Abstract
Early evaluation of treatment efficacy in invasive aspergillosis (IA), a leading cause of morbidity and mortality in hematological patients, remains a challenge. We conducted a prospective study to evaluate the performance of different markers in predicting the outcome of patients with IA. Both clinical and biological criteria were assessed 7, 14, 21, and 45 days after inclusion in the study, and mortality was assessed at day 60. The association between baseline data and their evolution and the day 45 response to treatment was analyzed. A total of 57 patients (4 with proven, 44 with probable, and 9 with possible aspergillosis according to the revised EORTC/MSG [European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group] definitions) were included. At day 45, 30 patients (53%) were determined to be responders, 25 (44%) were nonresponders, and 2 were not able to be evaluated. Twenty patients died within the 60 days of follow-up. We found that a poor day 45 outcome was associated with patients who had high baseline serum galactomannan (GM) antigen levels and those receiving steroids at the time of IA. A consistently negative serum GM index was associated with a good outcome, and the day 14 clinical evaluation was predictive of the day 45 outcome. No association was found between Aspergillus antibodies or DNA detection and patients' outcome. We conclude that the GM index value at diagnosis of IA, GM index kinetics, and clinical evaluation at day 14 are good markers for predicting the outcome of patients with IA and should be taken into account for adapting antifungal treatment.
INTRODUCTION
Although therapeutic strategies have improved in the last several years, invasive aspergillosis (IA) remains an important cause of mortality and morbidity in patients with hematological malignancies (20, 21).
Various antifungal drugs are now available, and their efficacy is currently being evaluated, especially in combination; however, the optimal therapy for IA is still unknown (26). One limitation is the inability to make an early assessment of the impact of the administered treatment on patient outcome and thus to permit early changes to the antifungal treatment.
The treatment response is usually assessed by both clinical symptoms of IA and the evolution of radiological findings (8, 10, 11). However, fever and abnormalities during physical examination are not consistently present (24, 25), and sequential evaluation of lung computed tomography (CT) scans raises some challenges. Indeed, while little is known about the evolution of a lung CT scan in nonneutropenic patients with IA, Caillot et al. demonstrated that the early increase in the size of the radiological lesion attributable to aspergillosis on a CT scan was not correlated with an unfavorable outcome in neutropenic patients (4, 5).
In this context, the use of surrogate markers that could substitute for clinical events as tools to provide objective outcome measures has recently been recommended (24). However, until now, no published studies have proposed reliable markers to be used for this purpose (24), although some data support serial serum galactomannan (GM) measurements as promising (1). In addition, other non-culture-based laboratory assays (e.g., PCR [19] or specific recombinant antibody-based assays [23]) that need further validation may be of some interest for assessing the therapeutic response.
In the current prospective study, we investigated whether the kinetics of serum GM values, PCR, Aspergillus-specific antibodies, and sequential clinical evaluations could predict, early in the disease, the outcomes of both neutropenic and nonneutropenic hematological patients with IA.
MATERIALS AND METHODS
Study design.
This multicenter, prospective, observational study was designed to evaluate the performance of several markers in predicting the outcomes of patients with IA. The protocol was approved by the ethics committee of the Saint Louis teaching hospital in Paris, France. All patients provided informed consent. All consecutive patients who had proven or probable IA according to the revised EORTC/MSG (European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group) definitions between May 2005 and February 2007 were included (9). In an appropriate clinical setting, patients with abnormalities on a lung CT scan other than those retained for the diagnosis of IA in the revised definition, associated with a microbiological criteria (i.e., positive antigen and/or mycology and not PCR or antibodies), and for whom an alternative diagnosis was ruled out were considered probable IA (in accordance with the 2002 definition [2, 22]). Patients with possible IA were also included in the study if an alternative diagnosis had been ruled out (28). A data review committee assessed the diagnosis of IA for each case (P. Ribaud, A. Bergeron, A. Sulahian, and K. Chagnon). Lung CT scans were reviewed by the four members of the committee, and the conclusion was reached by consensus. The first part of the review process consisted of the description of the lung CT scans, blinded to mycological results. Subsequently, the committee had knowledge of serum and bronchoalveolar lavage (BAL) fluid GM levels and of the mycological examination, leading to the classification of IA as possible, probable, or proven.
The day of inclusion in the study was the first day of curative antifungal treatment (i.e., baseline). Although the main endpoint for the evaluation of the response was 45 days later (1), the response to treatment was also assessed at days 7, 14, and 21, and mortality was assessed at day 60. The response was evaluated as previously described (10). Complete responses were defined by the resolution of all clinical signs and symptoms and more than 90% of the lesions due to IA that were visible on radiology. Partial responses were defined by clinical improvement and greater than 50% improvement in findings on radiology. Stable responses were defined by the absence of change from baseline or an improvement of less than 50%. Failure of therapy was defined by a worsening of the disease. Patients with a complete or partial response were considered responders, and the other patients were considered nonresponders. The death of patients for whom the response could not be evaluated was also considered to be a nonresponse. Death was attributed to IA when a progression of IA was noted without any other identified cause of death. We chose day 45 as the primary outcome as deaths occurring after 6 weeks are mostly due to causes other than IA (24, 29). A data review committee assessed response in each case (P. Ribaud, A. Bergeron, A. Sulahian, and K. Chagnon). Lung CT scans were reviewed by the four members of the committee, and the conclusion for each of the responses was reached by consensus.
Antigen detection technique.
The GM antigen was detected using a sandwich immunocapture enzyme-linked immunosorbent assay (ELISA) technique (Platelia Aspergillus EIA; Bio-Rad, Marnes la Coquette, France) (27). The results were considered positive if the index value was ≥0.5 in both the serum and the BAL fluid. The serum GM was detected at baseline, twice a week from day 0 to day 15, and then once a week until day 60. To avoid false positivity of GM antigen due to the administration of piperacillin and tazobactam, each batch of this antibiotic was tested for GM. Only negative batches were administered to the patients of our study.
Lung computed tomography scan and fiberoptic bronchoscopy.
High-resolution lung CT scans were performed on each patient using multidetector computed tomography scanners.
Fiberoptic bronchoscopy was performed within 24 h of the first lung CT scan. The site of BAL was determined using the lung CT scan, and BAL was performed using four 50-ml aliquots of sterile saline solution. An extensive examination for pathogens was performed on the BAL fluid. A direct examination and culture for fungi were systematically performed on BAL fluid, bronchial aspirate, bronchial biopsy specimen, and sputum (when available). Each patient had a single BAL.
Detection of Aspergillus antibodies.
Serum antibodies were detected at baseline and then at days 14, 28, 42, and 60. Antibodies directed against the three major Aspergillus protein antigens, RNase, dipeptidyl peptidase V, and mycelial catalase 1, were evaluated using ELISAs as previously described (23). Anti-Aspergillus fumigatus IgG antibodies were evaluated using an indirect immunoenzymatic technique (Virion AES, France). Results of ≥12 arbitrary units/ml were considered positive, and results of <8 were considered negative. Physicians were blinded to the antibody results.
Aspergillus DNA detection.
Real-time PCR was performed on serum, whole blood, and blood buffy coat at the same time points as GM detection. Blood was collected into sterile vacuum collection tubes. Blood buffy coats were obtained from 7 ml of blood drawn into EDTA tubes using Histopaque 1119 (Sigma-Aldrich, Saint-Quentin-Fallavier, France). After a 30-min incubation with 10 units of lyticase (Sigma-Aldrich), DNA was extracted by using the QIAamp DNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
A real-time PCR assay that targets the 28S rRNA gene of A. fumigatus was performed as previously described (6) by using primers 5′-CTCGGAATGTATCACCTCTCGG-3′ and 5′-TCCTCGGTCCAGGCAGG-3′ and the TaqMan probe 5′-6-carboxyfluorescein-TGTCTTATAGCCGAGGGTGCAATGCG-6-carboxytetramethylrhodamine-3′. Additionally, a real-time PCR assay that targets a consensus sequence of the 18S rRNA genes of Aspergillus spp. was performed as previously described (14) by using primers 5′-TTGGTGGAGTGATTTGTCTGCT-3′ and 5′-TCTAAGGGCATCACAGACCTG-3′ and the TaqMan probe 5′-6-carboxyfluorescein-TCGGCCCTTAAATAGCCCGGTCCGC-6-carboxytetramethylrhodamine-3′. Both real-time PCR assays were performed on an Applied Biosystems 7500 PCR system (Applied Biosystems, Foster City, CA). For quantification, six serial 10-fold dilutions of A. fumigatus DNA were included in each amplification run, and the PCR results were converted into picograms of fungal DNA per milliliter by interpolation from the standard dilution curve.
When no amplification was observed after 45 PCR cycles, the sample was considered negative by PCR. The presence of PCR inhibitors was tested in each sample with TaqMan exogenous internal positive-control reagents (Applied Biosystems). The absence of Aspergillus DNA contamination during the process was assessed by negative PCR results on the negative controls included in each PCR set and on blood samples from immunocompetent control subjects collected using the same vacuum collection tubes (data not shown). Physicians were blinded to the patients' PCR results.
Statistical analysis.
Data are presented as frequency and percent or median and range. The association of baseline patient characteristics and the evolution of biological markers with day 45 response to treatment was analyzed using Fisher's exact tests and Wilcoxon rank-sum tests. In these analyses, patients with an undetermined day 45 response were excluded, but sensitivity analyses where they were treated as responders or nonresponders were subsequently performed. The slope of evolution of biological markers during the 10 days following inclusion was computed using individual predictions from a mixed linear model. Associations between variables at baseline were assessed by Spearman's rank correlation coefficient or Somers' Dxy rank correlation coefficient and Wilcoxon rank-sum test. The association between the baseline GM index and survival up to day 60 was analyzed using a Cox model. Patients alive at 60 days were censored at that time. All tests were two sided, and P values of ≤0.05 were considered statistically significant. Analyses were performed using R 2.6.2 statistical software (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient population and outcome.
A total of 57 patients from four participating centers were included; 4 had proven IA, 44 had probable IA, and 9 had possible IA (Table 1).
Table 1.
Characteristics of patients with invasive aspergillosis
| Characteristica | Data [no., no. (%), or median (range)] for: |
|||
|---|---|---|---|---|
| All patients (n = 57) | Patient(s) with IAa defined according to EORTC as: |
|||
| Proven (n = 4) | Probable (n = 44) | Possible (n = 9) | ||
| Female gender | 27 (47) | 1 (25) | 22 (50) | 4 (44) |
| Age (yr) | 52 (10–78) | 38 (14–52) | 52 (10–78) | 56 (17–64) |
| Disease | ||||
| AML | 24 (42) | 1 (25) | 15 (34) | 8 (89) |
| ALL | 6 (11) | 0 (0) | 5 (11) | 1 (11) |
| CML | 1 (2) | 1 (25) | 0 (0) | 0 (0) |
| Other myeloproliferative disorder | 1 (2) | 0 (0) | 1 (2) | 0 (0) |
| CLL | 3 (5) | 0 (0) | 3 (7) | 0 (0) |
| Malignant lymphoma | 8 (14) | 1 (25) | 7 (16) | 0 (0) |
| MM | 4 (7) | 0 (0) | 4 (9) | 0 (0) |
| AA | 2 (4) | 0 (0) | 2 (5) | 0 (0) |
| Other | 8 (14) | 1 (25) | 7 (16) | 0 (0) |
| Phase: 1st CR, 1st chronic phase | 28 (51) | 1 (25) | 24 (57) | 2 (22) |
| Autologous HSCT | 7 (12) | 1 (25) | 4 (9) | 2 (22) |
| Allogeneic HSCT | 23 (40) | 1 (25) | 20 (45) | 6 (67) |
| If yes, reduced intensity conditioning | 10 | 0 | 10 | 0 |
| If yes, GVHD | 17 | 1 | 15 | 1 |
| Acute grade of ≥2 | 10 | 0 | 10 | 0 |
| Extensive chronic | 6 | 0 | 6 | 0 |
| Receiving CS | 31 (54) | 4 (100) | 26 (59) | 1 (11) |
| If yes, dose | ||||
| <0.5 mg/kg of body wt | 5 (16) | 1 (25) | 4 (15) | 0 (0) |
| 0.5–1 mg/kg | 10 (32) | 1 (25) | 8 (31) | 1 (100) |
| 1–2 mg/kg | 14 (45) | 2 (50) | 12 (46) | 0 (0) |
| >2 mg/kg | 2 (6) | 0 (0) | 2 (8) | 0 (0) |
| Cumulative CS dose the week before diagnosis (mg/kg) | 2 (0–31) | 5 (1–7) | 3 (0–31) | 0 (0–4) |
| Received immunosuppressive therapy for <1 month | 20 (35) | 2 (50) | 17 (39) | 1 (11) |
| Mycophenolate mofetil | 8 | 1 | 7 | 0 |
| CsA | 16 | 1 | 14 | 1 |
| ATG | 2 | 1 | 1 | 0 |
| Other | 8 | 1 | 7 | 0 |
| Leukocyte count/mm3 | ||||
| <100 | 27 (47) | 1 (25) | 19 (43) | 7 (78) |
| 100–500 | 10 (18) | 2 (50) | 8 (18) | 0 (0) |
| >500 | 20 (35) | 1 (25) | 17 (39) | 2 (22) |
| Leucopenia (<500/mm3) for more than 15 days during the last 3 months | 41 (72) | 2 (50) | 30 (68) | 9 (100) |
| Antifungal therapy in the last 15 days | 34 (60) | 3 (75) | 26 (59) | 5 (56) |
| As curative therapy | 2 | 1 | 1 | 0 |
| As empirical therapy | 27 | 2 | 20 | 5 |
| Primary prophylaxis | 4 | 0 | 4 | 0 |
| Secondary prophylaxis | 1 | 0 | 1 | 0 |
| Antifungal treatment | ||||
| Amphotericin B | 2 (4) | 0 (0) | 2 (5) | 0 (0) |
| Liposomal amphotericin B | 6 (11) | 2 (50) | 4 (9) | 0 (0) |
| Voriconazole | 3 (5) | 0 (0) | 3 (7) | 0 (0) |
| Caspofungin | 23 (40) | 1 (25) | 17 (39) | 5 (56) |
| Other | 2 (4) | 0 (0) | 2 (5) | 0 (0) |
| Concomitant pulmonary infection | 16 (28) | 2 (50) | 14 (32) | 0 (0) |
| Bacterial | 9 | 1 | 8 | |
| Viral | 6 | 1 | 5 | |
| Fungal | 1 | 0 | 1 | |
| Lung CT scan imaging | ||||
| Halo sign | 33 (58) | 3 (75) | 22 (50) | 8 (89) |
| Air crescent sign | 3 (5) | 0 (0) | 3 (7) | 0 (0) |
| Cavity | 5 (9) | 0 (0) | 4 (9) | 1 (11) |
| Other signs | 34 (60) | 3 (75) | 25 (57) | 6 (67) |
AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphoid leukemia; MM, multiple myeloma; AA, aplastic anemia; CR, complete response; HSCT, hematological stem cell transplantation; GVHD, graft versus host disease; CS, corticosteroids; CsA, cyclosporine; ATG, antithymoglobulin; CT, computed tomography; IA, invasive aspergillosis.
The results of the microbiological evaluation at baseline are summarized in Table 2. Only five patients had a positive fungal load in the serum, whole blood, or buffy coat using the A. fumigatus PCR; A. fumigatus grew in cultures from the respiratory samples of only two of these five patients, but GM antigen was detected in four of the five. Eighteen patients had a positive fungal load at baseline in the serum, whole blood, or buffy coat using the Aspergillus spp. PCR; among these 18 patients, Aspergillus spp. grew in culture from the respiratory samples of 7 patients, but GM antigen was detected in 14 patients. Antibodies directed against RNase, dipeptidyl peptidase V, and mycelial catalase 1 as diagnostic markers for aspergillosis were all negative. Of note, all 9 patients with possible IA had underlying acute leukemia, versus 25% and 45% with proven and probable IA, respectively; all had prolonged neutropenia and suggestive radiological signs of IA (halo sign in 8 and cavity in 1) but no detectable GM at baseline (Table 2).
Table 2.
Laboratory evaluations at diagnosis
| Parametera | No. of patients with data available | Data [no., no. (%), or median (range)] for: |
|||
|---|---|---|---|---|---|
| All patients with data available | Patients with: |
||||
| Proven IAa | Probable IA | Possible IA | |||
| Serum GM antigen | 57 | ||||
| Value | 0.67 (0.02–>10) | 2.98 (0.67–8.85) | 0.81 (0.06–>10) | 0.16 (0.02–0.32) | |
| Patients with value of ≥0.5 | 30 (53) | 4 (100) | 26 (59) | 0 (0) | |
| BAL fluid GM antigen | 29 | ||||
| Value | 1.67 (0–9.80) | 2.51 (1.00–5.54) | 1.84 (0.00–9.80) | 0.17 (0.07–0.24) | |
| Patients with value of ≥0.5 | 18 (62) | 4 (100) | 14 (64) | 0 (0) | |
| Anti-Aspergillus antibodies | 56 | ||||
| Value | 3.2 (0.1–181) | 2.6 (1.4–20.0) | 3.7 (0.2–181.0) | 3.2 (0.1–9.3) | |
| Patients with value of ≥12 | 10 (18) | 1 (25) | 9 (21) | 0 (0) | |
| Mycological evaluation at diagnosis | 45 | ||||
| Positive samples | 28 (62) | 3 (100) | 25 (71) | 0 (0) | |
| BA | 36 | 21 (58) | 3 (100) | 18 (69) | 0 (0) |
| BAL fluid | 37 | 17 (46) | 3 (100) | 14 (52) | 0 (0) |
| Sputum | 20 | 15 (75) | 1 (100) | 14 (74) | 0 (0) |
| Species | 28 | ||||
| A. fumigatus | 22 (49) | 3 (100) | 19 (54) | 0 (0) | |
| A. flavus | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| A. nidulans | 2 (4) | 0 (0) | 2 (6) | 0 (0) | |
| A. niger | 2 (4) | 0 (0) | 2 (6) | 0 (0) | |
| Others | 2 (4) | 0 (0) | 2 (6) | 0 (0) | |
| PCR at diagnosis | |||||
| A. fumigatus real-time PCR | 39 | ||||
| Positive | 5 (13) | 0 (0) | 4 (14) | 1 (12) | |
| Range of positive values (pg of fungal DNA/ml) | 0.86–12.2 | ||||
| Aspergillus spp. real-time PCR | 38 | ||||
| Positive | 18 (47) | 2 (67) | 10 (37) | 6 (75) | |
| Range of positive values (pg of fungal DNA/ml) | 0.01–340 | ||||
GM, galactomannan; BAL, bronchoalveolar lavage; BA, bronchial aspirate; IA, invasive aspergillosis.
Voriconazole alone was used as the first-line treatment in 63% of patients (n = 36). The other first-line treatments were as follows: liposomal amphotericin B for 13 patients (23%), 4 of whom also had caspofungin and 2 of whom also had voriconazole; caspofungin alone for 3 patients; and both voriconazole and caspofungin for 5 patients. At day 60, only 18% of patients were still on first-line antifungal treatment. The median duration of the first-line treatment was 16 days (1st to 3rd quartile, 7 to 41). Treatment modifications were due to the progression or absence of regression of IA CT scan lesions (44%), toxicity (28%), or administration convenience (28%). Overall, only five patients did not receive voriconazole during the first 45 days of the study. Due to these numerous modifications, biological markers were not analyzed in relation to treatment schedules.
At day 45, 30 patients (53%) were assessed as responders (10 had a complete response and 20 had a partial response) and 25 (44%) were nonresponders, while 2 patients were unable to be evaluated. A total of 20 patients (35%) died within the 60 days of follow-up, of whom only 2 died from IA; the others died from other causes but still had IA. On the day of death, 12 of these patients had progressive IA, 1 had a partial response to treatment, 1 had stable disease, and 4 were unable to be evaluated.
Associations between baseline characteristics and outcome.
A significant positive association was found between a poor day 45 evaluation and patients who were receiving a high cumulative dose of prednisone the week before diagnosis of IA (Tables 3 and 4). No association was found between the baseline level of Aspergillus antibodies, the presence of a halo sign at diagnosis, or allogeneic hematological stem cell transplantation (HSCT) and the outcome (Tables 3 and 4). All of these results were confirmed using sensitivity analyses.
Table 3.
Factors at baseline associated with day 45 evaluation for all patients
| Variablea | Data [no., no. (%), or median (range)] for indicated patient(s) |
Pb | ||
|---|---|---|---|---|
| Responder (n = 3) | Nonresponder (n = 2) | Undetermined (n = 2) | ||
| Age (yr) | 52.1 (15.6–77.8) | 45.3 (9.8–71.9) | 31.5 and 63.9 | 0.53 |
| Patient risk category | 0.29 | |||
| Allogeneic HSCT | 10 (33) | 13 (52) | 0 (0) | |
| AL | 15 (50) | 7 (28) | 1 (50) | |
| Others | 5 (17) | 5 (20) | 1 (50) | |
| EORTC-defined IA | 0.060 | |||
| Proven | 2 (7) | 2 (8) | 0 (0) | |
| Probable | 20 (67) | 22 (88) | 2 (100) | |
| Possible | 8 (27) | 1 (4) | 0 (0) | |
| Leukocyte count of <100/mm3 at diagnosis | 15 (50) | 12 (48) | 0 (0) | 1.00 |
| Receiving corticosteroids | 11 (37) | 18 (72) | 2 (100) | 0.014 |
| Cumulative dose of prednisone the week before diagnosis (mg/kg of body wt) | 0.0 (0.0–11.0) | 7.0 (0.0–28.0) | 7.0 and 31.0 | 0.002 |
| Received immunosuppressive therapy for <1 month | 8 (27) | 12 (48) | 0 (0) | 0.16 |
| Received antimold therapy for <15 days | 15 (50) | 19 (76) | 0 (0) | 0.057 |
| Concomitant pulmonary infection | 8 (27) | 8 (32) | 0 (0) | 0.77 |
| Serum GM antigen value | 0.4 (0.0–9.3) | 1.1 (0.1–11.0) | 0.2 and 4.8 | 0.039 |
| Patient(s) with value of ≥0.5 | 14 (47) | 15 (60) | 1 (50) | 0.42 |
| BAL fluid GM antigen value | 3.4 (0.1–9.8) | 0.4 (0.0–7.0) | 0.096 | |
| Patients with value of ≥0.5 | 10 (71) | 8 (53) | 0.45 | |
| Anti-Aspergillus antibody value | 3.3 (0.1–181.0) | 3.2 (1.0–108.0) | 0.2 and 8.1 | 0.79 |
| Patients with value of ≥12 | 4 (14) | 6 (24) | 0 (0) | 0.49 |
| Positive mycological examination of respiratory samples | 13 (57) | 14 (67) | 1 (100) | 0.55 |
| Positive PCR Aspergillus fumigatus | 2 (9) | 3 (20) | 0 (0) | 0.36 |
| Positive PCR Aspergillus spp | 13 (57) | 4 (29) | 1 (100) | 0.17 |
| First-line treatment with voriconazole | 22 (73) | 20 (80) | 1 (50) | 0.75 |
| Halo sign | 19 (63) | 14 (56) | 0 (0) | 0.59 |
HSCT, hematopoietic stem cell transplantation; GVHD, graft versus host disease; AL, acute leukemia; CS, corticosteroids; GM, galactomannan.
Fisher's exact test or Wilcoxon rank-sum test for responders versus nonresponders.
Table 4.
Factors at baseline associated with day 45 evaluation after excluding possible IA
| Variablea | Data [no., no. (%), or median (range)] for indicated patient(s) |
Pb | ||
|---|---|---|---|---|
| Responder (n = 22) | Nonresponder (n = 24) | Undetermined (n = 2) | ||
| Age (yr) | 52.1 (15.6–77.8) | 45.0 (9.8–71.9) | 31.5 and 63.9 | 0.27 |
| Patient risk category | 0.64 | |||
| Allogeneic HSCT | 9 (41) | 13 (54) | 0 (0) | |
| AL | 8 (36) | 6 (25) | 1 (50) | |
| Others | 5 (23) | 5 (21) | 1 (50) | |
| EORTC-defined IA | 1.00 | |||
| Proven | 2 (9) | 2 (8) | 0 (0) | |
| Probable | 20 (91) | 22 (92) | 2 (100) | |
| Leukocyte count <100/mm3 at diagnosis | 9 (41) | 11 (46) | 0 (0) | 0.77 |
| Receiving corticosteroids | 10 (45) | 18 (75) | 2 (100) | 0.069 |
| Cumulative dose of prednisone (mg/kg of body wt) the week before diagnosis | 0.0 (0.0–11.0) | 7.0 (0.0–28.0) | 7.0 and 31.0 | 0.018 |
| Received immunosuppressive therapy for <1 month | 7 (32) | 12 (50) | 0 (0) | 0.24 |
| Received antimold therapy for <15 days | 11 (50) | 18 (75) | 0 (0) | 0.13 |
| Concomitant pulmonary infection | 8 (36) | 8 (33) | 0 (0) | 1.00 |
| Serum GM antigen value | 0.8 (0.1–9.3) | 1.3 (0.1–11.0) | 0.2 and 4.8 | 0.064 |
| Patients with value of ≥0.5 | 14 (64) | 15 (62) | 1 (50) | 1 |
| BAL fluid GM antigen value | 3.4 (0.1–9.8) | 0.8 (0.0–7.0) | 0.027 | |
| Patients with value of ≥0.5 | 10 (83) | 8 (57) | 0.22 | |
| Anti-Aspergillus antibody value | 3.3 (1.0–181.0) | 3.4 (1.0–108.0) | 0.2 and 8.1 | 0.9 |
| Patients with value of ≥12 | 4 (19) | 6 (25) | 0 (0) | 0.73 |
| Positive mycological examination of respiratory samples | 13 (76) | 14 (70) | 1 (100) | 0.72 |
| Positive PCR for Aspergillus fumigatus | 1 (6) | 3 (21) | 0 (0) | 0.32 |
| Positive PCR for Aspergillus spp. | 8 (50) | 3 (23) | 1 (100) | 0.25 |
| First-line treatment with voriconazole | 12 (82) | 20 (83) | 1 (50) | 1.00 |
| Halo sign | 12 (55) | 13 (54) | 0 (0) | 1.00 |
HSCT, hematopoietic stem cell transplantation; GVHD, graft versus host disease; AL, acute leukemia; CS, corticosteroids; GM, galactomannan.
Fisher's exact test or Wilcoxon rank-sum test for responders versus nonresponders.
We found no evidence of association at baseline between serum GM antigen level and mycological evaluation (Somers' Dxy = 0.025, P = 0.90), Aspergillus antibodies (Spearman rho = 0.007, P = 0.96), or PCR fungal load (Spearman rho = −0.02, P = 0.90 for A. fumigatus and Spearman rho = −0.14, P = 0.40 for Aspergillus spp.).
In addition, the GM level at baseline was associated with survival at day 60 (hazard ratio of 1.25 per unit increase of GM index [95% confidence interval, 1.10 to 1.43]).
Associations between kinetics of biological markers and the outcome of patients at day 45.
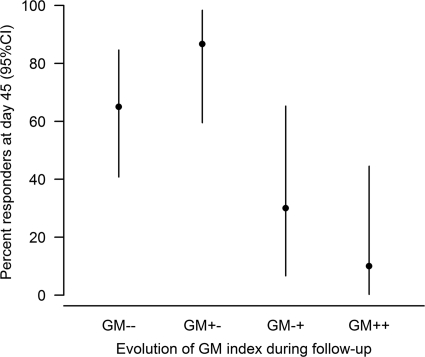
We indeed found a strong association between the evolution of GM levels and outcome at day 45 with four profiles of evolution (Fig. 1 and Table 5).
Fig 1.
Association between the evolution of serum galactomannan (GM) during the follow-up and clinical response at day 45. GM−−, patients with a negative serum GM index at baseline and whose GM index remained negative throughout the follow-up (n = 20); GM+−, patients with a positive baseline GM index that became consistently negative during the follow-up (n = 16); GM−+, patients who had a transient negative GM index during the follow-up and a subsequent positive GM index (n = 11); GM++, patients with a positive GM index at baseline that remained positive during the follow-up (n = 10). 95% CI, 95% confidence interval.
Table 5.
Association between the evolution of serum galactomannan during the follow-up and positive clinical response at day 45
| GM evolutiona | OR (95% CI)b |
|
|---|---|---|
| All patients | Possible IA excluded | |
| GM −/− | 1 | 1 |
| GM +/− | 3.50 (0.61–20.1) | 6.50 (1.00–42.2) |
| GM −/+ | 0.23 (0.04–1.18) | 0.29 (0.04–1.98) |
| GM +/+ | 0.06 (0.01–0.57) | 0.11 (0.01–1.17) |
GM, serum galactomannan; GM−/−, patients with a negative serum GM index at baseline and whose GM index remained negative throughout the follow-up (n = 20 including 8 possible IA); GM+/−, patients with a positive baseline GM index that became consistently negative during the follow-up (n = 16 including no possible IA); GM−/+, patients who had a transient negative GM index during the follow-up and a subsequent positive GM index (n = 11 including 1 possible IA); GM+/+, patients with a positive GM index at baseline that remained positive during the follow-up (n = 10 including no possible IA).
OR, odds ratio; 95% CI, 95% confidence interval; IA, invasive aspergillosis.
The response rates were significantly different between the four groups of patients (P = 0.0003). Among the 20 patients who had a negative serum GM index at baseline and whose GM index remained negative throughout the follow-up (12 patients with probable IA and 8 patients with possible IA), 65% were responders. Among the 11 patients who had a transient negative GM index during the follow-up and a subsequent positive GM index, 30% were responders (all responders had a negative GM index at diagnosis). Among the patients with possible IA, one became positive for serum GM antigen during follow-up (day 7) and another did not reach the positivity threshold (0.5) but had a serum GM antigen level of 0.48 at day 6.
Among the 16 patients with a positive baseline GM index that became consistently negative during the follow-up, 87% were responders. Among the 10 patients with a positive GM index at baseline that remained positive during the follow-up, only 10% were responders.
We then focused on the evolution of serum GM values during the 10 days following the inclusion to identify an early marker of the outcome and found no association between the slope of GM values or the area under the curve; the association between serum GM values and the outcome was only strongly associated with the baseline GM value.
No other association between the kinetics of any other biological marker and the outcome of patients was found. Regardless of the PCR method used (A. fumigatus or Aspergillus spp.), a progressive increase in the fungal load was noted for 10 patients during the 45 days of follow-up. This increase was not correlated with the outcome. Of note, a positive fungal load was identified at baseline in six of the nine patients with possible IA. In addition, no antibodies directed against recombinant antigens were identified in any patient at any time during the follow-up and no significant association was found between the kinetics of anti-A. fumigatus IgG and patient outcomes. Interestingly, four patients with possible IA had positive anti-Aspergillus antibodies during follow-up (at day 3, 4, 6, and 6), whereas they were negative at baseline.
Sequential clinical evaluations and outcome.
The clinical evaluation at day 14 was significantly associated with the day 45 outcome, as 76% of patients who were responders at day 14 were responders at day 45, and 73% of patients who had stable disease at day 14 were responders at day 45. Conversely, only 17% of patients who had progressive disease at day 14 were responders at day 45 (P = 0.003). A similar association was not found between clinical evaluation at day 7 and day 45 outcome.
DISCUSSION
In this prospective study, we found the following: (i) a poor day 45 outcome was strongly associated with a high baseline serum GM index; (ii) a consistently negative serum GM index during the follow-up was associated with a good outcome, in contrast to either a steady or an emerging positive GM index; and (iii) the day 14 clinical evaluation was predictive of the day 45 outcome.
Monitoring the serum GM antigen level was crucial for predicting the outcome of patients with IA. Our data prospectively confirmed a recent retrospective study showing a strong association between a high baseline serum GM index and a poor outcome (15).
Several previous studies, mostly retrospective, focused on the kinetics of GM index values during the follow-up of patients with IA and are of two types: those that demonstrated a correlation between a persistently positive or negative GM index and the survival outcome (16, 18, 30) and those that analyzed GM index decay (3, 15). The first category of study showed that the survival of patients whose serum GM index titers normalized was significantly better than that of those whose titers remained persistently positive (18, 30). In the second category of study, we had previously found that an increase in the GM index of 1.0 over the baseline value during the first week of observation was predictive of treatment failure (3). Koo et al. also found that both the GM value at baseline and the 1-week GM decay were predictive of mortality, independent of other risk factors for mortality and antifungal exposure (15). One limitation of these studies is that they only included patients with a positive serum GM index at diagnosis, whereas a significant proportion of patients with IA have a negative serum GM index (7, 11). Similar to the results of other studies, 47% of our patients had a negative GM index, although the diagnosis of IA was retained. In this context, we showed for the first time that the prognosis of patients with a negative GM index at diagnosis was favorable; however, this outcome was less favorable than that of those whose positive GM index at diagnosis became negative during the follow-up. We confirmed that a persistently positive GM index was clearly associated with a poor outcome (16, 18, 30). One could postulate that patients with a persistently negative serum GM would not have IA. However, 60% of these patients had probable IA; among the other 40% with possible IA, six of eight patients had macronodules with a halo sign, one had an air crescent sign, and one had macronodules and consolidation with a history of probable IA. Furthermore, all cases were discussed in a data review committee in which particular attention was paid to exclude any differential diagnosis.
In the current study, in contrast to the previous retrospective studies, we could not find any association between the early modification of serum GM index values and the outcome (3, 15). One explanation may be that a significant proportion of our patients had a high level of serum GM at diagnosis, leading to a low probability of further increase within the days following the introduction of antifungal treatment.
Because death occurring during an antifungal treatment for IA is frequently unrelated to IA (24), we chose to consider not only survival but also the sequential clinical response for evaluation of the outcome of IA. However, reliable evaluations of the radiological response of patients with IA have to face several difficulties: (i) in neutropenic patients, the volume of aspergillosis lesions usually increases during neutrophil recovery (4), with a maximum at day 7 after diagnosis of IA, and then decreases after day 14 (5); (ii) radiographic abnormalities do not necessarily reflect infection (4, 17); and (iii) the early evolution of lung CT scans in hematological nonneutropenic patients with IA is unknown. Indeed, sequential clinical evaluation also has some limitations but has never been prospectively assessed in relation to the day 45 outcome. In the current study, we found that the evaluation of the clinical response at day 14 was associated with the day 45 outcome. Indeed, few patients who had progressive disease at day 14 were responders at day 45. Although this finding must be confirmed in further prospective studies, we think that it is of great importance for early adjustment of the antifungal treatment.
As recommended in our study, we included patients who had clinical manifestations fully consistent with fungal etiology but for whom there was no mycological evidence available, although a reasonable attempt has been made to exclude an alternative etiology (i.e., patients with newly defined possible IA) (9). From the moment a well-defined category of possible IA is retained, evaluation of new mycological tools (other than microscopic/culture mycology or galactomannan) should be encouraged to reinforce the diagnosis of IA. Indeed, we found that 66% of the patients with possible IA had a positive PCR at baseline. We also found that although anti-Aspergillus IgG results were negative at diagnosis in all patients with possible IA, they became positive in 44% of these patients during the follow-up. Further studies are needed to evaluate whether PCR and/or anti-Aspergillus antibodies may upgrade the diagnosis of IA in patients with possible IA.
Due to low and transient fungal loads in blood, Aspergillus PCR was unreliable to predict the outcomes of patients in our study. As reported in other studies, our understanding of DNA release and kinetics in fungal infections is still poor, and Aspergillus DNAemia is likely to be only transient (12, 13).
The detection of antibodies, which was originally used for diagnosis rather than outcome (23), was not contributive in our group of deeply immunocompromised patients. No antibody response against Aspergillus recombinant antigens was found, and the rates of IgG antibody responses assessed by the virion AES test were not discriminant between responders and nonresponders (P > 0.99).
Finally, we conclude that the GM index value at diagnosis, the GM index kinetics, and clinical evaluation at day 14 during the follow-up of IA are the best markers to predict the outcome in patients and thus should be taken into account for adapting antifungal treatment.
ACKNOWLEDGMENTS
This work was supported by La Ligue Contre le Cancer and MSD Laboratories.
We thank Caroline Auvray for her substantial contribution in data retrieval.
Potential conflicts of interest include the following. A.B. has been a board member for Schering-Plough and has served in the speakers' bureau for Schering-Plough and Pfizer. R.P. has served as a consultant for Pierre Fabre Oncologie. J.M. has served in the speakers' bureau for Merck Sharp and Dohme-Chibret. M.C. has received travel accommodations for herself and her institution from Pfizer and Gilead and travel accommodations for her institution from Astellas and Merck Sharp and Dohme-Chibret and has worked as a consultant for Conidia. E.R. has been a board member for Genzyme. B.B. has received honoraria from Merck Sharp and Dohme-Chibret for manuscript preparation. C.L. has worked as a consultant for Pfizer and Gilead and has served in the speakers' bureau for Merck Sharp and Dohme-Chibret. S.T. has been a board member for Astellas. P.R. has received advisory board fees from Schering-Plough and Pfizer and has served in the speakers' bureau for Gilead, Merck Sharp and Dohme-Chibret, Pfizer, and Schering-Plough. All other authors have no conflicts.
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Anaissie EJ. 2007. Trial design for mold-active agents: time to break the mold—aspergillosis in neutropenic adults. Clin. Infect. Dis. 44:1298–1306 [DOI] [PubMed] [Google Scholar]
- 2. Ascioglu S, et al. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7–14 [DOI] [PubMed] [Google Scholar]
- 3. Boutboul F, et al. 2002. Invasive aspergillosis in allogeneic stem cell transplant recipients: increasing antigenemia is associated with progressive disease. Clin. Infect. Dis. 34:939–943 [DOI] [PubMed] [Google Scholar]
- 4. Caillot D, et al. 2001. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J. Clin. Oncol. 19:253–259 [DOI] [PubMed] [Google Scholar]
- 5. Caillot D, et al. 2010. Computer tomography in pulmonary invasive aspergillosis in hematological patients with neutropenia: a useful tool for diagnosis and assessment of outcome in clinical trials. Eur. J. Radiol. 74:e172–e175 [DOI] [PubMed] [Google Scholar]
- 6. Challier S, Boyer S, Abachin E, Berche P. 2004. Development of a serum-based TaqMan real-time PCR assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 42:844–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cordonnier C, et al. 2009. Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis. Clin. Microbiol. Infect. 15:81–86 [DOI] [PubMed] [Google Scholar]
- 8. Cornely OA, et al. 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin. Infect. Dis. 44:1289–1297 [DOI] [PubMed] [Google Scholar]
- 9. De Pauw B, et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herbrecht R, et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 11. Herbrecht R, et al. 2010. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: a European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant. 45:1227–1233 [DOI] [PubMed] [Google Scholar]
- 12. Hope WW, Walsh TJ, Denning DW. 2005. Laboratory diagnosis of invasive aspergillosis. Lancet Infect. Dis. 5:609–622 [DOI] [PubMed] [Google Scholar]
- 13. Hummel M, et al. 2010. Aspergillus PCR testing: results from a prospective PCR study within the AmBiLoad trial. Eur. J. Haematol. 85:164–169 [DOI] [PubMed] [Google Scholar]
- 14. Kami M, et al. 2001. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin. Infect. Dis. 33:1504–1512 [DOI] [PubMed] [Google Scholar]
- 15. Koo S, Bryar JM, Baden LR, Marty FM. 2010. Prognostic features of galactomannan antigenemia in galactomannan-positive invasive aspergillosis. J. Clin. Microbiol. 48:1255–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maertens J, et al. 2009. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer 115:355–362 [DOI] [PubMed] [Google Scholar]
- 17. Miceli MH, Maertens J, Buve K. 2007. Immune reconstitution inflammatory syndrome in cancer patients with pulmonary aspergillosis recovering from neutropenia: proof of principle, description, and clinical and research implications. Cancer 110:112–120 [DOI] [PubMed] [Google Scholar]
- 18. Miceli MH, et al. 2008. Strong correlation between serum aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin. Infect. Dis. 46:1412–1422 [DOI] [PubMed] [Google Scholar]
- 19. Millon L, et al. 2005. Use of real-time PCR to process the first galactomannan-positive serum sample in diagnosing invasive aspergillosis. J. Clin. Microbiol. 43:5097–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neofytos D, et al. 2010. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl. Infect. Dis. 12:220–229 [DOI] [PubMed] [Google Scholar]
- 21. Nivoix Y, et al. 2008. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin. Infect. Dis. 47:1176–1184 [DOI] [PubMed] [Google Scholar]
- 22. Nucci M, et al. 2010. Probable invasive aspergillosis without prespecified radiologic findings: proposal for inclusion of a new category of aspergillosis and implications for studying novel therapies. Clin. Infect. Dis. 51:1273–1280 [DOI] [PubMed] [Google Scholar]
- 23. Sarfati J, et al. 2006. Recombinant antigens as diagnostic markers for aspergillosis. Diagn. Microbiol. Infect. Dis. 55:279–291 [DOI] [PubMed] [Google Scholar]
- 24. Segal BH, et al. 2008. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin. Infect. Dis. 47:674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaukat A, et al. 2005. Invasive filamentous fungal infections in allogeneic hematopoietic stem cell transplant recipients after recovery from neutropenia: clinical, radiologic, and pathologic characteristics. Mycopathologia 159:181–188 [DOI] [PubMed] [Google Scholar]
- 26. Steinbach WJ, Juvvadi PR, Fortwendel JR, Rogg LE. 2011. Newer combination antifungal therapies for invasive aspergillosis. Med. Mycol. 49:S77–S81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stynen D, Goris A, Sarfati J, Latge JP. 1995. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33:497–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wingard JR, et al. 2010. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 116:5111–5118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wingard JR, Ribaud P, Schlamm HT, Herbrecht R. 2008. Changes in causes of death over time after treatment for invasive aspergillosis. Cancer 112:2309–2312 [DOI] [PubMed] [Google Scholar]
- 30. Woods G, et al. 2007. Serum Aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis: a study of 56 patients with hematologic cancer. Cancer 110:830–834 [DOI] [PubMed] [Google Scholar]