Abstract
We report a case of sepsis caused by Rhodococcus corynebacterioides, identified using 16S rRNA gene sequencing, in a myelodysplastic syndrome patient who had undergone hematopoietic stem cell transplantation. This is the first report of R. corynebacterioides infection in a human.
CASE REPORT
A 64-year-old man was hospitalized because of a high fever. Three years earlier, he was diagnosed with myelodysplastic syndrome and underwent allogeneic hematopoietic stem cell transplantation. Since that time, he had been taking low-dose prednisolone to control chronic graft-versus-host disease. One year posttransplant, he experienced a cerebral hemorrhage, which caused left hemiplegia. Due to repeated aspiration pneumonia, a central venous catheter and port system were implanted in his chest wall to administer antibiotics and for nutritional support. The cause of the high fever at presentation was diagnosed as a bloodstream infection caused by Candida albicans. Removal of the catheter system and administration of micafungin resulted in the patient's recovery; the elevated serum β-d-glucan level returned to below the detectable limit.
Two weeks later, the patient again developed a fever (39.7°C). His white blood cell count was 2.1 × 109 cells/liter, and his C-reactive protein (CRP) level was 159 mg/liter. Blood cultures were collected, and empirical treatment with cefepime (1 g twice daily) was initiated. After 3 days of incubation, Gram-positive rods were detected in the blood cultures, which were unidentifiable by routine microbiological examinations. Pseudomonas aeruginosa was detected in his sputum, but this bacterium was not thought to be the causative agent of his sepsis, because he showed no findings suggestive of pneumonia and because the bacterium had been repeatedly detected before. Following the initiation of antibiotic treatment with cefepime, the patient became afebrile, his CRP level decreased to 47 mg/liter, and repeated blood cultures were negative. However, 18 days later, the high fever (39.2°C) recurred, and CRP level was again elevated to 106 mg/liter. Blood cultures again showed Gram-positive rods. Treatment was changed to cefozopran (2 g twice daily). However, his condition deteriorated, and he died 9 days later.
Microbiological data.
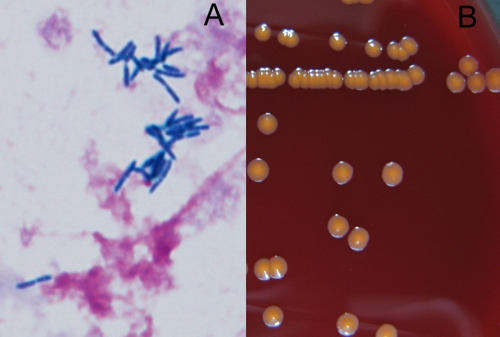
The blood cultures were positive for Gram-positive rods in aerobic bottles of the Bactec 9240 system (BD, Franklin Lakes, NJ) twice, as described above, after 52 h and 88 h of incubation, respectively. Gram staining of the specimens revealed long and slightly bent Gram-positive rods in pairs, V forms, and palisade arrangements (Fig. 1A). The specimens from the bottles were plated onto Trypticase soy agar II with 5% sheep blood (BD) and incubated at 35°C in air supplemented with 5% CO2. After 72 h, odorless, orange-colored, smooth, and nonhemolytic colonies, 4 mm in diameter, were observed (Fig. 1B). The colonies were positive for the catalase test and negative for the oxidase test. The RapID CB Plus system (Remel Inc., Lenexa) was used for identification. The isolates were identified as Turicella otitidis (microcode 0007511; probability level, 98.29%). However, the colonial features described above were clearly different from those of T. otitidis, colonies of which are whitish, convex, and creamy with entire edges (7). Therefore, we performed molecular identification by PCR amplification and sequencing analysis of the 16S rRNA gene using DNA extracted from the isolates. The universal primers 8UA (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1485B (5′-ACGGGCGGTGTGTRC-3′) were used as described previously (11). We performed sequencing analysis using a GenBank BLAST search and sequence editing and phylogenetic analysis using CLUSTAL W (neighbor-joining method) with Treeview. The sequence of the 16S rRNA gene (GenBank accession number AB685427) was 99.86% identical (1,440 bp over the entire 1,442-bp fragment) with that of the type strain of Rhodococcus corynebacterioides (DSM 20151; accession number NR 041873). Based on this result, we identified the isolate as R. corynebacterioides. The features of the isolate were consistent with the reported features of R. corynebacterioides, such as the rods in V forms and palisade arrangement and orange-colored colonies (12). The antibiotic susceptibility of the bacterium was determined by the broth microdilution method approved by the Clinical and Laboratory Standards Institute (4) using a commercially prepared microtiter plate containing a series of lyophilized antimicrobials (DP34; Eiken Chemicals, Japan). MICs are shown in Table 1.
Fig 1.
(A) Gram staining of Rhodococcus corynebacterioides isolated from blood cultures (oil immersion, ×1,000); (B) colonial appearance of R. corynebacterioides on a sheep blood agar plate after 72 h of incubation in air supplemented with 5% CO2.
Table 1.
MICs for Rhodococcus corynebacterioides isolated from the present patient
| Antimicrobial agent | MIC (μg/ml) |
|---|---|
| Benzylpenicillin | 0.5 |
| Ampicillin | 1 |
| Ampicillin-sulbactam | 2 |
| Cefazolin | 1 |
| Ceftriaxone | >2 |
| Cefepime | 1 |
| Imipenem | ≤0.06 |
| Meropenem | 0.5 |
| Clarithromycin | ≤0.12 |
| Azithromycin | 0.25 |
| Clindamycin | 0.5 |
| Minocycline | ≤0.12 |
| Levofloxacin | ≤1 |
| Moxifloxacin | ≤0.5 |
| Vancomycin | 0.5 |
| Trimethoprim-sulfamethoxazole | >2/38 |
Rhodococcus, which belongs to the family Nocardiaceae, is a genus of aerobic, nonmotile, non-spore-forming, Gram-positive bacteria. The morphology can range from coccoid to bacillary depending on species and specimen type. Colonies are salmon-pink to red colored and teardrop shaped or coalescent mucoid (3, 5, 13). There are 34 named species in the genus. Most of these bacteria are nonpathogenic and found in a broad range of environments, such as soil, groundwater, animal dung, and plants. The most common pathogenic species in the genus is Rhodococcus equi. This bacterium is known to be a pathogen that causes pulmonary abscesses in horses. Recently, R. equi infection in immunocompromised patients has been reported (5). So far, 30 cases of R. equi infection in transplant patients have been described, and the clinical diagnosis in 24 of these 30 cases was pneumonia or a lung abscess (13). Besides R. equi infection, a case with Rhodococcus erythropolis causing bloodstream infection (2) and Rhodococcus rhodochrous infection causing a corneal ulcer have been reported (3, 8).
R. corynebacterioides was originally named Corynebacterium rubrum (6). Later, it was reclassified as Nocardia corynebacterioides based on its physiologic, chemical, and ultrastructural characteristics (12). Recently, the bacterium was again reclassified as R. corynebacterioides based on phylogenetic data from 16S rRNA gene sequencing and chemotaxonomic data (14). The colonial morphology and Gram stain findings of R. corynebacterioides are similar to those of other species of the genus Rhodococcus. It has been reported that R. corynebacterioides can be distinguished from other species by its physiologic characteristics, such as assimilation of various sugars as carbon sources and utilization of various amino acids as simultaneous carbon and nitrogen sources (14). However, these characteristics cannot be examined in routine hospital laboratories, and so 16S rRNA gene sequencing is generally needed to positively identify R. corynebacterioides.
We believe that R. corynebacterioides was the cause of the high fever in the present patient, because the bacterium was detected in blood cultures twice at the same time that he developed a high fever and CRP levels increased. Because we had not seen orange-colored colonies like this before and because we thought that the result from the identification kit was incorrect, sequencing analysis of the 16S rRNA gene was performed, leading to the positive identification of the isolate.
We were unable to specify the cause underlying his sepsis, as he had no active lesions in the lungs, eyes, or skin. We were also unable to specify the source of the infection, because he was not exposed to farming environments, to which the patients with R. equi infection were often exposed (13). Recently, it was reported that R. corynebacterioides was recovered from the mouth of healthy volunteers (9). This may be suggestive of a possible source for R. corynebacterioides in immunocompromised patients.
Antimicrobials such as imipenem, vancomycin, and quinolones are recommended for the treatment of R. equi infection (5, 13). The MICs for R. corynebacterioides observed in this case were almost identical to those reported for Rhodococcus species (1, 2, 10). In this patient, cefepime, which seemed susceptible in the susceptibility testing, was administered; however, this treatment was unsuccessful.
To the best of our knowledge, this is the first reported case of R. corynebacterioides infection in a human. Because this species is not well known and is difficult to identify, it may previously have been overlooked or misidentified. Therefore, molecular identification by 16S rRNA gene sequencing is useful for the positive identification of R. corynebacterioides infection. To clarify the clinical features of R. corynebacterioides infection and antimicrobial susceptibility patterns, the accumulation of more cases is required.
ACKNOWLEDGMENTS
We thank N. Furuhata, Y. Haruyama, N. Ichimura, I. Takebe, R. Takahashi, and M. Hagihara (Department of Clinical Laboratory) for their technical assistance. We also thank Y. Umezawa, A. Arai, and O. Miura (Department of Hematology) for providing us with the patient's clinical information.
Footnotes
Published ahead of print 28 December 2011
REFERENCES
- 1. Asoh N, et al. 2003. Emergence of rifampin-resistant Rhodococcus equi with several types of mutations in the rpoB gene among AIDS patients in northern Thailand. J. Clin. Microbiol. 41:2337–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baba H, et al. 2009. First case of bloodstream infection caused by Rhodococcus erythropolis. J. Clin. Microbiol. 47:2667–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell KS, Philp JC, Aw DW, Christofi N. 1998. The genus Rhodococcus. J. Appl. Microbiol. 85:195–210 [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2010. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline M45-A2, 2nd ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Conville PS, Witebsky FG. 2011. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic Actinomycetes, p 443–471 In Versalovic J, et al. (ed), Manual of clinical microbiology, 10th ed ASM Press, Washington, DC [Google Scholar]
- 6. Crowle AJ. 1962. Corynebacterium rubrum nov. spec., a Gram-positive non-acid-fast bacterium of unusually high lipid content. Antonie Van Leeuwenhoek 28:182–192 [DOI] [PubMed] [Google Scholar]
- 7. Funke G, Bernard KA. 2011. Coryneforme Gram-positive rods, p 413–442 In Versalovic J, et al. (ed), Manual of clinical microbiology, 10th ed ASM Press, Washington, DC [Google Scholar]
- 8. Gopaul D, Ellis C, Maki A, Jr, Joseph MG. 1988. Isolation of Rhodococcus rhodo-chrous from a chronic corneal ulcer. Diagn. Microbiol. Infect. Dis. 10:185–190 [DOI] [PubMed] [Google Scholar]
- 9. Hung WL, Wade WG, Boden R, Kelly DP, Wood AP. 2011. Facultative methylotrophs from the human oral cavity and methylotrophy in strains of Gordonia, Leifsonia, and Microbacterium. Arch. Microbiol. 193:407–417 [DOI] [PubMed] [Google Scholar]
- 10. Jacks SS, Giguère S, Nguyen A. 2003. In vitro susceptibilities of Rhodococcus equi and other common equine pathogens to azithromycin, clarithromycin, and 20 other antimicrobials. Antimicrob. Agents Chemother. 47:1742–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masaki T, et al. 2006. Mycobacterium kumamotonense sp. nov. recovered from clinical specimen and the first isolation report of Mycobacterium arupense in Japan: novel slowly growing, nonchromogenic clinical isolates related to Mycobacterium terrae complex. Microbiol. Immunol. 50:889–897 [DOI] [PubMed] [Google Scholar]
- 12. Serrano JA, Tablante RV, de Serrano AA, de San Blas GC, Imaeda T. 1972. Physiological, chemical and ultrastructural characteristics of Corynebacterium rubrum. J. Gen. Microbiol. 70:339–349 [DOI] [PubMed] [Google Scholar]
- 13. Yamshchikov AV, Schuetz A, Lyon GM. 2010. Rhodococcus equi infection. Lancet Infect. Dis. 10:350–359 [DOI] [PubMed] [Google Scholar]
- 14. Yassin AF, Schaal KP. 2005. Reclassification of Nocardia corynebacterioides Serrano et al. 1972 (approved lists 1980) as Rhodococcus corynebacterioides comb. nov. Int. J. Syst. Evol. Microbiol. 55:1345–1348 [DOI] [PubMed] [Google Scholar]