Abstract
The correct diagnosis and monitoring of HIV-1 group O (HIV-O) infection are essential for appropriate patient management, for the prevention of mother-to-child transmission, and for the detection of dual HIV-M/HIV-O infections. HIV-O RNA quantification is currently possible with two commercial kits (from Abbott and Roche), which quantify HIV-M and HIV-O strains indifferently; therefore, they cannot be used for the specific identification of HIV-O infection. We designed a new real-time quantitative reverse transcription PCR (RT-qPCR assay) (INT-O), which we compared with our previous version, LTR-O, and with the Abbott RealTime HIV-1 kit. Specificity was assessed with 27 HIV-1 group M strains and the prototype strain of group P. Clinical performances were analyzed by using 198 stored plasma samples, representative of HIV-O genetic diversity. Analytical sensitivity, repeatability, and reproducibility were also determined. The detection limit of the INT-O assay was 40 copies/ml, and its specificity was 100%. The repeatability and reproducibility were excellent. Analysis of clinical samples showed a good correlation between the INT-O and LTR-O assays (r = 0.8240), with an improvement of analytical sensitivity. A good correlation was also obtained between the INT-O and Abbott assays (r = 0.8599) but with significantly higher values (0.19 logs) for the INT-O method, due to marked underquantifications for some patients. These results showed that HIV-O genetic diversity still has an impact on RNA quantification. The new assay, INT-O, allows both the specific diagnosis of HIV-O infection and the quantification of diverse HIV-O strains. Its detection limit is equivalent to that of commercial kits. This assay is cheap and suitable for use in areas in which strains of HIV-1 groups M and O cocirculate.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) is divided into four groups, groups M, N, O, and P, the most recently described group (18, 28). HIV-1 group O (HIV-O) infection is endemic in West Central Africa and particularly in Cameroon, where it accounts for about 1% of HIV diagnoses (2, 32). Cases of HIV-O infection have also been reported in countries with links to this region (12, 20, 24, 25). Due to close historical links, a larger number of HIV-O-infected patients are found in France, where a network created to identify and monitor HIV-O (RES-O) has detected 130 cases since the first described case (1, 7, 14).
HIV-O strains are highly divergent from the major group M, leading to their designation as “outliers.” These strains also display marked intragroup genetic diversity: they are divided into three clades (clades A, B, and C), but numerous divergent strains lie outside these clades (21). This genetic diversity has important implications for the diagnosis and monitoring of HIV-O infection, including a risk of false negativity and viral load underestimations (6, 11, 16). Monitoring has been improved by the development of an in-house HIV-O-specific real-time PCR method (10) and a commercial kit covering both HIV-M and HIV-O (RealTime HIV-1; Abbott Molecular, Rungis, France) (11). More recently, a new version (v2.0) of the Cobas TaqMan HIV-1 assay (Roche, Meylan, France) has also been validated for the quantification of both HIV-M and HIV-O (23). The availability of commercial assays capable of quantifying the viral load for both HIV-1 groups M and O is a welcome development. Paradoxically, however, this improved performance makes it more difficult to detect cases of HIV-O infection. Indeed, group O infection was previously identified on the basis of discrepancies between seropositivity and molecular or immunologic findings, such as a failure to detect viral RNA in HIV-seropositive patients with low CD4 cell counts, particularly in patients originating from areas where this disease is endemic or who had been in contact with persons from these areas.
In areas in which HIV-M and HIV-O cocirculate, a failure to diagnose HIV-O infection specifically before treatment initiation can have harmful consequences due to the natural resistance of HIV-O to nonnucleoside reverse transcriptase inhibitors (NNRTIs) efavirenz and nevirapine (8, 19, 27). The specific identification of HIV-O is therefore necessary (i) to avoid a rapid virological failure if an NNRTI is included in the treatment, resulting in a situation equivalent to bitherapy for the patient, and the use of NNRTIs in treatments for the prevention of mother-to-child transmission, (ii) for the follow-up of children born to infected mothers (4, 9), and (iii) for therapeutic management (resistance analysis) that needs specific HIV-O tools, as previously described (5, 6, 32). Finally, we and others have shown that dual HIV-M/HIV-O infection/superinfection can occur in areas in which both groups circulate, including France (3, 17, 29, 30, 33), necessitating the precise individual monitoring of each strain to determine the course of infection in the absence of treatment and, above all, the specific response of the strain to treatment.
Moreover, available commercial kits are costly, and alternative techniques are therefore needed for use in regions where the disease is endemic. We previously developed a quantitative reverse transcription PCR (RT-qPCR) method targeting the long terminal repeat (LTR) region for the specific detection and quantification of HIV-O RNA (9, 10). However, this “LTR-O” assay had a detection limit of 200 copies/ml and a specificity of only 85% due to the amplification of some HIV-M variants.
The aim of this work was to design and evaluate a new, more sensitive and specific RT-qPCR assay targeting the integrase gene of HIV-O that is suitable for use in all countries in which HIV-M and HIV-O cocirculate.
MATERIALS AND METHODS
The primers and probe targeting the integrase gene were defined with Oligo 6 (Medprobe, Oslo, Norway) and AlleleID 4 (Premier Biosoft International, Palo Alto, CA) software and were synthesized by Eurogentec (Seraing, Belgium), based on an alignment of 17 complete sequences of HIV-O. Primers pol-O-4958-U-24 (5′-TCTATTACAGAGACAGCAGAGAYC-3′) and pol-O-5012-L-21 (5′-CTACTGCTCCYTCACCTTTCC-3′), at positions 4958 and 5012 of strain ANT (GenBank accession number L20587), generated a product of 75 bp. A highly specific hydrolysis probe, S-pol-O-4988-L23 (5′-ACAGGAGYTGKGCCGGTCCTTTC-3′), binding to position 4988 was selected. A reporter fluorescein dye (6-carboxyfluorescein [FAM]) was attached to its 5′ end, and a nonfluorescent quencher (Eclipse Dark Quencher) was linked to the 3′ end, for detection.
Viral RNA was extracted from 200 μl of plasma with a magnetic bead technology (MagNa Pure; Roche, Meylan, France) and the Total Nucleic Acid kit, according to the manufacturer's instructions. The RNA was eluted in 50 μl of RNase-free water. The ABI 7900HT instrument (Applied Biosystems, Courtaboeuf, France) was used to amplify and quantify the PCR products after each cycle. PCR was performed with the RNA Ultrasense One-Step Quantitative RT-PCR kit (Invitrogen, Cergy Pontoise, France). The enzyme mix (2.5 μl) was added to 10 μl of 5× reaction mix, 0.5 μM each primer, 0.5 μM probe, and 1 μl of Rox. RNA (30 μl) was added to 20 μl of this mixture, and amplification was carried out as follows: 50°C for 15 min (1 cycle) and 95°C for 2 min (1 cycle), followed by denaturation at 95°C for 15 s and annealing at 60°C for 30 s (50 cycles). The standard for quantification was a culture supernatant of an HIV-1 group O strain (YBF32), which was quantified 3 times with the RealTime HIV-1 assay (Abbott) and with our previously described RT-qPCR assay. The supernatant was diluted in HIV-negative human plasma, aliquoted, and stored at −80°C. The standard extract was diluted from 2 × 105 to 20 copies/ml to obtain the quantification range.
We determined the specificity of the new RT-qPCR assay by testing the diluted culture supernatants of 27 HIV-1 group M strains with different subtypes or circulating recombinant forms (CRFs) (subtype A, n = 2; subtype B, n = 10; subtype C, n = 1; subtype D, n = 2; subtype F, n = 1; CRF01, n = 2; CRF02, n = 6; CRF06, n = 1; CRF11, n = 2) and the prototype strain (RBF168) of HIV-1 group P. Group and subtype determinations were based on 627 bp of the reverse transcriptase gene and 300 bp of the protease gene, as previously described (15, 18). We evaluated the performance of the assay with respect to the genetic diversity of the HIV-O strains by analyzing eight supernatants of clade A (n = 5), clade B (n = 2), and nontypeable (n = 1) strains. All these HIV-M, -P, and -O samples were RNA positive, with about 5 log10 copies/ml, in the RealTime HIV-1 assay (Abbott).
The sensitivity of the new real-time PCR method was determined by testing 20 replicates each of serial dilutions of the YBF32 standard, 1.2, 2.4, and 4.8 copies/30 μl, corresponding to 10, 20, and 40 copies/ml, after multiplication by 1.66 to take into account the volume of the extract used (30 μl) and after multiplication by 5 to convert the number of copies per 200 μl of extracted plasma into the number of copies per ml. The dynamic range over which the reaction was linear was evaluated by testing duplicate dilutions of the standard from 20 to 2 × 107 copies/ml. For repeatability (intra-assay variation) assessments, a dilution of the YBF32 standard containing around 5 log10 copies/ml was subjected to 20 extractions and RT-PCR assays in the same experiment. For the determination of reproducibility and the definition of a future reproducibility control (interrun variability), one frozen YBF32 culture supernatant containing an unknown amount of viral RNA was aliquoted and studied under the usual experimental conditions, with a new range for each run, in 20 experiments.
The clinical performance of the new assay was then compared with those of the RealTime HIV-1 kit (Abbott) and our previously used in-house method. Briefly, in the RealTime HIV-1 assay, 600 μl of plasma is extracted with an m2000sp automaton. The dynamic range extends from 40 copies/ml to 10 million copies/ml (1.6 log to 7 log copies/ml). In the LTR-O in-house RT-qPCR method (9), 200 μl of plasma is extracted with a MagNa Pure automaton and amplified with a LightCycler 1.5 device (Roche Diagnostics); the dynamic range is 200 copies/ml to 2 million copies/ml (2.3 log to 6.3 log copies/ml).
We studied 198 clinical plasma samples from 77 patients, collected into EDTA or acid citrate dextrose (ACD), between 2005 and 2009. Of these samples, 151 were collected from 35 patients living in France, including two vertically infected children, two treatment-naive patients, and four patients from whom serial samples were obtained during follow-up. The remaining 47 samples (42 patients) were collected during diagnosis and follow-up at the Centre Pasteur du Cameroun in Yaoundé, Cameroon. Sixteen of the Cameroonian patients were receiving antiretroviral treatment. Group O infection had been diagnosed with specific peptide-based assays, as previously described (22), and confirmed by the nucleotide sequencing (13, 32) of several regions. Fifty-six strains belonged to HIV-O clade A, 4 belonged to clade B, and 5 belonged to clade C, whereas 12 strains were outside these clades. Comparative studies of the new assay were performed with all 198 samples for our previously used technique and with 149 samples for the Abbott kit. The Wilcoxon matched-pairs test was used to compare the distributions for each of the three techniques. Bland and Altman curves were used to represent the degree of agreement between two techniques. The mean values for each sample, obtained by two techniques, were plotted on the x axis, and the difference between the values obtained with these two techniques was plotted on the y axis.
RESULTS
The specificity of the new integrase O RNA quantification (INT-O) assay was excellent: all the group O strains were correctly identified, and none of the group P or group M strains gave a false-positive result.
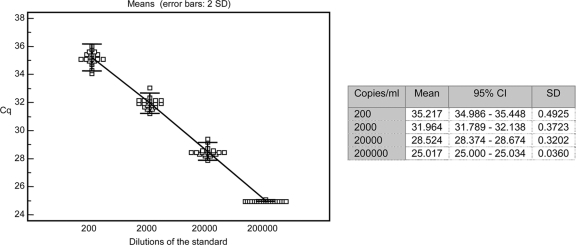
The INT-O assay gave detection rates of 100% for dilutions of HIV-O supernatants containing 4.8 copies/30 μl, 95% at 2.4 copies/30 μl, and 65% at 1.2 copies/30 μl. The quantification cutoff was thus set at 4.8 copies/30 μl, corresponding to 40 RNA copies/ml. The slope of the standard curve including standard plots from 20 to 2 × 107 copies/ml (evaluating the dynamic range) was −3.25, with an r2 coefficient of 0.992. The repeatability experiment gave a mean value of 5.25 log10 copies/ml (standard deviation [SD], 0.06 log10 copies/ml) for the diluted standard. Reproducibility experiments with four serial dilutions of the standard gave standard deviations of the cycle quantification (Cq) of 0.49 at 200 copies/ml and 0.036 at 200,000 copies/ml (Fig. 1) and a mean value for the reproducibility control of 4.09 log10 copies/ml with an SD of 0.23 log10 copies/ml.
Fig 1.
Reproducibility of 20 measurements for each dilution of the standard curve. Cq, cycle quantification; CI, confidence interval.
Qualitative analyses showed that the 14 samples that were not detected with the LTR-O assay either contained more than 40 copies/ml in the INT-O assay (n = 10; median, 236 copies/ml; range, 52 to 3,128 copies/ml) or were detectable but not quantifiable (n = 4) (Table 1). For five samples in which the virus was undetectable with the INT-O assay, the virus was detected but not quantifiable with the LTR-O assay. For two samples in which the virus was undetectable with the Abbott method, a positive result was obtained with the INT-O assay (67 and 461 copies/ml). However, these samples had to be diluted before testing with the Abbott technique, due to a lack of sample, raising the theoretical detection limit to 400 copies/ml instead of 40 copies/ml. Six other samples in which virus was detected with the Abbott kit were quantifiable with the INT-O assay (median, 336 copies/ml; range, 82 to 4,290 copies/ml) (Table 1). For seven samples in which the virus was detected but not quantifiable with the Abbott method, the virus was undetectable with the INT-O assay.
Table 1.
Qualitative analysis of the results obtained with plasma samplesa
| Assay | Result | No. of samples in agreement with INT-O viral load assay result of: |
||
|---|---|---|---|---|
| Undetectable | Detected | Quantifiable | ||
| LTR-O viral load | Undetectable | 88 | 4 | 10 |
| Detected | 5 | 5 | ||
| Quantifiable | 86 | |||
| HIV RealTime | Undetectable | 76 | 2 | |
| Detected | 7 | 1 | 6 | |
| Quantifiable | 1 | 56 | ||
Quantifiable indicates a positive result (>40 copies/ml for the INT-O and HIV RealTime methods and >200 copies/ml for the LTR-O assay), and detected indicates results of between 0 and 40 copies/ml (Abbott and INT-O methods) and between 0 and 200 copies/ml (LTR-O method); these values indicate RNA detection but are not linear.
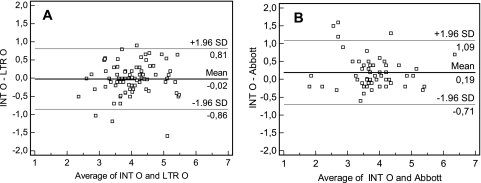
Eighty-five of the 86 samples (52 patients) that yielded values of more than 40 copies/ml with both the INT-O and LTR-O methods could be compared (one sample above the linear range, i.e., >1 × 106 copies/ml, was excluded). The correlation coefficient was an r value of 0.8240 (data not shown). The distributions did not differ significantly between the two techniques (P = 0.7855). There were 5 differences exceeding 0.7 logs, 3 of which were due to viral load underestimations by the INT-O assay and two of which were due to viral load underestimations by the LTR-O assay; these differences were not clade dependent (Fig. 2A).
Fig 2.
Degree of agreement in log copies/ml between the viral loads determined by the INT-O, LTR-O, and Abbott RealTime HIV-1 methods. For Bland and Altman curves, the mean values for each sample obtained by the 2 techniques are plotted on the x axis. The differences between the values obtained by the 2 techniques are plotted on the y axis. The solid lines show the mean differences between the values, and the dotted lines show the mean differences plus or minus 1.96 SD (95% limits of agreement). (A) Degree of agreement between viral loads determined by the INT-O and LTR-O methods. (B) Degree of agreement between viral loads determined by the INT-O and Abbott RealTime methods.
A comparison of the results for 56 samples (24 patients) obtained with the INT-O and Abbott techniques showed that six samples (5 patients) gave results more than 0.7 logs lower by the Abbott method (non-clade-dependent differences) (Fig. 2B). The correlation coefficient was an r value of 0.8599. The distributions differed significantly between the two techniques (P < 0.05), with higher values (0.19 logs) for the INT-O method than for the Abbott RealTime assay. This difference was due mostly to the marked underestimation of the viral load (by 1.19 to 1.63 logs) for three samples (3 patients) by the Abbott technique. The distribution of viral loads was no longer different when these three samples were excluded (data not shown).
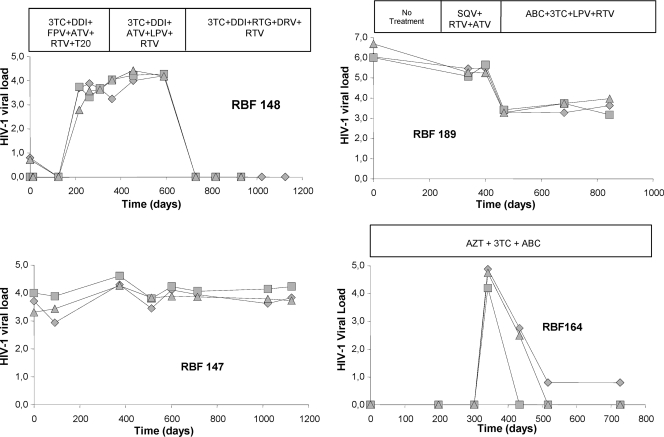
We determined the performances of the HIV-O viral load assays during treatment follow-up by analyzing serial samples from four patients (Fig. 3). The three techniques gave similar curves for three patients, whereas the values for patient RBF147 differed by up to 1 log for some samples, although these differences disappeared in the next sample. Undetectability was observed later for patient RBF164 with the INT-O technique than with the LTR-O technique, demonstrating the markedly higher sensitivity of the new assay. In subsequent samples, the Abbott method continued to give a signal (between 0 and 40 copies/ml), while INT-O values remained undetectable; however, these signals did not indicate virological failure, as the viral load did not increase during the 7 months of follow-up.
Fig 3.
Viral loads during follow-up for 4 patients. ♦, Abbott assay; ■, LTR-O method; ▲, INT-O method. 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; AZT, zidovudine; DDI, didanosine; DRV, darunavir; FPV, fosamprenavir; LPV, lopinavir; RTG, raltegravir; RTV, ritonavir; SQV, saquinavir; T20, enfuvirtide.
DISCUSSION
We describe a new specific RT-qPCR assay for the detection and quantification of HIV-1 group O RNA in plasma. This version is more specific and sensitive than our previously used assay. The excellent specificity of this assay makes it suitable for use in the molecular diagnosis of HIV-O infection, based on an algorithm defined previously for regions where these variants circulate (32). This specific identification is particularly important due to the genetic characteristics of HIV-O compared to the “classical” subtypes or CRFs of HIV-M; these properties have impacts on treatment decisions and on the follow-up of HIV-O infection. The identification and monitoring of HIV-O in a context of dual HIV-M/HIV-O infection in regions where these two groups of viruses cocirculate are also necessary. We recently reported that such dual infections and recombinants are not particularly rare (30), that they can spread outside the endemic region of Cameroon (17, 31), and that they require specific patient management. As HIV-O strains are naturally resistant to nonnucleoside inhibitors (8, 19, 27), patients with HIV-O single infections or HIV-O/HIV-M dual infections with HIV-O who are prescribed NNRTI-containing combinations may actually be receiving only one or two active drugs, creating a risk for the rapid emergence and spread of resistance against the non-NNRTI drugs.
The Roche and Abbott commercial assays are now able to quantify the loads of both HIV-M and HIV-O strains, but neither of these tests can distinguish between the two groups. Paradoxically, this makes it more difficult to diagnose HIV-O infection, which was previously suspected on the basis of discrepancies between seropositivity and molecular or immunologic status. As it has been clearly demonstrated that other commercial assays using Nasba (Nuclisens EasyQ; bioMérieux) or b-DNA (Versant HIV-1 RNA 3.0; Siemens) technologies underestimate the viral load or fail to detect HIV-O strains (26), we compared the INT-O assay to the Abbott kit (the gold standard for HIV-M/HIV-O quantification at the time of the study) and to our previous assay, LTR-O, which provided the initial values. The total volume required for testing made it impossible to include the new Roche Cobas TaqMan v2 assay in the comparison.
The analytical and clinical performances of the INT-O assay are excellent and comparable to those of the Abbott kit. Like most real-time PCR methods, our assay is rapid, sensitive, and reproducible and has a large linear dynamic range, with a detection limit of 40 copies/ml, similar to that of the reference commercial assays. This excellent performance is associated with a lower cost (around 10 euros per well), and the assay is suitable for use in all laboratories with the capacity to perform quantitative PCR. Quantitative analyses of clinical samples showed a good correlation between the results obtained with our two in-house assays, INT-O and LTR-O, except for the low values obtained with the less sensitive LTR-O assay (detection limit, 200 copies/ml). A good correlation between the INT-O and Abbott assays was also obtained. The seven samples in which virus was undetectable by the INT-O assay but detectable by the Abbott assay must be interpreted with caution, because the values obtained lie below the threshold values for the techniques, in a zone of nonreproducibility. In contrast, for some samples quantifiable above 40 copies/ml with the INT-O assay (from 82 to 4,290 copies/ml), the virus was merely “detected” with the Abbott method, highlighting the difficulty of obtaining reliable results for low levels of viral RNA but demonstrating that low levels of replication could be not detected with the commercial assay. For samples with a quantifiable viral load, the Abbott RealTime method gave slightly lower values than did the INT-O assay, mostly due to marked viral load underestimations for three patients. These findings confirm our previous results obtained with culture supernatants (11) and show that none of the available techniques is perfect, owing to the stronger intragroup genetic diversity of HIV group O strains than of group M strains. Thus, the commercial assay is unsuitable for the monitoring of certain patients, and the results obtained at baseline (before treatment) must, where possible, be compared when trying to select the best assay for monitoring a given HIV-O-infected patient. We already use this approach in practice, making it possible to monitor the patients with the most appropriate tool. If such testing is not initially carried out, follow-up based on the monitoring of the viral load is of limited value for these patients. Confirmation is also required for the first sample found to be below the detection limit, as virological failure may remain undetected if the test sensitivity is inadequate.
In conclusion, we have validated a new specific and sensitive assay for the diagnosis and monitoring of HIV-O infection. This simple method is affordable and suitable for use in laboratories in all countries in which HIV groups O and M cocirculate. Our results demonstrate the importance of such an alternative technique, given the marked genetic diversity of group O viruses.
ACKNOWLEDGMENTS
We thank the Institut de Veille Sanitaire and Rouen University Hospital for financial support.
Sylvie Lambert, Fanny Lermechain, and Sébastien Delannoy performed the technical part of this work. We also thank all the physicians and biologists involved in the RES-O HIV-O surveillance network in France.
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Agut H, et al. 1992. Isolation of atypical HIV-1-related retrovirus from AIDS patient. Lancet 340:681–682 [DOI] [PubMed] [Google Scholar]
- 2. Ayouba A, et al. 2001. HIV-1 group O infection in Cameroon, 1986 to 1998. Emerg. Infect. Dis. 7:466–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brand D, et al. 2004. First identification of HIV-1 groups M and O dual infections in Europe. AIDS 18:2425–2428 [PubMed] [Google Scholar]
- 4. Chaix-Baudier ML, et al. 1998. First case of mother-to-infant HIV type 1 group O transmission and evolution of C2V3 sequences in the infected child. French HIV Pediatric Cohort Study Group. AIDS Res. Hum. Retroviruses 14:15–23 [DOI] [PubMed] [Google Scholar]
- 5. Depatureaux A, et al. 2010. Baseline genotypic and phenotypic susceptibilities of HIV-1 group O to enfuvirtide. Antimicrob. Agents Chemother. 54:4016–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Depatureaux A, et al. 2011. Impact of HIV-1 group O genetic diversity on genotypic resistance interpretation by algorithms designed for HIV-1 group M. J. Acquir. Immune Defic. Syndr. 56:139–145 [DOI] [PubMed] [Google Scholar]
- 7. Depatureaux A, et al. 19 June 2010, posting date Specific diagnosis and follow-up of HIV-1 group O infection: RES-O data. Med. Mal. Infect. [Epub ahead of print.] doi:10.1016/j.medmal.2010.04.011. (In French.) [DOI] [PubMed]
- 8. Descamps D, et al. 1997. Susceptibility of human immunodeficiency virus type 1 group O isolates to antiretroviral agents: in vitro phenotypic and genotypic analyses. J. Virol. 71:8893–8898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gueudin M, et al. 2004. Virologic diagnosis and follow-up of children born to mothers infected by HIV-1 group O. J. Acquir. Immune Defic. Syndr. 36:639–641 [DOI] [PubMed] [Google Scholar]
- 10. Gueudin M, et al. 2003. Plasma viral RNA assay in HIV-1 group O infection by real-time PCR. J. Virol. Methods 113:43–49 [DOI] [PubMed] [Google Scholar]
- 11. Gueudin M, et al. 2007. Evaluation of the Roche Cobas TaqMan and Abbott RealTime extraction-quantification systems for HIV-1 subtypes. J. Acquir. Immune Defic. Syndr. 44:500–505 [DOI] [PubMed] [Google Scholar]
- 12. Hampl H, et al. 1995. First case of HIV-1 subtype 0 infection in Germany. Infection 23:369–370 [DOI] [PubMed] [Google Scholar]
- 13. Leoz M, et al. 2008. Integrase polymorphism and HIV-1 group O diversity. AIDS 22:1239–1243 [DOI] [PubMed] [Google Scholar]
- 14. Loussert-Ajaka I, et al. 1995. Variability of human immunodeficiency virus type 1 group O strains isolated from Cameroonian patients living in France. J. Virol. 69:5640–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plantier JC, et al. 2005. HIV-1 resistance genotyping on dried serum spots. AIDS 19:391–397 [DOI] [PubMed] [Google Scholar]
- 16. Plantier JC, et al. 2009. Census and analysis of persistent false-negative results in serological diagnosis of human immunodeficiency virus type 1 group O infections. J. Clin. Microbiol. 47:2906–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plantier JC, et al. 2004. HIV-1 group M superinfection in an HIV-1 group O-infected patient. AIDS 18:2444–2446 [PubMed] [Google Scholar]
- 18. Plantier JC, et al. 2009. A new human immunodeficiency virus derived from gorillas. Nat. Med. 15:871–872 [DOI] [PubMed] [Google Scholar]
- 19. Quinones-Mateu ME, Soriano V, Domingo E, Menendez-Arias L. 1997. Characterization of the reverse transcriptase of a human immunodeficiency virus type 1 group O isolate. Virology 236:364–373 [DOI] [PubMed] [Google Scholar]
- 20. Rayfield MA, et al. 1996. HIV-1 group O virus identified for the first time in the United States. Emerg. Infect. Dis. 2:209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roques P, et al. 2002. Phylogenetic analysis of 49 newly derived HIV-1 group O strains: high viral diversity but no group M-like subtype structure. Virology 302:259–273 [DOI] [PubMed] [Google Scholar]
- 22. Simon F, et al. 2001. Synthetic peptide strategy for the detection of and discrimination among highly divergent primate lentiviruses. AIDS Res. Hum. Retroviruses 17:937–952 [DOI] [PubMed] [Google Scholar]
- 23. Sire JM, et al. 2011. Comparative RNA quantification of HIV-1 group M and non-M with the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 v2.0 and Abbott Real-Time HIV-1 PCR assays. J. Acquir. Immune Defic. Syndr. 56:239–243 [DOI] [PubMed] [Google Scholar]
- 24. Soriano V, et al. 1996. First case of HIV-1 group O infection in Spain. Vox Sang. 71:66. [DOI] [PubMed] [Google Scholar]
- 25. Sullivan PS, et al. 2000. Human immunodeficiency virus (HIV) subtype surveillance of African-born persons at risk for group O and group N HIV infections in the United States. J. Infect. Dis. 181:463–469 [DOI] [PubMed] [Google Scholar]
- 26. Swanson P, et al. 2005. Impact of human immunodeficiency virus type 1 (HIV-1) genetic diversity on performance of four commercial viral load assays: LCx HIV RNA Quantitative, AMPLICOR HIV-1 MONITOR v1.5, VERSANT HIV-1 RNA 3.0, and NucliSens HIV-1 QT. J. Clin. Microbiol. 43:3860–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tuaillon E, et al. 2004. Phenotypic susceptibility to nonnucleoside inhibitors of virion-associated reverse transcriptase from different HIV types and groups. J. Acquir. Immune Defic. Syndr. 37:1543–1549 [DOI] [PubMed] [Google Scholar]
- 28. Vallari A, et al. 2011. Confirmation of putative HIV-1 group P in Cameroon. J. Virol. 85:1403–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vergne L, et al. 2003. Biological and genetic characteristics of HIV infections in Cameroon reveals dual group M and O infections and a correlation between SI-inducing phenotype of the predominant CRF02_AG variant and disease stage. Virology 310:254–266 [DOI] [PubMed] [Google Scholar]
- 30. Vessiere A, et al. 2011. HIV-1 M/O dual infections and recombinant forms circulating in Cameroon and France, poster B125. Abstr. 18th Conf. Retroviruses Oppor. Infect., Boston, MA [Google Scholar]
- 31. Vessiere A, et al. 2010. First evidence of a HIV-1 M/O recombinant form circulating outside Cameroon. AIDS 24:1079–1082 [DOI] [PubMed] [Google Scholar]
- 32. Vessiere A, et al. 2010. Diagnosis and monitoring of HIV-1 group O-infected patients in Cameroun. J. Acquir. Immune Defic. Syndr. 53:107–110 [DOI] [PubMed] [Google Scholar]
- 33. Yamaguchi J, et al. 2004. HIV infections in northwestern Cameroon: identification of HIV type 1 group O and dual HIV type 1 group M and group O infections. AIDS Res. Hum. Retroviruses 20:944–957 [DOI] [PubMed] [Google Scholar]