Abstract
Genotype 3 hepatitis E viruses (HEVs) are distributed across the world and are now considered to be an emerging public health concern in industrialized countries. At least 10 genotype 3 subtypes have been identified in humans and animals worldwide. It was recently reported that the sensitivities of HEV RNA assays differ greatly. We have assessed the influence of genotype 3 diversity on the performances of two HEV RNA assays: one targeting the ORF3 gene and the other targeting the ORF2 gene. We tested a panel of 5 HEV-positive reference samples of genotypes 3a, 3b, 3c, 3e, and 3f at 10-fold serial dilutions. The HEV RNA concentrations obtained with both reverse transcription (RT)-PCRs were correlated, but the RT-PCR based on ORF2 underestimated the HEV RNA concentrations. The mean [ORF3 − ORF2] difference was 1.41 log copies/ml. We also tested 34 clinical specimens of genotypes 3c (n = 15), 3e (n = 4), and 3f (n = 15), representing the most prevalent subtypes in Europe. The mean [ORF3 − ORF2] differences were 1.41 log copies/ml for genotype 3c, 0.96 log copies/ml for genotype 3e, and 0.70 log copies/ml for genotype 3f. The bias between the 2 RT-PCR assays was significantly greater for genotype 3c than for genotype 3f (P = 0.007). We therefore recommend the use of an RT-PCR protocol based on ORF3 to quantify HEV RNA of genotype 3 strains.
INTRODUCTION
Hepatitis E virus (HEV) is the causative agent of acute or fulminant hepatitis in many resource-limited regions of the world. It is transmitted primarily by the fecal-oral route (36). It is now also considered to be an emerging concern in industrialized countries (8, 41). HEV is a nonenveloped, single-stranded, positive-sense RNA virus and a member of the family Hepeviridae (10). Its genome consists of a single-stranded, positive-sense RNA, approximately 7.2 kb long, which is capped and polyadenylated (18). It contains a short 5′ untranslated region (UTR), three open reading frames (ORFs) (ORF1, ORF2, and ORF3), and a 3′ UTR. ORF1 encodes nonstructural proteins, including methyltransferase, papain-like cysteine protease, helicase, and RNA-dependent RNA polymerase (25). ORF2 encodes the viral capsid protein. ORF3 encodes a small, phosphorylated protein, 113 or 114 amino acids long, that is involved in virion morphogenesis and release (11, 12).
Analyses of the nucleotide sequences of HEV strains have revealed extensive genomic diversity, leading to the identification of 4 main genotypes and several subtypes within each genotype (31). HEV genotypes 1 and 2 are restricted to humans and are associated with epidemics in developing countries, whereas HEV genotypes 3 and 4 are zoonotic and are responsible for sporadic cases. While genotype 4 is restricted to parts of Asia, genotype 3 is present worldwide (31). However, the HEV genotype 3 subtypes each have distinct geographic distributions. Genotype 3a and 3b strains are found more frequently in North America and Japan (31, 37), while genotype 3c, 3e, and 3f strains are the most prevalent subtypes in European countries (28, 31, 42).
HEV genotype 3 is an emerging cause of acute and chronic hepatitis in immunocompromised patients (21, 26), and it poses concerns for food and environmental safety worldwide (35). The optimal diagnosis of an HEV infection relies on a combination of serological tests and nucleic acid amplification techniques (NATs) (9). An accurate quantitative assay of HEV RNA is also necessary for pathophysiological studies (22) and to monitor the HEV loads of chronically infected patients on antiviral therapy (2, 19, 20).
Several in-house conventional or real-time reverse transcription (RT)-PCRs based on the amplification of the ORF2 or ORF3 gene were recently evaluated (3). Those investigators found that the sensitivities of the majority of the assays differed greatly. We have therefore investigated the influence of genotype 3 diversity on the performances of two quantitative real-time RT-PCRs: one based on the amplification of a fragment within ORF2 and the other based on the amplification of a fragment within the ORF3 gene.
MATERIALS AND METHODS
HEV reference strain panel.
Genotype 3a, 3b, 3c, 3e, and 3f strains were selected. The genotype 3a and 3b strains came from previous studies performed with the Paul Ehrlich Institute and were provided by Keiji Nastubayashi from the Japanese Red Cross Hokkaido Blood Center, and the genotype 3c, 3e, and 3f strains were from blood samples collected from patients with acute or chronic infections in the Midi-Pyrenees area, France (27, 32). Each strain was characterized by the sequencing of a 189-nucleotide (nt) ORF2 fragment, as previously described (28). Each sample was tested undiluted or diluted 1/10 and 1/100 in HEV-negative plasma. Each dilution was quantified in duplicate in 4 distinct runs.
Clinical specimens.
We tested 34 plasma samples from French patients with French isolates: 15 from patients infected with HEV genotype 3c, 15 from patients infected with genotype 3f, and 4 from patients infected with genotype 3e. Each strain was characterized by the sequencing of a 189-nt ORF2 fragment, as previously described (28).
HEV RNA extraction.
HEV RNA was extracted from blood samples (850 μl) with the Total Nucleic Acid Isolation (TNAI) kit on the Cobas Ampliprep instrument according to the manufacturer's instructions (Roche Diagnostics, France).
Real-time PCR based on ORF3.
One-step real-time RT-PCR was performed with the LightCycler 480 instrument (Roche Diagnostics, France). The following primers and probe targeting the ORF2/ORF3 overlapping region were used to amplify a 70-nt fragment: forward primer HEVORF3-S (5′-GGTGGTTTCTGGGGTGAC-3′), reverse primer HEVORF3-AS (5′-AGGGGTTGGTTGGATGAA-3′), and the probe 5′–6-carboxyfluorescein (FAM)–TGATTCTCAGCCCTTCGC–6-carboxytetramethylrhodamine (TAMRA)–3′ (17). For RT-PCR, the 50-μl reaction mix contained 1 μl of SuperScript III Platinum One-Step quantitative RT-PCR system medium (Invitrogen), 15 μl of RNA, primers (200 nM), probes (150 nM), and 40 U/reaction RNase Out (Invitrogen). Reverse transcription was carried out at 50°C for 15 min, followed by denaturation at 95°C for 1 min. DNA was amplified with 50 PCR cycles at 95°C (20 s) and 58°C (40 s). The amplification efficiency calculated with a standard curve was 2.02. The limit of detection was 100 copies/ml.
Real-time PCR based on ORF2.
One-step real-time RT-PCR was performed with the LightCycler 480 instrument (Roche Diagnostics) as previously described (26, 34). Primers and probes targeting a 140-nt fragment within the ORF2 gene were forward primer HEVORF2-S1 (5′-GACAGAATTRATTTCGTCGGCTGG-3′), reverse primer HEVORF2-A2 (5′-CCCTTRTCCTGCTGNGCATTCTCGACAGA-3′), and probe HEVORF2-S2 (5′-FAM-GTYGTCTCRGCCAATGGCGAGC-TAMRA-3′). The 50-μl RT-PCR mix contained 1 μl SuperScript III Platinum One-Step quantitative RT-PCR system medium (Invitrogen), 15 μl of RNA, primers (200 nM) and probes (150 nM), and 40 U/reaction RNase Out (Invitrogen). Reverse transcription was carried out at 42°C for 15 min, followed by denaturation at 95°C for 1 min. DNA was amplified with 50 PCR cycles at 95°C (20 s) and 60°C (1 min). The amplification efficiency calculated with a standard curve was 1.96. The limit of detection was 100 copies/ml.
RNA standards.
Two transcribed RNA standards were constructed from a sample from a patient infected with genotype 3f (GenBank accession number EU495148): one was based on the amplification of a fragment within the ORF3 gene (70 nt), and the other was based on the amplification of a fragment within the ORF2 gene (140 nt). Each of the resulting cDNAs was purified and inserted and cloned into the PCR-II vector using Topo TA cloning for sequencing (Invitrogen). The positive clone, screened by colony PCR, was confirmed by digestion with a restriction enzyme and sequencing. The vector was cut down with EcoRI and cloned into transcriptional vector pGEM.3Z with the same enzyme sites. pGEM.3Z was linearized with SmaI and retrotranscribed by T7 RNA polymerase to obtain a positive strand for use as an RNA standard for quantitative RT-PCR. The transcribed RNA standard was titrated by measuring the optical density with a spectrophotometer. A standard curve was generated from serial 10-fold dilutions of the standard.
Statistical analysis.
The Spearman test was used to test the correlation between the 2 RT-PCR assays. One-way analysis of variance (ANOVA) was used to test for differences in quantification between the 2 RT-PCR assays.
RESULTS
Reference strain panel.
We assayed 5 samples, containing genotype 3a, 3b, 3c, 3e, and 3f strains, undiluted or diluted, with the ORF2-based real-time RT-PCR and with the ORF3-based real-time RT-PCR. The reproducibility of each RT-PCR assay was good (Table 1). The mean standard deviations were 0.14 log copies/ml (range, 0.03 to 0.44 log copies/ml) for the ORF2-based RT-PCR and 0.13 log copies/ml (range, 0.07 to 0.21 log copies/ml) for the RT-PCR based on ORF3.
Table 1.
HEV RNA results for the panel of genotype 3a, 3b, 3c, 3e, and 3f strainsa
| Strain genotype | Sample dilution | ORF2 |
ORF3 |
Difference in [ORF3 − ORF2] (log copies/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of samples tested/no. of positive samples detected | Mean viral load (log copies/ml) | SD (log copies/ml) | CV (%) | No. of samples tested/no. of positive samples detected | Mean viral load (log copies/ml) | SD (log copies/ml) | CV (%) | |||
| 3a | Undiluted | 8/8 | 4.20 | 0.19 | 4.42 | 8/8 | 5.51 | 0.21 | 3.73 | 1.32 |
| 1/10 | 8/8 | 3.08 | 0.12 | 3.74 | 8/8 | 4.08 | 0.18 | 4.39 | 1.0 | |
| 1/100 | 8/8 | 2.24 | 0.11 | 4.81 | 8/8 | 3.07 | 0.15 | 4.99 | 0.83 | |
| 3b | Undiluted | 8/8 | 5.13 | 0.03 | 0.57 | 8/8 | 5.65 | 0.21 | 3.66 | 0.52 |
| 1/10 | 8/8 | 4.05 | 0.11 | 2.71 | 8/8 | 4.31 | 0.18 | 4.06 | 0.26 | |
| 1/100 | 8/8 | 2.98 | 0.09 | 2.88 | 8/8 | 3.22 | 0.09 | 2.82 | 0.25 | |
| 3c | Undiluted | 8/8 | 3.78 | 0.11 | 2.93 | 8/8 | 5.63 | 0.09 | 1.65 | 1.85 |
| 1/10 | 8/8 | 2.66 | 0.18 | 6.86 | 8/8 | 4.54 | 0.12 | 2.63 | 1.83 | |
| 1/100 | 4/8 | 1.02 | 0.27 | 25.9 | 8/8 | 3.44 | 0.17 | 4.79 | 2.42 | |
| 3e | Undiluted | 8/8 | 3.45 | 0.18 | 5.58 | 8/8 | 5.66 | 0.07 | 1.31 | 2.21 |
| 1/10 | 8/8 | 1.97 | 0.44 | 22.2 | 8/8 | 4.54 | 0.10 | 2.17 | 2.57 | |
| 1/100 | 1/8 | 0.60 | 8/8 | 3.44 | 0.09 | 2.55 | 2.84 | |||
| 3f | Undiluted | 8/8 | 5.12 | 0.10 | 2.05 | 8/8 | 6.14 | 0.13 | 2.04 | 1.02 |
| 1/10 | 8/8 | 3.99 | 0.07 | 1.74 | 8/8 | 5.10 | 0.12 | 2.27 | 1.11 | |
| 1/100 | 8/8 | 2.88 | 0.17 | 5.8 | 8/8 | 4.04 | 0.1 | 2.36 | 1.15 | |
| All samples | 109/120 | 3.15 | 0.14b | 6.5c | 120/120 | 4.56 | 0.13b | 3.03c | 1.41d | |
CV, coefficient of variation.
Mean standard deviation.
Mean coefficient of variation.
Mean difference.
However, only one of the eight 1/100-diluted genotype 3e samples was detected with the RT-PCR based on ORF2. Similarly, only 4 of the eight 1/100-diluted genotype 3c samples were detected with the RT-PCR based on ORF2. All the other samples scored positive with both assays (Table 1).
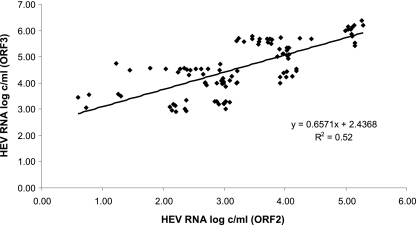
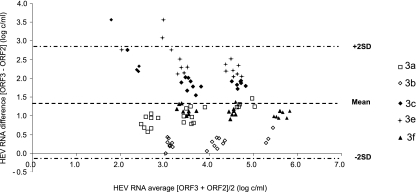
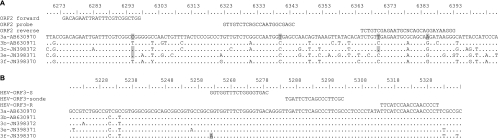
The virus loads in the 109 positive samples measured by each assay are shown in Fig. 1. The ORF3 RT-PCR and ORF2 RT-PCR results were linearly associated (R2 = 0.52) and correlated (ρ = 0.69; P < 0.001) (Fig. 1). The ORF2 RT-PCR gave a mean HEV RNA concentration of 3.15 log copies/ml, and the ORF3 RT-PCR assay gave a mean HEV RNA concentration of 4.56 log copies/ml. The mean deviation between the ORF3 and the ORF2 RT-PCR results was 1.41 log copies/ml. Bland-Altman analysis showed that differences in virus load were independent of the concentration of HEV RNA (Fig. 2). However, the average deviation varied with the HEV subtype. The mean [ORF3 − ORF2] differences were 2.54 log copies/ml for the genotype 3e samples, 2.03 log copies/ml for the genotype 3c samples, 1.09 log copies/ml for the genotype 3f samples, 1.05 log copies/ml for the genotype 3a samples, and 0.34 log copies/ml for the genotype 3b samples. HEV genetic polymorphisms may generate mismatches between HEV RNA and primers or probes, which could contribute to underquantification by the RT-PCR based on ORF2 (Fig. 3).
Fig 1.
HEV RNA concentrations of the genotype 3a, 3b, 3c, 3e, and 3f reference strains measured by the ORF3 and ORF2 RT-PCRs.
Fig 2.
Bland-Altman plot for bias analysis between RT-PCRs based on ORF3 and ORF2 for the panel of genotype 3a, 3b, 3c, 3e, and 3f reference strains. SD, standard deviation.
Fig 3.
Alignment of reference sequences showing the positions of the primers and probes in the HEV ORF2 (A) and HEV ORF3 (B) regions. Nucleotides in gray indicate mismatches with the primer or probe. Numbers refer to the corresponding nucleotide positions of HEV (GenBank accession number M73218).
Clinical specimens.
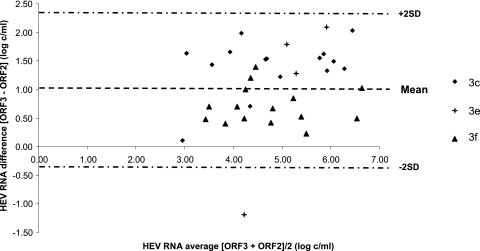
We also tested 34 clinical samples with both assays in order to eliminate any potential bias due to assaying only the 5 reference strains. These samples included samples of genotypes 3c (n = 15), 3e (n = 4), and 3f (n = 15), which are the most prevalent genotype 3 subtypes found in Europe. The ORF3 RT-PCR and ORF2 RT-PCR results were correlated (ρ = 0.82; P < 0.001). The ORF3 RT-PCR gave a mean HEV RNA concentration of 5.40 log copies/ml, and the ORF2 RT-PCR assay gave a mean HEV concentration of 4.36 log copies/ml. The mean deviation between the ORF3 and the ORF2 RT-PCR results was 1.04 log copies/ml. Data for the Bland-Altman analysis are shown in Fig. 4. Again, the average deviation varied with the HEV subtype. The mean [ORF3 − ORF2] differences were 1.41 log copies/ml for genotype 3c, 0.96 log copies/ml for genotype 3e, and 0.70 log copies/ml for genotype 3f. The average deviation between the 3 subtypes was significantly different (P = 0.009). The bias between the 2 RT-PCRs was significantly greater for the genotype 3c samples than for the genotype 3f samples (P = 0.007).
Fig 4.
Bland-Altman plot for bias analysis between the RT-PCRs based on ORF3 and ORF2 for the 34 clinical samples. SD, standard deviation.
DISCUSSION
We have assessed the influence of HEV genotype 3 diversity on the performances of two HEV RNA quantitative assays. The RT-PCR assays were correlated, but we found substantial differences in the quantities of RNA of the main genotype 3 subtypes detected by the two real-time RT-PCRs.
Several protocols for real-time PCR targeting the ORF3 region (13, 17, 40) or the ORF2 region (1, 16, 34, 47) have been developed for the detection of HEV RNA over the past 10 years. The performances of assays based on the amplification of HEV RNA nucleic acid were recently investigated by using a panel of HEV-containing plasma samples (3). The panel was comprised of 22 HEV-positive plasma samples representing 10-fold serial dilutions of HEV subtypes 3a, 3b, 3f, and 4c obtained from blood donors. Only 2 of the 20 laboratories that tested the panel used an RT-PCR protocol that targeted the ORF1 region of the HEV genome; the other methods targeted the ORF2 and ORF3 regions. That study demonstrated that real-time RT-PCRs are more sensitive than nested PCRs, but the sensitivities of the majority of the assays differed enormously (100-fold to 1,000-fold), independent of the virus strains (3). Among protocols targeting ORF3, we selected one developed by Jothikumar et al., because it was the most frequently used protocol in a recent evaluation performed by Baylis et al. (17). Among protocols targeting ORF2, we selected a method developed previously by Mansuy et al., because it was previously used in our laboratory for HEV RNA detection and quantification (26, 34).
The great genetic diversity of RNA viruses makes it very difficult to design appropriate primers and probes for use in the development of molecular diagnostic assays. The performance of quantitative assays for RNA viruses is influenced by their genetic diversity (15, 23, 43). For the 34 clinical samples, the sequence identity between the different subtypes in the ORF2 region ranged from 83% to 90.4% (data not shown). Based on the genotype 3c, 3e, and 3f complete genome sequences available in the GenBank database, the sequence identities between the different subtypes ranged from 82.4% to 90% in the ORF2 region and ranged from 89.5% to 95.2% in the ORF3 region. This variability between subtypes has prompted us to assess its influence on HEV RNA quantification. Our sequence alignments have shown several mismatches, mainly for the primers and the probe targeting ORF2. Nevertheless, the ORF2 assay showed a similar sensitivity in the detection of genotype 3a and 3f standard preparations despite a higher number of mismatches between the ORF2 primers and probe and the 3a strain than between the ORF2 primers and probe and the 3f strain. This may be because other critical parameters, such as RNA conformation, could be similar for the two TaqMan detection systems.
We evaluated the capacities of two different TaqMan real-time RT-PCRs to detect and quantify HEV genotype 3 subtypes. Our assays of serial 10-fold dilutions of genotype 3a, 3b, 3c, 3e, and 3f reference samples indicated that the ORF2-based RT-PCR was less sensitive than the ORF3-based RT-PCR. The RT-PCR based on ORF2 rarely detected the 100-fold-diluted genotype
3c and 3e samples, and our assays of several clinical plasma samples showed that the RT-PCR based on ORF2 significantly underestimated the HEV RNA concentration in genotype 3c samples compared to the genotype 3f samples. These data agree well with findings described previously by Ward et al. (46), who studied the performances of 4 real-time RT-PCRs based on ORF3 and ORF2 by testing Canadian swine genotype 3 samples. Those authors compared the threshold cycle (CT) values obtained with the real-time RT-PCR tests and found that the RT-PCR test based on ORF3 was the most sensitive test for the detection of swine HEV strains (46). Unfortunately, those researchers did not determine the HEV subtypes, and their assays were only semiquantitative.
Our study comparison of two HEV RNA assays was focused on HEV genotype 3, the most prevalent genotype in industrialized countries. However, the primers and the probe for the RT-PCR based on the ORF2 region, adapted from our previously reported in-house protocol (26, 34), were designed in order to detect the 4 main genotypes of HEV. They were used to conduct several previous studies of HEV infections (19–21, 26, 32–34), and they allow us to detect HEV genotype 1 and 4 infections (32). Similarly, we have used the primers and the probes targeting the ORF3 region designed previously by Jothikumar et al. (17). Those researchers validated the capacity of these primers to detect HEV isolates representing genotypes 1 to 4 with several samples. However, further studies are needed to address whether the quantitative HEV RNA assays are influenced by the HEV genetic diversity for all the genotypes.
The extraction protocol can affect the sensitivities of molecular tests (4, 14), but we used automated extraction with the Cobas Ampliprep instrument to extract the HEV RNA used for both assays. This automated extraction is more reliable, standardized, reproducible, and time-saving than manual extraction for the preparation of nucleic acids (24, 44). This procedure also limits the risk of cross-contamination in the laboratory.
The diagnosis of an HEV infection in a patient requires accurate and sensitive tools. Because serological tests may lack sensitivity (9, 29), the detection of virus genomic RNA in serum or stool samples by RT-PCR is a crucial marker of an acute or a chronic HEV infection. The ubiquitous nature of HEV genotype 3 strains in domestic pigs and wild boars also raises public health concerns for zoonotic infection through direct contacts with infected animals (5, 7, 38) or the consumption of contaminated animal products (6, 30, 45). In addition, genotype 3 HEV-contaminated coastal, drinking, irrigation, and sewage waters could all be sources for human infections (35). This reinforces the need for a highly sensitive RT-PCR protocol for the detection of HEV in food or water samples. The development of sensitive assays may reveal that HEV is far more common in industrialized countries than was previously thought (39). Additionally, an underestimation of HEV RNA quantification could result in false-negative results in treated immunocompromised patients (2, 19, 20) and thus may be the cause of a relapse if the treatment is stopped too early.
In conclusion, our findings indicate that an RT-PCR protocol based on ORF3 provides the most suitable tool for assaying the HEV RNA of genotype 3 strains.
Footnotes
Published ahead of print 28 December 2011
REFERENCES
- 1. Ahn JM, Rayamajhi N, Gyun Kang S, Sang Yoo H. 2006. Comparison of real-time reverse transcriptase-polymerase chain reaction and nested or commercial reverse transcriptase-polymerase chain reaction for the detection of hepatitis E virus particle in human serum. Diagn. Microbiol. Infect. Dis. 56:269–274 [DOI] [PubMed] [Google Scholar]
- 2. Alric L, Bonnet D, Laurent G, Kamar N, Izopet J. 2010. Chronic hepatitis E virus infection: successful virologic response to pegylated interferon-alpha therapy. Ann. Intern. Med. 153:135–136 [DOI] [PubMed] [Google Scholar]
- 3. Baylis SA, Hanschmann KM, Blumel J, Nubling CM. 2011. Standardization of hepatitis E virus (HEV) nucleic acid amplification technique-based assays: an initial study to evaluate a panel of HEV strains and investigate laboratory performance. J. Clin. Microbiol. 49:1234–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bianchi S, Dal Vecchio A, Vilarino ML, Romalde JL. 2011. Evaluation of different RNA-extraction kits for sensitive detection of hepatitis A virus in strawberry samples. Food Microbiol. 28:38–42 [DOI] [PubMed] [Google Scholar]
- 5. Christensen PB, et al. 2008. Time trend of the prevalence of hepatitis E antibodies among farmers and blood donors: a potential zoonosis in Denmark. Clin. Infect. Dis. 47:1026–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colson P, et al. 2010. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 202:825–834 [DOI] [PubMed] [Google Scholar]
- 7. Colson P, Kaba M, Bernit E, Motte A, Tamalet C. 2007. Hepatitis E associated with surgical training on pigs. Lancet 370:935. [DOI] [PubMed] [Google Scholar]
- 8. Dalton HR, Bendall R, Ijaz S, Banks M. 2008. Hepatitis E: an emerging infection in developed countries. Lancet Infect. Dis. 8:698–709 [DOI] [PubMed] [Google Scholar]
- 9. Drobeniuc J, et al. 2010. Serologic assays specific to immunoglobulin M antibodies against hepatitis E virus: pangenotypic evaluation of performances. Clin. Infect. Dis. 51:e24–e27 [DOI] [PubMed] [Google Scholar]
- 10. Emerson SU, et al. 2005. Hepevirus, p 853–857 In Fauquet CM, Mayo MA, Maniloff J, Desselberg U, Ball LA. (ed), Virus taxonomy. Eighth report of the International Committee on Taxonomy of Viruses Elsevier Academic Press, London, United Kingdom [Google Scholar]
- 11. Emerson SU, Nguyen H, Torian U, Purcell RH. 2006. ORF3 protein of hepatitis E virus is not required for replication, virion assembly, or infection of hepatoma cells in vitro. J. Virol. 80:10457–10464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emerson SU, et al. 2010. Release of genotype 1 hepatitis E virus from cultured hepatoma and polarized intestinal cells depends on open reading frame 3 protein and requires an intact PXXP motif. J. Virol. 84:9059–9069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enouf V, et al. 2006. Validation of single real-time TaqMan PCR assay for the detection and quantitation of four major genotypes of hepatitis E virus in clinical specimens. J. Med. Virol. 78:1076–1082 [DOI] [PubMed] [Google Scholar]
- 14. Gartner BC, Fischinger JM, Litwicki A, Roemer K, Mueller-Lantzsch N. 2004. Evaluation of a new automated, standardized generic nucleic acid extraction method (total nucleic acid isolation kit) used in combination with cytomegalovirus DNA quantification by COBAS AMPLICOR CMV MONITOR. J. Clin. Microbiol. 42:3881–3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gueudin M, et al. 2007. Evaluation of the Roche Cobas TaqMan and Abbott RealTime extraction-quantification systems for HIV-1 subtypes. J. Acquir. Immune Defic. Syndr. 44:500–505 [DOI] [PubMed] [Google Scholar]
- 16. Gyarmati P, et al. 2007. Universal detection of hepatitis E virus by two real-time PCR assays: TaqMan and Primer-Probe Energy Transfer. J. Virol. Methods 146:226–235 [DOI] [PubMed] [Google Scholar]
- 17. Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. 2006. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 131:65–71 [DOI] [PubMed] [Google Scholar]
- 18. Kabrane-Lazizi Y, Meng XJ, Purcell RH, Emerson SU. 1999. Evidence that the genomic RNA of hepatitis E virus is capped. J. Virol. 73:8848–8850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamar N, et al. 2010. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin. Infect. Dis. 50:e30–e33 [DOI] [PubMed] [Google Scholar]
- 20. Kamar N, et al. 2010. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis E virus infection. Gastroenterology 139:1612–1618 [DOI] [PubMed] [Google Scholar]
- 21. Kamar N, et al. 2008. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 358:811–817 [DOI] [PubMed] [Google Scholar]
- 22. Kar P, et al. 2008. Does hepatitis E viral load and genotypes influence the final outcome of acute liver failure during pregnancy? Am. J. Gastroenterol. 103:2495–2501 [DOI] [PubMed] [Google Scholar]
- 23. Klungthong C, et al. 2010. The impact of primer and probe-template mismatches on the sensitivity of pandemic influenza A/H1N1/2009 virus detection by real-time RT-PCR. J. Clin. Virol. 48:91–95 [DOI] [PubMed] [Google Scholar]
- 24. Knepp JH, Geahr MA, Forman MS, Valsamakis A. 2003. Comparison of automated and manual nucleic acid extraction methods for detection of enterovirus RNA. J. Clin. Microbiol. 41:3532–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koonin EV, et al. 1992. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: delineation of an additional group of positive-strand RNA plant and animal viruses. Proc. Natl. Acad. Sci. U. S. A. 89:8259–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Legrand-Abravanel F, et al. 2010. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J. Infect. Dis. 202:835–844 [DOI] [PubMed] [Google Scholar]
- 27. Legrand-Abravanel F, et al. 2011. Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg. Infect. Dis. 17:30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Legrand-Abravanel F, et al. 2009. Hepatitis E virus genotype 3 diversity, France. Emerg. Infect. Dis. 15:110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Legrand-Abravanel F, et al. 2009. Good performance of immunoglobulin M assays in diagnosing genotype 3 hepatitis E virus infections. Clin. Vaccine Immunol. 16:772–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li TC, et al. 2005. Hepatitis E virus transmission from wild boar meat. Emerg. Infect. Dis. 11:1958–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu L, Li C, Hagedorn CH. 2006. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev. Med. Virol. 16:5–36 [DOI] [PubMed] [Google Scholar]
- 32. Mansuy JM, et al. 2009. Acute hepatitis E in south-west France over a 5-year period. J. Clin. Virol. 44:74–77 [DOI] [PubMed] [Google Scholar]
- 33. Mansuy JM, et al. 2009. Molecular evidence of patient-to-patient transmission of hepatitis E virus in a hematology ward. Clin. Infect. Dis. 48:373–374 [DOI] [PubMed] [Google Scholar]
- 34. Mansuy JM, et al. 2004. Hepatitis E in the south west of France in individuals who have never visited an endemic area. J. Med. Virol. 74:419–424 [DOI] [PubMed] [Google Scholar]
- 35. Meng XJ. 2011. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 161:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meng XJ, Anderson DA, Arankalle VA. 2012. Hepeviridae. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy: classification and nomenclature of viruses. Ninth Report of the International Committee on Taxonomy of Viruses Elsevier, London, United Kingdom [Google Scholar]
- 37. Meng XJ, et al. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. U. S. A. 94:9860–9865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meng XJ, et al. 2002. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 40:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miyamura T. 2011. Hepatitis E virus infection in developed countries. Virus Res. 161:40–46 [DOI] [PubMed] [Google Scholar]
- 40. Orru G, et al. 2004. Detection and quantitation of hepatitis E virus in human faeces by real-time quantitative PCR. J. Virol. Methods 118:77–82 [DOI] [PubMed] [Google Scholar]
- 41. Purcell RH, Emerson SU. 2008. Hepatitis E: an emerging awareness of an old disease. J. Hepatol. 48:494–503 [DOI] [PubMed] [Google Scholar]
- 42. Rutjes SA, et al. 2009. Sources of hepatitis E virus genotype 3 in The Netherlands. Emerg. Infect. Dis. 15:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarrazin C, et al. 2006. Comparison of conventional PCR with real-time PCR and branched DNA-based assays for hepatitis C virus RNA quantification and clinical significance for genotypes 1 to 5. J. Clin. Microbiol. 44:729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stelzl E, et al. 2002. Evaluation of an automated sample preparation protocol for quantitative detection of hepatitis C virus RNA. J. Clin. Microbiol. 40:1447–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tei S, Kitajima N, Takahashi K, Mishiro S. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371–373 [DOI] [PubMed] [Google Scholar]
- 46. Ward P, et al. 2009. Comparative analysis of different TaqMan real-time RT-PCR assays for the detection of swine hepatitis E virus and integration of feline calicivirus as internal control. J. Appl. Microbiol. 106:1360–1369 [DOI] [PubMed] [Google Scholar]
- 47. Zhao C, et al. 2007. Comparison of real-time fluorescent RT-PCR and conventional RT-PCR for the detection of hepatitis E virus genotypes prevalent in China. J. Med. Virol. 79:1966–1973 [DOI] [PubMed] [Google Scholar]