Abstract
In 2009, three children were hospitalized in Rochester, NY, with sequence-confirmed G8P[4] rotavirus gastroenteritis—the first U.S. detection of this uncommon strain more typically found in Africa. Continued monitoring of G8P[4] and other rotavirus genotypes not represented in current vaccines is essential to assess whether vaccination will result in an increase in prevalence of these strains.
CASE REPORTS
Case 1.
In mid-April 2009, a 25-month-old girl was admitted to the Golisano Children's Hospital at University of Rochester Medical Center with a 3-day history of diarrhea and vomiting (12 to 20 episodes per day). Her maximum daily temperature was 38.3°C. She was previously healthy, although she was born prematurely after a 33-week gestation; she was breast-fed for the first 3 months of life. She lived in the Rochester, NY, metropolitan area and did not have any unusual animal, dietary, or travel exposures in the preceding 3 months. The child had not been vaccinated against rotavirus. Moderate dehydration and lethargy were noted upon physical examination; there were no other abnormalities. After intravenous rehydration in the hospital for 24 h, she was discharged to her home. At admission to the hospital, the child was enrolled, with parental informed consent, into an ongoing, active, prospective, population-based surveillance for acute gastroenteritis among children <5 years old (Centers for Disease Control and Prevention New Vaccine Surveillance Network [CDC NVSN]), the details of which have been previously published (23–26, 32). A stool sample taken during her hospitalization was positive for rotavirus antigen by enzyme immunoassay (Premier Rotaclone; Meridian Biosciences, Inc.). Her parents noted that one of her two siblings (each 3 to 4 years of age) experienced a few days of fever and emesis several days after the patient became ill, suggesting possible intrafamilial spread; however, no samples were taken from that child by their physician, and the sibling was not eligible for study enrollment. The patient's stool specimen was analyzed at the CDC by real-time reverse transcription-PCR (RT-PCR) genotyping and nucleotide sequencing of partial VP7 and VP4 genes as previously described (3, 14). BLAST searches of public databases using VP7 and VP4 sequences identified the strain as genotype G8P[4].
Case 2.
In mid-April 2009, a 34-month-old girl was admitted to the Golisano Children's Hospital at University of Rochester Medical Center with a 5-day history of diarrhea and vomiting (3 to 10 episodes per day). Her maximum daily temperature was 37.7°C. She was previously healthy, born after a full-term gestation and breast-fed for the first 12 months of life. She lived in the Rochester, NY, metropolitan area and did not have any unusual animal, dietary, or travel exposures in the preceding 3 months. The child had not been vaccinated against rotavirus. Moderate dehydration and lethargy were noted upon physical examination; there were no other abnormalities. After intravenous rehydration in the hospital for 24 h, she was discharged to her home. The child was enrolled into NVSN surveillance at admission. Stool testing was positive for rotavirus antigen by enzyme immunoassay, and subsequent partial VP7 and VP4 gene analysis at the CDC revealed that the isolate was genotype G8P[4]. Her parents noted that her 5-year-old sister became ill with diarrhea and vomiting 4 days after the patient's admission (and was herself admitted to the hospital for 3 days), suggesting possible intrafamilial spread; however, no samples were taken from this contact.
Case 3.
During late March of 2009, a 54-month-old boy was admitted to the Golisano Children's Hospital at University of Rochester Medical Center with a 2-day history of diarrhea and vomiting (15 to 30 episodes per day). His maximum daily temperature was 37.7°C. He had a complex past medical history of global developmental delay, poor growth, intermittent hematochezia, and coagulopathy of uncertain origin. He lived in the Rochester, NY, metropolitan area and did not have any unusual dietary or travel exposures in the preceding 3 months; the child did have contact with horses in a therapeutic riding program during this time. The child had not been vaccinated against rotavirus. Moderate dehydration and his baseline levels of hypotonia and choreiform movements were noted upon physical examination; there were no other abnormalities. Intravenous rehydration was administered, and the child improved over the next 1 to 2 days, although his hospitalization was prolonged for 14 days because of evaluation and institution of therapy for newly diagnosed ulcerative colitis and von Willebrand disease. The child was enrolled in the NVSN at admission. Stool testing was positive for rotavirus antigen by enzyme immunoassay, and subsequent partial VP7 and VP4 gene analysis at the CDC revealed that the isolate was genotype G8P[4].
Licensed U.S. rotavirus vaccines include a pentavalent bovine-human reassortant vaccine containing G1 to G4 and P[8] types (RotaTeq; Merck & Co., Inc.) and a monovalent attenuated human G1P[8] vaccine (Rotarix; GlaxoSmithKline, Inc.) (6). Eighty-five percent of U.S. circulating rotavirus strains have a G or P antigen that is contained in both U.S.-licensed rotavirus vaccines (6, 12). However, >40 human G and P antigen combinations have been reported among the >160 known rotavirus strains (13, 20), and uncommon strains (e.g., G9P[8], G12P[8], and G8P[4]) may suddenly appear in a new geographic area (12, 14, 15, 19, 25, 26). The G9P[8] genotype in particular has emerged to become a globally prevalent strain in a relatively short time since its first description (13, 15, 17, 21).
In ongoing postlicensure surveillance through the CDC NVSN, three children with G8P[4] rotavirus gastroenteritis were detected in Rochester, NY, during the 2009 winter season (December 2008 through June 2009). The 3 G8P[4] rotavirus strains were detected in a total of 183 enrolled children with acute gastroenteritis, 54 (30%) of whom had rotavirus infection. Fifty (94%) of the remaining 51 rotavirus strains were typically found U.S. strains with G or P antigens that are contained in the licensed rotavirus vaccines.
Although not statistically significant, these 3 children were 13 months older on average than the 50 infected with more-typical rotavirus strains (37.3 ± 14.7 months versus 24.2 ± 12.3 months, respectively; P = 0.26). No other demographic or clinical differences among these children were found, although all were ill enough to be hospitalized (Table 1). None of the children had unusual travel, dietary, or animal contact; some G8 rotavirus strains infecting humans are thought to reflect a bovine origin (7, 11, 13, 22, 27). This suggests endemic circulation and transmission of the G8P[4] strains in the community, a hypothesis supported by the possible intrafamilial spread in two of the case patients. All 3 children had severe gastroenteritis at onset, although 2 of 3 rapidly recovered with intravenous rehydration and supportive care. None had received any doses of rotavirus vaccines (each was born between 2004 and 2007).
Table 1.
Characteristics of children with G8P[4] rotavirus acute gastroenteritis
| Characteristic | Values for each case (strain) |
||
|---|---|---|---|
| 1 (US09Ro103) | 2 (US09Ro104) | 3 (US09Ro045) | |
| Age (mo) | 24.8 | 33.5 | 53.5 |
| Duration of symptoms at enrollment (days) | 3 | 5 | 2 |
| Maximum no. of daily episodes of diarrhea/maximum no. of daily episodes of vomiting | 12/20 | 3/10 | 30/15 |
| Length of hospital stay (days) | 1 | 1 | 14a |
| Previous rotavirus vaccination | None | None | None |
| Travel or contact with international travelers | None | None | None |
| Animal exposure | None | None | Horse |
| Unusual dietary exposures | None | None | None |
| Possible household spreadb | Yes | Yes | No |
Child subsequently diagnosed with inflammatory bowel disease (see text).
Based on parental report of illness.
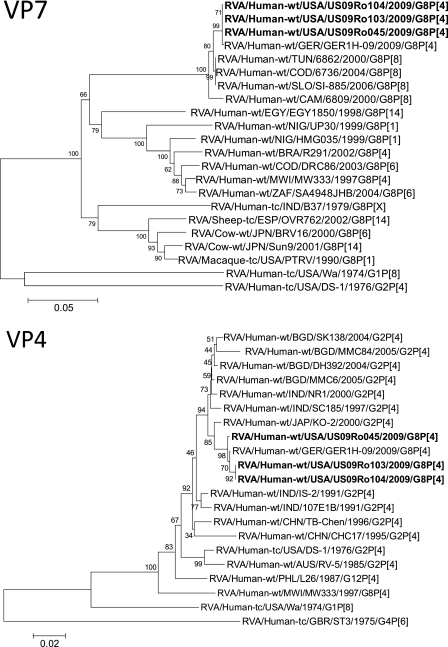
This is the first sequence-confirmed U.S. detection of G8P[4], a strain previously found mainly in Africa (especially Malawi) and sporadically in Europe, Brazil, and Indonesia (7, 8, 11, 34). Sequencing and phylogenetic analysis of partial VP7 and VP4 gene sequences from these 3 G8P[4] strains, designated US09Ro045, US09Ro103, and US09Ro104, revealed that all 3 exhibited 99 to 100% identity with the VP7 and VP4 genes of the 2009 German rotavirus strain GER1H-09 (Fig. 1) and shared phylogenetic lineage with it (27). Full-genome sequencing of these 3 strains is under way (our unpublished data). Although some G8 rotavirus strains have previously been mistyped as G12 strains by genotyping (1), this was not the case for the 3 strains reported herein which were identified and confirmed as genotype G8 by sequencing.
Fig 1.
Phylograms indicating genetic relationships of partial VP7 and VP4 nucleotide sequences of the rotavirus strains described in this report (RVA/Human-wt/USA/US09Ro103/2009/G8P[4], RVA/Human-wt/USA/US09Ro104/2009/G8P[4], and RVA/Human-wt/USA/US09Ro045/2009/G8P[4]) (in bold) with representatives of known rotavirus G8 and P[4] genotypes. Evolutionary relationships were inferred by using the neighbor-joining method (30) in the MEGA4 program (33). The p-distance model was used to compute the evolutionary distances. The horizontal branch lengths are proportional to the numbers of nucleotide substitutions per site, with the lengths defined by each scale bar. Numbers next to the nodes are percentages of bootstrap support based on 1,000 replicates. The rotavirus group, species of origin, country of identification, common name, year of identification, and G and P genotypes have been indicated for each of the strains.
The emergence of G8P[4] strains, which are now being detected in the United States, in several locations in Europe (1, 15, 27) raises questions about the effectiveness of current rotavirus vaccines, which share neither G nor P types with G8P[4] viruses. Similar questions have been raised in the context of a greater prevalence of G2P[4] strains observed after routine implementation of the monovalent G1P[8] rotavirus vaccine in Brazil and in parts of Australia and Belgium (4, 17, 35). However, immunity to rotavirus is believed to be polygenic and likely involves antigens in addition to G and P antigens (9). It is somewhat reassuring that the monovalent G1P[8] vaccine appears to provide good cross-protection against heterologous G8P[4] (18) and G2P[4] (5, 10, 16) rotavirus strains. Nevertheless, mathematical modeling suggests that relatively small differences in effectiveness against particular rotavirus strains could notably alter rotavirus disease dynamics over extended periods of time (2, 28, 29, 31). Thus, continued monitoring is critical to assess whether G8P[4] rotavirus or strains with other unusual genotypes become more prevalent in the United States and globally following the implementation of rotavirus vaccines.
ACKNOWLEDGMENTS
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Aladin F, Nawaz S, Iturriza-Gomara M, Gray J. 2010. Identification of G8 rotavirus strains determined as G12 by rotavirus genotyping PCR: updating the current genotyping methods. J. Clin. Virol. 47:340–344 [DOI] [PubMed] [Google Scholar]
- 2. Atchison C, Lopman B, Edmunds WJ. 2010. Modelling the seasonality of rotavirus disease and the impact of vaccination in England and Wales. Vaccine 28:3118–3126 [DOI] [PubMed] [Google Scholar]
- 3. Banyai K, et al. 2011. Sequencing and phylogenetic analysis of the coding region of six common rotavirus strains: evidence for intragenogroup reassortment among co-circulating G1P[8] and G2P[4] strains from the United States. J. Med. Virol. 83:532–539 [DOI] [PubMed] [Google Scholar]
- 4. Carvalho-Costa FA, et al. 2009. Rotavirus genotype distribution after vaccine introduction, Rio de Janeiro, Brazil. Emerg. Infect. Dis. 15:95–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Correia JB, et al. 2010. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J. Infect. Dis. 201:363–369 [DOI] [PubMed] [Google Scholar]
- 6. Cortese MM, Parashar UD. 2009. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 58:1–25 [PubMed] [Google Scholar]
- 7. Cunliffe NA, et al. 2000. Molecular and serologic characterization of novel serotype G8 human rotavirus strains detected in Blantyre, Malawi. Virology 274:309–320 [DOI] [PubMed] [Google Scholar]
- 8. Cunliffe NA, et al. 2010. Epidemiology of rotavirus infection in children in Blantyre, Malawi, 1997-2007. J. Infect. Dis. 202(Suppl):S168–S174 [DOI] [PubMed] [Google Scholar]
- 9. Desselberger U, Huppertz HI. 2011. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J. Infect. Dis. 203:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Vos B, et al. 2009. Live attenuated human rotavirus vaccine, RIX4414, provides clinical protection in infants against rotavirus strains with and without shared G and P genotypes: integrated analysis of randomized controlled trials. Pediatr. Infect. Dis. J. 28:261–266 [DOI] [PubMed] [Google Scholar]
- 11. Esona MD, et al. 2009. Genomic characterization of human rotavirus G8 strains from the African rotavirus network: relationship to animal rotaviruses. J. Med. Virol. 81:937–951 [DOI] [PubMed] [Google Scholar]
- 12. Gentsch JR, et al. 2009. G and P types of circulating rotavirus strains in the United States during 1996-2005: nine years of prevaccine data. J. Infect. Dis. 200(Suppl 1):S99–S105 [DOI] [PubMed] [Google Scholar]
- 13. Gentsch JR, et al. 2005. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J. Infect. Dis. 192(Suppl 1):S146–S159 [DOI] [PubMed] [Google Scholar]
- 14. Hull JJ, et al. 2011. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr. Infect. Dis. J. 30:S42–S47 [DOI] [PubMed] [Google Scholar]
- 15. Iturriza-Gomara M, et al. 2011. Rotavirus genotypes co-circulating in Europe between 2006 and 2009 as determined by EuroRotaNet, a pan-European collaborative strain surveillance network. Epidemiol. Infect. 139:895–909 [DOI] [PubMed] [Google Scholar]
- 16. Justino MC, et al. 2011. Effectiveness of the monovalent G1P[8] human rotavirus vaccine against hospitalization for severe G2P[4] rotavirus gastroenteritis in Belem, Brazil. Pediatr. Infect. Dis. J. 30:396–401 [DOI] [PubMed] [Google Scholar]
- 17. Kirkwood CD, Boniface K, Barnes GL, Bishop RF. 2011. Distribution of rotavirus genotypes after introduction of rotavirus vaccines, Rotarix(R) and RotaTeq(R), into the National Immunization Program of Australia. Pediatr. Infect. Dis. J. 30:S48–S53 [DOI] [PubMed] [Google Scholar]
- 18. Madhi SA, et al. 2010. Effect of human rotavirus vaccine on severe diarrhea in African infants. N. Engl. J. Med. 362:289–298 [DOI] [PubMed] [Google Scholar]
- 19. Matthijnssens J, et al. 2009. Rotavirus disease and vaccination: impact on genotype diversity. Future Microbiol. 4:1303–1316 [DOI] [PubMed] [Google Scholar]
- 20. Matthijnssens J, et al. 2011. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 156:1397–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthijnssens J, et al. 2010. Phylodynamic analyses of rotavirus genotypes G9 and G12 underscore their potential for swift global spread. Mol. Biol. Evol. 27:2431–2436 [DOI] [PubMed] [Google Scholar]
- 22. Matthijnssens J, et al. 2006. G8 rotavirus strains isolated in the Democratic Republic of Congo belong to the DS-1-like genogroup. J. Clin. Microbiol. 44:1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Payne DC, et al. 2008. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics 122:1235–1243 [DOI] [PubMed] [Google Scholar]
- 24. Payne DC, et al. 2011. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006-2009. Clin. Infect. Dis. 53:245–253 [DOI] [PubMed] [Google Scholar]
- 25. Payne DC, et al. 2010. Corrected 2007 rotavirus hospitalization rates. Pediatr. Infect. Dis. J. 29:287–288 [DOI] [PubMed] [Google Scholar]
- 26. Payne DC, et al. 2009. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr. Infect. Dis. J. 28:948–953 [DOI] [PubMed] [Google Scholar]
- 27. Pietsch C, Petersen L, Patzer L, Liebert UG. 2009. Molecular characteristics of German G8P[4] rotavirus strain GER1H-09 suggest that a genotyping and subclassification update is required for G8. J. Clin. Microbiol. 47:3569–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pitzer VE, et al. 2011. Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence. J. R. Soc. Interface 8:1584–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pitzer VE, et al. 2009. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science 325:290–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 31. Sato T, Nakagomi T, Naghipour M, Nakagomi O. 2010. Modeling seasonal variation in rotavirus hospitalizations for use in evaluating the effect of rotavirus vaccine. J. Med. Virol. 82:1468–1474 [DOI] [PubMed] [Google Scholar]
- 32. Staat MA, et al. 2011. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics 128:e267–e275 [DOI] [PubMed] [Google Scholar]
- 33. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 34. Todd S, Page NA, Duncan Steele A, Peenze I, Cunliffe NA. 2010. Rotavirus strain types circulating in Africa: review of studies published during 1997-2006. J. Infect. Dis. 202(Suppl):S34–S42 [DOI] [PubMed] [Google Scholar]
- 35. Zeller M, et al. 2010. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 28:7507–7513 [DOI] [PubMed] [Google Scholar]