Abstract
We present a case of fever, brain abscesses, and Gemella morbillorum bacteremia after anti-tumor necrosis factor alpha (TNF-α) therapy in a 21-year-old acne inversa patient currently taking long-term dapsone. To the best of our knowledge, this is the first report describing such a case. During antimicrobial therapy, the patient developed systemic varicella infection with severe thrombocytopenia.
CASE REPORT
A21-year-old HIV-negative male patient with acne inversa (hidradenitis suppurativa) presented at the Dermatology Department of the Donauspital, Vienna, with reduced general condition (corresponding to a Karnofsky performance status [15] of 30%) and high fever of 2 weeks' duration. Eight weeks previously, he had undergone surgical treatment of fistulating and scarring lesions in the right axilla. He was taking oral dapsone as a long-term treatment for his underlying skin disease and in the past had received immunomodulating therapy with anti-tumor necrosis factor alpha (TNF-α) inhibitors as a treatment of last resort for his underlying medical condition, on the basis of several reports of successful treatment of acne inversa (4, 14). He had undergone four treatments with the monoclonal anti-TNF-α antibody infliximab (5 mg/kg body weight intravenously, last administration 14 months prior to admission) and etanercept (50 mg/week subcutaneously, 26 months prior to admission). At the time of admission, the patient was not receiving any immunomodulating or immunosuppressant medication.
Although leukocyte levels were not raised, the blood sedimentation rate was elevated (82 mm after 1 h; 107 mm after 2 h), and C-reactive protein (CRP) levels showed elevation to 133.9 mg/liter (normal range, 0 to 5 mg/liter). The initial clinical assessment revealed no site of infection or lesions for entry of bacteria. The patient was admitted to the Dermatology Department of the Donauspital, Vienna, because he was febrile (39°C) and showed significantly elevated parameters indicative of severe bacterial infection as described above. Antimicrobial treatment with 900 mg intravenous clindamycin 3 times daily was initiated by the attending physicians. Because the patient did not improve after 3 days on clindamycin, oral moxifloxacin was added, 400 mg once daily. In the meantime, the long-term acne inversa therapy with dapsone, 50 mg daily for 2 years, was stopped because of minor methemoglobinemia of 3.4% methemoglobin (normal range, <0.8%).
During the first week of hospitalization, a total of five blood culture sets (a set comprises one aerobic and one anaerobic bottle) were drawn. Seven days after admission, with CRP rising to 152.1 mg/liter and persisting fever, the fifth blood culture set was reported as positive after incubation for 24 h in a continuous-monitoring culture system (BacT/Alert; Becton-Dickinson, Organon Teknika, Durham, NC), with the aerobic and anaerobic bottles being positive simultaneously. Gram stain revealed Gram-positive cocci in clusters and short chains, resembling streptococci. After subculture on Columbia agar and chocolate agar, small colonies became visible after incubation for 48 h: they were catalase negative. The bacteria were identified as Gemella morbillorum by means of the Api 20 streptococcus and Api rapid ID STREP identification system (bioMérieux, Vitek Inc., Hazelwood, MO) with good confidence (95.3% and 94.7%). Routine antimicrobial susceptibility testing with disc diffusion on Mueller-Hinton agar with 5% sheep blood showed the organism to be resistant to clindamycin, ciprofloxacin, and gentamicin (Table 1). The MIC of penicillin was determined with the Etest and showed the bacteria to be sensitive at 0.016 mg/liter. Four blood culture sets drawn some days previously remained negative.
Table 1.
Disk diffusion antimicrobial susceptibility testing of Gemella morbillorum cultured from the blood of a patient with brain abscesses
| Antimicrobial agents | Sensitivitya |
|---|---|
| Penicillin | S |
| Aminopenicillin | S |
| Aminopenicillin-clavulanic acid | S |
| Cefazolin | S |
| Cefamandole | S |
| Cefotaxime | S |
| Gentamicin | R |
| Erythromycin | S |
| Clindamycin | R |
| Vancomycin | S |
| Teicoplanin | S |
| Doxycycline | S |
| Ciprofloxacin | R |
| Moxifloxacin | S |
S, sensitive; R, resistant.
This was the only time that blood cultures (one anaerobic and one aerobic blood culture bottle) were positive for bacterial growth. This was retrospectively at a time of insufficient antimicrobial therapy against the Gemella morbillorum according to the antimicrobial resistance profile. However, during the time of sufficient antimicrobial therapy against Gemella morbillorum, no additional blood culture was positive. The initial, unsuccessful treatment regimen with clindamycin and moxifloxacin was replaced by intravenous doripenem, 500 mg 3 times daily. In the following 5 days, inflammation parameters regressed. Diagnostic colonoscopy and multiple transthoracic cardiac sonographies after the positive blood culture result gave no information regarding the focus of G. morbillorum bacteremia. The patient had no apparent skin lesion that could have served as an entry point for the bacteria. Skin swabs of acne inversa lesions with mild signs of inflammation at the axilla and gluteal region before and during the inpatient stay were negative for G. morbillorum but were positive for group B and viridans streptococci at the axilla and for Proteus mirabilis, Pseudomonas aeruginosa, and Corynebacterium sp. at the gluteal region.
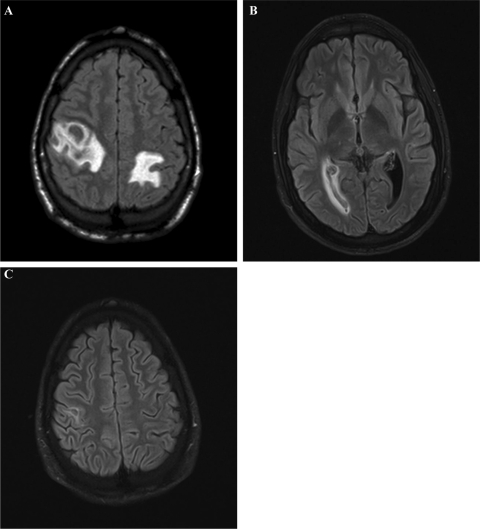
Two weeks after admission, the patient reported increasing headaches and paresthesia of the right arm. Magnetic resonance tomography (MRT) of the neurocranium showed multiple septic abscesses with a perifocal edema (Fig. 1A). The patient's overall condition worsened; therefore, treatment with intravenous dexamethasone (4 g, 3 times a day) was started, and the antimicrobial treatment was changed to intravenous linezolid, 600 mg 3 times a day, because of its superior tissue penetration. Despite this aggressive treatment, the general condition of the patient deteriorated further, necessitating his transfer to an intensive care unit (ICU) at Vienna General Hospital on day 17 after admission. At the ICU, the antimicrobial treatment with linezolid was continued with the addition of intravenous ampicillin (4 g, 3 times a day). That high-dose combination antimicrobial therapy resulted in substantial improvement in the patient's overall condition, allowing his transfer to the Division of Infectious Diseases and Tropical Medicine in the same hospital. The patient's headaches subsequently subsided, and his ability to move his right arm improved slowly.
Fig 1.
(A) MRT of the neurocranium 14 days after admission, showing prominent abscesses with perifocal edema; (B) MRT of the neurocranium 12 weeks after admission: slight periventricular edema due to a clot in the right posterior horn of the brain; (C) MRT of the neurocranium 12 weeks after admission: almost complete regression of the abscesses.
In week 4 after admission, about 1 week after his stay in the ICU and ongoing intravenous glucocorticoid and antimicrobial therapy, increasing lactate dehydrogenase, liver, and pancreatic enzymes were the prominent laboratory changes, without signs of systemic inflammation (CRP in normal range). The patient also developed pancytopenia, in particular a severe thrombocytopenia with a nadir platelet count of 28 × 109/liter. In the following days, he developed clinical symptoms, such as nausea and abdominal pain. Because of the clinical worsening, with an additional skin rash on the face and upper body, plus fever up to 39°C, linezolid was stopped on the basis of speculation of heavy linezolid-induced side effects. Shortly afterward, however, the patient showed the typical skin lesions of primary varicella zoster virus infection, proven indirectly by seroconversion and directly by PCR from serum (2.41 × 106 genome copies/μl) and cutaneous lesions. He was not vaccinated against varicella zoster virus in the past. The patient responded well to treatment with intravenous acyclovir (10 mg/kg body weight 3 times daily for 18 days) but developed PCR-confirmed segmental herpes zoster in the left C3 dermatome only 20 days after the last acyclovir dose. This was again treated with intravenous acyclovir (10 mg/kg body weight 3 times daily for 2 days) followed by 9 days of oral valacyclovir (1 g, 3 times a day). In addition, in week 4 postadmission, thrush was observed and responded well to topical treatment with amphotericin B solution.
Since multiple control MRTs showed only a slight improvement, approximately 6 weeks after the initial admission, the treatment with ampicillin was modified to include intravenous fosfomycin (8 g, 3 times a day). Following this change in medication, the brain abscesses showed a significant reduction, making an already planned brain biopsy unnecessary. However, the patient did not tolerate high-dose fosfomycin well, making it necessary to return to ampicillin monotherapy, ultimately leading to a recurrence of fever on day 57 after admission. Because of this development, the antimicrobial treatment was again modified to ampicillin plus intravenous cefotaxime (3 g, 3 times daily), leading to a swift recovery.
Sometime between weeks 6 and 12, the patient developed a clot in the right posterior horn of the brain, leading to a slight left shift of the septum pellucidum and a periventricular edema (Fig. 1B). However, since he did not develop neurologic symptoms, surgical intervention was not considered.
Although there had been an interval of at least 14 months between the last course of immunomodulating therapy and the onset of symptoms, the patient showed multiple clinical signs of immunocompromisation, as described.
In addition, flow cytometry analysis on a FACScan flowcytometer (BectonDickinson, Heidelberg, Germany) revealed subnormal counts for natural killer cells (5%; threshold, 6%) and B memory cells (2%; threshold, 3%) and reduced expression of complement receptor 3 (CR3; CD18/CD11b) on granulocytes (33% of normal values). Although these findings are not compelling evidence for immunocompromisation, they could offer an explanation for the multiple illnesses observed over a short period. Nevertheless, detected levels of immunoglobulin isotypes IgG, IgM, and IgA were within their normal serum ranges during the period overall.
On day 61, the patient developed a drug-induced fever, probably due to the prolonged administration of ampicillin. This required a final modification of the antimicrobial treatment to monotherapy with oral doxycycline, 300 mg per day, which was well tolerated by the patient. On day 71, the patient was discharged from our hospital with a Karnofsky performance status (15) of 100%, with almost complete regression of the abscesses (Fig. 1C), and without residual neurologic impairment. To further monitor the clot in the right posterior horn of the brain, regular computerized axial tomography scans were scheduled. At subsequent scheduled check-ups, the patient's neurologic status was normal.
Although definitive proof is lacking for G. morbillorum being the causative agent of the patient's brain abscesses, in light of the diagnostic findings, the preceding immunomodulating therapy, and after a thorough research of the available literature, we think that “brain abscesses caused by G. morbillorum, mediated by previous immunomodulating therapy,” is the most probable diagnosis.
G. morbillorum is a Gram-positive, catalase-negative, facultative anaerobic coccus. It can usually be found as a commensal organism on mucous membranes and is part of the standard oropharyngeal flora. There have been several reports of G. morbillorum as a causative agent in a number of infections, including endocarditis (1, 3, 11, 17, 20), septic abscesses (8, 10, 18), peritonitis (9), and brain abscesses or meningitis (2, 12, 13, 19). Often there is a definite entry point, such as dental defect, oral piercing, or gastrointestinal carcinomas (3, 5, 6, 7, 16). To the best of our knowledge, our case is the first bacteremia due to G. morbillorum that could be associated with anti-TNF-α as acne inversa therapy.
Footnotes
Published ahead of print 21 December 2011
REFERENCES
- 1. Al Chekakie MO, Heroux A, Montpetit M, Nemeh H. 2009. Gemella morbillorum prosthetic valve endocarditis. Congest Heart Fail. 15:291–292 [DOI] [PubMed] [Google Scholar]
- 2. Benedetti P, Rassu M, Branscombe M, Sefton A, Pellizzer G. 2009. Gemella morbillorum: an underestimated aetiology of central nervous system infection? J. Med. Microbiol. 58:1652–1656 [DOI] [PubMed] [Google Scholar]
- 3. Carano N, et al. 2010. Infective endocarditis following body piercing: Presentation of one case due to Gemella morbillorum and review of the literature. Med. Sci. Monit 16:CS124–128 [PubMed] [Google Scholar]
- 4. Cusack C, Buckley C. 2006. Etanercept: effective in the management of hidradenitis suppurativa. Br. J. Dermatol. 154:726–729 [DOI] [PubMed] [Google Scholar]
- 5. Estivals M, et al. 2007. [Necrotizing pneumonia due to Gemella morbillorum in an immunocompetent patient]. Rev. Mal Respir. 24:1143–1146 [DOI] [PubMed] [Google Scholar]
- 6. Famularo G, De Simone C, Minisola G, Nicotra GC. 2006. Pneumonia and sepsis caused by Gemella morbillorum: an unusual association. Intern. Med. 45:1253–1254 [DOI] [PubMed] [Google Scholar]
- 7. FitzGerald SF, Moloney AC, Maurer BJ, Hall WW. 2006. Gemella endocarditis: consider the colon. J. Heart Valve Dis. 15:833–835 [PubMed] [Google Scholar]
- 8. Garcia-Bordes L, et al. 2010. An unusual case of spondylodiscitis. Spine (Phila Pa 1976). 35:E167–171 [DOI] [PubMed] [Google Scholar]
- 9. Guney I, Isik A, Altintepe L, Er C, Kurdoglu MG. 2009. Gemella morbillorum peritonitis in a CAPD patient. Perit. Dial. Int. 29:674–675 [PubMed] [Google Scholar]
- 10. Hsu CY, Su YC, Wang TL, Chong CF, Chen CC. 2007. Gemella morbillorum liver abscess. Scand. J. Infect. Dis. 39:637–638 [DOI] [PubMed] [Google Scholar]
- 11. Hull JE. 2010. Multisystem organ failure due to Gemella morbillorum native valve endocarditis. Mil. Med. 175:923–925 [DOI] [PubMed] [Google Scholar]
- 12. Liberto MC, et al. 2006. An unusual case of brain abscess by Gemella morbillorum. Jpn. J. Infect. Dis. 59:126–128 [PubMed] [Google Scholar]
- 13. Lopes A, et al. 2007. Cerebellar abscess by Gemella morbillorum in a patient with inter-atrial communication. Arq. Neuropsiquiatr. 65:1022–1025 [DOI] [PubMed] [Google Scholar]
- 14. Mekkes JR, Bos JD. 2008. Long-term efficacy of a single course of infliximab in hidradenitis suppurativa. Br. J. Dermatol. 158:370–374 [DOI] [PubMed] [Google Scholar]
- 15. Mor V, Laliberte L, Morris JN, Wiemann M. 1984. The Karnofsky performance status scale. An examination of its reliability and validity in a research setting. Cancer 53:2002–2007 [DOI] [PubMed] [Google Scholar]
- 16. Raposeiras Roubin S, Cabarcos Ortiz De Barron A, Maldonado Bakovic AM, Barreiro Pardal C. 2010. [Hepatic abscesses in a sepsis context due to Gemella morbillorum: base for gastric adenocarcinoma diagnosis]. Rev. Clin. Esp. 210:146–147 [DOI] [PubMed] [Google Scholar]
- 17. Taimur S, Madiha R, Samar F, Bushra J. 2010. Gemella morbillorum endocarditis in a patient with a bicuspid aortic valve. Hellenic J. Cardiol. 51:183–186 [PubMed] [Google Scholar]
- 18. Valipour A, Koller H, Setinek U, Burghuber OC. 2005. Pleural empyema associated with Gemella morbillorum: report of a case and review of the literature. Scand. J. Infect. Dis. 37:378–381 [DOI] [PubMed] [Google Scholar]
- 19. Villegas E, et al. 2008. Meningitis by Gemella morbillorum with associated pituitary apoplexy: a case report. Eur. J. Intern. Med. 19:e101–102 [DOI] [PubMed] [Google Scholar]
- 20. Zakir RM, Al-Dehneh A, Dabu L, Kapila R, Saric M. 2004. Mitral bioprosthetic valve endocarditis caused by an unusual microorganism, Gemella morbillorum, in an intravenous drug user. J. Clin. Microbiol. 42:4893–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]