Abstract
Studies of U.S. epidemics of community- and health care-associated methicillin-resistant Staphylococcus aureus (MRSA) suggested differences in MRSA strains in adults and those in children. Comprehensive population-based studies exploring these differences are lacking. We conducted a prospective cohort study of inpatients in Orange County, CA, collecting clinical MRSA isolates from 30 of 31 Orange County hospitals, to characterize differences in MRSA strains isolated from children compared to those isolated from adults. All isolates were characterized by spa typing. We collected 1,124 MRSA isolates from adults and 159 from children. Annual Orange County population estimates of MRSA inpatient clinical cultures were 119/100,000 adults and 22/100,000 children. spa types t008, t242, and t002 accounted for 83% of all isolates. The distribution of these three spa types among adults was significantly different from that among children (χ2 = 52.29; P < 0.001). Forty-one percent of adult isolates were of t008 (USA300), compared to 69% of pediatric isolates. In multivariate analyses, specimens from pediatric patients, wounds, non-intensive care unit (ICU) wards, and hospitals with a high proportion of Medicaid-insured patients were significantly associated with the detection of t008 strains. While community- and health care-associated MRSA reservoirs have begun to merge, significant differences remain in pediatric and adult patient populations. Community-associated MRSA spa type t008 is significantly more common in pediatric patients.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a major global cause of morbidity and mortality, imposing serious economic costs on patients and hospitals (1, 6, 7, 13, 26, 53). Prior to the mid-1990s, MRSA was largely a health care-associated pathogen, causing infection predominantly in people with frequent or recent contact with health care facilities (health care-associated MRSA [HA-MRSA]). In the United States, the rates of MRSA carriage (both asymptomatic and symptomatic) are estimated to be 6 to 12% in general hospital patient populations and 9 to 24% in intensive care unit populations (ICUs) (23, 32, 50). Although HA-MRSA has long been the primary cause of MRSA infections, community-associated MRSA (CA-MRSA), which often causes infections among healthy children and young adults with no exposure to the health care setting, is becoming increasingly prevalent. The first reports of MRSA isolated from patients with no identifiable risk factors came from Australia and the United States in the 1990s (5, 21, 56). Since then, the prevalence of CA-MRSA has rapidly increased, with reports of CA-MRSA infection from virtually every geographic region of the world (55, 59). The incidence of life-threatening invasive infections owing to CA-MRSA is increasing, and CA-MRSA appears to be particularly virulent among children (38). Moreover, CA-MRSA has caused outbreaks in the hospital setting (4, 41, 51), with some reports suggesting that it may be replacing HA-MRSA (8, 46, 49, 52).
In the United States, the predominant community-associated MRSA clone is now USA300 (defined by spa typing and multilocus sequence typing [MLST] as t008 and ST8, respectively), having rapidly disseminated and replaced USA400 (t128/ST1) since its appearance in 2000. USA300 has several characteristics that may offer a selective advantage over other MRSA clones, both community associated (e.g., USA400) and health care associated (e.g., USA100 [t002/ST5]). These advantages include (i) a smaller staphylococcal cassette chromosome mec (SCCmec) element (usually type IV) than those of health care-associated strains (usually SCCmec types I to III), which is more readily transmissible and may be an advantage in terms of the DNA replication speed; (ii) fewer antibiotic resistance genes than health care-associated strains, resulting in a fitness benefit due to the carriage of smaller or fewer genes; and (iii) a higher growth rate, which may lead to successful colonization by outcompeting health care-associated strains (8, 43). Furthermore, the linkage of an arginine catabolic mobile element with SCCmec type IV in USA300 likely confers increased fitness and/or pathogenicity (11). Finally, high levels of expression of regulatory genes associated with the virulence factors Panton-Valentine leukocidin and alpha-toxin have been shown for USA300 versus USA400 isolates, which may contribute to the invasiveness of USA300 (39).
The phenotypic and genotypic differences between HA- and CA-MRSA strains have been well documented (2, 10, 12, 33, 43), yet there are few studies that have directly explored the differences in MRSA strains isolated from adults and those isolated from children. Park et al. (44) previously compared a small number of adult and pediatric MRSA isolates in a South Korean hospital and found a predominance of CA-MRSA isolates among children. A better understanding of the frequency of community- versus health care-associated MRSA clones among adults and children, and in particular the USA300 clone, may inform strategies to prevent transmission and disease.
Children may have different exposures to MRSA, as they constitute a largely healthy population that is most likely to incur MRSA infection through skin and soft tissue injuries related to sports and other play activities (16). This is in contrast to the chronically and critically ill adult population, which frequents hospitals and may encounter health care-associated MRSA strains more readily. Furthermore, children may experience different antimicrobial drug selection pressure compared to that of adults due to differences in common disease syndromes and different guidance on antibiotic therapy (9, 44).
Defining the characteristics of MRSA strains in adults and children would provide insight into the spread of MRSA strains, particularly since there is growing evidence that community and health care MRSA reservoirs are mixing (28, 30, 34, 49, 52). Furthermore, few studies of adult or pediatric MRSA strains have involved a population-based sample of strains. We conducted a prospective cohort study of inpatients in a large metropolitan county to characterize differences in pediatric and adult MRSA strains.
MATERIALS AND METHODS
Study.
We conducted a population-based, prospective collection of clinical isolates of MRSA from 30 of 31 hospitals in Orange County, CA. This study was approved by the Institutional Review Board of the University of California Regents.
Isolate collection.
Clinical (nonscreening) isolates of MRSA from unique adult patients (≥18 years of age) and unique pediatric patients (<18 years of age) were collected from hospital microbiology laboratories. Hospitals were instructed to collect MRSA isolates from unique patients up to a total of 100 isolates or for a duration of 12 months, whichever came first. In order to have a representative sample of Orange County MRSA isolates, we limited isolates in this study to those collected for a uniform duration of time from adult hospitals. Since the largest adult hospitals reached 100 isolates over a 5-month period, we restricted the period of all adult isolate collections to 5 months. All pediatric hospitals required a 12-month collection period. Nearly all adult isolates were collected between December 2008 and April 2009. Pediatric isolates were collected between October 2008 and September 2009. Isolates from patients not admitted to hospitals were excluded from the study. Samples were batched and delivered to the Orange County Public Health Laboratory using soy agar slants. For the repeated confirmation of MRSA, isolates were plated onto selective medium for MRSA (BD CHROMagar). MRSA strains were stored at −65°C in 15% glycerol Brucella broth.
Specimen data and hospital characteristics.
Specimen data, including patient age in years, specimen source (wound, blood, urine, sputum, or other), specimen location (ICU or non-ICU), and time of specimen collection with respect to admission date (hospital onset [HO], ≥3 days after admission; community onset [CO], <3 days after admission), were collected. Hospital characteristics were obtained from a California hospital data set (42), which included annual admissions, hospital type (acute care versus long-term acute care [LTAC] facility), percentage of Medicaid-insured patients, and percentage of Hispanic patients. Population estimates of adults and children in Orange County were obtained from the 2010 U.S. Census (57).
Laboratory methods and molecular typing.
All strains were shipped to Imperial College London in the United Kingdom for spa typing and stored at −80°C. Cells were harvested on blood agar plates (Oxoid) and incubated at 37°C overnight. DNA was extracted by using a Qiagen DNeasy Blood & Tissue kit. DNA samples were eluted in 200 μl of elution buffer (10 mM Tris-Cl, 0.5 mM EDTA [pH 9.0]) and stored at −20°C. Following the sequencing of the spa region, spa types were determined by using Ridom StaphType v2.1 (Ridom GmbH, Würzburg, Germany) (20). To assess spa type diversity and relatedness, cluster analysis of spa types was performed separately for adult and pediatric isolates by using the Based upon Repeat Pattern (BURP) algorithm, a built-in feature of the StaphType software (35). MLST and SmaI pulsed-field gel electrophoresis (PFGE) were performed on a subset of the isolates (n = 171), to confirm MRSA strain types, according to methods described previously (14, 48). This subset included one isolate of each spa type and, for the 10 most common spa types, one isolate from each of the hospitals in which these spa types were present. Isolates were selected by using a random number generator. For PFGE, DNA profiles were analyzed by using BioNumerics software (version 5.0, 2007; Applied Maths). PFGE types were defined using a similarity coefficient of 78%, and USA100 to USA800 strains were used as references.
Statistical analyses.
Annual adult and pediatric population estimates of hospitalized patients with clinical MRSA cultures were calculated by spa type, accounting for the duration of countywide collection. We further calculated the percentage of MRSA strains from adult versus pediatric patients that were due to the most common spa types (t008, t242, and t002) and compared them by using χ2 tests. Specimen data for t008, t242, and t002 isolates were compared by using χ2 or Fisher's exact tests and, for patient age, the Wilcoxon Mann-Whitney test. Simpson's index of diversity (1 − D) was used to compare the genetic diversities of MRSA strains among adults and children. 1−D gives an unbiased measure of the probability of drawing two different spa types given the distribution of spa types in a sample (19). The 95% confidence intervals (CIs) were calculated as described previously (18). We conducted bivariate tests to evaluate the association of spa type t008 with individual variables, including age (adult/pediatric), specimen source (particularly wound and blood), time of specimen collection (community or hospital onset), and ward type (non-ICU/ICU). We also tested hospital-level variables, including annual admissions (greater or less than 10,000), LTAC facility, percentage of Hispanic patients, and percentage of Medicaid-insured patients. For multivariate analyses, variables with a P value of <0.1 were entered into a generalized linear mixed model clustered by hospital and were retained at an α value of ≤0.05 (xtmelogit, STATA release 11, 2009; Stata Corp.).
RESULTS
A total of 1,124 adult and 159 pediatric MRSA isolates were collected over the 5- and 12-month periods, respectively. A summary of the characteristics of the clinical MRSA strains collected is shown in Table 1. The median age of adults was 67 years (interquartile range [IQR], 50 to 81 years), and that of children was 2 years (IQR, 1 to 9 years).
Table 1.
Characteristics of clinical MRSA strains isolated from adult and pediatric patients
| Characteristic | No. (%) of isolates |
||
|---|---|---|---|
| Adulta | Pediatricb | Total/overall | |
| Total MRSA isolates | 1,124 (87.6) | 159 (12.4) | 1,283 (100) |
| Specimen source ofc: | |||
| Wound/abscess | 488 (43.4) | 81 (55.9) | 569 (44.8) |
| Sputum | 331 (29.4) | 27 (18.6) | 358 (28.2) |
| Urine | 109 (9.7) | 4 (2.8) | 113 (8.9) |
| Blood | 104 (9.3) | 7 (4.8) | 111 (8.8) |
| Otherd | 92 (8.2) | 26 (17.9) | 118 (9.3) |
| Intensive care unit collectione | 187 (16.7) | 17 (11.8) | 204 (16.1) |
| Hospital onset | 399 (35.5) | 40 (25.2) | 439 (34.2) |
Collected for 5 months from hospitals serving adults.
Collected for 12 months from hospitals serving children.
Fourteen missing pediatric entries.
According to brief notes in the data set, “other” specimen sources included the following anatomical locations or types of specimens: 5 ear; 5 eye; 3 buttock; 2 each of finger, leg, pleural, and skin; and 1 each of gastrointestinal, sinus, perineum, spleen, and umbilical for pediatric specimen sources and 8 leg; 7 foot, knee, and medical device related; 6 groin; 5 abdominal, spinal, and stool; 4 gastric; 4 hand; 3 back, pleural, and tissue; 2 each of ankle, body fluid, buttock, ear, eye, stump, synovial fluid, and unknown; and 1 each of drainage, gallbladder, hip, humerus, ileal crest, lung, pancreatic fluid, skin, and stoma.
Nineteen missing entries (4 adult and 15 pediatric).
t008, t242, and t002 were the predominant spa types in Orange County, accounting for 83% of all isolates (Table 2). The distribution of these spa types among adults (t008, 41%; t242, 23%; t002, 19%) was significantly different from that among children (t008, 69%; t242, 9%; t002, 6%) (χ2 = 52.29; P < 0.001). Annual population estimates of clinical inpatient MRSA infections were 119/100,000 adults and 22/100,000 children. Annual estimates by spa type were 48/100,000 adults and 15/100,000 children for t008, 27/100,000 adults and 2/100,000 children for t242, and 22/100,000 adults and 1/100,000 children for t002.
Table 2.
Ten most frequently found spa types among adult and pediatric patients in Orange County, CAa
| Rank | Adult patients |
Pediatric patients |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| spa type | MLST | No. of isolates | % of isolates | Cumulative % | spa type | MLST | No. of isolates | % of isolates | Cumulative % | |
| 1 | t008 | 8 | 457 | 40.7 | 40.7 | t008 | 8 | 110 | 69.2 | 69.2 |
| 2 | t242 | 5 | 260 | 23.1 | 63.8 | t242 | 5 | 14 | 8.8 | 78 |
| 3 | t002 | 5 | 211 | 18.8 | 82.6 | t002 | 5 | 9 | 5.7 | 83.7 |
| 4 | t024 | 8 | 19 | 1.7 | 84.3 | t024 | 8 | 3 | 1.9 | 85.5 |
| 5 | t037 | 8 | 15 | 1.3 | 85.6 | t045 | 5 | 2 | 1.3 | 86.8 |
| 6 | t127 | 1 | 14 | 1.3 | 86.8 | t068 | 8 | 2 | 1.3 | 88.1 |
| 7 | t088 | 105 | 12 | 1.1 | 87.9 | t2689 | 8 | 2 | 1.3 | 89.3 |
| 8 | t1737 | 5 | 11 | 1 | 88.9 | t324 | 72 | 2 | 1.3 | 90.6 |
| 9 | t306 | 5 | 6 | 0.5 | 89.4 | t622 | 8 | 2 | 1.3 | 91.8 |
| 10 | t126 | 72 | 5 | 0.4 | 89.9 | 13 others | 1 each | 0.6 each | 100 | |
The total numbers of spa types were 89 for adult patients and 22 for pediatric patients. Simpson's index of diversity (1-D) values were 75% (95% CI, 73%, 76%) for adult patients and 51% (95% CI, 41%, 60%) for pediatric patients. MLST, multilocus sequence type.
According to MLST, the t008 isolates in our study were of the prototypic community clone USA300 (t008/ST8), and the t002 isolates were of the prototypic hospital clone USA100 (t002/ST5), with t242 isolates being identified as ST5 (Table 2). Comparison of t242 and t002 isolates for the following parameters revealed no significant difference: the proportion from each specimen source, the proportion of hospital and community onset, the proportion collected in ICU and non-ICU wards, and the age distribution of patients (all P > 0.05). Conversely, t008 isolates were significantly different from t242 and t002 isolates in the same tests (P < 0.001). t242 and t002 isolates shared the most common specimen source, sputum (34% and 38%, respectively), whereas wounds were the most common specimen source of t008 isolates (56%). PFGE of a sample of t242 and t002 isolates showed them to be predominantly USA100 isolates (data not shown).
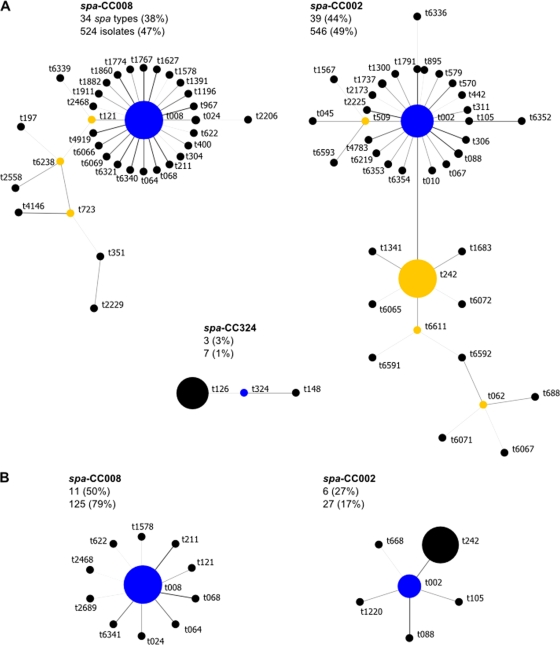
BURP analysis of the spa types clustered the majority of adult isolates (97%) into three spa clonal complexes (spa-CCs) and most pediatric isolates (96%) into two spa-CCs (Fig. 1). spa types were clustered with either t008 (spa-CC008; community-associated strains) or t002 (spa-CC002; health care-associated strains), but in adults, a further spa-CC with founder t324 was identified (spa-CC324). Isolates in this spa-CC were characterized as ST72 isolates. For both adult and pediatric MRSA isolates, MLST results showed that all isolates in spa-CC008 were of ST8 and that all isolates in spa-CC002 were of either ST5 or a single-locus variant, ST105 (spa types t045, t088, and t1791 for the latter). According to the BURP algorithm, spa types that differ from all other spa types in the sample by more than 4 repeats, and thus which cannot be clustered into a spa-CC, are termed singletons. For adults, 10 (11.2%) spa types (40 [3.6%] isolates) were classified as singletons, and for children, 4 (18.2%) spa types (6 [3.8%] isolates) were classified as singletons. spa types of less than 5 repeats in length were excluded from the BURP analysis because no reliable evolutionary history can be inferred from “short” spa types (35). For adults, 2 (2.2%) spa types (2 [0.2%] isolates) were excluded, and for children, 1 (4.5%) spa type (1 [0.6%] isolate) was excluded. The estimated genetic diversity of MRSA isolates was significantly higher among adults than among children (1-D = 75% versus 51%) (Table 2).
Fig 1.
Relatedness of spa types among adult (A) and pediatric (B) MRSA isolates according to the Based upon Repeat Pattern (BURP) algorithm. Clusters of linked spa types correspond to spa clonal complexes (spa-CCs). spa types are clustered into a spa-CC when their repeat patterns differ by no more than 4 repeats. The BURP algorithm sums up “costs” (a measure of relatedness based on the repeat pattern) to define a founder score for each spa type in a spa-CC. The founder (blue node) is the spa type with the highest founder score in its spa-CC, and the subfounder (yellow node) is the spa type with the second highest founder score. spa-CC008 has founder t008. Each node represents a spa type. The node size represents the number of clustered strains that belong to that spa type. The shading of the branches represents the costs (similarities in repeat patterns) between two spa types; the darker the branch, the lower the cost (more similar repeat patterns).
In bivariate analyses, pediatric patients, wound specimens, isolation in a non-ICU ward, community-onset timing of collection, and isolation from a hospital with >10,000 annual admissions were associated with t008 (USA300) isolates (Table 3). In addition, admission to a hospital with a high proportion of Medicaid-insured patients or a high proportion of Hispanic patients was linearly associated with the recovery of t008 isolates. In multivariate analyses, isolates from pediatric patients, wounds, non-ICU wards, and hospitals with a high proportion of Medicaid-insured patients remained significantly associated with spa type t008 (Table 4). Isolates from hospitals with a high proportion of Hispanic patients were significantly more likely to be t008 isolates (20% higher odds of being a spa type t008 isolate per 10% increase in numbers of Hispanic patients). However, this finding was collinear with hospitals with a high proportion of Medicaid-insured patients and thus was removed from the multivariate model.
Table 3.
Bivariate analyses of variables associated with spa type t008
| Variable | % of t008 isolates |
χ2 | P | |
|---|---|---|---|---|
| Those with characteristic | Those without characteristic | |||
| Individual | ||||
| Pediatric | 69.81 | 40.75 | 47.67 | <0.001 |
| Community onset | 48.10 | 37.13 | 14.09 | <0.001 |
| Non-ICU | 47.17 | 27.45 | 27.00 | <0.001 |
| Blood specimen | 40.54 | 44.30 | 0.58 | 0.446 |
| Wound specimen | 60.04 | 32.34 | 96.28 | <0.001 |
| LTAC | 35.21 | 44.88 | 2.54 | 0.111 |
| Hospital level | ||||
| >10,000 annual admissions | 38.40 | 51.52 | 22.00 | <0.001 |
| Medicaid-insured patientsa | 1.34 (1.21–1.48) | <0.001 | ||
| Hispanic patientsa | 1.29 (1.15–1.44) | <0.001 | ||
Odds ratio per 10% increase.
Table 4.
Multivariate analysis of variables associated with spa type t008
| Variable | Odds ratio | SE | 95% CI | P |
|---|---|---|---|---|
| Patient/isolate characteristic | ||||
| Wound specimen | 2.64 | 0.34 | 2.06, 3.39 | <0.001 |
| Pediatric | 2.07 | 0.52 | 1.26, 3.40 | 0.004 |
| Non-ICU | 1.77 | 0.32 | 1.24, 2.54 | 0.002 |
| Hospital characteristic | ||||
| % Medicaid-insured patientsa | 1.24 | 0.06 | 1.13, 1.35 | <0.001 |
Odds ratio per 10% increase.
DISCUSSION
We conducted a prospective cohort study of inpatients in a large metropolitan county in Californian, collecting all clinical MRSA isolates from 30 of 31 hospitals in order to characterize differences in pediatric and adult MRSA strains. To our knowledge, this is the first study to assess adult and pediatric MRSA isolates from a population-based sample across a large region.
Countywide, adult and pediatric clinical MRSA isolates were dominated by three spa types, two of which were consistent with the prototypic community- and health care-associated clones prevalent in the United States (t008 [USA300] and t002 [USA100]). t008 (USA300) was the most common single clone among both adult and pediatric isolates. Nevertheless, t008 comprised a large majority of pediatric isolates, whereas adult isolates were nearly equally divided among community- and health care-associated clones. Most other spa types were shown by BURP to be related to these two dominant clones. The two spa clonal complexes spa-CC008 and spa-CC002 can therefore be thought of as two distinct groups of isolates representing the major community- and health care-associated MRSA strains prevalent in the United States.
Interestingly, t242/ST5 was slightly more common than t002/ST5 among both adult and pediatric isolates, despite the predominance of the t002/ST5 hospital clone in the United States. Given the similarities of t242 and t002 isolates in this study, and the fact that t242 differs from t002 by only one nucleotide (resulting in a different spa repeat pattern by one spa repeat), t242/ST5 presumably represents a minor variant of USA100 that has become prevalent in Orange County hospitals. t242 has been reported infrequently in the literature (24, 25, 60), with just one study reporting t242 at an endemic level in an Italian hospital (45).
The additional spa clonal complex identified among adult isolates included a community-onset isolate identified as a t324/ST72 isolate, an invasive community-associated MRSA clone reported for elderly patients in South Korea from 2006 to 2007, just before our isolate collection began (29). According to the U.S. Census Bureau, 17.9% of the Orange County population is Asian, approximately 2.9% of which is Korean (57).
There was significantly more genetic diversity among adult MRSA isolates than among pediatric isolates. This could simply represent the greater time that health care-associated clones have had to diversify at the spa locus than community-associated clones, which have emerged only in the past 2 decades. The greater MRSA diversity among adults could also be due to different degrees of contact; for example, adults may have more diverse MRSA encounters (travel, work, social venues, and health care facilities) than young children (schools and day care centers).
The population estimates of clinical MRSA isolates in Orange County show that there was a 6-fold-higher frequency of inpatient MRSA clinical cultures among adults than among children. This pattern was consistent among the three most common spa types, t008, t242, and t002, and is likely a combination of more frequent hospitalizations among adults (many of whom were elderly, with a median age of 67 years) and more frequent MRSA carriage.
In multivariate analyses, community-associated MRSA clone t008 (USA300) was associated with pediatric patients. In contrast to adults, children are often healthier and are more likely to encounter MRSA in the community through exposure to high-density environments, such as schools, day cares, camps, and sporting activities, where close contact may facilitate the spread of community MRSA strains. In agreement with data from previous studies, we found that USA300 was associated with wounds, which is the most common presentation for hospitalization due to community-acquired MRSA infection (2, 17). USA300 was also associated with hospitals that treat a large fraction of Medicaid-insured patients, suggesting that community MRSA infections may be more prevalent among patients from economically disadvantaged or high-density areas.
USA300 was also associated with isolation from non-ICU wards, suggesting that this community strain is occurring in healthier hosts or is producing infections that are less severe than those caused by traditional health care-associated strains. Nevertheless, there is ample evidence that community strains are capable of producing fulminant infections (15, 37, 52). An understanding of what component of invasiveness is due to host comorbidities versus pathogen virulence factors is an area of active research.
Interestingly, we did not find that the isolation of t008 was associated with community-onset clinical isolates (clinical culture isolated less than 3 days after admission). This finding is likely due to the fact that the majority of health care-associated carriage or infection is found upon readmission to hospitals (27). It could also be explained by community-associated strains that have become endemic in some hospitals (49, 52).
Community- and health care-associated MRSA strains are becoming increasingly difficult to distinguish epidemiologically as community-associated strains continue to penetrate hospital MRSA reservoirs. Furthermore, it remains unclear whether community clones are adding to or replacing traditional health care-associated MRSA strains (3, 8, 22, 49). The implication of the blurred line between community- and health care-associated MRSA strains may be that efforts to control MRSA transmission within hospitals will not be effective in controlling community influx into hospitals. Simultaneous community strategies to limit MRSA spread are needed. However, much is still unknown about the acquisition and transmission of CA-MRSA, so improved knowledge is needed to better guide infection control strategies. Further studies are needed to ascertain whether community strategies to reduce transmission in children and young adults would produce benefits across the entire age spectrum.
One limitation of our study is that few individual-level characteristics were available. Also, our study did not account for the different policies in place at each hospital with regard to when to obtain clinical cultures. These differences could affect MRSA detection at each hospital and, possibly, the type of MRSA strains isolated, if clinical cultures were more likely to be obtained for sicker, older patients. Moreover, our results could have been affected by the potential seasonality of MRSA infections and infection types due to the different collection periods for adult and pediatric isolates (largely winter and spring for adult collections, compared to all seasons for pediatric collections). A seasonality of S. aureus infections, particularly skin infections, has been observed in pediatric and adult patients in temperate and tropical environments, with a predominance of infections during summer and autumn (31, 36, 54, 58). A recent study in Rhode Island found a 2- to 3-fold-increased incidence of MRSA infections (both CA- and HA-MRSA) in pediatric patients during the second two quarters of the year over the last decade (36). However, in that same study, adult CA-MRSA infections showed less seasonal variation than did pediatric infections, and no variation was observed among adult HA-MRSA infections. Some studies observed no significant seasonality of S. aureus infections, but those studies focused on bacteremia (40, 47). The collection of both adult and pediatric MRSA isolates for the same time period, i.e., 12 months, would have accounted for any potential seasonality effects and/or other factors that could affect the type and diversity of MRSA strains isolated.
Mandatory screening of high-risk inpatients was not in place in California until 2009; therefore, our population estimates are likely underestimates. In addition, our estimates should not be construed as measures of MRSA infection among inpatients. Clinical isolates often represent carriage without infection. Finally, our estimates of the index of diversity for adult and pediatric MRSA isolates may have been influenced by differing sample sizes (18).
In conclusion, our study found that in a large county, MRSA isolates from hospitalized children were more likely to be of spa type t008 (USA300). This community-associated spa type was associated with children, wounds, non-ICU care, and admission to a hospital with a high percentage of Medicaid-insured patients. Despite the association of t008 isolates with children, t008 was still the most common spa type among adult patients, suggesting that community-based interventions are needed to stem the influx of t008 isolates into hospitals. We also found evidence for a prevalent variant of the USA100 clone (t242/ST5), which has not been reported elsewhere. While community- and hospital-associated MRSA reservoirs have begun to merge, significant differences remain in pediatric and adult patient populations, which may provide an impetus for different age-based strategies to reduce transmission and disease.
ACKNOWLEDGMENTS
This work was supported by the University of California Irvine School of Medicine, the Biotechnology and Biological Sciences Research Council (grant number BB/D52637X/1), and the Wellcome Trust (grant number 089472/Z/09/Z).
We thank Diane Kim for her contributions to this study. We also extend special thanks to the participating microbiology laboratories throughout Orange County.
We do not have an association that might pose a conflict of interest.
Footnotes
Published ahead of print 28 December 2011
REFERENCES
- 1. Abramson MA, Sexton DJ. 1999. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect. Control Hosp. Epidemiol. 20:408–411 [DOI] [PubMed] [Google Scholar]
- 2. Bassetti M, Nicco E, Mikulska M. 2009. Why is community-associated MRSA spreading across the world and how will it change clinical practice? Int. J. Antimicrob. Agents 34(Suppl 1):S15–S19 [DOI] [PubMed] [Google Scholar]
- 3. Bootsma M, Hota B, Diekmann O, Weinstein RA, Bonten M. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr K-1680. [Google Scholar]
- 4. Bratu S, et al. 2005. Community-associated methicillin-resistant Staphylococcus aureus in hospital nursery and maternity units. Emerg. Infect. Dis. 11:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. JAMA 282:1123–1125 [PubMed] [Google Scholar]
- 6. Cosgrove SE, et al. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36:53–59 [DOI] [PubMed] [Google Scholar]
- 7. Cosgrove SE, et al. 2005. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect. Control Hosp. Epidemiol. 26:166–174 [DOI] [PubMed] [Google Scholar]
- 8. D'Agata EM, Webb GF, Horn MA, Moellering RC, Jr, Ruan S. 2009. Modeling the invasion of community-acquired methicillin-resistant Staphylococcus aureus into hospitals. Clin. Infect. Dis. 48:274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. David MZ, et al. 2006. Contrasting pediatric and adult methicillin-resistant Staphylococcus aureus isolates. Emerg. Infect. Dis. 12:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diep BA, Carleton HA, Chang RF, Sensabaugh GF, Perdreau-Remington F. 2006. Roles of 34 virulence genes in the evolution of hospital- and community-associated strains of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 193:1495–1503 [DOI] [PubMed] [Google Scholar]
- 11. Diep BA, et al. 2008. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 197:1523–1530 [DOI] [PubMed] [Google Scholar]
- 12. Eady EA, Cove JH. 2003. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Curr. Opin. Infect. Dis. 16:103–124 [DOI] [PubMed] [Google Scholar]
- 13. Engemann JJ, et al. 2003. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 36:592–598 [DOI] [PubMed] [Google Scholar]
- 14. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frazee BW, Salz TO, Lambert L, Perdreau-Remington F. 2005. Fatal community-associated methicillin-resistant Staphylococcus aureus pneumonia in an immunocompetent young adult. Ann. Emerg. Med. 46:401–404 [DOI] [PubMed] [Google Scholar]
- 16. Frei CR, Makos BR, Daniels KR, Oramasionwu CU. 2010. Emergence of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections as a common cause of hospitalization in United States children. J. Pediatr. Surg. 45:1967–1974 [DOI] [PubMed] [Google Scholar]
- 17. Fridkin SK, et al. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436–1444 [DOI] [PubMed] [Google Scholar]
- 18. Grundmann H, Hori S, Tanner G. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39:4190–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grundmann H, et al. 2010. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7:e1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harmsen D, et al. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herold BC, et al. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598 [DOI] [PubMed] [Google Scholar]
- 22. Hota B, et al. 2007. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch. Intern. Med. 167:1026–1033 [DOI] [PubMed] [Google Scholar]
- 23. Huang SS, et al. 2007. Improving methicillin-resistant Staphylococcus aureus surveillance and reporting in intensive care units. J. Infect. Dis. 195:330–338 [DOI] [PubMed] [Google Scholar]
- 24. Johnson JK, et al. 2007. Skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus USA300 clone. Emerg. Infect. Dis. 13:1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kinnevey P, Shore A, Rossney A, Coleman D. 2010. Abstr. 20th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr P1712. [Google Scholar]
- 26. Klein E, Smith DL, Laxminarayan R. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 13:1840–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klevens RM, et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 28. Kourbatova EV, et al. 2005. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am. J. Infect. Control 33:385–391 [DOI] [PubMed] [Google Scholar]
- 29. Lee SS, Kim YJ, Chung DR, Jung KS, Kim JS. 2010. Invasive infection caused by a community-associated methicillin-resistant Staphylococcus aureus strain not carrying Panton-Valentine leukocidin in South Korea. J. Clin. Microbiol. 48:311–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu C, et al. 2008. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004-2005. Clin. Infect. Dis. 46:1637–1646 [DOI] [PubMed] [Google Scholar]
- 31. Loffeld A, Davies P, Lewis A, Moss C. 2005. Seasonal occurrence of impetigo: a retrospective 8-year review (1996–2003). Clin. Exp. Dermatol. 30:512–514 [DOI] [PubMed] [Google Scholar]
- 32. Lucet JC, et al. 2009. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch. Intern. Med. 169:1372–1378 [DOI] [PubMed] [Google Scholar]
- 33. Ma XX, et al. 2002. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 46:1147–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG. 2007. Community-associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg. Infect. Dis. 13:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mellmann A, et al. 2007. Based upon Repeat Pattern (BURP): an algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mermel LA, Machan JT, Parenteau S. 2011. Seasonality of MRSA infections. PLoS One 6:e17925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller LG, et al. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445–1453 [DOI] [PubMed] [Google Scholar]
- 38. Moellering RC., Jr 2006. The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann. Intern. Med. 144:368–370 [DOI] [PubMed] [Google Scholar]
- 39. Montgomery CP, et al. 2008. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J. Infect. Dis. 198:561–570 [DOI] [PubMed] [Google Scholar]
- 40. Morin CA, Hadler JL. 2001. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J. Infect. Dis. 184:1029–1034 [DOI] [PubMed] [Google Scholar]
- 41. O'Brien FG, Pearman JW, Gracey M, Riley TV, Grubb WB. 1999. Community strain of methicillin-resistant Staphylococcus aureus involved in a hospital outbreak. J. Clin. Microbiol. 37:2858–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Office of Statewide Health Planning and Development 6 June 2011, accession date Patient discharge data public data set 2005. Office of Statewide Health Planning and Development, Sacramento, CA: http://www.oshpd.ca.gov/HID/Products/PatDischargeData/PublicDataSet/index.html [Google Scholar]
- 43. Okuma K, et al. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 40:4289–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park JY, et al. 2007. A comparison of adult and pediatric methicillin-resistant Staphylococcus aureus isolates collected from patients at a university hospital in Korea. J. Microbiol. 45:447–452 [PubMed] [Google Scholar]
- 45. Parlato C, Cavallerio P, Fossati L, Allice T, Serra R. 2009. Abstr. 19th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr P1574. [Google Scholar]
- 46. Patel M, et al. 2008. Emergence of USA300 MRSA in a tertiary medical centre: implications for epidemiological studies. J. Hosp. Infect. 68:208–213 [DOI] [PubMed] [Google Scholar]
- 47. Perencevich EN, et al. 2008. Summer peaks in the incidences of Gram-negative bacterial infection among hospitalized patients. Infect. Control Hosp. Epidemiol. 29:1124–1131 [DOI] [PubMed] [Google Scholar]
- 48. Pfaller MA. 1998. Chromosomal restriction fragment analysis by pulsed-field gel electrophoresis: application to molecular epidemiology, p 651–657 In Isenberg HD. (ed), Essential procedures for clinical microbiology. ASM Press, Washington, DC [Google Scholar]
- 49. Popovich KJ, Weinstein RA, Hota B. 2008. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin. Infect. Dis. 46:787–794 [DOI] [PubMed] [Google Scholar]
- 50. Robicsek A, et al. 2008. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann. Intern. Med. 148:409–418 [DOI] [PubMed] [Google Scholar]
- 51. Saiman L, et al. 2003. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin. Infect. Dis. 37:1313–1319 [DOI] [PubMed] [Google Scholar]
- 52. Seybold U, et al. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42:647–656 [DOI] [PubMed] [Google Scholar]
- 53. Shurland S, Zhan M, Bradham DD, Roghmann MC. 2007. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 28:273–279 [DOI] [PubMed] [Google Scholar]
- 54. Szczesiul JM, Shermock KM, Murtaza UI, Siberry GK. 2007. No decrease in clindamycin susceptibility despite increased use of clindamycin for pediatric community-associated methicillin-resistant Staphylococcus aureus skin infections. Pediatr. Infect. Dis. J. 26:852–854 [DOI] [PubMed] [Google Scholar]
- 55. Tristan A, et al. 2007. Global distribution of Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus, 2006. Emerg. Infect. Dis. 13:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Udo EE, Pearman JW, Grubb WB. 1993. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J. Hosp. Infect. 25:97–108 [DOI] [PubMed] [Google Scholar]
- 57. U.S. Census Bureau 6 June 2011, accession date United States census 2010 demographic profile data for Orange County, California. US Census Bureau, Washington, DC: http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_DP_DPDP1&prodType=table [Google Scholar]
- 58. Van De Griend P, et al. 2009. Community-associated methicillin-resistant Staphylococcus aureus, Iowa, USA. Emerg. Infect. Dis. 15:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wallin TR, Hern HG, Frazee BW. 2008. Community-associated methicillin-resistant Staphylococcus aureus. Emerg. Med. Clin. North Am. 26:431–455 [DOI] [PubMed] [Google Scholar]
- 60. Weese JS, Avery BP, Reid-Smith RJ. 2010. Detection and quantification of methicillin-resistant Staphylococcus aureus (MRSA) clones in retail meat products. Lett. Appl. Microbiol. 51:338–342 [DOI] [PubMed] [Google Scholar]