Abstract
Cryptococcus species are known agents of opportunistic infections in healthy and immunocompromised hosts. Here we describe the first case of Cryptococcus uzbekistanensis causing bone marrow infection in an elderly Asian man with undiagnosed T cell lymphoma presenting with fever of unknown origin, pancytopenia, and exposure to chicken manure.
CASE REPORT
An 83-year-old Cantonese man was referred to Stanford Hospital from an outside hospital for specialized care in July 2009. He was a resident of the San Francisco Bay area, having emigrated from Vietnam more than 30 years preceding the present illness and without recent travel history. He had presented to his primary care physician 3 weeks earlier in June 2009 with a complaint of a 1-week duration of low-grade fevers and left lower quadrant abdominal pain. Of note, he had a history of vague abdominal pains 3 months preceding the present illness, at which time complete blood counts had been unrevealing. Repeat workup in early June 2009 had revealed pancytopenia (leukocytes [WBC], 2,300/μl; hemoglobin, 12 g/dl; platelets, 52,000/μl) as well as elevated liver enzymes. Abdominal imaging by computed tomography (CT) had revealed a 3.6-cm solid mass in the distal portion of the duodenum. A CT-guided biopsy of the mass had been nondiagnostic, and esophagogastroduodenoscopy had revealed diffuse gastritis.
He was subsequently hospitalized at a community hospital, where evaluation by bone marrow biopsy was unrevealing for malignancy and by cultures was negative for mycobacteria and fungi. Blood and urine cultures were also unrevealing for bacterial or fungal microorganisms. Bone marrow findings at the time had included relative hypercellularity for age at 40%, as well as left shifted hyperplastic myelopoiesis and erythropoiesis, with adequate iron stores despite anemia. The patient was discharged home on empirical oral antibiotic therapy with metronidazole and levofloxacin for a presumed enteric infection in the setting of leukopenia. However, he continued to have fevers, abdominal pain, and weakness and was rehospitalized 4 days later and then transferred to Stanford Hospital for further studies.
Upon transfer to Stanford Hospital in early July 2009, he was ill appearing, febrile, and tachycardic, with respiratory distress associated with hypoxia and bibasilar crackles on examination of lung fields. Laboratory studies revealed persistent cytopenias (WBC, 2,400/μl [comprising 89% neutrophils, 1% lymphocytes, and 4% monocytes on manual differential]; hemoglobin, 9.9 g/dl; platelets, 38,000/μl). Peripheral blood analysis of B and T lymphocyte subsets showed profound lymphopenia at 14/μl. Renal function was relatively preserved with serum creatinine of 1.0 mg/dl, though mild elevations of hepatic enzymes were again noted (total bilirubin, 0.8 g/dl; aspartate transaminase [AST], 83; alanine aminotransferase [ALT], 60; alkaline phosphatase, 175; albumin, 2.1 g/dl). He was treated initially empirically with intravenous vancomycin and later switched to imipenem, levofloxacin, and metronidazole, given continuing fevers.
Serological and/or molecular testing for human immunodeficiency virus type 1, Coxiella burnetii, Legionella spp., Coccidioides immitis, West Nile virus, cytomegalovirus, Epstein-Barr virus (EBV), human herpesvirus 6, and parvovirus B19 were negative, whereas testing for hepatitis B virus revealed evidence for immunity after exposure based on seropositivity for antibodies to HBc and HBs antigens (Ag) but absent antigenemia for HBsAg. A urinary antigen test for Histoplasma and a serum cryptococcal antigen test were both negative. Testing for tuberculosis by QuantiFERON gold in-tube assay was indeterminate. Repeat abdominal imaging by CT revealed a persistent duodenal mass interpreted as confluent adenopathy.
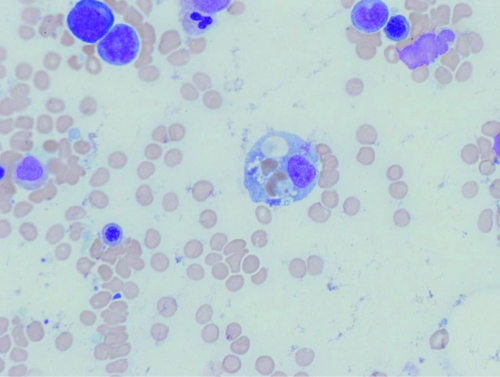
On hospital day 7, a repeat bone marrow biopsy was performed. Microscopic examination of the aspirate smears and sections of the trephined core biopsy specimen revealed hypocellularity, erythrophagocytosis (Fig. 1), and mast cell hyperplasia. Flow cytometric studies revealed no evidence of B or T cell lymphoma, evidenced by light chain restriction or anomalous antigen expression. However, a relative expansion of a natural killer (NK) cell population was noted, expressing CD56, CD2, CD7 and variably expressing CD16, comprising more than 60% of cells within the lymphocyte gate. No aberrant coexpression of CD25 or CD2 on mast cells was observed to suggest a mast cell neoplasm. Cytogenetic studies by conventional banding revealed no clonal karyotypic anomalies.
Fig 1.
Morphological evidence for hemophagocytosis. Bone marrow aspirate stained with hematoxylin and eosin (magnification, ×1,000).
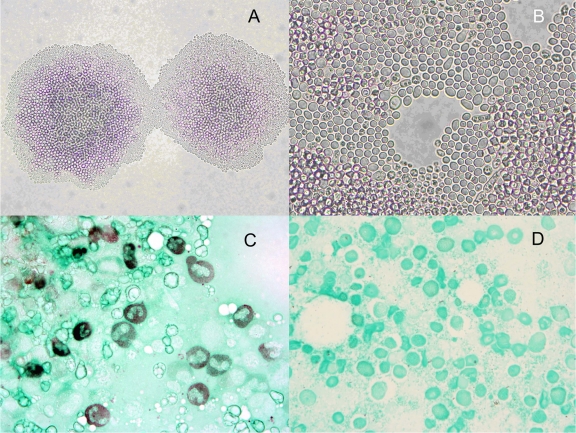
Staining of bone marrow aspirate with Gomori methenamine silver (GMS) showed misshapen budding yeast cells, 5 to 10 μm in diameter (Fig. 2C). Fungal culture of the bone marrow aspirate in an Isolator blood lysis tube (Wampole, Cranbury, NJ) incubated at 35°C for 24 h grew 50 to 100 colonies of white creamy colonies of yeast on a Sabouraud dextrose agar plate and fewer colonies on potato dextrose agar with 50 μg/ml chloramphenicol. Microscopic examination of colony growth on cornmeal agar demonstrated round yeast forms of 5 μm in diameter (Fig. 2A and B), which was morphologically consistent with Cryptococcus species. Urease and phenoloxidase activity was negative, and the organism was not identifiable on the Vitek 2 automated identification system (bioMérieux, Durham, NC). Nucleic acid sequencing of the D1/D2 region 26S rRNA gene was performed on the isolate and showed 100% (529 bp out of 529 bp) similarity to that of Cryptococcus uzbekistanensis reference strain C654 (NCBI GenBank EF116916.1).
Fig 2.
Morphological features of Cryptococcus uzbekistanensis. (A and B) Growth on cornmeal agar after incubation at 35°C (panel A, magnification, ×100; panel B, magnification, ×400). Round budding yeast cells are 5 μm in diameter. (C and D) Bone marrow aspirate (panel C, patient; panel D, control) stained with GMS (magnification, ×1,000).
The patient was started on high-dose fluconazole. On hospital day 15, endoscopic ultrasound and fine-needle aspiratation (FNA) of the duodenal mass were performed, and tissue sections showed yeast forms but no malignant cells. Cultures of this duodenal aspirate grew Candida albicans and Candida glabrata, which were thought to represent colonization rather than invasive fungal disease.
The patient's condition waxed and waned with intermittent fevers and persistently negative blood cultures. He developed increasing multiorgan dysfunction, including pulmonary infiltrates, renal failure, and spontaneous bleeding, including hemodynamically significant retroperitoneal hemorrhage as well as mucosal bleeding. On hospital day 26, he began having episodes of hemoptysis and epistaxis associated with hypofibrinogenemia (nadir, 110 mg/dl) and thrombocytopenia (nadir, 19,000/μl), requiring regular transfusion of fresh frozen plasma, cryoprecipitate, and platelets. Broad-spectrum antibiotic coverage was restarted, including empirical coverage for tuberculosis and influenza. The patient then developed increasing respiratory distress and expired on hospital day 31.
At autopsy, a large T cell lymphoma was diagnosed in multiple sites, including duodenal mass, the bone marrow, lymph nodes, and the spleen. In addition, acute necrotizing tracheobronchial-pulmonary aspergillosis was diagnosed, which was the likely cause of death. Immunohistochemical studies of the spleen and paraduodenal nodule showed a diffuse infiltrate of large cells with high nucleus-to-cytoplasm ratio, clumped chromatin, and increased mitotic rate. These cells stained positively for Ki67 (>90%), CD3, CD5, T cell receptor (TCR) beta, LAT (linker for activation of T cells), and CD45RB but were absent staining for CD20, (linker for activation of T cells) EBV by immunohistochemical staining or in situ hybridization, CD138, CD30, PAX5, CD138, CD163, and CD56. Collectively, these findings support an undiagnosed aggressive peripheral T cell lymphoma, not otherwise specified (PTCL-NOS), involving the spleen, lymph nodes, and eventually the bone marrow.
The present illness fulfills the diagnosis of hemophagocytic lymphohistiocytosis (HLH), based on the patient's presenting and evolving features comprising at least five of the eight recognized criteria (3). These features included hemophagocytosis, fever, bicytopenia, hyperferritinemia, and hypofibrinogenemia. Notably, he did not have clinically significant splenomegaly and was not assessed for hypertriglyceridemia, functionally defective NK cells, or elevation of soluble interleukin-2 (IL-2) receptor levels.
Cryptococcus uzbekistanensis is a round noncapsulated yeast that was first identified in 1999 after being isolated by Chernov and colleagues from the desert soil near Bukhara, Uzbekistan. Its microbiological features were subsequently characterized by Fonseca and colleagues (2). They describe the colonies on yeast-mold agar as cream to pinkish-cream in color and slightly glossy and smooth, with soft to butyrous texture. In broth culture, cells grow in a coccoid shape measuring 3 to 5 by 5 to 10 μm (2).
Review of the medical and veterinary literature indicates that C. uzbekistanensis has never been documented to cause an infection in humans or any other animal host. Therefore, this report represents the first isolation of C. uzbekistanensis from the bone marrow of an immunocompromised patient with pancytopenia and PTCL. It is not entirely clear whether C. uzbekistanensis or the patient's lymphoma was the true underlying cause of his symptoms on presentation.
Of interest, the patient's bone marrow biopsy did identify erythrophagocytosis, a hallmark of acquired HLH. This syndrome has been associated with both lymphomas and infection (1, 4). Although the pathophysiology of acquired HLH and its contribution to infection with C. uzbekistanensis are not entirely clear in this case, this syndrome is known to be associated with immunosuppression, leaving the host susceptible to opportunistic infections. The reverse can also occur; there is a report of Cryptococcus neoformans meningoencephalitis leading to hemophagocytic syndrome in a child (8).
A systematic review by Khawcharoenporn and colleagues showed that non-neoformans (and non-gattii) cryptococcal infections occur sporadically, with Cryptococcus laurentii and Cryptococcus albidus accounting for 80% of the reported cases (5). Impaired cellular immunity due to various underlying conditions was a major risk factor for non-neoformans cryptococcal infections. The environmental sources had a wide geographic distribution and included air, water, wood, soil, pigeon excreta, and various human foods.
While there have been no reports of isolation of C. uzbekistanensis from a human host, there have also been no reports of its isolation from environmental sources in North America. The original report on the isolation of C. uzbekistanensis came from Uzbekistan in Central Asia (2). There was a report of C. uzbekistanensis by the U.S. military isolating the yeast from dust samples in the Middle East (6) and an unpublished report by Liu and Xu isolating it from the soil of wine-growing regions of Gansu Province in China. These reports suggest a strictly Asian distribution for C. uzbekistanensis, but they do not preclude that it also exists outside Asia. It is possible that the patient in this report became infected or colonized with C. uzbekistanensis in the distant past when he was still living in Vietnam and only recently developed an active infection when he became immunocompromised in a manner described for dimorphic fungal organisms such as Histoplasma capsulatum (9). Alternatively, he may have been exposed to C. uzbekistanensis recently in the United States, after he had developed lymphoma and associated immunosuppression. Further investigation of his social history revealed recent exposure to chicken manure while preparing homemade fertilizer for his garden. Given the well-known association between C. neoformans and pigeon droppings (7), it is tempting to hypothesize that the chicken manure was the source of his exposure to C. uzbekistanensis. But if that were the case, one would expect to see more cases of C. uzbekistanensis infection in the immunosuppressed population.
In summary, Cryptococcus uzbekistanensis, a previously unknown pathogen, has now been identified in an 83-year-old Asian man with pancytopenia and a peripheral T cell lymphoma. While the organism has never been isolated in North America, and the circumstances of our patient's infection are not certain, this organism represents a sporadic cause of cryptococcal infection in the immunocompromised host.
Footnotes
Published ahead of print 21 December 2011
REFERENCES
- 1. Filipovich AH. 2009. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology Am. Soc. Hematol. Educ. Program 2009:127–131 [DOI] [PubMed] [Google Scholar]
- 2. Fonseca A, Scorzetti G, Fell JW. 2000. Diversity in the yeast Cryptococcus albidus and related species as revealed by ribosomal DNA sequence analysis. Can. J. Microbiol. 46:7–27 [PubMed] [Google Scholar]
- 3. Henter JI, et al. 2007. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 48:124–131 [DOI] [PubMed] [Google Scholar]
- 4. Janka GE. 2007. Hemophagocytic syndromes. Blood Rev. 21:245–253 [DOI] [PubMed] [Google Scholar]
- 5. Khawcharoenporn T, Apisarnthanarak A, Mundy LM. 2007. Non-neoformans cryptococcal infections: a systematic review. Infection 35:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyles MB, et al. 2008. Medical geology: dust exposure and potential health risks in the Middle East. Geochim. Cosmochim. Acta 72:A576 [Google Scholar]
- 7. Nielsen K, De Obaldia AL, Heitman J. 2007. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot. Cell 6:949–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Numata K, Tsutsumi H, Wakai S, Tachi N, Chiba S. 1998. A child case of haemophagocytic syndrome associated with cryptococcal meningoencephalitis. J. Infect. 36:118–119 [DOI] [PubMed] [Google Scholar]
- 9. Seider K, Heyken A, Luttich A, Miramon P, Hube B. 2010. Interaction of pathogenic yeasts with phagocytes: survival, persistence and escape. Curr. Opin. Microbiol. 13:392–400 [DOI] [PubMed] [Google Scholar]