Abstract
An alternative method for the cultivation of Lawsonia intracellularis, an obligate intracellular bacterium and the causative agent of proliferative enteropathy, was developed using an Original Space Bag inflated with a mixture of gas containing 10% hydrogen, 10% carbon dioxide, and 80% nitrogen. The flexibility of this protocol allows the testing of various environmental conditions for static cultivation of this bacterium and the development of diagnostic techniques.
TEXT
Lawsonia intracellularis is a fastidious and obligate intracellular bacterium and the causative agent of proliferative enteropathy, or ileitis. The disease has been observed in a variety of animal species, including nonhuman primates, but its occurrence in hamsters, pigs, and horses has been best described (2). Dividing cells in culture and strict environmental conditions are required for isolation and cultivation of L. intracellularis in vitro (6). The conventional method for isolation and cultivation of this bacterium in monolayers is well established and uses various methods of supplying hydrogen for infection followed by incubation in a tri-gas incubator with 83.2% nitrogen, 8.8% carbon dioxide, and 8% oxygen at 37°C (3, 8). The cost of these requirements has limited the maintenance of this microorganism in vitro to only a few research institutes. Furthermore, since this disease was first reported (1), there have been only a dozen or so L. intracellularis isolates cultured worldwide. This study describes an alternative method for cultivation of L. intracellularis in cell monolayers that provides the necessary atmospheric conditions for growth without tri-gas incubators. This alternative protocol presents new opportunities for testing different environmental conditions for isolation and cultivation of this organism. Additionally, it allows the development of diagnostic approaches, including an immunoperoxidase monolayer assay, indirect immunofluorescence, and MIC testing.
The L. intracellularis isolate PHE/MN1-00 (ATCC PTA-3457), previously isolated from a pig with the hemorrhagic form of proliferative enteropathy, was used for evaluating growth and in vitro infection under two different sets of environmental conditions (conventional and alternative). This isolate was grown in murine fibroblast-like McCoy cells (ATCC CRL 1696), maintained in a cell culture system, and stored at −72°C until use, as described previously (3). Frozen bacteria were thawed and grown in cell culture for three continuous passages in order to allow the bacteria to recover from the frozen stage. In these three passages, the bacteria were grown using both the conventional and alternative methods (Fig. 1), as described below. The infection was monitored during every passage using immunoperoxidase staining with a polyclonal antibody specific for L. intracellularis (3). After three passages, 16-well, glass-bottom tissue culture plates containing 1-day-old McCoy cells (30% confluence) were infected (day 0) with bacterial suspensions containing approximately 104 L. intracellularis organisms/well. Murine fibroblast-like McCoy cells were grown in Dulbecco's modified Eagle medium (DMEM; Gibco Invitrogen Corporation) with 1% l-glutamine (Gibco Invitrogen Corporation), 7% fetal bovine serum (FBS; Sigma Chemical), and 0.5% amphotericin B (Cellgro; Mediatech), without antibiotics or medium replacements throughout the study (3). The infected cells were incubated using two different methods. For the conventional method, the cells were placed in the tri-gas incubator with 83.2% nitrogen gas, 8.8% carbon dioxide, and 8% oxygen gas and a temperature of 37°C (6). Tissue culture plates were removed and flushed with hydrogen gas daily. For the alternative method, the plates were placed in an Original Space Bag (Storage Packs, San Diego, CA) measuring 54 cm by 85 cm, which was hermetically closed. The air inside the bag was then removed by vacuum pump to a pressure of 100 mm Hg. Afterward, the bag was inflated through a cuff containing a 0.22-μm filter connected to a gas cylinder containing 10% hydrogen, 10% carbon dioxide, and 80% nitrogen gas. Finally, the bag was incubated at 37°C for 8 days (Fig. 1). The atmosphere inside the bag was replaced as described above every 24 h. Carbon dioxide and oxygen gas percentages were monitored in both protocols using CO2 and O2 indicators (Fyrite gas analyzer) at the initiation of incubation and every 24 h during the 8 days. The CO2 and O2 levels in the bag were measured daily after inflation and before replacement of the gas mixture and were found to be stable. The CyQuant cell proliferation assay, a fluorescence-based approach for determining numbers of cultured cells (5), was used to monitor the growth of infected and noninfected cells for both incubation methods. In addition, the population doubling time (days) of the McCoy cells was calculated using the algorithm provided at http://www.doubling-time.com (11), based on the intensity of the fluorescence demonstrated in the CyQuant assay. Infection and growth of L. intracellularis were monitored by direct counting of heavily infected cells (HIC), identified by immunocytochemistry staining with polyclonal antibody specific for L. intracellularis (3), and by quantitative PCR (qPCR), as previously described (10). The level of infection was also monitored by calculating the estimated population doublings of the HIC, which had been counted previously. The number of HIC and the number of L. intracellularis organisms were determined using four replicates (wells) in 16-well tissue culture plates. The average from four replicates was used in the statistical analysis. The Wilcoxon signed-rank test was performed using SAS software (9.1) to assess differences between both incubation methods. A P value of <0.05 was considered significant.
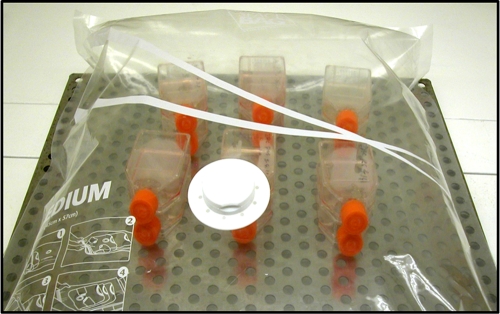
Fig 1.
Alternative incubation with 12 T25 tissue culture flasks in the Original Space Bag.
During the 8 days of incubation, the levels of carbon dioxide and oxygen gas were constant (8.8% CO2; 8.0% O2) in the tri-gas incubator (conventional method). In the alternative method, CO2 and O2 levels ranged from 7.0 to 8.0% and from 5.5 to 6.5%, respectively (Table 1). The CyQuant cell proliferation assay was used to measure cellular DNA via fluorescent dye binding in noninfected and infected cells every 24 h for 8 days of incubation. Noninfected cells had similar growth rates and no significant difference on average of estimated population doubling (calculated by day) between the conventional protocol at 5% CO2 and 37°C (1.83 ± 0.18), the tri-gas incubator (1.79 ± 0.06), and the plastic bag (1.76 ± 0.08). These results showed that noninfected McCoy cells are able to be grown in these bags and able to support the cultivation of L. intracellularis. The bag described in this study was able to support up to 12 T25 (Fig. 1) or six T175 tissue culture flasks (Corning). However, we have observed that larger bags from the same manufacturer support more flasks.
Table 1.
Carbon dioxide and oxygen gas levels during 8 days of incubation in a conventional tri-gas incubator and the Original Space Bag
| Days of incubation | Tri-gas incubator |
Original Space Bag |
||
|---|---|---|---|---|
| % O2 | % CO2 | % O2 | % CO2 | |
| 1 | 8.0 | 8.8 | 6.0 | 7.0 |
| 2 | 8.0 | 8.8 | 5.5 | 7.0 |
| 3 | 8.0 | 8.8 | 6.0 | 7.5 |
| 4 | 8.0 | 8.8 | 6.0 | 7.5 |
| 5 | 8.0 | 8.8 | 6.5 | 8.0 |
| 6 | 8.0 | 8.8 | 6.5 | 8.0 |
| 7 | 8.0 | 8.8 | 6.0 | 7.5 |
| 8 | 8.0 | 8.8 | 6.0 | 7.5 |
Enterocyte proliferation is the primary lesion associated with L. intracellularis infection in vivo (2). Although previous studies have not reported cellular proliferation in vitro (7, 9), there is no information regarding cell growth during in vitro infection. The CyQuant cell proliferation assay was used to compare the growth curves of infected and noninfected cells in the conventional and alternative methods (Fig. 2). The results did not show statistical differences (P < 0.05) between infected and noninfected cells in either incubation method. Oh et al. (9) described upregulation of cell cycle genes in infected McCoy cells; however, cell growth was not measured in that experiment. Furthermore, epithelial growth factors and their interactions with the lamina propria during in vivo infections can play a critical role in pathogenesis, which is still poorly understood.
Fig 2.
Quantification of infected and noninfected cells in the tri-gas and bag incubation systems using a CyQuant cell proliferation assay. Fluorescence intensities are based on cellular DNA content during days of incubation. Infected cells were measured from day 0 (arrow).
The number of heavily infected cells increased progressively and reached a peak on day 7 postinfection in both the conventional and alternative incubation methods. There was no significant difference (P < 0.05) in the number of HIC throughout incubation (Fig. 3). No significant difference was found in the estimated population doubling of HIC using the conventional (2.8 ± 0.2) and the alternative (3.1 ± 0.3) protocols. Similar to the immunocytochemistry results, the greatest number of L. intracellularis organisms per well was observed on day 7 postinfection by quantitative PCR (Fig. 3). In addition, the quantitative PCR showed no significant difference between the conventional and alternative methods of incubation. There was no positive reaction for the noninfected cell cultures (negative control) in the qPCR. Previous studies have described L. intracellularis cultivation in 5 to 7 days using a tri-gas incubator, including experiments to validate diagnostic approaches (4) and to investigate the pathogenesis of proliferative enteropathy (9). However, these studies failed to quantify the numbers of L. intracellularis organisms or the number of HIC in the infected cultures.
Fig 3.
Infection and growth monitoring of L. intracellularis in cell culture plates during 8 days of incubation. (Left) Direct counting of heavily infected cells by immunocytochemistry, with infected cells in the alternative bag incubation system over time shown below the graph. (Right) Copies of the aspartate ammonia lyase gene of L. intracellularis measured by quantitative PCR.
Similar to the conventional method, incubation in the bag provided environmental conditions that enable L. intracellularis to infect and multiply in the cells. Based on these results, we believe this approach can be used for the static cultivation and, potentially, isolation of this bacterium without requiring a tri-gas incubator. In addition, our experience has shown no difference in the cell growth or level of the infection when the gas inside the bag is replaced at least twice (on the second and fifth days postinfection) during incubation. This alternative method has been also successfully reproduced (J. S. V. Oliveira and R. M. C. Guedes, unpublished data) in an independent trial. This fact has confirmed the usefulness and feasibility of the present method.
The flexibility of this method allows the testing of various environmental conditions for L. intracellularis cultivation and production of antigens for the development of diagnostic techniques. Additionally, this affordable technology gives research institutes an opportunity to explore the this proliferative-disease agent, which has intriguing and unique properties among bacterial pathogens.
ACKNOWLEDGMENT
F. A. Vannucci was supported by the Brazilian government sponsoring agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
Published ahead of print 4 January 2012
REFERENCES
- 1. Biester HE, Schwarte LH. 1931. Intestinal adenoma in swine. Am. J. Pathol. 7:175–185 [PMC free article] [PubMed] [Google Scholar]
- 2. Gebhart CJ, Guedes RMC. 2010. Lawsonia intracellularis, p 503–512 In Gyles CL, Prescott JF, Songer JG, Thoen CO. (ed), Pathogenesis of bacterial infections in animals, 4th ed Blackwell Publishing, Ames, IA [Google Scholar]
- 3. Guedes RMC, Gebhart CJ. 2003. Preparation and characterization of polyclonal and monoclonal antibodies against Lawsonia intracellularis. J. Vet. Diagn. Invest. 15:438–446 [DOI] [PubMed] [Google Scholar]
- 4. Guedes RMC, Gebhart CJ, Deen J, Winkelman NL. 2002. Validation of an immunoperoxidase monolayer assay as a serologic test for porcine proliferative enteropathy. J. Vet. Diagn. Invest. 14:528–530 [DOI] [PubMed] [Google Scholar]
- 5. Jones LJ, Gray M, Yue ST, Haugland RP, Singer VL. 2001. Sensitive determination of cell number using the CyQUANT® cell proliferation assay. J. Immunol. Methods 254:85–98 [DOI] [PubMed] [Google Scholar]
- 6. Lawson GH, McOrist S, Jasni S, Mackie RA. 1993. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J. Clin. Microbiol. 31:1136–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McOrist S, Gebhart CJ, Bosworth BT. 2006. Evaluation of porcine ileum models of enterocyte infection by Lawsonia intracellularis. Can. J. Vet. Res. 70:155–159 [PMC free article] [PubMed] [Google Scholar]
- 8. McOrist S, Gebhart CJ, Boid R, Barns S. 1995. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int. J. Syst. Bacteriol. 45:820–825 [DOI] [PubMed] [Google Scholar]
- 9. Oh YS, Lee JB, McOrist S. 2010. Microarray analysis of differential expression of cell cycle and cell differentiation genes in cells infected with Lawsonia intracellularis. Vet. J. 184:340–345 [DOI] [PubMed] [Google Scholar]
- 10. Wattanaphansak S, Gebhart CJ, Anderson JM, Singer RS. 2010. Development of a polymerase chain reaction assay for quantification of Lawsonia intracellularis. J. Vet. Diagn. Invest. 22:598–602 [DOI] [PubMed] [Google Scholar]
- 11. Widera D, et al. 2009. Adult palatum as a novel source of neural crest-related stem cells. Stem Cells 27:1899–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]