Abstract
Heteroresistance to antimicrobial agents may affect susceptibility test results and therapeutic success. In this study, we investigated heteroresistance to cephalosporins and penicillins in Acinetobacter baumannii, a major pathogen causing nosocomial infections. Two A. baumannii isolates exhibited heteroresistance to ampicillin-sulbactam, ticarcillin-clavulanic acid, cefepime, and cefpirome, showing a distinct colony morphology of circular rings within the inhibition halos. Pulsed-field gel electrophoresis (PFGE) and outer membrane protein (OMP) analysis demonstrated that subpopulations around the disks/Etest strips and the original strains all belonged to the same PFGE type and OMP profile. Population analysis profile (PAP) showed the presence of heteroresistant subpopulations with high cefepime resistance levels in two isolates (008 and 328). Interestingly, A. baumannii 008 contained two peaks: one was grown in the presence of up to 1 μg of cefepime/ml, the other apparently occurred when the concentration of cefepime was raised to 256 μg/ml. After serial passages without exposure to cefepime, the PAP curve maintained the same trend observed for the original strain of A. baumannii 008. However, the PAP curve showed a shift to relatively lower cefepime resistance (from 256 to 64 μg/ml) in A. baumannii 328 after 10 passages in antibiotic-free Mueller-Hinton agar plates. Convergence to a monotypic resistance phenotype did not occur. Growth rate analysis revealed that slower growth in resistant subpopulations may provide a strategy against antibiotic challenge. To our knowledge, this is the first report of heteroresistance to cephalosporins and penicillins in A. baumannii.
INTRODUCTION
Acinetobacter baumannii, a Gram-negative, glucose-nonfermentative coccobacilli, is a major cause of nosocomial infections such as bloodstream, urinary tract, and wound infections, and ventilator-associated pneumonia worldwide (8, 10, 11). Treatment of A. baumannii infection has been challenging because this pathogen is resistant to multiple classes of antimicrobial agents (8, 10, 11). β-Lactams, including penicillins and cephalosporins, and fluoroquinolones have poor activity against A. baumannii (8, 10). Recently, combination therapy has been found to be effective in treatment of A. baumannii infection (8, 10). However, the increasing incidence of multidrug-resistant (MDR) and pan-drug-resistant (PDR) A. baumannii isolates leads to limited therapeutic options (8, 10, 11).
Heteroresistance is defined as the occurrence of antimicrobial resistance where different subpopulations within a genetically homogeneous isolate exhibit various susceptibilities to antimicrobial agents (16, 22). Many studies have reported heteroresistance to methicillin, daptomycin, or vancomycin in staphylococci and enterococci (1, 5, 23), heteroresistance to colistin, carbapenems, or piperacillin-tazobactam in A. baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa (12–14, 18–21, 24), and heteroresistance to penicillin in Streptococcus pneumoniae (16). This indicates that this phenomenon is not rare and is considered to be a precursor stage, which may lead to the emergence of a resistant strain (6). Moreover, heteroresistance is believed to be a strategy of natural evolution to antimicrobial resistance, since it provides bacteria with an opportunity to explore growth in the presence of antibiotics before the acquisition of resistance by the major proportion of the microbial population (6, 16). Heteroresistance generates diagnostic and therapeutic problems; therefore, careful interpretation of susceptibility results from disk or Etest methods is mandatory in order to prevent misreporting and treatment failure.
In the present study, we found two A. baumannii isolates exhibiting heteroresistance to ampicillin-sulbactam, ticarcillin-clavulanic acid, cefepime, and cefpirome. They showed a distinct colony morphology of a circular ring within the inhibition halos. Based on our knowledge, this is the first report of heteroresistance to cephalosporins and penicillins in A. baumannii.
MATERIALS AND METHODS
Brief clinical history.
Patient A, a 59-year-old woman with lung cancer, was admitted to the intensive care unit and received mechanical ventilator support for progressive respiratory failure. Cefotaxime and then cefepime plus levofloxacin were administered for community-acquired pneumonia. On day 15 of hospitalization, empirical imipenem was given for persistent sepsis. Ten days later, PDR A. baumannii (AB328) was isolated from the sputum, which was considered as airway carrier. On the next day, vancomycin-resistant Enterococcus was isolated from the urine. PDR A. baumannii with the same antibiogram as the previous strain was cultured from the sputum on days 31 and 36. On day 52, she was transferred to the respiratory care center with clinical and microbiological resolution without ventilator support.
Patient B, a 42-year-old woman, was admitted to the neurological ward due to basilar artery occlusion. Fever appeared on the second day of hospitalization, and cefotaxime plus vancomycin were administered. On day 6, A. baumannii was isolated from the sputum. The strain was susceptible to ampicillin-sulbactam, gentamicin, ciprofloxacin, co-trimoxazole, ceftazidime, cefepime, cefpirome, imipenem, and meropenem and resistant to piperacillin. On day 12, she was intubated for ventilatory support because of progressive respiratory distress and then was transferred to the intensive care unit. On day 14, oxacillin-resistant Staphylococcus aureus and a strain of A. baumannii (AB008) resistant to ampicillin-sulbactam, ticarcillin-clavulanic acid, cefepime, and cefpirome was cultured from the sputum. The strain of A. baumannii was considered as pathogen for ventilator-associated pneumonia and cultured from the sputum on day 16, which showed additional resistance to gentamicin, ciprofloxacin, co-trimoxazole, and ceftazidime. Meropenem was given, and this patient had no recurrence of A. baumannii during hospitalization. She was subsequently transferred to a general care ward and then discharged on day 34.
Bacterial isolates.
Two A. baumannii isolates with heteroresistance to cephalosporins and penicillins were collected between December 2010 and January 2011 from the Department of Pathology, National Cheng Kung University Hospital, Tainan, Taiwan. These strains obtained from sputum were identified by colony morphology, Gram stain, biochemical tests, and the Vitek 2 system (bioMérieux, Marcy l'Etoile, France). The reference strain A. baumannii ATCC 19606 was purchased from the American Type Culture Collection (Manassas, VA).
Antimicrobial susceptibility testing.
Susceptibility to ampicillin-sulbactam, cefepime, and cefpirome for A. baumannii isolates was determined by the disk diffusion, agar dilution, and Etest methods on Mueller-Hinton agar based on the Clinical and Laboratory Standards Institute (CLSI) guideline (2, 3). Escherichia coli ATCC 25922 was used as the quality control strain. The resistance breakpoints for these antimicrobial agents were determined according to the recommendations of the CLSI (4).
PFGE.
Pulsed-field gel electrophoresis (PFGE) of ApaI-digested genomic DNA samples of A. baumannii isolates was carried out with a CHEF Mapper XA apparatus (Bio-Rad Laboratories, Hercules, CA) according to the instruction manual. PFGE patterns were interpreted in accordance with the criteria of Tenover et al. (25).
Population analysis profiles (PAP).
Bacteria were inoculated into 5 ml of Mueller-Hinton broth (BBL Microbiology Systems) and incubated overnight at 37°C. Overnight cultures were diluted in 1× phosphate-buffered saline (pH 7.4), and 100 μl (∼108 CFU) was spiral plated on Mueller-Hinton agar plates containing cefepime (Sigma Chemical Co., St. Louis, MO) concentrations ranging from 0 to 1,024 μg/ml or 0 to 512 μg/ml, respectively. A 10−6 dilution of the culture was spread onto Mueller-Hinton agar plates without antibiotic for determination of CFU/ml. Colonies were counted after 48 h of incubation at 37°C. The analysis was conducted in three replicates, and A. baumannii ATCC 19606 was used as the control.
PAP for the stability of homogeneously resistant population.
PAP for the homogeneously resistant population (HOM*) were performed as previously described (16, 26). Single colonies, called HOM*1, were selected from agar plates containing the highest cefepime concentrations at which bacterial growth was detectable. These colonies were subcultured for 10 passages on Mueller-Hinton agar plates without antibiotics and frozen at −80°C until PAP analysis to test the stability of cefepime resistance.
Growth rate analysis.
Bacteria were inoculated into 5 ml of Mueller-Hinton broth and incubated overnight at 37°C. Overnight cultures of A. baumannii strains were diluted 1:100 in Mueller-Hinton broth, and growth curves were performed in triplicate, incubating the cultures for 10 h at 37°C with shaking at 200 rpm. Bacterial growth was monitored by measuring the optical density of the culture at 600 nm.
OMP analysis.
Bacterial outer membrane proteins (OMPs) were isolated and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using SDS–10% PAGE with 6 M urea (7). Purified OMPs were obtained by treatment of the cell envelopes with 2% sodium-N-lauryl sarcosinate (Sigma Chemical Co.). A. baumannii ATCC 19606 was used as the control.
DNA isolation, PCR amplification, and direct sequencing.
Total DNA of A. baumannii was extracted and suspended in 500 μl of 1× Tris-EDTA buffer. Cell suspensions were transferred to boiling water for 20 min. Cell debris was removed by centrifugation, and 2 μl of supernatant was used as a source of template DNA in a 50-μl PCR. Strains were analyzed for β-lactamase genes (blaCTX-M, blaOXA, blaSHV, and blaTEM), using the primers described previously (27, 28). The purified PCR products were directly sequenced using the automated ABI Prism 3730 DNA sequencer (Applied Biosystems, Foster City, CA).
RESULTS
Antimicrobial susceptibility test of A. baumannii strains.
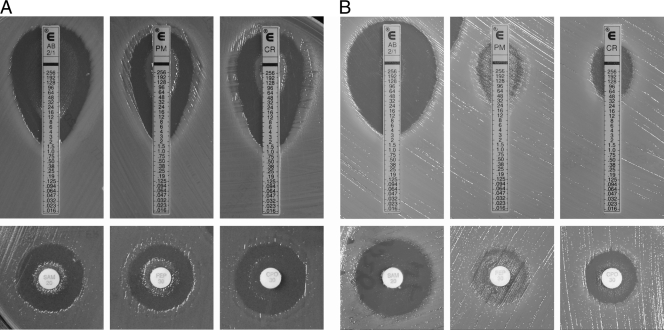
Two A. baumannii isolates, 008 and 328, showing heteroresistance to ampicillin-sulbactam, ticarcillin-clavulanic acid, cefepime, and cefpirome, collected from 2010 to 2011 were investigated. Both the disk diffusion and the Etest results showed unusual phenotypes in these two isolates (Fig. 1). A large number of resistant subpopulations were observed in the inner zone of inhibition, around the disks and Etest strips, with an ampicillin-sulbactam, cefepime, and cefpirome MIC of >256 μg/ml (Fig. 1). The same results were obtained when the assay was repeated two to three times by selecting the inner and outer colonies and retesting the ampicillin-sulbactam, cefepime, and cefpirome disk diffusion tests. MICs remained in these ranges, and a subpopulation of resistant isolates also grew around the disks and Etest strips.
Fig 1.
Characteristics of heteroresistant A. baumannii strains in penicillin and cephalosporin disk and Etest susceptibility tests. The initial MICs of A. baumannii 008 (A) and A. baumannii 328 (B) to ampicillin-sulbactam (AB; SAM), cefepime (PM; FEP), and cefpirome (CR; CPO) are shown. Heteroresistance to these antimicrobial agents was detected by the disk diffusion and Etest methods, respectively.
The in vitro activities of antimicrobial agents against A. baumannii isolates are presented in Table 1. A. baumannii 328 was resistant to all of the tested antimicrobial agents; in contrast, the susceptibility to antimicrobials differed among different isolates in A. baumannii 008. The original isolates (days 6 and 12) were susceptible to ampicillin-sulbactam, cefepime, and cefpirome and intermediate to ticarcillin-clavulanic acid. However, heteroresistance to these agents in A. baumannii 008 was detected after 2 days (day 14). This strain was resistant to almost all of the tested agents on day 16, except for imipenem and meropenem (Table 1).
Table 1.
In vitro activity of antimicrobial agents against A. baumannii isolatesa
| Antibiotic |
A. baumannii 008 at day: |
A. baumannii 328 | |||
|---|---|---|---|---|---|
| 6 | 12 | 14 | 16 | ||
| Ampicillin-sulbactam | S | S | R | R | R |
| Piperacillin | R | I | I | R | R |
| Piperacillin-tazobactam | I | I | I | I | R |
| Ticarcillin-clavulanic acid | I | I | R | R | R |
| Co-trimoxazole | S | S | S | R | R |
| Gentamicin | S | S | S | R | R |
| Ciprofloxacin | S | S | S | R | R |
| Ceftazidime | S | S | S | R | R |
| Cefepime | S | S | R | R | R |
| Cefpirome | S | S | R | R | R |
| Imipenem | S | S | S | S | R |
| Meropenem | S | S | S | S | R |
S, susceptible; I, intermediate; R, resistant.
PFGE and OMP profiles and analysis of β-lactamase genes in A. baumannii strains.
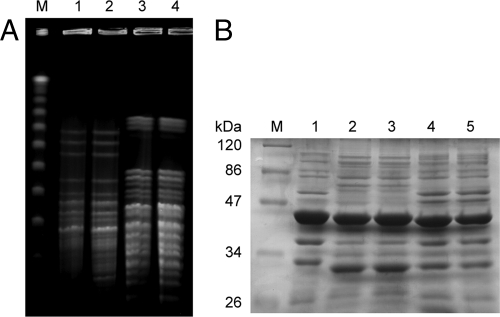
Two A. baumannii isolates, compared to their subpopulations from around the Etest strips and the original strains, all belonged to the same PFGE type and OMP profiles (Fig. 2). The original isolates (A. baumannii 008 and 328) and their subpopulations around the Etest strips did not carry blaCTX-M, blaSHV, or blaTEM. Only blaOXA-72 was found in an A. baumannii 328 isolate. However, both heteroresistant populations of A. baumannii 328 showed identical DNA sequences of blaOXA-72.
Fig 2.
PFGE and bacterial OMP profiles of A. baumannii isolates. (A) Lanes: M, lambda ladder; 1 and 2, cefepime-resistant and -susceptible A. baumannii 008 isolates; 3 and 4, cefepime-resistant and -susceptible A. baumannii 328 isolates. (B) Lanes: M, protein molecular mass marker; 1, A. baumannii ATCC 19606; 2 and 3, cefepime-resistant and -susceptible A. baumannii 008 isolates; 4 and 5, cefepime-resistant and -susceptible A. baumannii 328 isolates.
PAP for A. baumannii strains.
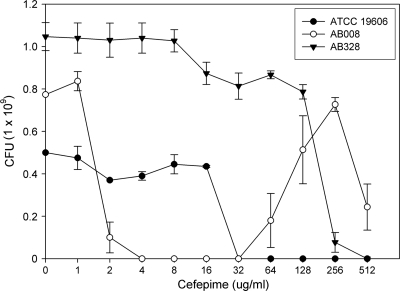
PAP confirmed the presence of heteroresistant subpopulations with high cefepime resistance levels in A. baumannii isolates 008 and 328 (Fig. 3). Interestingly, an atypical profile was observed in A. baumannii 008: colonies disappeared in the presence of cefepime at 4 to 32 μg/ml and appear again at cefepime concentrations of ≥64 μg/ml. Moreover, A. baumannii 328 also contained highly resistant subpopulations that grew in the presence of up to 128 μg of cefepime/ml.
Fig 3.
Population analysis profiles (PAP) for A. baumannii strains. Cefepime was used to select subpopulations with higher resistance levels.
PAP for the stability of HOM* of A. baumannii isolates.
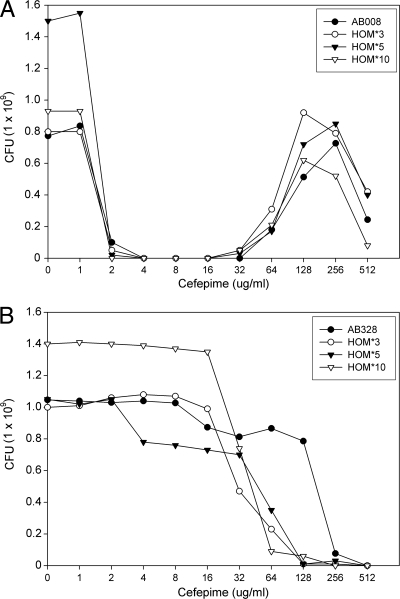
The stability of the heteroresistant phenotypes in A. baumannii isolates 008 and 328 was investigated. PAP of HOM*3, HOM*5, and HOM*10 strains showed the same trend as that observed for the original strain of A. baumannii 008, which indicates the heteroresistance is stable even without exposure to cefepime for several passages (Fig. 4A). With A. baumannii 328, after 10 passages in antibiotic-free Mueller-Hinton agar plates, the PAP curve showed a shift to relatively lower cefepime concentrations (from 256 to 64 μg/ml). However, the overall shape of PAP curves was maintained (Fig. 4B).
Fig 4.
PAP for the stability of homogeneously resistant population (HOM*) of A. baumannii isolates with heteroresistance to cefepime. HOM*3, HOM*5, and HOM*10 of A. baumannii 008 (A) and A. baumannii 328 (B) are strains derived from the plate with the highest cefepime concentration showing bacterial growth, and passaged 3, 5, and 10 times on Mueller-Hinton agar plates without antibiotics before PAP, respectively.
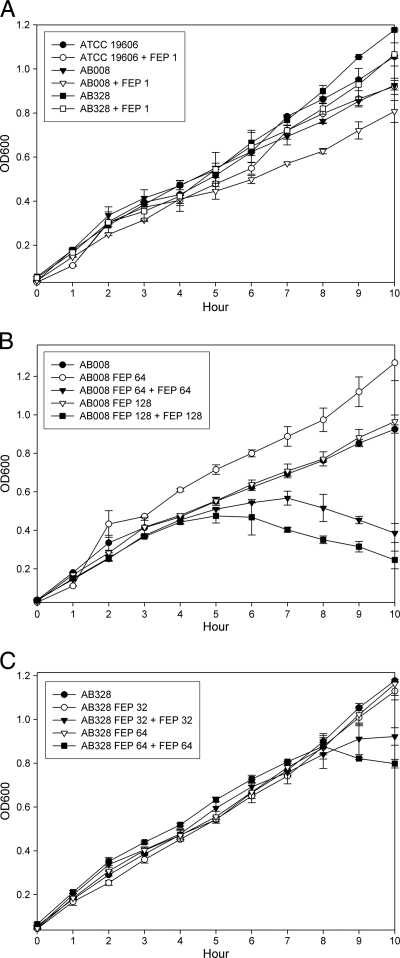
Growth rate analysis of A. baumannii strains.
Growth curves among ATCC 19606, A. baumannii 008, and A. baumannii 328 revealed that all of the tested strains displayed slower growth in the presence of 1 μg of cefepime/ml compared to strains without exposure to cefepime (Fig. 5A). Small colony size was observed in heteroresistant subpopulations of A. baumannii 008 and A. baumannii 328 growing on Mueller-Hinton agar plates containing ≥64-μg/ml concentrations of cefepime. However, these subpopulations did not grow more slowly than the original isolates when cultured in Mueller-Hinton broth without cefepime (Fig. 5B and C). Growth curves showed obviously slower growth among heteroresistant subpopulations that were repeatedly exposed to 64 or 128 μg of cefepime/ml compared to subpopulations without exposure to cefepime (Fig. 5B and C).
Fig 5.
Growth rate analyses of A. baumannii strains. (A) ATCC 19606, A. baumannii 008, and A. baumannii 328 were challenged with or without 1 μg of cefepime/ml. A. baumannii 008 (B), A. baumannii 328 (C), and its resistant subpopulations (growth on Mueller-Hinton agar plates containing 64 and 128 μg of cefepime/ml, respectively) were challenged with or without 64 or 128 μg of cefepime/ml.
DISCUSSION
Heteroresistance was first demonstrated in S. aureus to methicillin (23) and has been reported in Gram-positive bacteria to daptomycin, vancomycin, or penicillin (1, 5, 16) and in Gram-negative bacteria to colistin, carbapenems, and piperacillin-tazobactam (12–14, 18–21, 24). The present study investigated the heteroresistance to cephalosporins and penicillins in two A. baumannii isolates. A phenotype of heteroresistance was a distinct colony type: a circular ring within the inhibition halos around disks and Etest strips, which was not seen before.
Heteroresistance exhibits a resistance phenotype due to the presence of mixed populations of drug-resistant and -susceptible organisms in a genetically homogeneous isolate (1, 16, 22). These heteroresistant isolates were originally susceptible in vitro to low concentrations of antimicrobial agents and subsequently yielded heteroresistance after exposure to the agents (1). Both of our patients were treated with third generation cephalosporins during hospitalization, and this might be the reason a heteroresistant subpopulation to cephalosporins arose in these patients.
The most prevalent mechanism of β-lactam resistance in A. baumannii is enzymatic degradation by β-lactamases such as carbapenemases, including metallo-β-lactamases (IMP, SIM, and VIM) and the serine oxacillinases (OXA) (10, 11). These confer high-level resistance to carbapenems and all other β-lactams, with the exception of aztreonam (10, 11). A wide range of extended spectrum β-lactamases, including CTX-M, SHV, and TEM, also have been described (11). A. baumannii 008 did not carry β-lactamase genes that we detected; A. baumannii 328 presented only OXA-72. However, both heteroresistant populations of A. baumannii 328 revealed identical sequences of OXA-72. Loss of OMPs also may be involved in β-lactam resistance in A. baumannii (11). Two of our A. baumannii isolates, compared to their genetically homogeneous subpopulations, shared the same OMP profiles. Therefore, the exact mechanisms contributing to heteroresistance to cephalosporins and penicillins in A. baumannii still need to be determined.
Antibiotic-dependent growth was originally described by Miller and Bohnhoff, who showed that a meningococcus variant requires streptomycin for its growth (15). Streptomycin-dependent growth was also observed in Bacillus subtilis, Escherichia coli, Haemophilus influenzae type b, Mycobacterium ranae, and Pseudomonas aeruginosa (17, 29). Vancomycin-dependent enterococci were subsequently isolated (9). These differ from the usual antibiotic-resistant variants in that the antibiotic-dependent variant grows only in the presence of antibiotic. In the present study, PAP analysis showed the growth at a particular dose range of cefepime and ampicillin in A. baumannii 008 (Fig. 3), suggesting the presence of antibiotic dose-dependent heteroresistance in this isolate. However, the exact regulated mechanism needs to be further investigated.
Colonies that grew in the presence of the highest carbapenem concentration exhibited carbapenem MICs that were similar to those of the native isolates after seven daily subcultures in antibiotic-free medium (12, 21). High-level cefepime-resistant subpopulations of our two A. baumannii strains collected from isolates (HOM*) were stable and retained their high-level resistance trait upon repeated passages in medium without cefepime. The cefepime MICs of strains from the different passages of HOM* subcultures in cefepime-free medium were similar to their parental strains. This implies that heteroresistance may be induced by the antibiotic treatment; however, this phenotype can be maintained without antibiotic pressure in A. baumannii.
One of the characteristics in heteroresistance subpopulations was heterogeneity in colony size; however, MIC values and PAP did not differ between small and large colonies (16). Our resistant subpopulations exhibited a small colony size when grown on Mueller-Hinton plates containing cefepime concentrations of 64 μg/ml and higher. Growth rate analysis revealed that these strains with small colony sizes showed slower growth when repeatedly exposed to cefepime. It indicates that slower growth in resistant subpopulations may provide a strategy against antibiotic challenge.
Appearance of heteroresistance to cephalosporins and penicillins in A. baumannii isolated from our patients with airway colonization and ventilator-associated pneumonia, respectively, did not affect the clinical outcome. Combination therapy involving extended-spectrum penicillins (ampicillin and ticarcillin), broad-spectrum cephalosporins (ceftazidime and cefepime), or carbapenems (imipenem, meropenem, and doripenem) and aminoglycosides (amikacin and tobramycin) have been reported to be effective in treatment of A. baumannii infection (8, 10). Therefore, due to the presence of heteroresistance to cephalosporins and penicillins in A. baumannii subpopulations, one might choose carbapenems for treatment of A. baumannii infections. However, the similar but unknown mechanism for development of heteroresistance to cephalosporins and penicillins might also induce heteroresistance to carbapenem, which will result in treatment failure of A. baumannii infections and increase morbidity and mortality (12, 18, 22).
In conclusion, two A. baumannii isolates showed heteroresistance to ampicillin-sulbactam, ticarcillin-clavulanic acid, cefepime, and cefpirome. To our knowledge, this is the first report of heteroresistance to cephalosporins and penicillins in A. baumannii.
ACKNOWLEDGMENTS
We are very grateful to Robert M. Jonas for helpful comments on the manuscript.
This study was supported in part by grants NSC99-3112-B-006-015 from the National Science Council of Taiwan.
Footnotes
Published ahead of print 21 December 2011
REFERENCES
- 1. Alam MR, et al. 2001. Heteroresistance to vancomycin in Enterococcus faecium. J. Clin. Microbiol. 39:3379–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial disk susceptibility tests; M02–A10, approved standard-10th ed. CLSI, Wayne, PA [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; M07–A8, approved standard-8th ed. CLSI, Wayne, PA [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing, 21st informational supplement, M100–S21. CLSI, Wayne, PA [Google Scholar]
- 5. Cui L, et al. 2010. An RpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 54:5222–5233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falagas ME, Makris GC, Dimopoulos G, Matthaiou DK. 2008. Heteroresistance: a concern of increasing clinical significance? Clin. Microbiol. Infect. 14:101–104 [DOI] [PubMed] [Google Scholar]
- 7. Fernández-Cuenca F, et al. 2003. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:565–574 [DOI] [PubMed] [Google Scholar]
- 8. Fishbain J, Peleg AY. 2010. Treatment of Acinetobacter infections. Clin. Infect. Dis. 51:79–84 [DOI] [PubMed] [Google Scholar]
- 9. Fraimow HS, Jungkind DL, Lander DW, Delso DR, Dean JL. 1994. Urinary tract infection with an Enterococcus faecalis isolate that requires vancomycin for growth. Ann. Intern. Med. 121:22–26 [DOI] [PubMed] [Google Scholar]
- 10. Garnacho-Montero J, Amaya-Villar R. 2010. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr. Opin. Infect. Dis. 23:332–339 [DOI] [PubMed] [Google Scholar]
- 11. Gordon NC, Wareham DW. 2010. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int. J. Antimicrob. Agents 35:219–226 [DOI] [PubMed] [Google Scholar]
- 12. Ikonomidis A, et al. 2009. Heteroresistance to meropenem in carbapenem-susceptible Acinetobacter baumannii. J. Clin. Microbiol. 47:4055–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, et al. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:2946–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meletis G, Tzampaz E, Sianou EI, Tzavaras Sofianou D. 2011. Colistin heteroresistance in carbapenemase-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 66:946–947 [DOI] [PubMed] [Google Scholar]
- 15. Miller CP, Bohnhoff M. 1947. Two streptomycin-resistant variants of meningococcus. J. Bacteriol. 54:467–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morand B, Mühlemann K. 2007. Heteroresistance to penicillin in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 104:14098–14103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paine TF, Finland M. 1948. Observations on bacteria sensitive to, resistant to, and dependent upon streptomycin. J. Bacteriol. 56:207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pournaras S, Ikonomidis A, Markogiannakis A, Maniatis AN, Tsakris A. 2005. Heteroresistance to carbapenems in Acinetobacter baumannii. J. Antimicrob. Chemother. 55:1055–1056 [DOI] [PubMed] [Google Scholar]
- 19. Pournaras S, et al. 2005. Piperacillin-tazobactam-heteroresistant Pseudomonas aeruginosa from urinary infection, successfully treated by piperacillin-tazobactam. J. Antimicrob. Chemother. 61:757–758 [DOI] [PubMed] [Google Scholar]
- 20. Pournaras S, et al. 2007. Characterization of clinical isolates of Pseudomonas aeruginosa heterogeneously resistant to carbapenems. J. Med. Microbiol. 56:66–70 [DOI] [PubMed] [Google Scholar]
- 21. Pournaras S, et al. 2010. Characteristics of meropenem heteroresistance in Klebsiella pneumoniae carbapenemase (KPC)-producing clinical isolates of K. pneumoniae. J. Clin. Microbiol. 48:2601–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rinder H. 2001. Hetero-resistance: an under-recognized confounder in diagnosis and therapy? J. Med. Microbiol. 50:1018–1020 [DOI] [PubMed] [Google Scholar]
- 23. Ryffel C, Strässle A, Kayser FH, Berger-Bächi B. 1994. Mechanisms of heteroresistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 38:724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tato M, et al. 2010. Carbapenem heteroresistance in VIM-1-producing Klebsiella pneumoniae isolates belonging to the same clone: consequences for routine susceptibility testing. J. Clin. Microbiol. 48:4089–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tenover FC, et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tomasz A, Nachman S, Leaf H. 1991. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 35:124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan JJ, Tsai SH, Chuang CL, Wu JJ. 2006. OXA-type beta-lactamases among extended-spectrum cephalosporin-resistant Pseudomonas aeruginosa isolates in a university hospital in southern Taiwan. J. Microbiol. Immunol. Infect. 39:130–134 [PubMed] [Google Scholar]
- 28. Yan JJ, Wu JJ, Lee CC, Ko WC, Yang FC. 2010. Prevalence and characteristics of ertapenem-nonsusceptible Escherichia coli in a Taiwanese university hospital, 1999 to 2007. Eur. J. Clin. Microbiol. Infect. Dis. 29:1417–1425 [DOI] [PubMed] [Google Scholar]
- 29. Yegian D, Budd V. 1948. A variant of Mycobacterium ranae requiring streptomycin for growth. J. Bacteriol. 55:459–461 [DOI] [PMC free article] [PubMed] [Google Scholar]