Abstract
Eosinophilic fasciitis (EF) with generalized sclerodermiform skin lesions developed over a 19-month period in a previously healthy 23-year-old man. Although we confirmed EF by skin histology and laboratory tests, the recurrent fevers and the clinical observation of sclerotic prepuce with urethritis indicated further bacteriological analysis by conventional microbiological and DNA-based tests. Urethra cultures were positive for an arginine-hydrolyzing mycoplasma and Ureaplasma urealyticum. The patient also had serum IgM antibodies to Mycoplasma pneumoniae using enzyme-linked immunosorbent assay (ELISA)-based qualitative detection. Mycoplasma arginini was isolated from two independent venous blood serum samples and was identified by conventional microbiological tests and sequencing of the 16S rRNA and rpoB genes (GenBank sequence accession numbers HM179555 and HM179556, respectively). M. arginini genomic DNA also was detected by species-specific PCR in the skin lesion biopsy sample. Treatment with corticosteroids and long-term courses of selected antibiotics led to remission of skin symptoms and normalization of laboratory values. This report provides the first evidence of EF associated with mycoplasma infection and the second report of human infection with M. arginini and therefore suggests that this mycoplasma infection might have contributed to the pathogenesis of the disease.
CASE REPORT
A 23-year-old male, a former bodybuilder, sought medical treatment for progressive generalized skin tightening. His previous medical history was unremarkable. The patient had terminated sport exercises 19 months earlier due to a sudden, progressive skin induration, which led to restricted movement of wrists and ankles within several months, and cutaneous symptoms gradually extended to the extremities and to the upper body. At the beginning of his illness, he quit body building and stopped anabolic steroid consumption. Within 1 year, he lost 23 kg, he became febrile in the afternoons, and his skin disease slowly progressed. Serological tests for HIV, hepatitis C virus (HCV), and Lyme borreliosis were negative. His crista biopsy specimen revealed eosinophilia. The patient was treated with a very low dose of prednisone (10 mg/day orally [p.o.]) for the next 6 months without dermatological improvement. Any attempt to decrease the prednisone dosage resulted in return of arthralgia and fever.
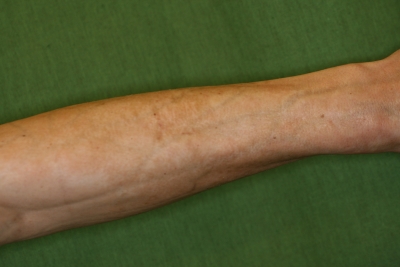
Upon initial examination, the patient suffered from generalized skin tightening and tautness on his whole trunk and on all the extremities except the fingers and toes. He also exhibited multiple cutaneous venous furrowing on the arms (Fig. 1). The shiny, brownish, firm skin with some porcelain-whitish patches was tightly bound to the underlying tissues, resulting in a restricted movement of the chest, wrists, elbows, ankles, and knees. The genital region was also involved; the indurated, sclerotic, depigmented prepuce led to phimosis and showed lichen sclerosus-like symptoms with an inflamed urethral orifice.
Fig 1.
Classical symptoms of eosinophilic fasciitis with venous furrowing on the arms of the patient.
Initial laboratory tests showed peripheral blood eosinophilia (12.3 × 109/liter; normal, 0.0 × 109 to 7.0 × 109/liter), high serum IgG concentration (50 g/liter; normal, 7 to 16 g/liter), elevated serum IgG1 concentration (29.5 g/liter; normal, 5.2 to 12.7 g/liter), increased IgE concentration (408 U/ml; normal, 0 to 100 U/ml), elevated C-reactive protein (CRP) (25.6 mg/liter; normal, 0.0 to 5.0 mg/liter), and elevated erythrocyte sedimentation rate (ESR) (61 mm/h; normal, 1 to 20 mm/h). Skin histology from the right upper arm revealed a mildly atrophic epidermis, with a fibrotic reticular dermis and homogenized, hyalinized connective tissue in the lower dermis. There was no clear border between the lower dermis and the subcutis, where almost all the subcutaneous fat was replaced by horizontally running, homogenized collagen fibers intermingled with chronic inflammation with lymphocytes, macrophages, a few eosinophils, and several plasma cells up to the fascial layer. Thus, the laboratory data, skin histology, and the clinical symptoms were in agreement with the diagnosis of eosinophilic fasciitis (EF).
Cardiac echocardiogram, electrocardiogram, and chest, skull, and barium swallow X-ray values were within normal limits. The bone scintigraphy showed increased tracer activity in the wrists, ankles, shoulders, elbows, and knees.
An arginine-hydrolyzing mycoplasma and Ureaplasma urealyticum were cultured (Mycoplasma Duo kit; Bio-Rad, France) from the urethra. The initial isolate of the mycoplasma was not identified or saved. Qualitative enzyme immunoassays (EIAs) (Ani Labsystems Ltd. Oy, Vantaa, Finland) for detection of serum IgA, IgG, and IgM antibodies to Borrelia burgdorferi and Mycoplasma pneumoniae were performed and showed that the patient had serum IgM antibodies to M. pneumoniae. A quantitative assay to determine the titer of these IgM antibodies was not done. Isolation of M. pneumoniae from the patient was not attempted. Since results from high-resolution computed tomography (CT) scanning of the lungs and pulmonary function tests were within normal limits, the presence of a possible extrapulmonary manifestation of mycoplasma infection (37) or the presence of IgM antibodies due to past exposure to M. pneumoniae (13, 28, 38) could perhaps explain this positive result in the IgM EIA.
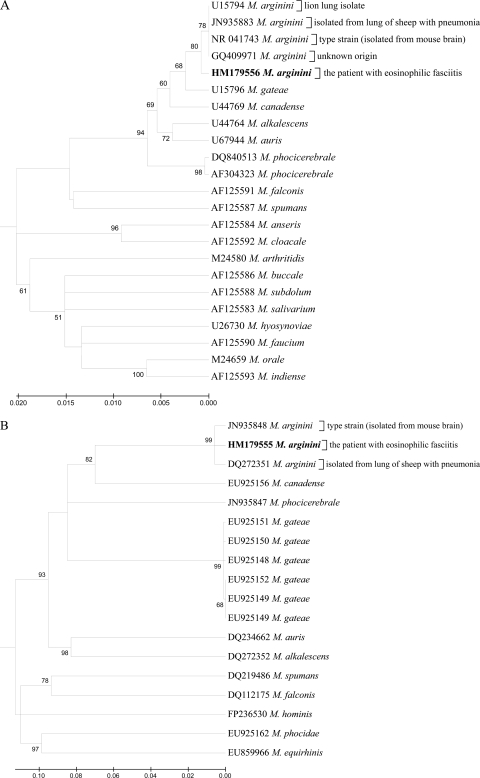
DNA extracted (MiniPrep Express Matrix kit; MP Biomedicals, Irvine, CA) from a deparaffinized skin biopsy specimen taken from the patient before the initiation of antibiotic therapy was PCR positive for the presence of mycoplasma. The skin DNA was tested by SYBR green real-time PCR with mycoplasma-specific universal primers as described previously (20, 35). On the basis of these findings, two independent venous blood serum specimens were collected from the patient at recurrent episodes of fever and were cultured (PPLO broth; BD Biosciences, Franklin Lakes, NJ) for the presence of mycoplasma. Both venous blood serum-inoculated cultures were positive for a pure growth of arginine-utilizing mycoplasma during incubation at 37°C with 5% CO2 for 5 to 7 days. Noninoculated broth cultures (negative control), as well as aliquots of the negative-control broth that were plated onto PPLO agar (BD Biosciences), remained negative. The organism was negative for glucose fermentation and urea hydrolysis, reduced 2,3,5-triphenyl tetrazolium chloride (TTC), and produced phosphatase, film, and spot. Previously published primers (36) were used to determine the complete 16S rRNA sequence, the internal transcribed spacers (ITS), and the partial 23S rRNA and RNA polymerase beta subunit (rpoB) genes, and the sequences were deposited at GenBank under accession numbers HM179555 and HM179556. The microorganism was identified as Mycoplasma arginini (Fig. 2) based on the sequencing data, as well as the cultural and biochemical tests. After the detection of viable mycoplasma in the patient's venous blood serum, mycoplasmal DNA from the skin biopsy specimen was reamplified with the species-specific primers for the rpoB gene (rpoB_argininiF, 5′-TTTGACGGGGTTGTAACATACGT-3′, and rpoB_argininiR, 5′-CAGCTAATCCTAGGTGTAATTCGAG-3′), and the PCR products were directly sequenced using the same primers. Therefore, the presence of DNA of M. arginini in the patient's skin biopsy specimen was confirmed, and the sequences of M. arginini cultured from the venous blood serum and PCR amplified from the skin sample were identical. Species-specific PCR detection of M. pneumoniae in the DNA extracted from the skin biopsy specimen was attempted using published primers (7, 18), but the result was negative. The in vitro antimicrobial susceptibility testing (17) was done, and the MICs were as follows: doxycycline, 0.25 mg/ml; erythromycin, 0.5 mg/ml; enrofloxacin, 1.0 mg/ml; lincomycin, 2.0 mg/ml; chlortetracycline, 4.0 mg/ml.
Fig 2.
Dendrograms showing phylogenetic relationships based on nucleotide sequence data for the 16S rRNA (A) and rpoB (B) genes for M. arginini isolated from the patient and closely related species of the family Mycoplasmataceae. The trees were constructed by the minimum evolution method in the MEGA 4 package. The bootstrap values presented at corresponding branches were evaluated from 1,000 replications. GenBank accession numbers are indicated for each strain used in creating the dendrograms.
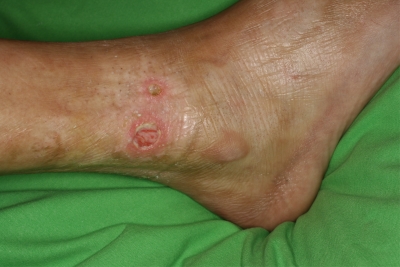
Based on the classical clinical picture and the confirmed diagnosis of EF, we initiated high doses of methylprednisone (1.5 mg/kg of body weight/day, p.o.). Because microbiological and PCR data indicated the presence of mycoplasma infection in urethra, skin lesions, and blood, when multiple 1/2- to 2-cm large unilateral and then bilateral skin ulcers developed above the ankles and progressed further on the tight skin (Fig. 3), intravenous (i.v.) erythromycin was added to the treatment regimen. For long-term therapy, methylprednisone treatment was gradually decreased (1.5 to 1.0 to 0.5 to 0.3 mg/kg/day, p.o.) in combination with a full dosage of i.v. clarithromycin or p.o. erythromycin, clarithromycin, or doxycycline. After 5 months of the combination treatment, the patient felt significantly better for the first time since the onset of his illness and his ulcers healed. Within the following 2 years, the patient's skin became soft, his movement was free and comfortable, and the phimosis was resolved without the otherwise refused circumcision. In the first year of that treatment, he received 1-week courses of doxycycline (100 mg, twice a day [BID], p.o.) in every second month and took methylprednisolone continuously (4 mg every second day, p.o.). Within the second year, the patient received doxycycline (100 mg, BID, p.o., for a week) in every third month and took 2.0 mg methylprednisone on every second day.
Fig 3.
Development of skin ulceration during high-dose steroid therapy.
EF is a scleroderma-like disease of unknown origin and is considered to be an autoimmune or recently also an autoinflammatory disease (26), supported by the fact that not only steroids but also immunosuppressive drugs, or biologics such as tumor necrosis factor alpha (TNF-α) inhibitors, seem to be an effective treatment (19, 22). Some of the autoimmune phenomena associated with EF are characteristic for the disease, like high serum IgG concentrations, while others such as hemolytic anemia; thrombocytopenia; aplastic anemia; arthralgia or arthritis; the presence of antinuclear, antithyroglobulin, and anti-thyroid peroxidase antibodies; livedo reticularis; and IgA nephropathy are rare (1, 3, 8, 24). Most of these autoimmune phenomena also have been associated with mycoplasma infections in humans (15, 37). While the etiology of EF has remained obscure, the following remarks should be noted: (i) the presence of granzyme B-positive CD8+ T lymphocytes observed in the EF skin lesions suggests a cytotoxic immune response to infectious or environmental agents (34); (ii) levotryptophan (LT) consumption, presumably containing biological or chemical impurities, has been associated with EF or eosinophilia-myalgia syndrome (EMS) (2, 8–12). Here we report a young EF patient with mycoplasma bacteremia (M. arginini) and possibly concurrent infection, or previously infected-recently recovered status, with another mycoplasma species (M. pneumoniae), who was successfully treated with high-dose corticosteroids in combination with long-term high doses of antibiotics.
The presence of a positive result for IgM antibodies to M. pneumoniae and explanation of their association with a possible concurrent M. pneumoniae infection of the respiratory tract in the patient are difficult due to the absence of clinical symptoms and the presence of normal chest X-ray and CT scan of the lungs and pulmonary function tests in the patient. The EIA (Ani Labsystems) used for detection of serum IgM antibodies to M. pneumoniae is based on the surface-exposed P1-enriched protein (31), which is the main virulence factor of M. pneumoniae, and the assay has been shown to have good performance in terms of sensitivity and specificity early in the course of infection (6). The possibility of cross-reactivity of anti-Mycoplasma arginini IgM antibodies with M. pneumoniae P1 protein in this test has never been investigated due to the absence of clinically documented importance of M. arginini in humans. Because IgM antibodies to M. pneumoniae may persist for several months in the circulation (13, 28, 38), the patient may have had the positive result in the EIA due to past exposure to M. pneumoniae.
We speculate that mycoplasma infection may have triggered EF in our patient. Non-EF skin manifestations during M. pneumoniae infection have been reported (16, 27, 30, 34, 37). The following known triggering factors were identified in our patient: (i) LT-rich food additive consumption derived partially from uncontrolled sources and (ii) strenuous exercises. We also wish to highlight some additional suspicious observations for this patient that may have contributed to EF: (i) regular use of p.o. and intramuscular (i.m.) anabolic steroids derived partially from uncontrolled animal sources, (ii) mycoplasma bacteremia, (iii) the presence of mycoplasma and ureaplasma urethral infection, and (iv) the presence of mycoplasma DNA in the diseased skin.
The fact that we have detected the presence of M. arginini in the blood and also in the skin lesion of the patient needs to be elucidated. This organism is normally associated with animals (5, 21), and the source of this M. arginini infection in the patient is unclear. The patient had regularly used nutrition supplements rich in proteins and amino acids (i.e., LT and arginine) for the past 10 years, and he reported the combinational use of anabolic steroid injections and tablets (testosterone enanthate, nandrolone phenylpropionate, clenbuterol, and methandrostenolone) for the past 3 years. All the products were from local distributors, and the majority of these nutritional supplements were derived from animal materials of uncontrolled sources. Although speculative, it is possible that the patient acquired the infection through the consumption of his dietary supplements and/or from hormone injections derived from uncontrolled animal materials. However, we were not able to identify this source retrospectively.
While most mycoplasma species are host specific, there are reports of the presence of mycoplasma in hosts that are not perceived as their normal habitat. Thus, in addition to M. arginini, rare human infections with Mycoplasma bovis (23), Mycoplasma phocicerebrale (4), and presumably animal hemotrophic mycoplasma species (14, 29, 32, 33) have been reported. In some cases, the “jump” of animal mycoplasma to humans may have a pathological impact, particularly under predisposing conditions such as immunodeficiency (25, 39). Mycoplasma infections have been associated with autoimmune disorders (15, 37), but their role in disease pathogenesis is unclear.
Our case is the second report of documented M. arginini infection in humans. The first human case was a fatal M. arginini septicemia with pneumonia reported in a 64-year-old slaughterhouse worker with advanced non-Hodgkin's lymphoma (39). Our EF patient may represent a case of induced autoimmunity, where the consumption of LT and anabolic steroids from uncontrolled sources could contribute to disease development. We think that the presence of these factors in the medical history might indicate a search for potential infectious triggers, including the presence of mycoplasma infections. Our data also suggest that complementary antibiotic therapy may be necessary in some patients with EF.
Nucleotide sequence accession numbers.
The sequences were deposited at GenBank under accession numbers HM179555 and HM179556.
ACKNOWLEDGMENTS
We thank Maureen K. Davidson, Donna K. F. Chandler, and Christine M. Anderson for critical review of this article.
This work was supported by the Hungarian Scientific Research Fund OTKA, ID 73296.
Footnotes
Published ahead of print 21 December 2011
REFERENCES
- 1. Antic M, Lautenschlager S, Itin PH. 2006. Eosinophilic fasciitis 30 years after—what do we really know? Report of 11 patients and review of the literature. Dermatology 213:93–101 [DOI] [PubMed] [Google Scholar]
- 2. Auerbach SB, Falk H. 1991. Eosinophilia-myalgia syndrome: CDC update. Cleve. Clin. J. Med. 58:215–217 [DOI] [PubMed] [Google Scholar]
- 3. Bachmeyer C, et al. 1999. Eosinophilic fasciitis following idiopathic thrombocytopenic purpura, autoimmune hemolytic anemia and Hashimoto's disease. Dermatology 199:282. [DOI] [PubMed] [Google Scholar]
- 4. Baker AS, Ruoff KL, Madoff S. 1998. Isolation of Mycoplasma species from a patient with seal finger. Clin. Infect. Dis. 27:1168–1170 [DOI] [PubMed] [Google Scholar]
- 5. Barile MF, DelGiudice RA, Carski TR, Gibbs CJ, Morris JA. 1968. Isolation and characterization of Mycoplasma arginini: spec. nov. Proc. Soc. Exp. Biol. Med. 129:489–494 [DOI] [PubMed] [Google Scholar]
- 6. Beersma MF, et al. 2005. Evaluation of 12 commercial tests and the complement fixation test for Mycoplasma pneumoniae-specific immunoglobulin G (IgG) and IgM antibodies, with PCR used as the “gold standard.” J. Clin. Microbiol. 43:2277–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernet C, Garret M, de Barbeyrac B, Bebear C, Bonnet J. 1989. Detection of Mycoplasma pneumoniae by using the polymerase chain reaction. J. Clin. Microbiol. 27:2492–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blauvelt A, Falanga V. 1991. Idiopathic and L-tryptophan-associated eosinophilic fasciitis before and after L-tryptophan contamination. Arch. Dermatol. 127:1159–1166 [PubMed] [Google Scholar]
- 9. Bohme A, Wolter M, Hoelzer D. 1998. L-tryptophan-related eosinophilia-myalgia syndrome possibly associated with a chronic B-lymphocytic leukemia. Ann. Hematol. 77:235–238 [DOI] [PubMed] [Google Scholar]
- 10. CDC 1990. Analysis of L-tryptophan for the etiology of eosinophilia-myalgia syndrome. MMWR Morb. Mortal. Wkly. Rep. 39:589–591 [PubMed] [Google Scholar]
- 11. CDC 1990. Analysis of L-tryptophan for the etiology of eosinophilia-myalgia syndrome. JAMA 264:1656. [PubMed] [Google Scholar]
- 12. CDC 1990. Update: eosinophilia-myalgia syndrome associated with ingestion of L-tryptophan—United States, as of January 9, 1990. JAMA 263:633. [PubMed] [Google Scholar]
- 13. Chamberlain P, Saeed AA. 1983. A study of the specific IgM antibody response in Mycoplasma pneumoniae infection in man. J. Hyg. (Lond.) 90:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. dos Santos AP, et al. 2008. Hemoplasma infection in HIV-positive patient, Brazil. Emerg. Infect. Dis. 14:1922–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feizi T, Loveless RW. 1996. Carbohydrate recognition by Mycoplasma pneumoniae and pathologic consequences. Am. J. Respir. Crit. Care Med. 154:S133–S136 [DOI] [PubMed] [Google Scholar]
- 16. Greco F, Sorge A, Salvo V, Sorge G. 2007. Cutaneous vasculitis associated with Mycoplasma pneumoniae infection: case report and literature review. Clin. Pediatr. (Phila.) 46:451–453 [DOI] [PubMed] [Google Scholar]
- 17. Hannan PC. 2000. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet. Res. 31:373–395 [DOI] [PubMed] [Google Scholar]
- 18. Kai M, et al. 1993. Rapid detection of Mycoplasma pneumoniae in clinical samples by the polymerase chain reaction. J. Med. Microbiol. 38:166–170 [DOI] [PubMed] [Google Scholar]
- 19. Khanna D, Agrawal H, Clements PJ. 2010. Infliximab may be effective in the treatment of steroid-resistant eosinophilic fasciitis: report of three cases. Rheumatology (Oxford) 49:1184–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kiss I, Matiz K, Kaszanyitzky E, Chavez Y, Johansson KE. 1997. Detection and identification of avian mycoplasmas by polymerase chain reaction and restriction fragment length polymorphism assay. Vet. Microbiol. 58:23–30 [DOI] [PubMed] [Google Scholar]
- 21. Leach RH. 1970. The occurrence of Mycoplasma arginini in several animal hosts. Vet. Rec. 87:319–320 [DOI] [PubMed] [Google Scholar]
- 22. Loupasakis K, Derk CT. 2010. Eosinophilic fasciitis in a pediatric patient. J. Clin. Rheumatol. 16:129–131 [DOI] [PubMed] [Google Scholar]
- 23. Madoff S, Pixley BQ, DelGiudice RA, Moellering RC., Jr 1979. Isolation of Mycoplasma bovis from a patient with systemic illness. J. Clin. Microbiol. 9:709–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miret C, Nonell F, Cervera R, Rodriguez-Flores E, Torres M. 2003. IgA nephropathy associated with eosinophilic fasciitis: report of a case. Clin. Exp. Rheumatol. 21:268. [PubMed] [Google Scholar]
- 25. Pitcher DG, Nicholas RA. 2005. Mycoplasma host specificity: fact or fiction? Vet. J. 170:300–306 [DOI] [PubMed] [Google Scholar]
- 26. Rieber N, Belohradsky BH. 2010. AHR activation by tryptophan—pathogenic hallmark of Th17-mediated inflammation in eosinophilic fasciitis, eosinophilia-myalgia-syndrome and toxic oil syndrome? Immunol. Lett. 128:154–155 [DOI] [PubMed] [Google Scholar]
- 27. Schalock PC, Dinulos JG. 2009. Mycoplasma pneumoniae-induced cutaneous disease. Int. J. Dermatol. 48:673–680 [DOI] [PubMed] [Google Scholar]
- 28. Skaug K, Eng J, Orstavik I, Haug KW. 1976. The diagnostic value of determination of IgM antibodies against Mycoplasma pneumoniae by the indirect immunofluorescent antibody test. Acta Pathol. Microbiol. Scand. B 84:170–176 [DOI] [PubMed] [Google Scholar]
- 29. Steer JA, et al. 2011. A novel hemotropic Mycoplasma (hemoplasma) in a patient with hemolytic anemia and pyrexia. Clin. Infect. Dis. 53:e147–e151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stockner I, et al. 2008. Non-episodic angioedema associated with eosinophilia following Mycoplasma pneumoniae infection. Clin. Rheumatol. 27:1573–1576 [DOI] [PubMed] [Google Scholar]
- 31. Suni J, Vainionpaa R, Tuuminen T. 2001. Multicenter evaluation of the novel enzyme immunoassay based on P1-enriched protein for the detection of Mycoplasma pneumoniae infection. J. Microbiol. Methods 47:65–71 [DOI] [PubMed] [Google Scholar]
- 32. Sykes JE, Lindsay LL, Maggi RG, Breitschwerdt EB. 2010. Human coinfection with Bartonella henselae and two hemotropic mycoplasma variants resembling Mycoplasma ovis. J. Clin. Microbiol. 48:3782–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tasker S, et al. 2010. Investigation of human haemotropic Mycoplasma infections using a novel generic haemoplasma qPCR assay on blood samples and blood smears. J. Med. Microbiol. 59:1285–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toquet C, et al. 2003. In situ immunophenotype of the inflammatory infiltrate in eosinophilic fasciitis. J. Rheumatol. 30:1811–1815 [PubMed] [Google Scholar]
- 35. van Kuppeveld FJ, et al. 1992. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl. Environ. Microbiol. 58:2606–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Volokhov DV, et al. 2007. Genetic analysis of housekeeping genes of members of the genus Acholeplasma: phylogeny and complementary molecular markers to the 16S rRNA gene. Mol. Phylogenet. Evol. 44:699–710 [DOI] [PubMed] [Google Scholar]
- 37. Waites KB, Balish MF, Atkinson TP. 2008. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol. 3:635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wreghitt TG, Sillis M. 1985. A micro-capture ELISA for detecting Mycoplasma pneumoniae IgM: comparison with indirect immunofluorescence and indirect ELISA. J. Hyg. (Lond.) 94:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yechouron A, Lefebvre J, Robson HG, Rose DL, Tully JG. 1992. Fatal septicemia due to Mycoplasma arginini: a new human zoonosis. Clin. Infect. Dis. 15:434–438 [DOI] [PubMed] [Google Scholar]