Abstract
The thermosensitive allelic mutations sm19-1 and sm19-2 of Paramecium tetraurelia cause defective basal body duplication: growth at the nonpermissive temperature yields smaller and smaller cells with fewer and fewer basal bodies. Complementation cloning of the SM19 gene identified a new tubulin, eta-tubulin, showing low homology with each of the other five tubulins, α to ɛ, characterized in P. tetraurelia. In order to analyze η-tubulin functions, we used a genetic approach to identify interacting molecules. Among a series of extragenic suppressors of the sm19-1 mutation, the su3-1 mutation was characterized as an E288K substitution in the β-PT2 gene coding for a β-tubulin, while the mutation nocr1 conferring nocodazole resistance and localized in another β-tubulin gene, β-PT3, was shown to enhance the mutant phenotype. The interaction between η-tubulin and microtubules, revealed by genetic data, is supported by two further types of evidence: first, the mutant phenotype is rescued by taxol, which stabilizes microtubules; second, molecular modeling suggests that η-tubulin, like γ- and δ-tubulins, might be a microtubule minus-end capping molecule. The likely function of η-tubulin as part of a complex specifically involved in basal body biogenesis is discussed.
The highly conserved α- and β-tubulins constitute the building blocks of microtubule arrays, and members of a third tubulin subfamily, γ-tubulin, involved in the polymerization of α/β dimers into microtubules, have been identified in all eukaryotes. In addition to these three ubiquitous subfamilies, several new members of the tubulin family have been discovered in the last few years: delta (14), epsilon (5), zeta (39), eta (30), and iota and theta (P. Dupuis-Williams, unpublished data; GenBank/EMBL/DDBJ accession numbers AJ427481 and AJ427480). Interestingly, while some of the new tubulins were identified through genome survey, two of them, δ and η, were identified by complementation cloning of mutations affecting basal body assembly/duplication, providing a first hint as to their function. In particular, a role of δ-tubulin in the C-tubule assembly of basal bodies, deduced from the properties of the UNI3 mutant of Chlamydomonas reinhardtii (14), was confirmed in Paramecium tetraurelia by the effect of δ-tubulin gene silencing (16).
A function of ɛ-tubulin in centriole/basal body assembly was recently demonstrated (4, 11, 13) and is consistent with the fact that ɛ-tubulin, like δ-tubulin, is found in widely distant organisms—mammals, green algae, protozoans—but is absent from the completely sequenced genomes of Saccharomyces cerevisiae, Drosophila melanogaster, and Caenorhabditis elegans, which do not possess such organelles or possess highly divergent ones. As for ζ-, η-, θ-, and ι-tubulins, they do not seem so far to have metazoan homologues, and their functions might be restricted to ciliates and flagellates. However, since η-tubulin is involved in basal body duplications but it has no homologue in other organisms, its function in basal body duplication might be accomplished by tubulins that have been identified by Blast but that as yet have no assigned function (for instance, tubulins with accession numbers XP_301543 and NP_079079 in the human genome). Understanding the function of η-tubulin should therefore help in identifying possible functional homologues in distant organisms and understanding the mechanism of basal body/centriole biogenesis.
An important step is to identify the molecular partners of these new tubulins. Based on in silico analysis, Inclan and Nogales (19) proposed that ɛ- and δ-tubulins were able to contact microtubular structure, but no experimental data have yet confirmed this assumption.
With the aim of a better knowledge of the function of η-tubulin and of its interacting proteins, we have taken a genetic approach. Two allelic thermosensitive mutations, sm19-1 and sm19-2, affecting gene SM19 of P. tetraurelia which encodes η-tubulin (30), lead at the nonpermissive temperature (35°C) to a progressive reduction in the number of basal bodies, accompanied by reduced cell length and modified cell shape (29). Mutant cells grown at the nonpermissive temperature showed a delocalization of γ-tubulin, also involved in basal duplication (30). This delocalization suggested that η-tubulin might contribute to tether γ-tubulin or γ-tubulin complexes to the nucleation site of basal bodies to initiate microtubule polymerization.
We show here that the two η-tubulin mutations interact with mutations in two different β-tubulin genes and that these β-tubulin mutations are expected to affect microtubule dynamics, two observations consistent with an η-tubulin-microtubule interaction. This interaction was supported both by physiological experiments, which show that taxol suppresses η-tubulin mutations, and by molecular modeling, which suggests that the η-tubulin could be a minus-end microtubule-associated protein. Altogether, our results suggest that η-tubulin could regulate the dynamic behavior of a microtubule subset or of a microtubule-containing complex involved in basal body duplication, by capping their minus end via a direct or indirect interaction with the β-tubulin subunit.
MATERIALS AND METHODS
Strains and culture conditions.
The wild-type strain used in these experiments and from which all the mutants were derived was the stock d4-2 of P. tetraurelia (34). sm19-1 was isolated after mutagenesis as a thermosensitive mutation resulting in a progressive reduction of the cell size throughout divisions at the nonpermissive temperature (35°C), and this phenotype was shown to result from defective basal body duplication (29). From the same mutagenesis, another allelic mutation, sm19-2, was isolated. The recessive mutation nd7 (33), which prevents trichocyst exocytosis, was used as a genetic marker in crosses. nocr1 is a semidominant mutation conferring resistance to nocodazole (37; P. Dupuis-Williams, C. Klotz, and J. Beisson, 37th American Society for Cell Biology Annual Meeting, Washington, D.C., 1997). Cells were grown in buffered wheat grass powder (Pines International Co.) infusion containing 0.4 μg of β-sitosterol (Merck) per ml, inoculated the day before use with Klebsiella pneumoniae according to the usual procedures (35). Culture temperature was 28°C or 35°C. Nocodazole (from Jansen Life of Science Product Piscataway, N.J.) was prepared as a stock solution (8 × 10−3 M) in dimethyl sulfoxides, kept at −20°C, and diluted in culture medium to the desired concentration (4 × 10−6 to 8 × 10−6 M) before use. Dimethyl sulfoxide at the same final concentration (0.1%) was added to control cultures. Taxol, kindly provided by D. Guénard, was prepared as a stock solution (5 × 10−3 M) in dimethyl sulfoxide kept at −20°C and diluted in the culture medium to the final concentration (2.5 × 10−6 M) before use.
Mutagenesis and selection of suppressors.
A total of 1.2 × 106 exponentially growing sm19-1 cells competent for autogamy were irradiated by UV (400 J/m2 for 80 s). After mutagenesis, autogamy was induced by starvation. This leads to the breakdown of the old macronucleus and to the formation of new micro- and macronuclei homozygous for all their genes, so that ex-autogamous cells can express the mutations that have been induced. In this experiment, the irradiated cells were distributed into two batches and refed to allow two divisions before autogamy. On a sample of isolated single autogamous cells, the percentage of lethality was estimated to be 30%. Each batch was distributed into 20-ml tubes and also into 500-ml flasks before testing their ability to grow at 35°C, which was the selective test. In each tube or flask, normal-size surviving cells were isolated and the corresponding clones were retested for the ability to grow at 35°C. Clones consistently growing at 35°C were retained as revertant lines.
Genetic analysis.
Genetic analysis was carried out according to standard procedures (35). Each revertant (R) was crossed with a wild-type strain carrying the nd7 marker to ascertain reciprocal genetic exchange at conjugation. For each cross, 20 to 30 pairs of conjugants were isolated, the exconjugants were separated, and the phenotype of the corresponding F1 clones was studied to ascertain heterozygosity. After autogamy, which involves a meiotic reassortment of the parental genes and restores homozygosity at all loci, 30 ex-autogamous F2 clones from each exconjugant of two pairs of selected F1 clones were studied for segregation of the parental genes. Then, to identify the clones homozygous for the suppressor genes, F2 clones were backcrossed with the sm19-1 parent (see Results). Allelism among the different suppressors was tested by carrying out all possible R × R crosses.
Measurement of cell length.
Cells were gathered in a drop of culture fluid and fixed by addition of a drop of Dippell's stain (9), a rapid technique previously shown to preserve the in vivo dimensions (Beisson and Rossignol, unpublished observation). Length was measured with an ocular micrometer adapted on a Zeiss light microscope at low magnification without a coverslip.
Sequencing methods and sequence alignment.
Macronuclear DNA was purified as previously described (10) and subjected to restriction by BglII, which allows individualization of the three β-tubulin genes β-PT1, β-PT2, and β-PT3 into fragments whose respective sizes are 3.6, 4, and 6 kb. After electrophoresing the restricted DNA on an agarose gel, the corresponding bands were excised, the DNA fractions were extracted on Ultrafree DA columns from Millipore (Bedford, Mass.), and subjected to PCR amplification with primers B02 (AATCCTTTTTCGTAACCGTCAC) and Bfin (GTTGTGATAAAAATCACTTAGATTA), which delimit the coding sequences of the three genes. The three PCR products were individually purified on S300 columns from Pharmacia to remove the amplification primers and either directly sequenced or cloned in a pBlueScript vector (Stratagene). The three genes of the wild-type and each mutant strain were sequenced on both strands either from the whole PCR amplification or after cloning, with primers designed from the β-PT1 sequence to generate overlapping sequences. The sequencing products were analyzed with an ABI 310 from Applied Biosystems.
The sequences of porcine and Paramecium tubulins were compared with ClustalW 1.8 (36) and Dialign 2.1 (22) and then adjusted manually.
Paramecium β-tubulin modeling.
A model of P. tetraurelia β-tubulin was constructed by homology. The modeling was carried out with the program Modeller (32). The sequence of pig β-tubulin being the closest sequence found in the Protein Data Base (3), its coordinates were used as the reference (entry 1TUB) (24). The structure stops at V440, since the C-terminal residues (E441 to Y451) are disordered in the crystal and were not given, and thus our model lacks the last 15 residues from A428 to Q442.
RESULTS
The two allelic mutations sm19-1 and sm19-2 specifically cause defective basal body duplication (29). The SM19 gene was cloned by complementation of the sm19-1 mutation: it encodes a tubulin, designated η-tubulin, in view of its low homology with each of the other five tubulins, α to ɛ, characterized in P. tetraurelia. The sm19-1 mutation leads to a 22-amino-acid insertion in the N-terminal part of the protein, and the sm19-2 mutation leads to a Y470C change in the C-terminal part of the protein (30). Both mutants have a wild-type phenotype at the permissive temperature (28°C), and the mutational defect is expressed only at the nonpermissive temperature.
In order to analyze the function of η-tubulin, we first attempted to inactivate the SM19 gene with the method developed for P. tetraurelia (31), which had been used successfully to silence γ-tubulin (28), δ-tubulin (16), and ɛ-tubulin (11). Microinjection of the coding sequence of the SM19 gene at high copy number had no detectable effect on the growth and phenotype of wild-type cells, whether at the permissive or nonpermissive temperature (data not shown).
Rescuing effect of taxol on the mutant phenotype.
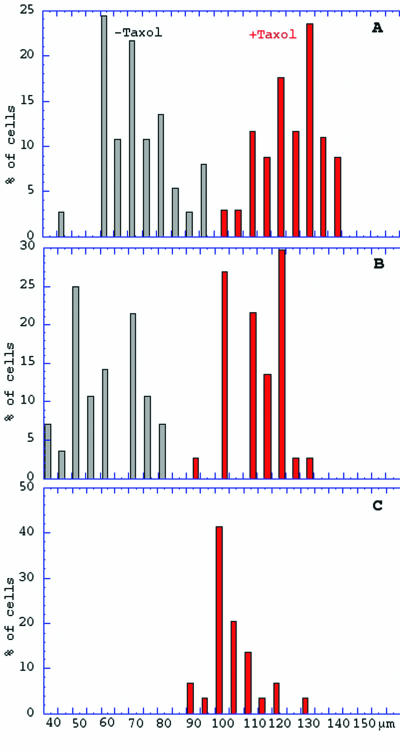
The abnormal presence of aggregates of γ-tubulin-containing material in the cytoplasm of the sm19-1 cells at the nonpermissive temperature suggested a possible interaction of η-tubulin with γ-tubulin and/or microtubular structure. In order to explore this hypothesis, we tested the effect of the microtubule-stabilizing drug taxol on the sm19-1 and sm19-2 strains at 35°C. At a dose of 2.5 × 10−5 M, which does not significantly affect wild-type cell growth, sm19-1 and sm19-2 cells were rescued and able to divide almost normally at 35°C. Figure 1 compares sm19-1 cell survival and sizes after 1, 3, and 10 divisions in the presence and in the absence of taxol. Similar results were obtained with sm19-2 cells. After three divisions (Fig. 1B), the taxol-treated cells remained significantly larger than the control sm19-1 cells without taxol. After 10 divisions (Fig. 1C), when the control cells had died, the taxol-treated sm19-1 cells still had a mean cell length of ca. 100 μm, while wild-type cells maintained a mean length of ca. 130 μm in the presence or in the absence of taxol (not shown). The phenotypical rescue observed supported the hypothesis that η-tubulin interacts with microtubular structure.
FIG. 1.
Effects of taxol on the growth of sm19-1 cells at 35°C; 100 individual sm19-1 cells were transferred from 28°C to 35°C in the presence or in the absence of taxol. Samples of 50 cells were fixed and measured after 1, 3, or ca. 10 divisions (A, B, and C, respectively). The mean lengths were compared by calculation of the error on the estimation of the mean at a 5% risk level. The means are 71.7 ± 11.6 without taxol and 123.6 ± 10.3 with taxol (A), 60.1 ± 11.8 without taxol and 111.4 ± 9.4 with taxol (B), and 104.5 ± 6 with taxol (C). The graphs (red for added taxol, black for control without taxol) show the percentage of cells in each length interval, from 40 to 150 μm. One can note a significantly larger size of mutant cells in the presence of taxol, although slightly smaller than that of wild-type cells, and the maintenance of a significant rescue of the phenotype at around 10 divisions, when control sm19-1 cells in the absence of taxol have long been dead. Wild-type control cells under the same conditions were also measured after 3 and ca. 10 divisions.
Selection of sm19-1 suppressors.
In order to identify η-tubulin-interacting proteins, we searched for extragenic suppressors of the sm19-1 mutation. Owing to their defective basal body duplication, sm19 cells grown at the nonpermissive temperature become smaller and smaller and die within 48 h: revertants able to multiply at 35°C can be easily screened. After UV mutagenesis of an sm19-1 population (see Materials and Methods), seven revertant lines named R2, R3, R7, R25, R35, R36, and R37 were isolated. Genetic analysis showed that they all carried an extragenic suppressor mutation and that all the suppressors appeared phenotypically wild type under the standard conditions of growth and observation. Crosses between revertant strains led to four loci named su1 (two alleles), su2 (two alleles), su3 (one allele), and su4 (two alleles).
Biochemical data obtained with R3 (see below) led us to focus on the su3-1 mutation. In the F2 progeny issued from the R3 × wild type cross, homozygous su3-1 clones were phenotypically wild type and thus indistinguishable from SU3 sister clones. Six of these wild-type phenotype F2 clones were back-crossed to the sm19-1 mutant. Those giving rise, in their F2 progeny, to 25% homozygous sm19-1 clones carried the su3-1 mutation and were used for further studies. In particular they were crossed by the sm19-2 strain. Interestingly, 50% of the F2 progeny issued from these crosses exhibited a wild-type phenotype, showing that the su3-1 mutation did not suppress the sm19-2 phenotype and was therefore an allele-specific suppressor of the mutation sm19-1. Other suppressors, like su2-2, from revertant R37, were active on both sm19-1 and sm19-2 alleles (data not shown).
Characterization of the su3-1 mutation.
Biochemical analyses were conducted on the suppressor strains and their content in tubulin isoforms was analyzed on two-dimensional electrophoregrams of postmicrosomal supernatants. An additional spot was detected among the β-tubulins of the R3 revertant (not shown) similar to that due to the nocr1 mutation localized in the β-PT3 gene (accession number AJ608919) (P. Dupuis-Williams, C. Klotz, and J. Beisson, 37th American Society for Cell Biology Annual Meeting, Washington, D.C., 1997; Dupuis-Williams et al., unpublished data). The patterns of α-tubulin isoforms from all the revertants were identical to those of wild-type and sm19-1 cells. The presence of the same supernumerary spot in cellular extracts from both the R3 revertant line and the isolated F2 su3-1 strain confirmed that the modified tubulin isoform correlated with the mutation and suggested that one of the three β-tubulin genes was affected in these strains. Crosses of nocr1 × su3-1 were carried out and showed that the two mutations segregated independently. Sequencing of the three β-tubulin genes from the su3-1 strain revealed a G to A transversion in the β-PT2 gene (accession number AJ608918), leading to a substitution for K of the E288 residue which is conserved in the β-tubulin family. This change is consistent with the less acidic position of the supernumerary spot observed in su3-1 extracts.
Genetic interactions between the su3-1, nocr1, and sm19 mutations.
The suppression of the sm19-1 phenotype by the su3-1 mutation and the fact that this effect is allele specific suggest an interaction between η-tubulin and β-tubulin. Further supporting evidence was obtained by phenotypic analysis of the double-mutants sm19-1 nocr1, sm19-2 nocr1, and su3-1 nocr1. The mutation nocr1 was shown to interact with the two alleles sm19-1 and sm19-2. While both types of double mutants displayed a nocodazole resistance similar to that of the nocr1 parent, at the nonpermissive temperature, double mutant cells exhibited an enhanced size reduction, due to a stronger inhibition of basal body duplication, compared to the sm19-1 or sm19-2 cells (Table 1). This revealed a negative interaction, direct or indirect, between η-tubulin and the product of the β-PT3 gene mutated in nocr1 cells.
TABLE 1.
Interaction between the sm19-1 and nocr1 mutationsa
| Clone | Mean length (μm) | Standard deviation | No. of cells |
|---|---|---|---|
| sm19-1 5c | 93 | 9 | 45 |
| sm19-1 3c | 107 | 10 | 45 |
| sm19-1 nocr1 3b | 66 | 12 | 45 |
| sm19-1 nocr1 5b | 73 | 9 | 45 |
The genotype of F2 clones issued from the cross sm19-1 nocr1 was identified on the basis of nocodazole resistance on sensitivity and of thermoresistance or sensitivity. sm19-1 and sm19-1 nocr1 clones were grown in parallel at 35°C, and cell lengths were measured after 5 to 6 divisions at the nonpermissive temperature. For two clones of each genotype, the values indicate the mean cell length (estimated at a 5% risk level), which was significantly smaller for the double mutant.
Since the effects of mutations in η-tubulin are modulated by mutations in both β-PT2 and β-PT3, some interaction could be expected between the su3-1 and the nocr1 mutations. Although both su3-1 and nocr1 cells display a normal growth rate at all temperatures, the double mutant su3-1 nocr1 cells show occasional morphological anomalies at 35°C; most importantly, the nocodazole resistance associated with the nocr1 mutation is suppressed in the double mutant su3-1 nocr1. At a nocodazole concentration of 2 × 10−6 M, which allows nocr1 cells to grow normally, double mutant cells undergo only a few divisions and display morphological abnormalities. This indicates that these two mutations interact genetically and that the products of the β-PT2 and β-PT3 genes coassemble into the same microtubular structures, as is likely since the three β-tubulin genes identified in P. tetraurelia encode identical β-tubulin isotypes (10). In both nocr1 and su3-1 cells, the coassembly of wild-type and mutant β-tubulin results in functional microtubules and no particular phenotype. Only in the presence of nocodazole, the resistance exhibited by the nocr1 strain reveals that incorporation of the mutated protein probably enhances microtubule stability. Conversely, the reduced resistance of the double mutant su3-1 nocr1 indicates that the coassembly of the two mutated β-tubulins results in a functional modification of the microtubular structure.
Localization of su3-1 and nocr1 mutations on the β-tubulin three-dimensional structure.
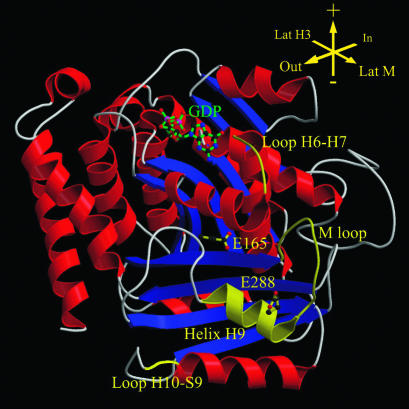
To examine how the mutations could affect β- and η-tubulins, we localized the su3-1 and nocr1 mutations on the currently available three-dimensional structural model of β-tubulin (24) and developed a predicted model for η-tubulin structure.
Figure 2 compares the β-tubulin sequences from P. tetraurelia (10) with pig α- and β-tubulins. The su3-1 mutation substitutes a lysine for the glutamic acid at position 288, which is well conserved in the β-tubulin family. On the currently available three-dimensional model (24), this conserved residue maps on the H9 helix located in the M-loop involved in interprotofilament contacts in the microtubular structure (Fig. 3). Its mutation could result in modification of tubulin-tubulin interactions. This assumption is supported by previous experimental data. The B2t8 male sterile mutation in D. melanogaster corresponds to the same E288K substitution and causes the formation, in homozygous mutant males, of aberrant microtubules, where sheets of protofilaments show S-curved cross-sections rather than closed microtubules, suggesting that the mutation impairs some interprotofilament bonds (15). The mutated tubulin is nevertheless able to coassemble with the wild-type protein to form normal microtubules in male heterozygotes. The same substitution in the unique β-tubulin gene of S. cerevisiae does not affect microtubule functions, but induces benomyl sensitivity (27), suggesting that modified interprotofilament contact might reduce microtubule stability. It is then reasonable to assume that the su3-1 substitution in P. tetraurelia also destabilizes the microtubule by weakening interactions between protofilaments.
FIG. 2.
Localization of the su3-1 and nocr1 mutations on the primary sequence of the Paramecium β-tubulin. Alignment of P. tetraurelia β-tubulin (beta-PT) with pig α- and β-tubulins is presented. Residues are numbered according to their position in each molecule. The substituted residues in su3-1 (E165) and nocr1 (E288) are marked by black boxes.
FIG. 3.
Localization of the su3-1 and nocr1 mutations on a three-dimensional model of P. tetraurelia β-tubulin.
The nocr1 mutation, which probably increases microtubule stability because it confers nocodazole resistance, yields an E165K substitution (Dupuis-Williams et al., unpublished data). Residue 165 maps in one of the alternated parallel β sheets, S5, constituting the GTP-binding region. On the recently refined structure (21), the homologous residue in pig β-tubulin (numbered Asn167) is expected to play, via its interaction with residues 200 and 202, an essential role in the structure and stability of the monomer and consequently of the microtubule, as demonstrated in Antarctic fish (8). Thus, it appears likely that the replacement in the β-tubulin of P. tetraurelia of glutamic acid 165 (homologous to the residue referred to as β:167 in pig tubulin; see Fig. 2) by a lysine, with a longer and a differently charged lateral chain, could alter the overall structure of the β-tubulin monomer and consequently interactions with molecular partners. Accordingly, since the E165K substitution is accompanied by a nocodazole-resistant phenotype in P. tetraurelia, it can be postulated that this conformational change could reduce the dynamics of the microtubule by modifying some tubulin-tubulin contacts. The fact that both the su3-1 and nocr1 mutations could modify physical interactions between tubulins in the microtubule is consistent with our observation that these mutations interact.
Localization of sm19-1 and sm19-2 mutations on a predicted structure of η-tubulin.
The establishment of the three-dimensional structures of heterodimeric tubulin (24) and of the microtubule (23) allowed us to localize on each α- and β-tubulin molecule the residues involved in lateral (H3 and ML surfaces) and longitudinal (plus end and minus end) contact between protofilaments. Based on sequence comparisons and on the assumption of a conserved three-dimensional architecture, predicted structural models of δ- and ɛ-tubulins have been proposed suggesting that these molecule could associate with some microtubular structure via tubulin-tubulin contact (19). We used the same strategy to model η-tubulin.
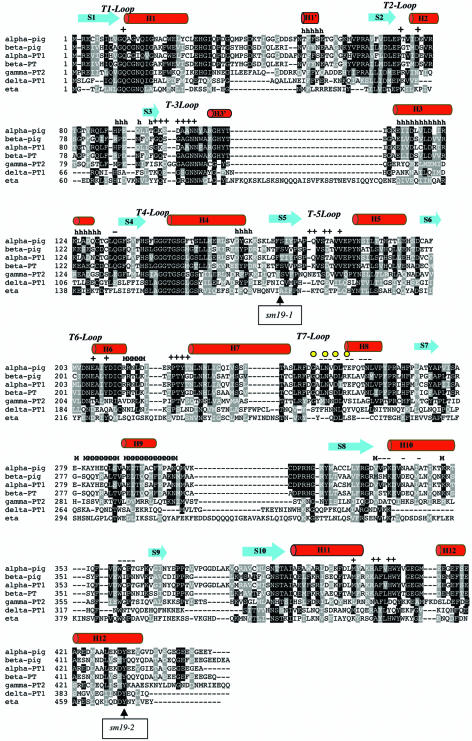
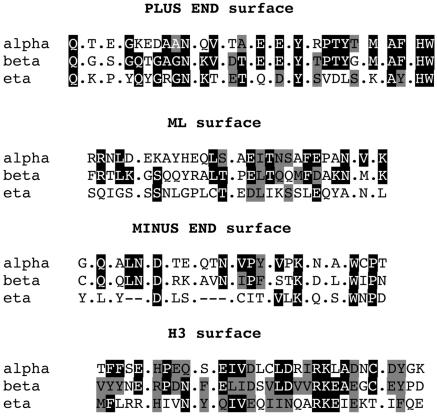
Figure 4 presents the alignment of the η-, α-, β-, γ-, and δ-tubulins of P. tetraurelia with pig α- and β-tubulins, whereas the alignments of the amino acids possibly located in the H3 (31 amino acids), ML (26 amino acids), plus end (27 amino acids), and minus end (26 amino acids) surfaces in the α, β, γ, and η molecules of P. tetraurelia (B) are presented in Fig. 5. As already noticed (19), the amino acids possibly involved in the longitudinal contacts appear more conserved than those involved in lateral contacts. Although some insertions and deletions are observed in the sequence of the η-tubulin, the residues corresponding to the structural elements of the tubulins are conserved in this molecule.
FIG. 4.
Sequence alignments of pig α- and β-tubulins and P. tetraurelia α (alpha-PT1), β (beta-PT), γ (gamma-PT2), δ (delta-PT1), and η (eta) tubulins. Residues are numbered according to their position in each molecule. Secondary structural elements observed in pig α- and β-tubulins are noted: helix in red (H) and strands in blue (S). The loops are also indicated. Identical residues are residues are boxed. Amino acids involved in tubulin-tubulin contacts in pig tubulins are indicated as follows: +, residues involved in contact at the plus end surface; −. residues involved in contact at the minus end; M, residues involved in contact at the ML surface; and H, residues involved in contact at the H3 surface. Yellow dots indicate the conserved GXXNXD motif. The localization of the mutations affecting the sm19-1 and sm19-2 alleles are indicated by arrows.
FIG. 5.
Comparison of residues possibly involved in tubulin-tubulin contact at ML, H3, plus-end, and minus-end surfaces in P. tetraurelia α-, β-, and η-tubulins as deduced from our alignments with pig α- and β-tubulins. Identical and similar residues are boxed in black and grey, respectively. Dots between residues indicate that homologous residues are missing in the primary sequence.
Two large insertions occur after the conserved GNNWAXG domain between the H3′ and H3 helix and in the loop connecting the H9 helix to the S8 sheet. The latter region, which appears very divergent between the different tubulin subfamilies, is supposed to be involved in the binding of proteins specific to each subfamily. Only two, three, and seven residues are inserted before the S3 sheet near residues involved in lateral contact, in the H6-H7 loop, and in the core of the H7 helix, respectively. As in α-, γ-, and δ-tubulins, several amino acids are inserted in the S9-S10 loop and mask the taxol-binding site in β-tubulin (2, 24). A part of the loop connecting H1 and S2, which is not conserved between the tubulin families, is deleted in η-tubulin. This deletion covers a great part of the loop connecting helix H1 to H1′, which was recently revealed in the refined structure of β-tubulin (21).
As already mentioned for δ-tubulin, we observed a great divergence in the T7 loop of η-tubulin and those of the other tubulin subfamilies. The consensus GXXNXD motif which, in α- and β-tubulins, comes into close contact with the γ-phosphate of the nucleotide belonging to the following tubulin subunit (24), is absent in η-tubulin and is not replaced by the YXXN-P which seems to be conserved in δ-tubulins. In addition, the residue homologous to the E254 which, in α-tubulin, is essential for the GTP hydrolysis step accompanying formation of the heterodimer (7), is replaced by a serine. These two observations indicate that η-tubulin is probably unable to catalyze the GTP hydrolysis accompanying the tubulin-tubulin dimerization process. This supports the assumption that, like δ-tubulin, η-tubulin is not capable of longitudinal self-assembly to form a protofilament-like oligomer.
As previously mentioned for δ-tubulin, several residues corresponding to amino acids at the minus end surface of α- and β-tubulins are missing, suggesting that η-tubulin is unable to interact at this surface with another tubulin via an α/β-tubulin-like contact. On the contrary, the residues corresponding to the plus end of the molecule, which would putatively interact with the minus end of an α/β tubulin dimer, show a significant amount of conservation, suggesting that this type of contact does exist. All these observations suggest that the η-tubulin is able to contact another tubulin at least at its plus end surface and support the assumption that it could be a minus end microtubule associated protein.
Localization of the η-tubulin mutations on the predicted model.
In η-tubulin, the sm19-1 and sm19-2 mutations lead to an insertion of 22 amino acids and to the replacement of the tyrosine 470 with a cysteine, respectively (30). We mapped these two mutations onto our model of η-tubulin. As shown in Fig. 4, the sm19-1 insertion disrupted a region corresponding to the S5 strand in the β-tubulin structure located in the first part of the protein corresponding to the GTP-binding domain. Recently (21), it was suggested that the robustness of the bonds connecting the neighboring strands located in this GTP binding domain of the β-tubulin play an important part in the conformation of the protein. It can thus be speculated that the insertion in sm19-1p might induce a conformational change of the η-tubulin molecule. However since sm19-1p is fully functional at 28°C, it appears that the structural defect is negligible at this temperature and develops only at high temperature (35°C).
The Y470C change in the sm19-2 mutation affects an amino acid which is conserved in the different tubulin families. The substitution of the corresponding residue in β-tubulin led A549 cells to become resistant to several stabilizing agents, suggesting that this residue is essential for microtubule stability (18). This tyrosine is located at the end of a potential helical structure corresponding to the H12 helix in α- and β- tubulins which was shown to be a part of the binding site of several microtubule-associated proteins (MAPs) (1, 17, 20). The conservation of this helix in η-tubulin suggests that, as it was already supposed for the γ-, δ-, and ɛ-tubulins (19), this tubulin may have maintained some binding surface for related motors. The sm19-2 mutation could thus modulate the affinity of η-tubulin for other partners besides tubulins and microtubules.
DISCUSSION
The SM19 gene, which encodes η-tubulin (30), is specifically involved in basal body duplication: thermosensitive sm19-1 and sm19-2 mutants, when grown at the nonpermissive temperature, can undergo a few divisions but yield cells more and more reduced in size, with a decreasing number of basal bodies but no apparent defect in other microtubule arrays (30). Under these conditions, a delocalization of γ-tubulin was observed, suggesting an interaction between η- and γ-tubulin and a role of η-tubulin as part of a complex specifically involved in basal body duplication (30). The data presented here provide genetic, physiological, and structural arguments for an interaction of η-tubulin with microtubules. A role of the other tubulins (δ and ɛ) in basal body/centriole duplication has also been documented (4, 11, 13), but their mode of action remains unknown. Among these basal body-specialized tubulins, η-tubulin is therefore the first with identified partners.
η-Tubulin interacts with β-tubulins: genetic evidence.
This interaction is demonstrated by several lines of evidences. First, a genetic interaction between η-tubulin and β-tubulin was revealed by the characterization of an extragenic suppressor of the sm19-1 mutation, su3-1, shown here to correspond to an E288K substitution in β-PT2, one of the three β-tubulin genes of P. tetraurelia (10). Furthermore, a direct interaction is suggested by the fact that su3-1 is an allele-specific suppressor, inactive on the sm19-2 mutation. Second, other genetic evidence of interactions between η-tubulin and β-tubulin was obtained by crossing the sm19-1 and sm19-2 strains with the nocr1 mutant, carrying a mutation in a different β-tubulin gene, β-PT3 (P. Dupuis-Williams, C. Klotz, and J. Beisson, 37th American Society for Cell Biology Annual Meeting, Washington, D.C., 1997); the double mutants displayed an aggravated phenotype at the nonpermissive temperature. Third, the loss of nocodazole resistance in the double mutant su3-1 nocr1 indicated an interaction between the two corresponding β-tubulins, which therefore are coexpressed and coassemble. The fact that η-tubulin interacts with each of the two β-tubulins suggests that it interacts with a microtubular structure. Finally, in further support of an interaction between η-tubulin and microtubules, we showed that taxol, known to stabilize microtubules, restores a sub-wild-type phenotype in sm19-1 and sm19-2 cells at 35°C.
The effect of taxol by itself could mean that any factor increasing microtubule stability might suffice to compensate for the deficiency of the mutant proteins, without implying a direct η/β molecular interaction. However, this interpretation is contradicted by the genetic data. First, the nocr1 mutation, which confers nocodazole resistance and therefore is likely to increase microtubule stability, has an aggravating rather than a rescuing effect on the mutant phenotype. Second, the su3-1 mutation corresponding to an E288K substitution known to rather destabilize microtubules, specifically rescues the sm19-1 mutation. Neither interaction can therefore be explained by the sole dynamic state of the microtubules, and both argue for a direct interaction between η- and β-tubulin. Finally, the structural likelihood of a direct interaction between η- and β-tubulin was examined. Using the approach developed to predict interactions between microtubules and γ-, δ-, and ɛ-tubulins (19), we proposed a sequence alignment allowing some structural inferences. The most significant features of η-tubulin, the absence of a residue essential for GTP hydrolysis and the singularity of the region potentially involved in longitudinal contact at the minus end, suggest that η-tubulin, like γ- and δ-tubulins, might be a microtubule minus-end capping molecule. However, like these tubulins, η-tubulin seems capable of lateral interactions with other tubulins.
In summary, it can be concluded that η-tubulin interacts, through η/β contacts, with some microtubular structure specific to the basal body or specifically involved in its assembly, which remains to be identified.
Possible structural basis of the η/β interactions.
The nature of the interacting mutations in β- and η-tubulins which could induce conformational changes of these molecules and thus modulate tubulin-tubulin contact also argues for a direct interaction between the two molecules. The mutation su3-1, which specifically suppress the sm19-1 mutation, localizes in helix H9 at the ML surface (Fig. 3), a region involved in lateral contacts between β-tubulins along the microtubule (23). The same substitution destabilizes microtubules and results in defective microtubule assembly in Drosophila melanogaster (15) or benomyl sensitivity in S. cerevisiae (27). The mutation nocr1 localizes at a site involved in a bond important for monomer stability during GTP hydrolysis (21) and, because it confers nocodazole resistance, it could be expected to increase microtubule stability by modifying tubulin-tubulin interactions.
The mutations sm19-1 and sm19-2 themselves are localized in regions corresponding to the S5 strand of the GTP-binding domain and to helix H12, respectively, two regions which could influence the stability of tubulin/tubulin interfaces, directly or through interactions with associated proteins. The main structural features of α- and β-tubulin being conserved in η-tubulin, it can be assumed that the sm19-1 and sm19-2 mutations are able to induce some conformational change of the η-tubulin.
The conclusion that η- and β-tubulins interact through lateral contacts is perfectly consistent with the observed rescue effect of the sm19-1 and sm19-2 phenotypes by taxol, known to influence microtubule stability through lateral contact modifications (23).
η-Tubulin might interact with other tubulins at least at three surfaces (plus end, H3, and ML surface), and this conformational change could modify interaction not only with β-tubulin but also with another tubulin. In particular, a modification of such tubulin-tubulin interaction between η- and γ-tubulin could explain the delocalization of the γ-tubulin observed at the nonpermissive temperature in the sm19 mutants (30). This hypothesis is compatible with the predicted structure of γ-tubulin, which could also contact multiple tubulin partners (19).
η-Tubulin and basal body duplication.
The genetic interaction observed between the sm19-1 allele and two mutations in β-tubulin which are both possibly involved in stability of the microtubule suggests that η-tubulin could regulate the dynamic behavior of some microtubular structure. Support for this hypothesis comes from the observation that the sm19-1 and sm19-2 mutations are expressed at 35°C, a temperature known to reduce this dynamics. The possible involvement of tubulin associated with the minus end of the microtubule in governing microtubule dynamics has been evoked previously (12) to explain the taxol hyperresistance conferred by the UNI3 mutation in the δ-tubulin of Chlamydomonas reinhardtii. It was also first suggested when γ-tubulin was discovered as an extragenic suppressor of a β-tubulin mutation which hyperstabilized the microtubules (25). The involvement of γ-tubulin in microtubule dynamics is now supported by a recent study which shows that a mutation in the γ-tubulin gene which does not affect microtubule nucleation perturbs chromosome segregation and cytoplasmic microtubule arrays in Schizosaccharomyces pombe (26).
Based on the observation that the phosphorylated state of γ-tubulin could also increase the numbers and change the dynamic properties of the microtubules in S. cerevisiae (40), several hypotheses were advanced to explain this regulation at the molecular level (38). Among them, one postulated that interaction between γ-tubulin and the heterodimeric tubulin could impose some conformational change at the minus end of the microtubule which could be transmitted from the minus end onward toward the dynamic plus end. Such a mechanism could also explain the regulation of the dynamics of some microtubule arrays by other tubulins possibly located at their minus ends and particularly the regulation of some microtubule structure involved in basal body duplication by η-tubulin.
A possible role in regulating microtubule dynamics thus seems to be a common feature of several divergent microtubule-associated tubulins. The diversity of these tubulins could thus reflect the diversity of the microtubular structures and the complexity of their regulation. In P. tetraurelia, at least 15 different microtubular networks were identified (6), which could explain the great diversity of the tubulins already discovered in this organism. No homologs of η-tubulin have been found in the human genome, but it cannot be excluded that one of the various unidentified divergent tubulins present in this genome and not yet functionally characterized can fulfill the function of η-tubulin.
In summary, our data support the conclusion that η-tubulin interacts directly with microtubules. If one takes into account that η-tubulin may also interact with γ-tubulin and essentially plays a specific role in basal body duplication, and since it does not seem to interact with any other microtubule arrays, the following hypothesis can be put forward. Considering that γ-tubulin is required for basal body duplication (28), the function of η-tubulin might be to tether γ-tubulin-containing complexes to the sites of basal body duplication, where it might either stabilize nascent microtubules in developing basal bodies or transduce a signal for basal body duplication.
Acknowledgments
We thank J. Cohen for helpful discussion and critical reading of the manuscript and Marine Froissard and Anne Marie Keller for constant moral support.
This work was supported by grant 5425 from the Association pour la Recherche contre le Cancer.
REFERENCES
- 1.Al-Bassam, J., R. S. Ozer, D. Safer, S. Halpain, and R. A. Milligan. 2002. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J. Cell Biol. 157:1187-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amos, L. A., and J. Lowe. 1999. How taxol stabilises microtubule structure. Chem. Biol. 6:R65-R69. [DOI] [PubMed] [Google Scholar]
- 3.Berman, H. M., J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov, and P. E. Bourne. 2000. The Protein Data Bank. Nucleic Acids Res. 28:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, P., T. H. Giddings, Jr., M. Winey, and T. Stearns. 2003. Epsilon -tubulin is required for centriole duplication and microtubule organization. Nat. Cell Biol. 5:71-76. [DOI] [PubMed] [Google Scholar]
- 5.Chang, P., and T. Stearns. 2000. Delta-tubulin and epsilon-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nat. Cell Biol. 2:30-35. [DOI] [PubMed] [Google Scholar]
- 6.Cohen, J., and J. Beisson. 1988. The cytoskeleton, p. 363-392. In G. H. Dunbe (ed.), Paramecium. Springer-Verlag, Berlin, Germany.
- 7.Dai, K., A. Mukherjee, Y. Xu, and J. Lutkenhaus. 1994. Mutations in ftsZ that confer resistance to SulA affect the interaction of FtsZ with GTP. J. Bacteriol. 176:130-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detrich, H. W., III, S. K. Parker, R. C. Williams, Jr., E. Nogales, and K. H. Downing. 2000. Cold adaptation of microtubule assembly and dynamics. Structural interpretation of primary sequence changes present in the alpha- and beta-tubulins of Antarctic fishes. J. Biol. Chem. 275:37038-37047. [DOI] [PubMed] [Google Scholar]
- 9.Dippell, R. 1955. A temporary stain for Paramecium and other protozoa. Stain Technol. 30:60-71. [DOI] [PubMed] [Google Scholar]
- 10.Dupuis, P. 1992. The beta-tubulin genes of Paramecium are interrupted by two 27 bp introns. EMBO J. 11:3713-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupuis-Williams, P., A. Fleury-Aubusson, N. G. de Loubresse, H. Geoffroy, L. Vayssie, A. Galvani, A. Espigat, and J. Rossier. 2002. Functional role of epsilon-tubulin in the assembly of the centriolar microtubule scaffold. J. Cell Biol. 158:1183-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutcher, S. K. 2001. Motile organelles: the importance of specific tubulin isoforms. Curr. Biol. 11:R419-R422. [DOI] [PubMed] [Google Scholar]
- 13.Dutcher, S. K., N. S. Morrissette, A. M. Preble, C. Rackley, and J. Stanga. 2002. Epsilon-tubulin is an essential component of the centriole. Mol. Biol. Cell 13:3859-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutcher, S. K., and E. C. Trabuco. 1998. The UNI3 gene is required for assembly of basal bodies of Chlamydomonas and encodes delta-tubulin, a new member of the tubulin superfamily. Mol. Biol. Cell 9:1293-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller, M. T., J. H. Caulton, J. A. Hutchens, T. C. Kaufman, and E. C. Raff. 1987. Genetic analysis of microtubule structure: a beta-tubulin mutation causes the formation of aberrant microtubules in vivo and in vitro. J. Cell Biol. 104:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garreau De Loubresse, N., F. Ruiz, J. Beisson, and C. Klotz. 2001. Role of delta-tubulin and the C-tubule in assembly of Paramecium basal bodies. BMC Cell Biol. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldsmith, M., L. Yarbrough, and D. van der Kooy. 1995. Mechanics of motility: distinct dynein binding domains on alpha- and beta-tubulin. Biochem. Cell Biol. 73:665-671. [DOI] [PubMed] [Google Scholar]
- 18.He, L., C. P. Yang, and S. B. Horwitz. 2001. Mutations in beta-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol. Cancer Ther. 1:3-10. [PubMed] [Google Scholar]
- 19.Inclan, Y. F., and E. Nogales. 2001. Structural models for the self-assembly and microtubule interactions of gamma-, delta- and epsilon-tubulin. J. Cell Sci. 114:413-422. [DOI] [PubMed] [Google Scholar]
- 20.Kikkawa, M., Y. Okada, and N. Hirokawa. 2000. 15 A resolution model of the monomeric kinesin motor, KIF1A. Cell 100:241-252. [DOI] [PubMed] [Google Scholar]
- 21.Lowe, J., H. Li, K. H. Downing, and E. Nogales. 2001. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 313:1045-1057. [DOI] [PubMed] [Google Scholar]
- 22.Morgenstern, B., A. Dress, and T. Werner. 1996. Multiple DNA and protein sequence alignment based on segment-to-segment comparison. Proc. Natl. Acad. Sci. USA 93:12098-12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogales, E., M. Whittaker, R. A. Milligan, and K. H. Downing. 1999. High-resolution model of the microtubule. Cell 96:79-88. [DOI] [PubMed] [Google Scholar]
- 24.Nogales, E., S. G. Wolf, and K. H. Downing. 1998. Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391:199-203. [DOI] [PubMed] [Google Scholar]
- 25.Oakley, C. E., and B. R. Oakley. 1989. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature 338:662-664. [DOI] [PubMed] [Google Scholar]
- 26.Paluh, J. L., E. Nogales, B. R. Oakley, K. McDonald, A. L. Pidoux, and W. Z. Cande. 2000. A mutation in gamma-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein pkl1p. Mol. Biol. Cell 11:1225-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Praitis, V., W. S. Katz, and F. Solomon. 1991. A codon change in beta-tubulin which drastically affects microtubule structure in Drosophila melanogaster fails to produce a significant phenotype in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:4726-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz, F., J. Beisson, J. Rossier, and P. Dupuis-Williams. 1999. Basal body duplication in Paramecium requires gamma-tubulin. Curr. Biol. 9:43-46. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz, F., N. Garreau de Loubresse, and J. Beisson. 1987. A mutation affecting basal body duplication and cell shape in Paramecium. J. Cell Biol. 104:417-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz, F., A. Krzywicka, C. Klotz, A. Keller, J. Cohen, F. Koll, G. Balavoine, and J. Beisson. 2000. The SM19 gene, required for duplication of basal bodies in Paramecium, encodes a novel tubulin, eta-tubulin. Curr. Biol. 10:1451-1454. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz, F., L. Vayssie, C. Klotz, L. Sperling, and L. Madeddu. 1998. Homology-dependent gene silencing in Paramecium. Mol. Biol. Cell 9:931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sali, A., and T. L. Blundell. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234:779-815. [DOI] [PubMed] [Google Scholar]
- 33.Skouri, F., and J. Cohen. 1997. Genetic approach to regulated exocytosis with functional complementation in Paramecium: identification of the ND7 gene required for membrane fusion. Mol. Biol. Cell 8:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonneborn, T. 1974. Paramecium aurelia. Plenum Publishing Corporation, New York, N.Y.
- 35.Sonneborn, T. M. 1970. Methods in Paramecium research. Methods Cell Physiol. 4:241-339. [Google Scholar]
- 36.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres, A., M. Rossignol, and J. Beisson. 1991. Nocodazole-resistant mutants in Paramecium. J. Protozool. 38:295-304. [Google Scholar]
- 38.Usui, T., and E. Schiebel. 2001. Regulating microtubule properties by modifying their organizing minus ends. Mol. Cell 8:931-932. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan, S., T. Attwood, M. Navarro, V. Scott, P. McKean, and K. Gull. 2000. New tubulins in protozoal parasites. Curr. Biol. 10:R258-R259. [DOI] [PubMed] [Google Scholar]
- 40.Vogel, J., B. Drapkin, J. Oomen, D. Beach, K. Bloom, and M. Snyder. 2001. Phosphorylation of gamma-tubulin regulates microtubule organization in budding yeast. Dev. Cell. 1:621-631. [DOI] [PubMed] [Google Scholar]