Abstract
Campylobacter spp. are a leading cause of bacterial gastroenteritis worldwide. The need for molecular subtyping methods with enhanced discrimination in the context of surveillance- and outbreak-based epidemiologic investigations of Campylobacter spp. is critical to our understanding of sources and routes of transmission and the development of mitigation strategies to reduce the incidence of campylobacteriosis. We describe the development and validation of a rapid and high-resolution comparative genomic fingerprinting (CGF) method for C. jejuni. A total of 412 isolates from agricultural, environmental, retail, and human clinical sources obtained from the Canadian national integrated enteric pathogen surveillance program (C-EnterNet) were analyzed using a 40-gene assay (CGF40) and multilocus sequence typing (MLST). The significantly higher Simpson's index of diversity (ID) obtained with CGF40 (ID = 0.994) suggests that it has a higher discriminatory power than MLST at both the level of clonal complex (ID = 0.873) and sequence type (ID = 0.935). High Wallace coefficients obtained when CGF40 was used as the primary typing method suggest that CGF and MLST are highly concordant, and we show that isolates with identical MLST profiles are comprised of isolates with distinct but highly similar CGF profiles. The high concordance with MLST coupled with the ability to discriminate between closely related isolates suggests that CFG40 is useful in differentiating highly prevalent sequence types, such as ST21 and ST45. CGF40 is a high-resolution comparative genomics-based method for C. jejuni subtyping with high discriminatory power that is also rapid, low cost, and easily deployable for routine epidemiologic surveillance and outbreak investigations.
INTRODUCTION
Campylobacter spp. are a leading cause of bacterial gastroenteritis worldwide (20), and most cases are thought to be the direct result of infection by C. jejuni or C. coli (20, 43). Risk factors for campylobacteriosis include exposure to contaminated water, milk, and various food products, such as poultry (1, 3, 6, 21). The development and implementation of effective control measures for these pathogens hinge on the identification of sources of infection. Although the ingestion of contaminated food or water and animal contact play a significant role in the epidemiology of campylobacteriosis, efforts to track sources of Campylobacter infection are hampered by the sporadic nature of campylobacteriosis (25), the infrequent association with outbreaks of disease, and widespread reservoirs that include water, livestock, domestic animals, and wildlife (8, 17, 46, 65, 67).
A number of different molecular subtyping methods, such as pulsed-field gel electrophoresis (PFGE), restriction fragment length polymorphism analysis of the flagellin gene (flaA RFLP), and the DNA sequencing of the flagellin gene short variable region (flaA SVR), have been used to identify genotypic clusters of Campylobacter in the context of molecular epidemiology (18, 33). More recently, a multilocus sequence typing (MLST) scheme for C. jejuni (12) has emerged as the leading method for subtyping on the strength of several key features. These include the high portability of nucleotide sequence-based typing data, which has greatly facilitated interlaboratory comparisons and has allowed for the establishment of a global MLST database, which has contributed to a greater understanding of the population structure and global epidemiology of C. jejuni and related organisms (42).
Numerous studies have shown the importance of molecular subtyping in tracing the molecular epidemiology of campylobacteriosis through the analysis of isolates from clinical, livestock, wildlife, and environmental sources (11, 24, 32, 35, 39, 40, 52, 54, 56). However, there are a number of inherent challenges to the conventional genotyping of campylobacter populations, which are characterized by high genetic diversity, weak clonality, and high levels of intraspecific recombination (13, 66). Routine PFGE subtyping is of limited value, because chromosomal rearrangements (4) and high genetic diversity (23, 45) may limit the clustering of related isolates. High rates of recombination may similarly affect genetic relationships inferred by methods such as flaA-based typing (42). Although MLST is able to generate relevant data for long-term epidemiological and population studies, it may lack sufficient resolution in short-term investigations aimed at identifying temporally and spatially related clusters of isolates arising from a common source, such as those that occur in outbreaks (55). In such cases, a secondary method such as flaA SVR analysis may be required for additional discrimination (9). In addition, recent evidence for significant genomic differences between isolates indistinguishable by MLST (63) serves to underscore the continuing need for genotyping methods with enhanced discrimination in the context of outbreak- and surveillance-based molecular epidemiologic investigations of Campylobacter spp.
In an effort to exploit recent advances in our understanding of Campylobacter genomics for genotyping and molecular epidemiology, we have developed a rapid, low-cost, and high-resolution comparative genomic fingerprinting (CGF) approach for C. jejuni that relies on the allelic assessment of multiple loci widely distributed around the genome, targeting genetic variability in the accessory genome content. We have performed an extensive validation of a 40-gene CGF assay (CGF40) by comparing it with MLST using a set of 412 isolates from C-EnterNet, the Canadian national integrated enteric pathogen surveillance program.
MATERIALS AND METHODS
Bacterial isolates used in this study.
Campylobacter jejuni isolates used in this study were obtained through the C-EnterNet sentinel site surveillance program (http://www.phac-aspc.gc.ca/c-enternet/index-eng.php) in the region of Waterloo, Ontario, Canada, and are described in the accompanying work by Clark et al. (9a). A total of 423 isolates were obtained through agricultural (n = 19), environmental (n = 16), retail (n = 117), and human clinical sources (n = 271) during this phase of the study. The agricultural and environmental surveillance components of the C-EnterNet program involved the sampling of food animal operations (swine, beef, dairy, and poultry) and surface water in the sentinel site. Meat samples were purchased from randomly selected stores/markets in the sentinel site. Human enteric samples were collected through the existing passive surveillance system, in which stool samples requested by physicians are submitted to private, hospital, or public health laboratories. Among the 423 C. jejuni isolates, 11 bore novel alleles and represented novel sequence types (ST); only the 412 remaining isolates with defined sequence types were analyzed for the testing and validation of the CGF method described here.
MLST analysis.
Multilocus sequence typing (MLST) was performed as previously described (9), which is based on the original protocols described by Dingle et al. (12). Genomic DNA was prepared using a PureGene genomic DNA purification kit (Gentra Systems, Minneapolis, MN). PCR amplicons were purified using Montage PCR centrifugal filter devices (Fisher Scientific, Edmonton, Canada) according to the manufacturer's instructions. Sequencing was performed at the Genomic Core Facility of the National Microbiology Laboratory of the Public Health Agency of Canada (Winnipeg) using BigDye Terminator 3.1 cycle sequencing chemistry (Applied Biosystems, Streetsville, Canada) on an ABI 3100 or 3730 DNA analyzer (Applied Biosystems). Sequences were assembled and checked for errors and overall quality in the SeqMan program within the Lasergene software suite (DNASTAR Inc., Madison, WI).
Selection of markers for CGF.
Prospective typing markers were selected based on five main criteria: (i) they were identified as likely absent from one or more of the 198 C. jejuni isolates from three microarray-based comparative genomic surveys (60, 62, 63) and preliminarily identified based on a bimodal log ratio distribution (i.e., genes with a distribution indicative of clear presence/absence rather than sequence divergence were selected) (61); (ii) they were classified as unbiased genes, identified by population frequency analysis and showing adequate carriage across the three data sets mentioned above (i.e., genes with very high presence or absence rates were avoided); (iii) they had a representative genomic distribution, including accessory genes from each of 16 major hypervariable regions in the C. jejuni genome (60); (iv) they provided the ability to capture strain-to-strain relationships inferred from whole-genome comparative genomic analysis (63); and (v) they were present in two or more of the completed and draft C. jejuni genomes that were publicly available at the time of assay design, which was done to identify regions free of single-nucleotide polymorphisms (SNPs) for PCR primer design. Of more than 200 prospective marker genes originally identified, 40 were subsequently selected for the development of the CGF40 assay (Fig. 1A). The rationale for the number of target genes used in this assay is based on simulations used to define the number of markers necessary to reproduce strain relationships inferred from whole-genome comparative genomic analysis and will be presented elsewhere.
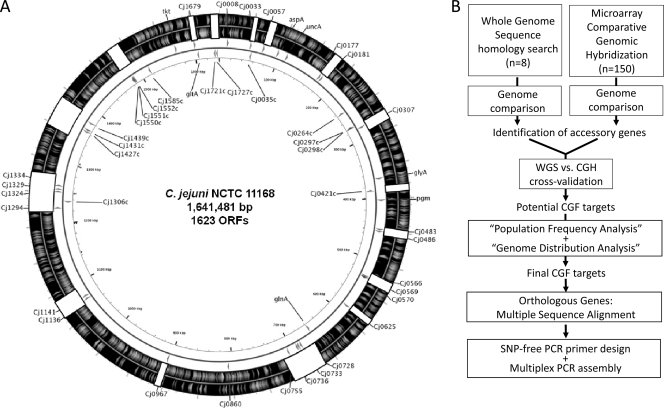
Fig 1.
Selection of markers for CGF assay. (A) Distribution of the CGF40 target genes around the C. jejuni NCTC11168 genome sequence. Arrowheads in the two innermost circles depict the location and direction of the target genes, and white blocks on the outermost circles depict the 16 hypervariable regions previously identified in the C. jejuni genome (60). The seven MLST loci (aspA, uncA, glyA, pgm, glnA, tkt, and gltA) also are depicted in the figure. This genome visualization was generated using CGView (58). (B) Binary accessory genes identified from comparative genomic analysis were checked for unbiased population frequency and genome distribution. Only genes that fulfilled these criteria and that had available multiple-sequence data were targeted for SNP-free PCR primer design, and they form part of the final set of markers used in the assay described here.
Design of the CGF40 assay.
An overview of the CGF assay design process is shown in Fig. 1B. Briefly, genome sequences for the seven C. jejuni completed and draft genomes publicly available at the time of assay development were obtained from NCBI: NCTC 11168 (accession no. AL111168) (47), RM1221 (accession no. CP000025) (19), 81-176 (accession no. CP000538, CP000549, and CP000550) (29), CF93-6 (accession no. AANJ00000000); HB93-13 (accession no. AANQ00000000), 84-25 (accession no. AANT00000000), and 260.94 (accession no. AANK00000000). Once the final list of CGF target genes was determined, the corresponding orthologous sequences were identified for each target by homology searching with BLAST (2) using the NCTC 11168 gene and the custom database for each genome. Multiple-sequence alignments for each set of orthologues was generated using ClustalX (37), and SNP-free PCR primers (Table 1) were designed for each of the prospective typing targets using Primer3 (53). After initial compatibility testing, the genes were assembled into the 8 multiplex PCRs, each targeting 5 loci, which together comprise the CGF40 assay used in this study (Fig. 2).
Table 1.
Primer pools for the 8 multiplex PCRs comprising the CGF40 assay
| Multiplex PCR no. and gene | Forward primer | Reverse primer | Amplicon size (bp) |
|---|---|---|---|
| 1 | |||
| Cj0298c | CTGTTTCTCGCGGAGCTAAA | CAACGACGGGTATTCCTGAT | 198 |
| Cj0728 | AGCGATAAGCCTGATGGAGA | TCTCTTTCTATGATGATGCCGTA | 296 |
| Cj0570 | CCAAACGATCACACCTATGC | AAAAGATTTGCCTTGGACATT | 405 |
| Cj0181 | AGGAGCGGAATTTACTTCCAT | GCTGCATAAGGGCAAGATTT | 486 |
| Cj0483 | CAGAATGCGGTGGAAGTGAT | GCTCATCACGCACTTCTTCTT | 612 |
| 2 | |||
| Cj0057 | CAATCAAGAAAATTTAAAACCCTTA | TTGGAATAGAACGCACTTCAGA | 175 |
| Cj0860 | ATAATGGGTTGGGCGAGTCT | AAAAGCAAAAATATCAAGCAAGC | 282 |
| Cj1431c | GAAGGGATGATGGATTTGGA | CACCCCCATTCTTTTTCGTA | 307 |
| Cj0733 | TGGCTTAAATTTTGTGTTGAAAAA | CATCCATTTTTCATAATCTTTACCC | 441 |
| Cj1427c | GCTCCACTTTGTAAAAGAAATCC | CTGGTTTCCAACCTGTAGCC | 613 |
| 3 | |||
| Cj0297c | GGCGTTGATATGGTTTTTATCC | GCTTTTAGCTAAGCCATCTTCTTC | 300 |
| Cj1727c | GGGCTTTTGGAATTCCTGTT | CCACGCCATGTTTAAAAGGT | 369 |
| Cj0264c | GCCTACACAGAAAATGGCAAA | TGTGGCAAAGTTGAGAGTGC | 406 |
| Cj0008 | TGAAAGCAAGACAGCTTGGA | CCGCTTTATCTGGATCTTGTTC | 524 |
| Cj1585c | TTGGAATTTAAGGCCTGTGC | TGTGCCGTGTTCAGCTTTTA | 630 |
| 4 | |||
| Cj1550c | AGGCACTGCGTTTGAAAAGT | TTTTAGTTGCACCACCAGAATG | 188 |
| Cj1329 | GGCTTAGGCTCTCGCTTAAAA | GCTCGGTTAAAATGTCTGCAT | 307 |
| Cj0177 | TTTATGTTGAGCGCTTGTGC | AAAAACCACATTGACAAATTGC | 399 |
| Cj1334 | TGCTTGTACATCGCCCTTTA | GAAATTTTGGAAAATCAAAAGGT | 462 |
| Cj0566 | GTTTCCACAAGCCTTAGAAAAA | TCCCCAAATCCTAGTAACTCCA | 558 |
| 5 | |||
| Cj0421c | ATCCAAATTTTCTTCACTTTCTTGC | TTTAGAATCCACCTTGAAAAACACG | 100 |
| Cj0033 | AATTGGGATAAAAGGGGTGAGAAT | TGAAGCCAAGTAAAACCAAAAACA | 206 |
| Cj0486 | TGCATTGGTAGCTAATATGGGAAA | AAAATAATAAGCACCTTGCGCATC | 301 |
| Cj0569 | CTGATTGGTGTGGATCTAGTGGAG | CAGAAAGGAAAACTACCGGATTTG | 399 |
| Cj0625 | AGCGATCATGAATGATGAAAATGT | CTATAGGACTTCTTGGCGTACAAGC | 498 |
| 6 | |||
| Cj0755 | TGAGCTTACTTTAAAAGGCGGTGT | GAAATCTGCCTTGGCCACTATAAC | 101 |
| Cj0736 | GGATCTGTAGCTTTTGATGGTGAA | CGATATTGGCTGAATCGATAAGAA | 205 |
| Cj0967 | CCGAACAAGGACAAGCCTTAGATA | TTTGAGAAAGATCGGTTGTGGTAA | 301 |
| Cj1141 | GCCAAAAGAGCAGGAGCTAAGATA | TGAAGCAAAACAAAAGGTATTTCA | 413 |
| Cj1136 | TATCTCGTTTAGCTGGGGTGGTAT | TGCCAAATTTTCCAAAGATCATAA | 510 |
| 7 | |||
| Cj1306c | ATAATTCGGGTTCGGCTGT | TTTTCTTTACGCGTCCAGTG | 151 |
| Cj1552c | GCCTGAAAGAACTTGCCATAATTT | GAGCTTGAAAGTTGGTTGCATAAA | 222 |
| Cj1439c | TCCCCCAAAATATACATTACTCTCG | TGATTATCGATATGGAGCTTTGGA | 307 |
| Cj1721c | AGCCGTAATTTGTTTGTCTTGGAT | GCGGGTTATCGTTTTAATGATTTG | 415 |
| Cj1679 | CAAGTTTTAATGGTCTTATGCTTGC | TTGGCTTCTTGTAGTGATTGAAAAA | 529 |
| 8 | |||
| Cj1294 | CAATATCCCTTTAATCGATGATGC | CTCTTCATTATCACTCACCACAGC | 160 |
| Cj1551c | AGCCCCTACCGATACAAATATCAT | CTTGGGGATATTGCAGAGATAACA | 241 |
| Cj0307 | TGCCACTTTCTGTAGTGCTTACAA | GCAAACCTTTTTCTAAGGCTTTTT | 347 |
| Cj1324 | TAATTTACAGCTATCCTTTTGCTATGG | AGCATGACCAAATTTAGGGTATTTC | 440 |
| Cj0035c | GAAGCAGTTCCGCTATGTTCTTTA | GGCATAGTATTTTTGCGACTTTGT | 541 |
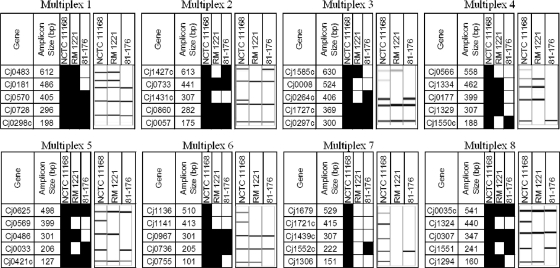
Fig 2.
Visualization of the eight 5-plex PCRs that comprise the CGF40 assay used in this study. Expected binary results for three genome-sequenced C. jejuni strains (NCTC 11168, RM1221, and 81-176) are shown (black, present band; white, absent band) along with gel representations of experimental data from the QIAxcel instrument.
CGF analysis.
To generate a CGF40 fingerprint, the 8 multiplex PCRs were performed on each isolate using the primer sets shown in Table 1. Each 5-plex reaction mix contained 1 U MP Taq DNA polymerase (Fisher Scientific, Nepean, Canada), 1× MP buffer, 2.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate (dNTP), 0.4 μM each of the 10 primers, and 1 μl DNA template (20 to 100 ng) in a 25-μl reaction mix. PCR conditions were the following: initial denaturation at 94°C for 5 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s; final extension step at 72°C for 5 min; and a hold at 4°C until samples were removed from the thermocycler. Multiplex PCRs were analyzed using standard gel electrophoresis on 2.0% agarose gels. Alternatively, PCRs were run using a QIAxcel high-throughput capillary electrophoresis system on DNA screening cartridges (Qiagen, Mississauga, Canada) using the AM320 separation method and a 20-s injection time. The 15- to 3,000-bp alignment marker was used as the internal standard, with band sizes determined based on the QX 100- to 3,000-bp DNA size marker (Qiagen, Mississauga, Canada); data were analyzed and visualized using the BioCalculator v3.0 software (Qiagen, Mississauga, Canada). Agarose gel- and QIAxcel-based PCR results were converted to binary values (i.e., 0 represents the absence of the marker and 1 indicates its presence) prior to import into Bionumerics (v.5.1; Applied Maths, Austin, TX).
Analysis of genotyping data. (i) MLST analysis.
The determination of alleles, ST, and clonal complex (CC) was performed by querying the C. jejuni MLST database (http://pubmlst.org/campylobacter/).
(ii) CGF40 analysis.
The clustering of the binary CGF40 data was performed using the simple matching distance coefficient and unweighted-pair group method using average linkages (UPGMA) of clustering in Bionumerics (v.5.1; Applied Maths, Austin, TX), using 100% fingerprint similarity for cluster definition. To obtain an estimate of strain relationships at lower levels of discrimination, data also were analyzed at the 90 and 95% similarity levels, which groups all types related at the ≥90 and ≥95% levels into single clusters without recalculating the dendrogram (see Table SA1 in the supplemental material).
(iii) Method comparison.
Molecular typing data were compared in BioNumerics (v.5.1; Applied Maths, Austin, TX) using custom scripts by Carriço et al. (7) for assessing the discriminatory power using the Simpson's index of diversity (ID) (31) and the concordance of methods using the Wallace coefficient (W) (64).
(iv) Comparison of genetic similarity estimates.
Pairwise comparisons between the 412 isolates in the data set were performed using a script written in VBA for MS Excel that computed two distinct genetic similarity estimates: (i) the number and/or proportion of matching MLST loci and (ii) the number and/or proportion of matching CGF40 loci. For the complete set of 84,666 nonredundant comparisons, see Table SA2 in the supplemental material.
(v) Analysis of cluster homogeneity.
The measurement of cluster homogeneity was performed by averaging the number of matching loci (from MLST and CGF40) among all pairs of isolates within the same CGF40 or MLST (CC and ST) cluster (see Table SA3 in the supplemental material).
RESULTS
CGF40 has higher discriminatory power than MLST.
Simpson's index of diversity (ID) (57), as proposed by Hunter and Gaston (31), along with confidence intervals proposed by Grundmann et al. (26), can be used to quantify and compare the discriminatory power of two typing methods (7). As can be seen in Table 2, based on results obtained with the C-EnterNet data set (n = 412), at 100% clustering stringency CGF40 yields a larger number of partitions and statistically significantly higher ID (282 partitions, ID = 0.994) than MLST at both the level of CC (48 partitions, ID = 0.873) and ST (116 partitions, ID = 0.935), indicating a higher discriminatory power.
Table 2.
Comparative assessment of discriminatory power using Simpson's index of discrimination
CGF40 and MLST share high levels of concordance.
Because of the wide difference in ID between CGF40 and MLST, global measures of concordance are unlikely to adequately reflect the agreement between the methods, and it is not surprising that the overall concordance between CGF40 and MLST as measured by the adjusted Rand coefficient (30) is only 0.144 and 0.082 at the CC and ST levels, respectively. The Wallace coefficient (W) (64) provides a more accurate estimate of concordance between two methods that differ in their ID by separately evaluating the concordance when each method is used as the primary method (7), thereby assessing the directionality of concordance. High Wallace coefficients were obtained when CGF40 was used as the primary typing method (CC, W = 0.983; ST, W = 0.866), which suggest that, despite large differences in ID, CGF40 is highly concordant with MLST (Table 2) and that isolates with identical CGF40 types tend to share the same CC and/or ST designations.
Genetic homogeneity among CGF40 and MLST clusters.
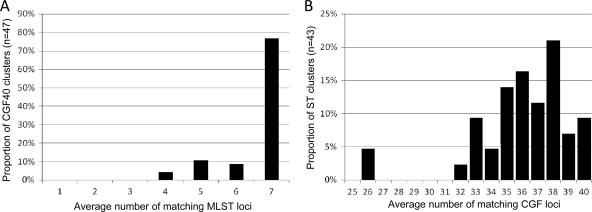
Despite the high directional concordance between MLST and CGF40, we were interested in further examining the composition of CGF40 multiple-isolate clusters with respect to the underlying MLST data. Of 47 CGF40 clusters ranging in size from n = 2 to 17 isolates, all had matching MLST locus averages of 4 or more (Fig. 3A; also see Table SA3, sheet 3, in the supplemental material), and 32 clusters were of homogeneous composition and corresponded to groups of isolates with matching alleles at all 7 MLST loci, thus sharing the same ST designation. Although the 15 remaining clusters were of heterogeneous composition and were comprised of isolates from two or more distinct STs, these were comprised mostly of single- and double-locus variants with respect to the central ST of the cluster (Table 3). For example, the largest heterogeneous CGF40 cluster (cluster 223; n = 17) had isolates from ST21 (n = 1) as well as from single-locus variants ST141 (n = 1) and ST982 (n = 15). Overall, when adjusted for cluster size, the average number of matching MLST loci was 6.698 among all CGF multiple-isolate clusters, with a slightly lower average of 6.299 among the heterogeneous clusters. Thus, CGF40 clusters generally are either homogeneous and comprised of isolates of the same ST or are heterogeneous but comprised of isolates with highly similar allelic profiles. With overall matching MLST locus averages of 6.53 and 5.85 at 95 and 90%, respectively, this relationship also holds for CGF40 clusters obtained at reduced cluster stringency (see Table SA3, sheet 3, in the supplemental material).
Fig 3.
Analysis of cluster homogeneity among multiple-isolate clusters. (A) Distribution of average numbers of matching MLST loci among multiple-isolate CGF40 clusters (n = 47). (B) Distribution of average numbers of matching CGF40 loci among multiple-isolate ST clusters (n = 43). The examination of clusters obtained using either method supports the high concordance between the two methods.
Table 3.
Allelic composition of isolates at the various MLST loci among heterogeneous CGF40 clusters at 100% stringencya
| CGF40 cluster | Cluster size (no. of isolates) | ST | No. of isolates carrying: |
||||||
|---|---|---|---|---|---|---|---|---|---|
| aspA | glnA | gltA | glyA | pgm | tkt | uncA | |||
| 171 | 15 | 982 | 2 | 1 | 2 | 3 | 2 | 1 | 5 |
| 1 | 21 | 2 | 1 | 1 | 3 | 2 | 1 | 5 | |
| 1 | 141 | 2 | 1 | 10 | 3 | 2 | 1 | 5 | |
| 48 | 7 | 459 | 1 | 2 | 3 | 3 | 5 | 9 | 3 |
| 1 | 3514 | 1 | 1 | 3 | 3 | 5 | 9 | 3 | |
| 90 | 6 | 45 | 4 | 7 | 10 | 4 | 1 | 7 | 1 |
| 1 | 3513 | 4 | 4 | 10 | 4 | 1 | 7 | 1 | |
| 206 | 4 | 922 | 1 | 1 | 2 | 83 | 2 | 3 | 6 |
| 1 | 441 | 7 | 1 | 2 | 83 | 2 | 3 | 6 | |
| 223 | 3 | 48 | 2 | 4 | 1 | 2 | 7 | 1 | 5 |
| 1 | 492 | 2 | 1 | 1 | 2 | 7 | 1 | 5 | |
| 92 | 2 | 45 | 4 | 7 | 10 | 4 | 1 | 7 | 1 |
| 1 | 766 | 4 | 17 | 10 | 4 | 1 | 7 | 1 | |
| 1 | 2522 | 168 | 17 | 5 | 4 | 1 | 7 | 1 | |
| 228 | 3 | 429 | 7 | 4 | 5 | 2 | 11 | 1 | 5 |
| 1 | 222 | 2 | 21 | 5 | 2 | 59 | 1 | 5 | |
| 37 | 3 | 1244 | 1 | 1 | 2 | 2 | 225 | 3 | 17 |
| 1 | 61 | 1 | 4 | 2 | 2 | 6 | 3 | 17 | |
| 172 | 2 | 21 | 2 | 1 | 1 | 3 | 2 | 1 | 5 |
| 2 | 8 | 2 | 1 | 1 | 3 | 2 | 1 | 6 | |
| 107 | 1 | 2249 | 4 | 7 | 10 | 4 | 1 | 3 | 1 |
| 1 | 45 | 4 | 7 | 10 | 4 | 1 | 7 | 1 | |
| 169 | 1 | 982 | 2 | 1 | 2 | 3 | 2 | 1 | 5 |
| 1 | 21 | 2 | 1 | 1 | 3 | 2 | 1 | 5 | |
| 144 | 1 | 3856 | 2 | 21 | 1 | 2 | 7 | 1 | 5 |
| 1 | 48 | 2 | 4 | 1 | 2 | 7 | 1 | 5 | |
| 34 | 1 | 1244 | 1 | 1 | 2 | 2 | 225 | 3 | 17 |
| 1 | 61 | 1 | 4 | 2 | 2 | 6 | 3 | 17 | |
| 73 | 1 | 2528 | 81 | 155 | 30 | 163 | 231 | 1 | 93 |
| 1 | 2527 | 81 | 155 | 30 | 82 | 231 | 3 | 93 | |
| 72 | 1 | 2528 | 81 | 155 | 30 | 163 | 231 | 1 | 93 |
| 1 | 1243 | 81 | 155 | 30 | 163 | 231 | 43 | 93 | |
Alleles that differ from those of the most frequent ST of the cluster are in boldface.
Given the low directional concordance when MLST was used as the primary typing method (W = 0.144), we also wanted to examine the composition of multiple-isolate ST clusters with respect to the underlying CGF40 data. Among 43 ST clusters ranging in size from n = 2 to 91, only 3 small clusters (ST429, n = 3; ST996, n = 2; and ST2525, n = 2) were comprised of isolates with identical CGF40 fingerprints, while the remaining clusters were of heterogeneous composition. Overall, 28 of 43 ST clusters (∼65%) had matching CGF locus averages of 35/40 or more (Fig. 3B; also see Table SA3, sheet 2, in the supplemental material). Thus, although most ST clusters were comprised of isolates from multiple CGF40 subtypes, these generally were very similar, with an average of 34.52 matching loci. For example, the 91 isolates of ST45, the largest ST cluster in the data set, belonged to 43 distinct CGF40 subtypes, with an average of 34.37 matching loci. This included a set of highly related CGF40 subtypes with an average of 38.26 matching loci and was unique to 22 isolates thought to be associated with an outbreak that occurred during the sampling period. Similarly, the 36 isolates from ST982, the second largest ST cluster in the data set, belonged to 20 distinct CGF40 subtypes with an average of 34.90 matching loci. Of note, the CGF data suggests that the isolates from ST982 (n = 36) and those from ST21 (n = 21) share a significant level of genetic relatedness, with an average of 34.03 matching loci; this observation is consistent with the fact that ST982 differs at a single locus from ST21. Thus, our data suggest that isolates from clusters obtained by CGF40 are generally from related subtypes when MLST is used as the secondary method for further characterizing them, and this observation also holds when MLST is used as the primary method.
Comparison of global CGF40- and MLST-based estimates of genetic similarity.
To assess the agreement between CGF40 and MLST independently of cluster or group definitions, the genetic similarity between each pair of isolates (n = 412) was estimated from either the CGF40 or MLST data. A total of 84,666 nonredundant pairwise comparisons then were used to comprehensively examine this relationship.
We first examined the relationship between CGF40 and MLST similarity among pairs of isolates with various levels of CGF40 similarity. Of the 537 pairs of isolates with identical CGF40 profiles, 465 (86.59%) had matches at all 7 MLST loci and only 5 (0.93%) had fewer than 4 matches. Conversely, among the 67,625 pairs with 30 or fewer matching CGF40 loci, only 761 (1.12%) had 7 matching loci, whereas 65,528 (96.89%) had fewer than 4 MLST matches. This relationship holds at various levels of CGF40 similarity (Fig. 4A), with pairs of isolates that share an increasing number of matching CGF40 loci also sharing an increasing number of identical alleles at the various MLST loci.
Fig 4.
Relationship between MLST- and CGF40-based genetic similarity assessments. (A) Distribution of matching MLST loci when CGF was used as the primary method. (B) Distribution of CGF matching loci when MLST was used as the primary method. In both cases, isolates that share a higher number of matching CGF40 loci tend toward higher levels of MLST similarity.
We then examined the relationship between CGF40 and MLST similarity among pairs of isolates with various levels of MLST similarity. Of the 5,514 pairs of isolates with identical MLST profiles, 2,642 (47.91%) had CGF40 profiles that had 36 or more matching loci (i.e., greater than 90% profile similarity), including 465 (8.43%) that had identical CGF40 profiles, and only 568 (10.30%) had fewer than 30 matching CGF40 loci. Conversely, among the 74,187 pairs with 3 or fewer matching MLST loci, only 548 (0.74%) had 36 or more matching loci, including 5 (0.007%) that had identical CGF40 profiles, whereas 62,630 (84.42%) had fewer than 30 matching loci. This relationship holds at various levels of MLST similarity (Fig. 4B), with pairs of isolates that share an increasing number of matching MLST loci also sharing an increasing number of matching CGF40 loci.
Taken together, these results suggest that the genetic similarity estimates obtained from both methods are in general agreement, with isolates that share high MLST similarity also tending to share high CGF40 similarity and vice versa.
DISCUSSION
Several molecular typing schemes have been developed and applied to the study of Campylobacter spp., with the recent emergence of MLST as a gold standard that has been useful in studies examining the population structure and molecular epidemiology of these important food-borne pathogens (42).
Although issues regarding the resolution of MLST for the purposes of short-term epidemiology (9, 55) pose a challenge to its applicability in some epidemiological contexts, perhaps a greater barrier precluding its use in routine epidemiologic surveillance is the relatively high cost and low throughput, which makes it difficult to implement in the analysis of the large number of isolates typically submitted to public health laboratories (14). For example, despite its status as the most frequently reported bacterial enteric disease in the country (22), Canada has yet to establish a PulseNet-like national program for real-time molecular epidemiologic surveillance of C. jejuni using comprehensive databases for the rapid sharing of molecular typing results performed by front-line state or provincial laboratories and for the identification of clusters of clinical isolates (i.e., clusters of cases of human disease) across jurisdictional boundaries (59). As a result, human clinical isolates submitted to public health laboratories are not subjected to routine subtyping using MLST or any other method. The continuing significance of campylobacteriosis underscores the need for rapid, cost-effective, high-throughput, and highly discriminatory methods for the subtyping of C. jejuni in support of the robust early detection of case clusters and the rapid identification of common sources of infection, many of which are currently considered to be sporadic (5).
A number of studies have demonstrated the potential of the high-resolution subtyping of Campylobacter using comparative genomics and its potential application in the context of molecular epidemiology or population surveys (28, 38, 50, 51). Despite these advances, the past decade has seen very limited transfer of genomic approaches to subtyping beyond proof-of-concept studies. The necessary compromise needed in balancing the resolution of a method with its cost and throughput, especially in a public health laboratory context, remains a significant obstacle to the application of genomic-based subtyping. Nevertheless, the increasing availability of genomic information represents a significant resource that can be used in the development of genotyping approaches that are informed by the most recent advances in our understanding of the genomics of Campylobacter spp.
Comparative genomic studies across a number of bacterial species have demonstrated extensive intraspecies genomic variability in the form of accessory genes that are present in some, but not all, members of a species (44), and this observation holds true for C. jejuni (15, 48, 60). Although many genes in the C. jejuni accessory genome remain putative genes with unassigned function, many encode determinants that are responsible for important phenotypic characteristics, including iron acquisition, capsule and lipooligosaccharide biosynthesis, flagellar modification, and restriction modification (27). In previous works, we were able to use a comparative genomic strategy to identify genes with a high degree of intraspecies variability that could be targeted for genotyping purposes (41, 60). CGF is based on the concept that the differential carriage of accessory genes can be used to generate high-resolution genomic fingerprints for genotyping purposes. We have previously shown that isolates that are indistinguishable by MLST can harbor significant genomic differences when examined at a greater resolution using comparative genomic hybridization (CGH) (63), and it is the potential for this increased resolution to be of use in an epidemiologic context that prompted the development of CGF. As is the case with MLST, CGF relies on the allelic assessment of multiple loci widely distributed around the genome, although CGF targets the variability that is inherent in accessory genome content, whereas MLST targets the sequence variability in core (i.e., housekeeping) genes. Despite this difference, we have recently demonstrated that the phylogenetic signal in comparative genomic data is highly consistent with that obtained with MLST in C. jejuni (63). In this light, the high concordance between the data from the CGF40 assay and MLST is not surprising and further demonstrates the feasibility of genomic-based approaches to subtyping C. jejuni.
We have previously described a CGF assay based on accessory genes identified from a comparative genomic survey of Escherichia coli O157:H7 (36) and have attempted to formalize the development of this approach to genotyping in terms of the number of target genes and their selection, leading to the development of the assay described here. This assay was designed by the selection of 40 highly informative accessory gene markers that were unbiased based on several criteria, including a moderate rate of carriage in a sample population from several large-scale comparative genomic surveys of C. jejuni (60, 62, 63); a representative genomic distribution with significant dispersal across the genome; and targeting all 16 major hypervariable regions previously identified in C. jejuni (48, 60). A significant difference between MLST and CGF is that whereas the former examines a small number of loci with high allelic diversity assayable by sequence determination, the latter examines a large number of loci with a binary allelic status and assayable by simple PCR. Nevertheless, the combinatorial effect of the 40 markers in the CGF40 assay yielded significantly higher levels of profile diversity than that observed with MLST (282 subtypes versus 116 subtypes). Moreover, in the accompanying work by Clark et al. (9a), we show that the use of either MLST, flaA SVR, or major outer membrane protein (MOMP) sequencing as a secondary typing method does not provide additional discriminatory power to CGF40 and show that, although it requires fewer PCRs than those required for a combined MLST-flaA SVR-MOMP typing approach, the CGF40 assay has greater discrimination than all three methods combined (ID = 0.995 versus 0.987).
High levels of discrimination, however, are not the sole criterion for the assessment of a subtyping method; the concordance analysis between MLST and CGF40 helps to properly contextualize the enhanced level of discrimination provided by the latter. A critical feature of the CGF approach appears to be that the number of loci used to generate a fingerprint allows for the detection of subtle genetic variation between otherwise highly related isolates. Thus, although isolates from the same ST are likely to have distinct CGF40 fingerprints, these are generally highly similar: 34 of 43 multiple-isolate ST clusters had average intracluster similarities of 35 or more matching CGF40 loci. The method therefore retains the ability to detect similarity between related isolates while at the same time providing the resolution to allow discrimination between isolates indistinguishable by MLST. This additional level of discrimination may be of particular use in resolving specific epidemiologic relationships obscured in studies that use MLST analysis alone, for example, among isolates from STs such as ST21 and ST45, which have been found to be the predominant STs in many studies (39), including this one. Although an aim of future studies will be to determine whether the high resolution afforded by CGF40 will provide an advantage in epidemiological investigations, it is worth noting that in this study, the 22 ST45 isolates associated with an outbreak, and thus epidemiologically distinct from the other ST45 isolates in the data set, share much higher similarity with each other (an average of 38.26 matching loci) than with the remaining ST45 isolates we analyzed (an average of 34.37 matching loci).
The need for methods for use in the characterization of campylobacter isolates that are accurate and reproducible and that can be transferred and readily compared among laboratories has led to the emergence of MLST as the leading method for the molecular subtyping of C. jejuni and C. coli in epidemiologic studies. However, despite recent efforts aimed at a streamlined implementation (34), the method remains difficult to implement in the context of surveillance due to cost and throughput and the need for access to DNA sequencing infrastructure. The CGF40 method described here is informed by comparative genomic principles and shows high concordance with MLST at both the CC and ST levels. At the same time, it exceeds the discriminatory power of MLST at a lower cost and with a faster turnaround time in its most basic implementation (i.e., a thermal cycler and conventional gel electrophoresis equipment), with the potential for increased throughput through automated gel electrophoresis instrumentation. Moreover, because the presence/absence of amplicons of the expected size is used to generate binary fingerprints, results are readily comparable across laboratories.
Although we have selected 40 unbiased marker genes for this assay, Cornelius et al. have recently developed an assay based on gene targets of known or putative status as epidemicity factors (10), 5 of which overlap with targets in the CGF40 assay and that could easily be adapted to an expanded CGF assay, paving the way for subtyping methods that can be of use in a range of epidemiologic applications and also to track the dissemination of clinically important traits. At the same time, Duong and Konkel (16) have recently outlined the importance of sequence variation in known and putative virulence factors that may alter the pathogenic potential of strains, underscoring the genotypic importance of SNPs beyond those found in core genes. The development of subtyping assays with enhanced biological/clinical/epidemiological relevance thus may require the development of methods that can simultaneously assess the content and/or the allelic variation in genes of interest.
The work we present here, along with recent work from other groups (10, 49), shows that comparative genomic-based approaches to genotyping can be implemented for routine surveillance. The wide-scale deployment of high-resolution genomic-based subtyping methods should provide an enhanced ability to identify genotypic clusters of C. jejuni in the context of outbreak and surveillance-based investigations and to provide valuable insights on the molecular epidemiology of these important pathogens.
Supplementary Material
ACKNOWLEDGMENTS
We thank the C-EnterNet team from the Public Health Agency of Canada for the bacterial isolates used in this study and also thank the New Brunswick Bacterial Enteric Reference Centre for the provision of additional isolates for analysis. We also acknowledge the contribution of members of the Genomics Core at the National Microbiology Laboratory (Public Health agency of Canada) for PCR primer synthesis and DNA sequencing in support of the MLST analysis.
This work was supported by the Government of Canada through funding by the Genomics Research and Development Initiative for CGF assay development and through support for the C-EnterNet surveillance program.
Footnotes
Published ahead of print 14 December 2011
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Allos BM. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201–1206 [DOI] [PubMed] [Google Scholar]
- 2. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker M, Wilson N, Edwards R. 2007. Campylobacter infection and chicken: an update on New Zealand's largest “common source outbreak. ” N. Z. Med. J. 120:U2717. [PubMed] [Google Scholar]
- 4. Barton C, Ng L-K, Tyler SD, Clark CG. 2007. Temperate bacteriophages affect pulsed-field gel electrophoresis patterns of Campylobacter jejuni. J. Clin. Microbiol. 45:386–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Best EL, Fox AJ, Frost JA, Bolton FJ. 2004. Identification of Campylobacter jejuni multilocus sequence type ST-21 clonal complex by single-nucleotide polymorphism analysis. J. Clin. Microbiol. 42:2836–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blaser MJ. 1997. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 176(Suppl. 2):S103–S105 [DOI] [PubMed] [Google Scholar]
- 7. Carriço JA, et al. 2006. Illustration of a common framework for relating multiple typing methods by application to macrolide-resistant Streptococcus pyogenes. J. Clin. Microbiol. 44:2524–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cawthraw SA, Lind L, Kaijser B, Newell DG. 2000. Antibodies, directed towards Campylobacter jejuni antigens, in sera from poultry abattoir workers. Clin. Exp. Immunol. 122:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark CG, et al. 2005. Use of the Oxford multilocus sequence typing protocol and sequencing of the flagellin short variable region to characterize isolates from a large outbreak of waterborne Campylobacter sp. strains in Walkerton, Ontario, Canada. J. Clin. Microbiol. 43:2080–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a. Clark CG, et al. 2012. Comparison of molecular typing methods useful for detecting clusters of Campylobacter jejuni and C. coli isolates through routine surveillance. J. Clin. Microbiol. 50:798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cornelius AJ, Gilpin B, Carter P, Nicol C, On SLW. 2010. Comparison of PCR binary typing (P-BIT), a new approach to epidemiological subtyping of Campylobacter jejuni, with serotyping, pulsed-field gel electrophoresis, and multilocus sequence typing methods. Appl. Environ. Microbiol. 76:1533–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denis M, et al. 2009. Comparison of genetic profiles of Campylobacter strains isolated from poultry, pig and Campylobacter human infections in Brittany, France. Pathol. Biol. 57:23–29 [DOI] [PubMed] [Google Scholar]
- 12. Dingle KE, et al. 2001. Multilocus sequence typing system for Campylobacter jejuni. J. Clin. Microbiol. 39:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dingle KE, Colles FM, Falush D, Maiden MCJ. 2005. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J. Clin. Microbiol. 43:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djordjevic SP, Unicomb LE, Adamson PJ, Mickan L, Rios R. 2007. Clonal complexes of Campylobacter jejuni identified by multilocus sequence typing are reliably predicted by restriction fragment length polymorphism analyses of the flaA gene. J. Clin. Microbiol. 45:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorrell N, et al. 2001. Whole genome comparison of Campylobacter jejuni human isolates using a low-cost microarray reveals extensive genetic diversity. Genome Res. 11:1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duong T, Konkel ME. 2009. Comparative studies of Campylobacter jejuni genomic diversity reveal the importance of core and dispensable genes in the biology of this enigmatic food-borne pathogen. Curr. Opin. Biotechnol. 20:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ekdahl K, Normann B, Andersson Y. 2005. Could flies explain the elusive epidemiology of campylobacteriosis? BMC Infect. Dis. 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitzgerald C, Sails AD, Fields PI. 2005. Campylobacter jejuni strain variation. In Ketley JM, Konkel ME. (ed.), Campylobacter jejuni: new perspectives in molecular and cellular biology. Horizon Bioscience, Wymondham, United Kingdom [Google Scholar]
- 19. Fouts DE, et al. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 3:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedman CR, Neimann JH, Wegner C, Tauxe RV. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrial nations, p. 121–138 In Nachamkin I, Blaser MJ. (ed.), Campylobacter. ASM Press, Washington, DC [Google Scholar]
- 21. Frost JA, Gillespie IA, O'Brien SJ. 2002. Public health implications of Campylobacter outbreaks in England and Wales, 1995–9: epidemiological and microbiological investigations. Epidemiol. Infect. 128:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galanis E. 2007. Campylobacter and bacterial gastroenteritis. CMAJ 177:570–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gerner-Smidt P, et al. 2006. PulseNet U. S. A.: a five-year update. Foodborne Pathog. Dis. 3:9–19 [DOI] [PubMed] [Google Scholar]
- 24. Gilpin BJ, et al. 2008. Comparison of Campylobacter jejuni genotypes from dairy cattle and human sources from the Matamata-Piako District of New Zealand. J. Appl. Microbiol. 105:1354–1360 [DOI] [PubMed] [Google Scholar]
- 25. Gormley FJ, et al. 2008. Has retail chicken played a role in the decline of human campylobacteriosis? Appl. Environ. Microbiol. 74:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grundmann H, Hori S, Tanner G. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gundogdu O, et al. 2007. Re-annotation and re-analysis of the Campylobacter jejuni NCTC11168 genome sequence. BMC Genomics 8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hannon SJ, et al. 2009. Genomics-based molecular epidemiology of Campylobacter jejuni isolates from feedlot cattle and from people in Alberta, Canada. J. Clin. Microbiol. 47:410–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hofreuter D, et al. 2006. Unique features of a highly pathogenic Campylobacter jejuni strain. Infect. Immun. 74:4694–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hubert L, Arabie P. 1985. Comparing partitions. J. Classification 2:193–218 [Google Scholar]
- 31. Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keller J, Wieland B, Wittwer M, Stephan R, Perreten V. 2007. Distribution and genetic variability among Campylobacter spp. isolates from different animal species and humans in Switzerland. Zoonoses Public Health 54:2–7 [DOI] [PubMed] [Google Scholar]
- 33. Klena JD, Konkel ME. 2005. Methods for epidemilogical analysis of Campylobacter jejuni, p. 165–179 In Ketley JM, Konkel ME. (ed.), Campylobacter jejuni: new perspectives in molecular and cellular biology. Horizon Bioscience, Wymondham, United Kingdom [Google Scholar]
- 34. Korczak BM, Zurfluh M, Emler S, Kuhn-Oertli J, Kuhnert P. 2009. Multiplex strategy for multilocus sequence typing, fla typing, and genetic determination of antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolates collected in Switzerland. J. Clin. Microbiol. 47:1996–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwan PSL, et al. 2008. Molecular epidemiology of Campylobacter jejuni populations in dairy cattle, wildlife, and the environment in a farmland area. Appl. Environ. Microbiol. 74:5130–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laing C, et al. 2008. Rapid determination of Escherichia coli O157:H7 lineage types and molecular subtypes by using comparative genomic fingerprinting. Appl. Environ. Microbiol. 74:6606–6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 38. Leonard EE, et al. 2003. Use of an open-reading frame-specific Campylobacter jejuni DNA microarray as a new genotyping tool for studying epidemiologically related isolates. J. Infect. Dis. 187:691–694 [DOI] [PubMed] [Google Scholar]
- 39. Levesque S, Frost E, Arbeit RD, Michaud S. 2008. Multilocus sequence typing of Campylobacter jejuni isolates from humans, chickens, raw milk, and environmental water in Quebec, Canada. J. Clin. Microbiol. 46:3404–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Litrup E, Torpdahl M, Nielsen EM. 2007. Multilocus sequence typing performed on Campylobacter coli isolates from humans, broilers, pigs and cattle originating in Denmark. J. Appl. Microbiol. 103:210–218 [DOI] [PubMed] [Google Scholar]
- 41. MacKinnon J, et al. 2005. Investigation of gene targets for epidemiologic and phylogenetic analysis of Campylobacter jejuni. Abstr. 13th Int. Workshop Campylobacter, Helicobacter Related Organisms, abstr. A56 [Google Scholar]
- 42. Maiden MCJ, Dingle KE. 2008. Population biology of Campylobacter jejuni and related organisms, p. 27–40 In Nachamkin I, Szymanski CM, Blaser MJ. (ed.), Campylobacter, 3rd ed ASM Press, Washington, DC [Google Scholar]
- 43. Mead PS, et al. 1999. Food-related illness and death in the United States. Emerging Infect. Dis. 5:607–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Medini D, Donati C, Tettelin H, Masignani V, Rappuoli R. 2005. The microbial pan-genome. Curr. Opin. Genet. Dev. 15:589–594 [DOI] [PubMed] [Google Scholar]
- 45. Michaud S, Menard S, Arbeit RD. 2005. Role of real-time molecular typing in the surveillance of Campylobacter enteritis and comparison of pulsed-field gel electrophoresis profiles from chicken and human isolates. J. Clin. Microbiol. 43:1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nelson W, Harris B. 2006. Flies, fingers, fomites, and food. Campylobacteriosis in New Zealand–food-associated rather than food-borne. N. Z. Med. J. 119:U2128. [PubMed] [Google Scholar]
- 47. Parkhill J, et al. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668 [DOI] [PubMed] [Google Scholar]
- 48. Pearson BM, et al. 2003. Comparative genome analysis of Campylobacter jejuni using whole genome DNA microarrays. FEBS Lett. 554:224–230 [DOI] [PubMed] [Google Scholar]
- 49. Price EP, Huygens F, Giffard PM. 2006. Fingerprinting of Campylobacter jejuni by using resolution-optimized binary gene targets derived from comparative genome hybridization studies. Appl. Environ. Microbiol. 72:7793–7803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quiñones B, et al. 2008. Comparative genomic analysis of clinical strains of Campylobacter jejuni from South Africa. PLoS One 3:e2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rodin S, et al. 2008. Performance of a 70-mer oligonucleotide microarray for genotyping of Campylobacter jejuni. BMC Microbiol. 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rotariu O, et al. 2009. Spatiotemporal homogeneity of Campylobacter subtypes from cattle and sheep across northeastern and southwestern Scotland. Appl. Environ. Microbiol. 75:6275–6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rozen S, Skaletsky H. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365–386 [DOI] [PubMed] [Google Scholar]
- 54. Rozynek E, Antos-Bielska M, Dzierzanowska-Fangrat K, Szczepańska B, Trafny EA. 2010. Genetic similarity of Campylobacter isolates in humans, food, and water sources in central Poland. Foodborne Pathog. Dis. 7:597–600 [DOI] [PubMed] [Google Scholar]
- 55. Sails AD, Swaminathan B, Fields PI. 2003. Utility of multilocus sequence typing as an epidemiological tool for investigation of outbreaks of gastroenteritis caused by Campylobacter jejuni. J. Clin. Microbiol. 41:4733–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sheppard SK, et al. 2009. Campylobacter genotypes from food animals, environmental sources and clinical disease in Scotland 2005/6. Int. J. Food Microbiol. 134:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simpson EH. 1949. Measurement of diversity. Nature 163:688 [Google Scholar]
- 58. Stothard P, Wishart DS. 2005. Circular genome visualization and exploration using CGView. Bioinformatics 21:537–539 [DOI] [PubMed] [Google Scholar]
- 59. Swaminathan B, Barrett TJ, Hunter SB, Tauxe RV. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerging Infect. Dis. 7:382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taboada EN, et al. 2004. Large-scale comparative genomics meta-analysis of Campylobacter jejuni isolates reveals low level of genome plasticity. J. Clin. Microbiol. 42:4566–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taboada E, Acedillo R, Luebbert C, Findlay W, Nash J. 2005. A new approach for the analysis of bacterial microarray-based comparative genomic hybridization: insights from an empirical study. BMC Genomics 6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Taboada E, et al. 2007. Comparative genomic analysis of Campylobacter jejuni associated with Guillain-Barré and Miller Fisher syndromes: neuropathogenic and enteritis-associated isolates can share high levels of genomic similarity. BMC Genomics 8:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taboada E, et al. 2008. Comparative genomic assessment of multi-locus sequence typing: rapid accumulation of genomic heterogeneity among clonal isolates of Campylobacter jejuni. BMC Evol. Biol. 8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wallace DL. 1983. A method for comparing two hierarchical clusterings: comment. J. Am. Stat. Assoc. 78:569–576 [Google Scholar]
- 65. Wesley IV, et al. 2000. Fecal shedding of Campylobacter and Arcobacter spp. in dairy cattle. Appl. Environ. Microbiol. 66:1994–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wilson DJ, et al. 2009. Rapid evolution and the importance of recombination to the gastroenteric pathogen Campylobacter jejuni. Mol. Biol. Evol. 26:385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao C, et al. 2001. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the greater Washington, DC, area. Appl. Environ. Microbiol. 67:5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.