.05). After adjustment for age and HIV transmission route, recent syphilis remained an independent factor associated with HCV seroconversion (odds ratio, 7.731; 95% confidence interval, 3.131 to 19.086; P < 0.01). In a nested case-control study, seroconverters had higher aminotranferase levels and were more likely to have CD4 ≥ 200 cells/μl and recent syphilis than nonseroconverters (P < 0.05). Among the 21 patients with HCV viremia, phylogenetic analysis revealed 7 HCV transmission clusters or pairs (4 within genotype 1b, 2 within genotype 2a, and 1 within genotype 3a). The incidence of HCV seroconversion that is associated with recent syphilis is increasing among HIV-infected patients in Taiwan.
INTRODUCTION
Hepatitis C virus (HCV) has been traditionally known to be transmitted parenterally while not efficiently transmitted through sexual contact (29, 32, 45). In a 10-year prospective study, 3 HCV infections were observed in 776 couples with discordant HCV serostatus, with an incidence rate of 0.37 per 1,000 person-years (PY); however, molecular investigations failed to document sexual transmission of HCV infection (45). Likewise, a long-term prospective study also demonstrated a low incidence of interspousal transmission of HCV, with an annual rate of 0.2% in Taiwan (19). In the Swiss HIV Cohort Study, the incidence of HCV seroconversion in patients with a history of injection drug use was 74 per 1,000 PY, whereas the incidence was only 2.3 per 1,000 PY in those without such a history (32).
After the introduction of highly active antiretroviral therapy (HAART) in 1996, sexually transmitted infections such as syphilis and lymphogranuloma venereum have been disproportionately diagnosed in HIV-infected men who have sex with men (MSM) in Western Europe (8), and several studies have demonstrated increased temporal trends in HCV incidence from 1 to 3 per 1,000 PY to >10 per 1,000 PY (43). Furthermore, outbreaks of sexually transmitted HCV infections have been reported among HIV-infected MSM in several European countries (3, 7, 13, 16, 36, 44), Australia (27), and North America (9, 24, 37). Phylogenetic analysis also revealed monophyletic transmission clusters of HCV within the MSM populations of major cities in England, France, The Netherlands, and Germany; this HCV incidence had already increased among HIV-infected MSM from the mid-1990s (44, 46).
Compared with patients with HCV monoinfection, HIV/HCV-coinfected patients have an accelerated rate of fibrosis progression (20), and such coinfections are associated with higher rates of end-stage liver disease-related morbidity and mortality (17, 38). Compared with HCV monoinfected patients, HIV-infected patients with chronic HCV infection have poorer treatment responses to pegylated interferon (IFN) and ribavirin. In HCV-monoinfected patients treated with pegylated IFN plus ribavirin, the overall sustained virologic response (SVR) is 41% to 64% (12, 18, 25, 34). In contrast, in HIV/HCV-coinfected patients treated with pegylated IFN plus ribavirin, the overall SVR in those infected with HCV genotype 1 is only 17% to 50% (4, 30, 31, 39). While more studies are needed to identify the optimal treatments for HIV-infected patients with acute HCV infection (33), the treatment responses seen with acute HCV infection in HIV-infected populations are promising, with an overall SVR of 46% to 95%, even in patients with genotype 1 or 4 infections (1, 43).
Given numerous studies reporting HCV infection as an emerging sexually transmitted infection among HIV-infected MSM in western countries (3, 7, 9, 13, 16, 24, 27, 36, 37, 44), whether such infections are emerging in Asia-Pacific countries is largely unknown. Additionally, identification of factors associated with recent HCV seroconversion in HIV-infected populations may offer an opportunity for medical practitioners to initiate anti-HCV treatment early to achieve a higher SVR. Thus, the present study aimed to assess the incidence of recent HCV infection and its associated factors among HIV-infected patients in Taiwan who acquired their HIV infections through sexual contacts instead of injecting drug use and to investigate whether HCV transmission clusters occurred.
MATERIALS AND METHODS
Setting.
In Taiwan, HIV-infected patients are provided free-of-charge access to HIV care, including HAART, which was introduced on 1 April 1997 at designated hospitals around the island. At National Taiwan University Hospital, the largest designated hospital providing inpatient and outpatient care in Taiwan, a computerized data collection form has been used since 1 January 1994 to collect the demographic and clinical data (date of birth, gender, and HIV transmission route) and sequential laboratory data (CD4 count, plasma HIV RNA load, serologic marker of hepatitis virus, and rapid plasma reagin [RPR] titer) of HIV-infected patients aged ≥15 years. Liver function tests were performed every 3 to 4 months. Information on infection risk for HIV transmission was collected only at baseline and not at follow-up. The decision to start HAART was made according to the national guidelines for HIV treatment.
The present study excluded HIV-infected injecting drug users (IDUs) and included only patients with a negative anti-HCV result at baseline who had undergone at least one subsequent anti-HCV antibody test to estimate the incidence of recent HCV seroconversion. The Research Ethics Committee of the hospital approved the study and waived the need of informed consent.
Nested case-control study.
A nested case-control study was performed to compare clinical characteristics of seroconverters to those of nonseroconverters. HCV seroconverters were selected as case patients, and 2 patients without HCV seroconversion who had a duration of follow-up similar to that of case patients were selected as controls. The two groups were matched for gender and age (±5 years).
Laboratory investigations.
Antibodies to HCV were determined with a third-generation enzyme immunoassay (Ax SYM HCV III; Abbott Laboratories, North Chicago, IL). A 336-bp fragment covering partial HCV NS5B (nucleotides [nt] 8294 to 8629 relative to HCV reference strain H77) was amplified by PCR; the PCR conditions are briefly described below. The first primer pair used was NS5B_513 (5′-CCA ATW SMC ACN ACC ATC ATG GC-3′) and aNS5B_1200 (5′-GAN ACR TTK GAK GAR CAW GAT GT-3′). The amplification conditions were 30 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 2 min and a final extension at 72°C for 7 min. A 1-μl aliquot of the first-round PCR product was used for the second-round PCR under conditions that were the same as those used for the first round. The second primer pair used was NS5B_755 (5′-TAT GAY ACC CGC TGY TTT GAC TC-3′) and aNS5B_1121 (5′-GCN GAR TAY CTV GTC ATA GCC TC-3′). The PCR results were sequenced. Phylogenetic analysis was performed to determine the HCV genotypes by the use of NS5B sequences amplified from HCV-infected patients, including IDUs, and patients with HCV infection at baseline (prevalent cases) who acquired HCV infections during the follow-up (incident cases) in our cohort (6). The study and reference sequences were aligned using the Clustal W program with minor manual adjustment. The tree was constructed by the neighbor-joining method based on the Kimura 2-parameter distance matrix listed in MEGA software (version 3.0) (22).
Definitions.
Recent HCV seroconversion was defined as the first positive anti-HCV detected within 1 year after the last negative anti-HCV. The date of seroconversion was assigned as the midpoint between the date of the last negative and that of the first positive anti-HCV result. The incidence rates of HCV seroconversion in 1994 to 2010, 1994 to 2000, 2001 to 2005, and 2006 to 2010 were estimated. Within the 6 months of HCV seroconversion or within the 6 months of the last anti-HCV test, patients with new RPR seroreactivity or a 4-fold increase in RPR titers were diagnosed as representing recent acquisition of syphilis (5).
Statistical analysis.
All statistical analyses were performed using SPSS software, version 16.0 (SPSS Inc., Chicago, IL). Categorical variables were compared using χ2 or Fisher's exact test and noncategorical variables using Student's t test or a Mann-Whitney U test. The rate of incidence of HCV seroconversion in each study period was calculated as the number of episodes of HCV seroconversion per 1,000 PY of observation. Poisson regression was used to compare incidence rates of HCV seroconversion among the three study periods (1994 to 2000, 2001 to 2005, and 2006 to 2010).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the 22 HCV NS5B partial sequences that were amplified from 21 seroconverters, which included two consecutive sequences amplified from the same individual (MSM8924), are JQ060115 to JQ060136. The accession numbers for the other sequences are DQ663598 to DQ663610, HM031193.1 to HM031218.1, and DQ666240 to DQ666268.
RESULTS
Of 2,507 HIV-positive patients seeking medical care at National Taiwan University Hospital (NTUH) between 1994 and 2010, 1,773 (70.7%) were MSM, 480 (19.1%) were heterosexuals, and 187 (7.5%) were IDUs. After the 187 IDUs were excluded, anti-HCV was positive at baseline in 6.5% (102/1,576) of MSM and 8.6% (36/420) of heterosexuals. The present study included a total of 892 patients (35.6%), including 731 MSM and 161 heterosexuals, with negative anti-HCV at baseline and at least one anti-HCV test result during follow-up whose only stated risk of HIV infection was sexual contact. Compared with 1,428 (2,507 − 187 − 892 = 1,428) non-IDU patients who were not included in this study, the 892 study subjects were younger (mean, 41 versus 42 years), less likely to be homosexuals (82.0% versus 73.0%), and more likely to be tested for syphilis (86.3% versus 77.7%) and had a higher plasma HIV RNA load (4.81 versus 4.64 log10 copies/ml) (all P < 0.05); no statistically significant differences were noted in HBsAg seropositivity, RPR titers, and CD4 counts (data not shown).
During the total follow-up duration of 4,270 PY, 30 patients (3.36%) had HCV seroconversion, with an overall incidence rate of 7.03 per 1,000 PY. The rate increased from 0 per 1,000 PY (0/283 PY) in 1994 to 2000 and 2.29 per 1,000 PY (3/1,309 PY) in 2001 to 2005 to 10.13 per 1,000 PY (27/2,667 PY) in 2006 to 2010 (P < 0.05). After exclusion of 161 heterosexuals, the overall incidence rate of HCV seroconversion was 9.25 per 1,000 PY (28/3,025.9 PY) in 731 HIV-infected MSM, which increased from 0 per 1,000 PY in 1994 to 2000 and 3.49 per 1,000 PY (3/858.79 PY) in 2001 to 2005 to 12.32 per 1,000 PY (25/2,029.38 PY) in 2006 to 2010 (P < 0.05).
Compared with 862 patients without HCV seroconversion, 30 HCV seroconverters were more likely to have recent syphilis (43.5% versus 9.4%; P < 0.001), whereas no statistically significant differences existed between the 2 groups in terms of age, gender, HIV transmission routes, HBsAg seropositivity, baseline RPR titers, CD4 counts, and plasma HIV RNA loads (Table 1). After adjustment for age and HIV transmission routes, recent syphilis remained an independent factor associated with HCV seroconversion (adjusted odds ratio, 7.731; 95% confidence interval, 3.131 to 19.086; P < 0.001) in multivariate analysis (Table 2).
Table 1.
Comparisons of demographic and clinical characteristics of 30 patients with HCV seroconversion and 862 patients without HCV seroconversion
| Characteristica | Value for group |
P value | |
|---|---|---|---|
| HCV seroconversion | No HCV seroconversion | ||
| No. of patients | 30 | 862 | |
| Mean age in yrs (SD) | 44 (16) | 43 (17) | 0.831 |
| % male (n) | 100 (30) | 95.2 (821) | 0.393 |
| % risk (n) | |||
| MSM | 93.3 (28) | 81.6 (703) | 0.099 |
| Heterosexuals | 6.7 (2) | 18.4 (159) | |
| % positive HBsAg (no. of patients with indicated result/total no. of patients) | 10.0 (3) | 20.0 (168/840) | 0.176 |
| Mean baseline CD4 cells/μl (SD) | 238 (222) | 237 (234) | 0.975 |
| % of patients with <200 CD4 cells/μl (no. of patients with indicated result/total no. of patients) | 44.8 (13/29) | 53.8 (434/807) | 0.342 |
| Mean baseline log10 PVL copies/ml (SD) | 4.93 (0.97) | 4.82 (1.01) | 0.580 |
| % of patients with >5 log10 PVL copies/ml (no. of patients with indicated result/total no. of patients) | 62.5 (15/24) | 52.3 (377/721) | 0.324 |
| % of patients with baseline RPR > 1:4 (no. of patients with indicated result/total no. of patients) | 17.9 (5/28) | 13.4 (99/741) | 0.570 |
| % of patients with recent syphilis acquisition (no. of patients with indicated result/total no. of patients) | 43.5 (10/23) | 9.4 (55/584) | <0.001 |
HBsAg, hepatitis B virus surface antigen; MSM, men having sex with men; RPR, rapid plasma reagin; PVL, plasma HIV RNA load; SD, standard deviation. Recent syphilis acquisition is defined as new RPR seroreactivity or a 4-fold increase in RPR titers within 6 months of HCV seroconversion or within 6 months of the last anti-HCV test.
Table 2.
Multivariable analysis for factors associated with HCV seroconversiona
| Variable | Reference category | OR (95% CI) | P value |
|---|---|---|---|
| Age | Continuous variable | 1.015 (0.993–1.037) | 0.174 |
| MSM | Heterosexuals | 2.030 (0.449–9.169) | 0.358 |
| Recent syphilis acquisition | No recent syphilis acquisition | 7.731 (3.131–19.086) | <0.001 |
OR, odds ratio; CI, confidence interval; MSM, men who have sex with men.
In the nested case-control study, 56 nonseroconverters who had similar observation durations were identified as controls for 30 seroconverters. Seroconverters were less likely to have baseline CD4 < 200 cells/μl but more likely to have recent syphilis acquisition and abnormal aminotransferase levels (all P < 0.05) (Table 3). There were no statistically significant differences between the 2 groups in terms of HIV transmission routes, HBsAg seropositivity, VDRL titers, total bilirubin levels, CD4 counts, plasma HIV RNA loads, and receipt of HAART at the last follow-up.
Table 3.
Comparisons of clinical characteristics of 30 HCV seroconverters and 56 nonseroconverters in the nested case-control study
| Characteristica | Value for group |
P value | |
|---|---|---|---|
| HCV seroconversion | No HCV seroconversion | ||
| No. of patients | 30 | 56 | |
| Mean age in yr (SD) | 42 (10) | 39 (9) | 0.240 |
| Age in yrs at last anti-HCV test | 39 (9) | 38 (9) | 0.630 |
| % male (n) | 100 (30) | 100 (56) | NA |
| % risk (n) | |||
| MSM | 93.3 (28) | 83.9 (47) | 0.315 |
| Heterosexuals | 6.7 (2) | 16.1 (9) | |
| % positive HBsAg (no. of patients with indicated result/total no. of patients) | 10.3 (3/29) | 22.2 (12/54) | 0.180 |
| Mean baseline CD4 cells/μl (SD) | 238 (222) | 205 (236) | 0.542 |
| % of patients with <200 CD4 cells/μl (no. of patients with indicated result/total no. of patients) | 44.8 (13/29) | 68.0 (34/50) | 0.043 |
| Mean baseline log10 PVL copies/ml (SD) | 4.93 (0.97) | 4.78 (1.15) | 0.572 |
| % of patients with >5 log10 PVL copies/ml (no. of patients with indicated result/total no. of patients) | 62.5 (15/24) | 52.3 (23/44) | 0.417 |
| % of patients with baseline RPR > 1:4 (no. of patients with indicated result/total no. of patients) | 17.9 (5/28) | 21.7 (10/46) | 0.687 |
| % of patients with recent syphilis acquisition at last follow-up (no. of patients with indicated result/total no. of patients) | 45.8 (11/24) | 12.5 (5/40) | 0.003 |
| Liver function tests at last follow-up | |||
| Mean AST level in U/liter (SD) (no. of patients with data available) | 113 (179) (24) | 37 (45) (39) | 0.051 |
| % of patients with AST > 37 U/liter (no. of patients with indicated result/total no. of patients) | 75 (18/24) | 25 (10/39) | <0.001 |
| Mean ALT level in U/liter (SD) (no. of patients with data available) | 194 (212) (20) | 38 (36) (28) | 0.004 |
| % of patients with ALT > 41 U/liter (no. of patients with indicated result/total no. of patients) | 85 (17/20) | 25 (7/28) | <0.001 |
| Mean total bilirubin level in mg/dl (SD) (no. of patients with data available) | 1.20 (0.87) (20) | 1.17 (1.04) (37) | 0.903 |
| % of patients with total bilirubin levels > 1.2 mg/dl (no. of patients with indicated result/total no. of patients) | 35 (7/20) | 27 (10/37) | 0.530 |
| Use of antiretroviral therapy at last follow-up | 76.7 (23) | 82.1 (46) | 0.543 |
| Mean baseline CD4 cells/μl (SD) | 477 (214) | 464 (220) | 0.784 |
| % of patients with <200 CD4 cells/μl (no. of patients with indicated result/total no. of patients) | 10.0 (3) | 9.3 (5/54) | 0.999 |
| Mean baseline log10 PVL copies/ml (SD) | 2.30 (1.10) | 2.22 (1.11) | 0.751 |
| % of patients with >5 log10 PVL copies/ml (no. of patients with indicated result/total no. of patients) | 3.3 (1) | 3.6 (2/55) | 0.999 |
ALT, alanine transaminase; AST, aspartate transaminase.
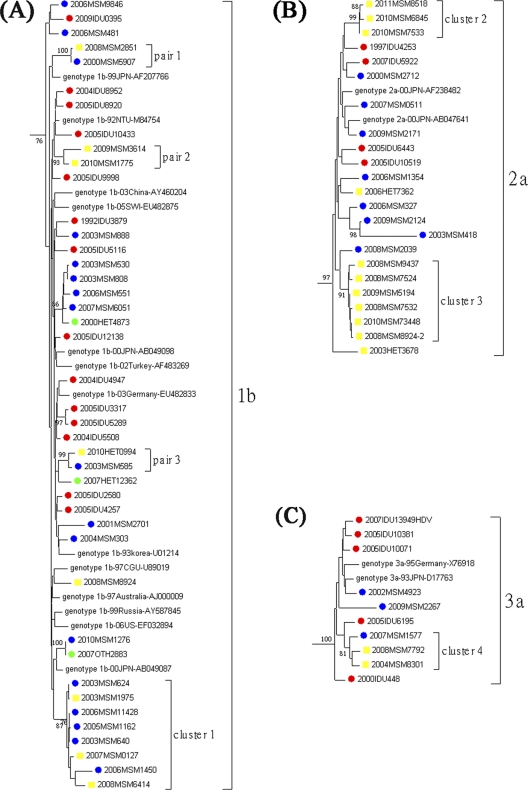
Among the 30 seroconverters, 21 (70%) had HCV viremia, with 7 strains of genotype 1b, 11 of genotype 2a, 2 of genotype 3a, and 1 of genotype 6a (Fig. 1). Phylogenetic analysis was performed to determine their relationship with prevalent HCV sequences amplified from other HIV-infected MSM (n = 33), heterosexuals (n = 8), and IDUs (n = 55). Some of the IDU sequences were excluded from analysis due to the size of the phylogenetic tree; such exclusion did not have any impacts on the outcomes (data not shown). As shown in Fig. 1 and in Fig. S1 in the supplemental material, 18 of 21 incidents of HCV infections (85.7%) clustered with at least one other sequence in the tree, resulting in 3 transmission pairs (pairs 1 to 3) and 4 clusters (clusters 1 to 4). The majority of cluster sequences were derived from MSM, except one derived from a heterosexual in pair 3. None of these sequences clustered with sequences amplified from IDUs. Three transmission pairs and cluster 1 were observed within genotype 1b. Clusters 2 and 3 were within genotype 2a, and all patients in those two clusters were recent HCV seroconverters. Cluster 4, consisting of 3 patients, was noted within genotype 3a. Two consecutive sequences were amplified from one of the study subjects, MSM8924, whose first sequence, 2008MSM8924, belonged to genotype 1b, whereas the second sequence, 2008MSM8924-2, amplified from a specimen collected 4 months later, was genotype 2a and belonged to cluster 3.
Fig 1.
Phylogenetic analysis of NS5B sequences amplified from HCV seroconverters. The 21 HCV sequences amplified from seroconverters are labeled in yellow. The 96 prevalent HCV sequences amplified from men who have sex with men (n = 33), heterosexuals (n = 8), and injecting drug users (IDUs) (n = 55) are labeled in blue, green, and red, respectively (see Fig. S1 in the supplemental material). Some HCV sequences from IDUs were excluded from analysis due to the size of the phylogenetic tree; such exclusion did not have any impact on the outcomes (data not shown). Only the HCV genoytypes with clusters/pairs are shown. Panels A, B, and C represent HCV genotypes 1b, 2a, and 3a, respectively. The horizontal branch was drawn in accordance with relative genetic distances. Bootstrap values of greater than 700 of 1,000 replicates were considered significant and are indicated at the nodes of the corresponding branches. The brackets at the right of the panels indicate the major sequence genotypes.
DISCUSSION
In this study, we demonstrated a significantly increasing trend of recent HCV seroconversion in HIV-infected patients who were not IDUs. With an overall incidence rate of 7.03 per 1,000 PY, the rate of recent HCV infection increased from 0 per 1,000 PY in 1994 to 2000 and 2.29 per 1,000 PY in 2001 to 2005 to 10.13 per 1,000 PY in 2006 to 2010. Our study also demonstrated that HCV seroconversion was independently associated with recent syphilis, suggesting the possibility of sexual HCV transmission. Furthermore, 85.7% of the incident HCV infections were found to form 3 transmission pairs and 4 clusters in the phylogenetic analysis (Fig. 1).
Since 2000, outbreaks of acute HCV infection have been documented among HIV-infected MSM in Western Europe, Australia, and North America (42). Molecular epidemiological techniques have confirmed the presence of recently evolved HCV transmission networks among HIV-infected MSM that have been linked to high-risk sexual behaviors (42). Our study is the first to describe the same phenomenon in Asia, highlighting the finding that sexually transmitted HCV infection also spreads in large urban settings outside countries traditionally classified as “Western” countries. In addition to the observation of two recent HCV transmission clusters among MSM (clusters 2 and 3 from samples collected during 2008 and 2010), we also observed one ongoing transmission of HCV genotype 1b among MSM over an extended period of time (from 2003 to 2008) (Fig. 1). For one of our study subjects, MSM8924, who was initially infected with HCV genotype 1b, an HCV genotype 2a sequence that belonged to cluster 3 was amplified from a blood specimen collected 4 months later. These data suggest that circulating HCV genotype 1b transmission is occurring among MSM in Taiwan.
Our findings are similar to observations of increasing incidence rates of HCV infections (7.9 to 17.5 per 1,000 PY) recently reported in European countries and North America (14, 15, 41, 44, 46). The majority of recent HCV infections in this study occurred in MSM, with an overall incidence rate of 9.25 per 1,000 PY. Other than HCV infections, outbreaks of lymphogranuloma venereum, rectal gonorrhea, and syphilis have been reported in HIV-infected MSM (8, 40). In a follow-up study in Taiwan, cases of syphilis in HIV-infected patients almost exclusively occurred in MSM, with an incidence of 5.8 per 100 PY and a recurrence rate of 17% (21). Improved survival coupled with serosorting among HIV-infected MSM has been proposed to be the cause of these outbreaks of sexually transmitted infections (8), and higher CD4 counts are identified as predictive of new-onset syphilis (21). Likewise, we also noted that a lower proportion of our HCV seroconverters had CD4 counts < 200 cells/μl at baseline, indicating that seroconverters might be in a healthier status after HAART (Table 3).
Phylogenetic analysis has shown a large international network of HCV transmission among HIV-infected MSM in European countries and Australia (42). HCV transmission clusters were also identified in our study (genotypes 1b, 2a, 3a, and 6a), with the majority of cluster transmissions observed within genotypes 1b and 2a (16/21 [76.2%]) (Fig. 1), which differ somewhat from the genotypes reported in Europe (genotypes 1a, 1b, 3a, and 4d) (42, 44) and those circulating among HIV-infected IDUs in Taiwan (genotypes 1a, 3a, and 6a) (23). Although we could not exclude the possibility of nasal ingestion of cocaine or amphetamines, which was previously reported to be associated with acute HCV infection (35), the findings of different circulating HCV strains between IDU and MSM populations suggest that transmission networks of recent HCV infection in our study population might be different from those of IDUs and that sexual transmission is likely, given its association with recent syphilis.
Compared with patients infected with HCV alone, HIV/HCV-coinfected patients have higher plasma HCV loads and are more likely to shed HCV RNA in the semen (2, 28). In addition, ulcerated sexually transmitted infections (such as herpes simplex virus type 2 infection or syphilis) and rough sexual practices (such as fisting) can facilitate the sexual transmission of HCV (8, 11, 14, 26, 35). Given the increasing trends of recent HCV infections in MSM in developed countries in several continents, counseling for preventing HCV transmission should be included in the public health and clinical approach to provision of information, education, and communication for sexually transmitted infections.
Given the dismal outcomes of chronic HCV infection and the encouraging treatment response of acute HCV infections to pegylated interferon and ribavirin in HIV-infected patients, early detection of acute HCV infections is imperative. Other than regular, periodic testing, tests for anti-HCV and HCV RNA load should be provided to patients with unexplained abnormal liver functions or to those diagnosed with recent sexually transmitted infections. Early treatment may be considered for patients who still have detectable HCV RNA 12 weeks after the onset of symptoms or 12 weeks after a putative exposure (1, 33).
Several limitations deserve to be acknowledged. Patients enrolled in the study were more likely to be tested for syphilis than patients not included, and not all study patients were tested for anti-HCV or HCV RNA load. Thus, we might underestimate or overestimate the incidence of HCV seroconversion and its association with recent syphilis. Second, our findings were limited to one single institution, and generalizing those findings to other institutions in Taiwan may not be appropriate. Two of the 30 seroconverters were heterosexuals rather than MSM. Because of the stigma related to homosexuality in Asian societies, it may not be easy to obtain the information on sexual preference. Since our study did not collect information on sexual behavior (such as number of sexual partners, sexual techniques, or concurrent ulcerative sexually transmitted infections) and whether MSM recreationally used drugs by injection (such as “crystal meth” [methamphetamine]) or nasal ingestion (such as cocaine) during follow-up, we were not able to give direct evidence that MSM-specific networks exist in Taiwan. However, given the identification of recent syphilis acquisition as a risk factor associated with HCV seroconversion in our study and those of others (10, 13, 14) and our findings shown in the phylogenetic tree, it is plausible that HCV was sexually transmitted.
In conclusion, we demonstrate the increasing incidence of recent HCV infections among HIV-infected MSM who are not IDUs in Taiwan and that recent syphilis acquisition is associated with HCV seroconversion. Counseling for safe sex to prevent HCV transmission and regular testing for sexually transmitted infections are crucial for successful long-term care of HIV-infected patients (33).
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Jürgen Rockstroh, Department of General Internal Medicine, University of Bonn, Germany, and Jia-Horng Kao, Director of Graduate Institute of Medicine, National Taiwan University College of Medicine, Taipei, Taiwan, for the critical review of the paper and to the Centers for Disease Control, Taiwan, for grant support (AIDS-97-1002 to C.-C.H.).
Footnotes
Published ahead of print 21 December 2011
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Boesecke C, Rockstroh JK. 2011. Treatment of acute hepatitis C infection in HIV-infected patients. Curr. Opin. HIV AIDS 6:278–284 [DOI] [PubMed] [Google Scholar]
- 2. Briat A, et al. 2005. Hepatitis C virus in the semen of men coinfected with HIV-1: prevalence and origin. AIDS 19:1827–1835 [DOI] [PubMed] [Google Scholar]
- 3. Browne R, et al. 2004. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sex. Transm. Infect. 80:326–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrat F, et al. 2004. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA 292:2839–2848 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 2006. Primary and secondary syphilis—United States, 2003–2004. MMWR Morb. Mortal. Wkly. Rep. 55:269–273 [PubMed] [Google Scholar]
- 6. Chang SY, et al. 2011. Molecular epidemiology of hepatitis D virus infection among injecting drug users with and without human immunodeficiency virus infection in Taiwan. J. Clin. Microbiol. 49:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danta M, et al. 2007. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS 21:983–991 [DOI] [PubMed] [Google Scholar]
- 8. Dougan S, Evans BG, Elford J. 2007. Sexually transmitted infections in Western Europe among HIV-positive men who have sex with men. Sex. Transm. Dis. 34:783–790 [DOI] [PubMed] [Google Scholar]
- 9. Fierer DS, et al. 2008. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J. Infect. Dis. 198:683–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fletcher S. 2003. Sexual transmission of hepatitis C and early intervention. J. Assoc. Nurses AIDS Care 14:87S–94S [DOI] [PubMed] [Google Scholar]
- 11. Fox J, et al. 2008. Increasing incidence of acute hepatitis C in individuals diagnosed with primary HIV in the United Kingdom. AIDS 22:666–668 [DOI] [PubMed] [Google Scholar]
- 12. Fried MW, et al. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975–982 [DOI] [PubMed] [Google Scholar]
- 13. Gambotti L, et al. 2005. Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001–2004. Euro. Surveill. 10:115–117 [DOI] [PubMed] [Google Scholar]
- 14. Ghosn J, et al. 2006. Increase in hepatitis C virus incidence in HIV-1-infected patients followed up since primary infection. Sex. Transm. Infect. 82:458–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giraudon I, et al. 2008. Increase in diagnosed newly acquired hepatitis C in HIV-positive men who have sex with men across London and Brighton, 2002–2006: is this an outbreak? Sex. Transm. Infect. 84:111–115 [DOI] [PubMed] [Google Scholar]
- 16. Götz HM, et al. 2005. A cluster of acute hepatitis C virus infection among men who have sex with men—results from contact tracing and public health implications. AIDS 19:969–974 [DOI] [PubMed] [Google Scholar]
- 17. Graham CS, et al. 2001. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin. Infect. Dis. 33:562–569 [DOI] [PubMed] [Google Scholar]
- 18. Hadziyannis SJ, et al. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140:346–355 [DOI] [PubMed] [Google Scholar]
- 19. Kao JH, et al. 2000. Low incidence of hepatitis C virus transmission between spouses: a prospective study. J. Gastroenterol. Hepatol. 15:391–395 [DOI] [PubMed] [Google Scholar]
- 20. Kim AY, Chung RT. 2009. Coinfection with HIV-1 and HCV—a one-two punch. Gastroenterology 137:795–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ko NY, et al. 2010. One-year follow-up of relapse to risky behaviors and incidence of syphilis among patients enrolled in the HIV case management program. AIDS Behav. 15:1067–1074 [DOI] [PubMed] [Google Scholar]
- 22. Kumar S, Tamura K, Jakobsen IB, Nei M. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245 [DOI] [PubMed] [Google Scholar]
- 23. Liu JY, et al. 2008. Extremely high prevalence and genetic diversity of hepatitis C virus infection among HIV-infected injection drug users in Taiwan. Clin. Infect. Dis. 46:1761–1768 [DOI] [PubMed] [Google Scholar]
- 24. Luetkemeyer A, et al. 2006. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 41:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manns MP, et al. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958–965 [DOI] [PubMed] [Google Scholar]
- 26. Marx MA, et al. 2003. Association of hepatitis C virus infection with sexual exposure in southern India. Clin. Infect. Dis. 37:514–520 [DOI] [PubMed] [Google Scholar]
- 27. Matthews GV, Hellard M, Kaldor J, Lloyd A, Dore GJ. 2007. Further evidence of HCV sexual transmission among HIV-positive men who have sex with men: response to Danta et al. AIDS 21:2112–2113 [DOI] [PubMed] [Google Scholar]
- 28. Matthews-Greer JM, et al. 2001. Comparison of hepatitis C viral loads in patients with or without human immunodeficiency virus. Clin. Diagn. Lab. Immunol. 8:690–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murphy EL, et al. 2000. Risk factors for hepatitis C virus infection in United States blood donors. NHLBI retrovirus epidemiology donor study (REDS). Hepatology 31:756–762 [DOI] [PubMed] [Google Scholar]
- 30. Núnez M, et al. 2007. Role of weight-based ribavirin dosing and extended duration of therapy in chronic hepatitis C in HIV-infected patients: the PRESCO trial. AIDS Res. Hum. Retroviruses 23:972–982 [DOI] [PubMed] [Google Scholar]
- 31. Pineda JA, et al. 2007. Influence of concomitant antiretroviral therapy on the rate of sustained virological response to pegylated interferon plus ribavirin in hepatitis C virus/HIV-coinfected patients. J. Antimicrob. Chemother. 60:1347–1354 [DOI] [PubMed] [Google Scholar]
- 32. Rauch A, et al. 2005. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV cohort study. Clin. Infect. Dis. 41:395–402 [DOI] [PubMed] [Google Scholar]
- 33. Rockstroh JK, et al. 2011. Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS 25:399–409 [DOI] [PubMed] [Google Scholar]
- 34. Sánchez-Tapias JM, et al. 2006. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology 131:451–460 [DOI] [PubMed] [Google Scholar]
- 35. Schmidt AJ, et al. 2011. Trouble with bleeding: risk factors for acute hepatitis C among HIV-positive gay men from Germany—a case-control study. PLoS One 6:e17781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Serpaggi J, et al. 2006. Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. AIDS 20:233–240 [DOI] [PubMed] [Google Scholar]
- 37. Taylor LE, et al. 2011. Incident hepatitis C virus infection among US HIV-infected men enrolled in clinical trials. Clin. Infect. Dis. 52:812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas DL, et al. 2000. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 284:450–456 [DOI] [PubMed] [Google Scholar]
- 39. Torriani FJ, et al. 2004. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N. Engl. J. Med. 351:438–450 [DOI] [PubMed] [Google Scholar]
- 40. Truong HM, et al. 2006. Increases in sexually transmitted infections and sexual risk behaviour without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting? Sex. Transm. Infect. 82:461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner JM, et al. 2006. Behavioural predictors of subsequent hepatitis C diagnosis in a UK clinic sample of HIV positive men who have sex with men. Sex. Transm. Infect. 82:298–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van de Laar T, et al. 2009. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology 136:1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van de Laar TJ, Matthews GV, Prins M, Danta M. 2010. Acute hepatitis C in HIV-infected men who have sex with men: an emerging sexually transmitted infection. AIDS 24:1799–1812 [DOI] [PubMed] [Google Scholar]
- 44. van de Laar TJ, et al. 2007. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J. Infect. Dis. 196:230–238 [DOI] [PubMed] [Google Scholar]
- 45. Vandelli C, et al. 2004. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am. J. Gastroenterol. 99:855–859 [DOI] [PubMed] [Google Scholar]
- 46. van der Helm JJ, et al. 2011. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS 25:1083–1091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.