Abstract
The accumulation of unfolded proteins in the endoplasmic reticulum (ER) triggers transcriptional and translational reprogramming. This unfolded protein response (UPR) protects cells during transient stress and can lead to apoptosis during prolonged stress. Two key mediators of the UPR are PKR-like ER kinase (PERK), which phosphorylates the α subunit of eukaryotic translation initiation factor 2 (eIF2α), resulting in decreased protein synthesis, and the α subunit of inositol-requiring enzyme 1 (IRE1α), which initiates cytoplasmic splicing of the mRNA encoding the transcription factor X-box binding protein 1 (XBP1). XBP1 induces transcription of genes involved in protein quality control. This report describes cross talk between these two pathways: phosphorylation of eIF2α was required for maximal induction of spliced XBP1 (XBP1s) protein levels via a mechanism that involved stabilization of XBP1s mRNA. By using mouse embryo fibroblasts deficient in UPR signaling pathways, we demonstrate that stress-induced stabilization of XBP1s mRNA requires cytoplasmic splicing of the mRNA and inhibition of its translation. Because the XBP1s protein promotes transcription of its own gene, the UPR-induced mRNA stabilization is part of a positive feedback loop that induces XBP1s protein accumulation and transcription of target genes during stress. We propose a model in which eIF2α phosphorylation-mediated control of mRNA turnover is a molecular switch that regulates the stress response transcription program and the ER's capacity for protein folding during stress.
INTRODUCTION
Nearly 30% of human genes encode proteins that are synthesized on membrane-bound ribosomes of the endoplasmic reticulum (ER). Folding of these proteins within the ER is a complex process that is aided by chaperones and enzymes for posttranslational modification (2). When protein synthesis exceeds the folding capacity of the ER, unfolded proteins accumulate, triggering a stress response (unfolded protein response [UPR]). During early and mild stress, the UPR inhibits global protein synthesis and also promotes the expression of proteins that assist in protein folding and cell survival (64). Severe and prolonged stress leads to apoptosis (8, 54).
Signaling in the UPR is mediated by the ER chaperone BiP (GRP78). Under basal conditions, this chaperone binds to and inhibits effectors of the UPR (4). During the UPR, BiP binds to unfolded proteins instead of the effectors, allowing activation of the response. In mammals, the UPR is induced by three effectors: (i) PKR-like ER kinase (PERK) phosphorylates the α subunit of eukaryotic translation initiation factor 2 (eIF2α), thereby inhibiting the translation of most mRNAs (15); (ii) membrane-bound ATF6 (activating transcription factor 6) is transported from the ER to the Golgi complex, where proteolytic cleavage releases a soluble fragment that is transported to the nucleus, where it then activates the expression of stress response genes (17); (iii) the transmembrane protein kinase and endonuclease IRE1α (α subunit of inositol-requiring enzyme 1) is activated by oligomerization and autophosphorylation. Activated IRE1α initiates the splicing of the mRNA for the transcription factor XBP1 (X-box binding protein 1). In this unconventional cytoplasmic splicing, IRE1α excises 26 nucleotides from the unspliced XBP1 (XBP1u) mRNA, followed by ligation of the cleaved fragments (6, 30, 69). The unspliced mRNA encodes XBP1u protein (33 kDa), which is rapidly degraded. The spliced mRNA encodes the spliced XBP1 (XBP1s) protein (54 kDa, as a result of a frameshift), which induces the expression of genes coding for ER proteins that facilitate protein folding and assist in clearing misfolded proteins.
XBP1s is an unstable protein (half-life of 22 min [6]) whose regulation is remarkably complex. Its expression is regulated by ATF6 and IRE1α (27, 69). XBP1 gene transcription is induced by XBP1s itself and activated ATF6 (69). As mentioned above, IRE1α must be activated to induce the splicing that synthesizes the XBP1s mRNA. In unstressed cells, the XBP1u mRNA is associated with ER-bound ribosomes via a mechanism that involves translation of a hydrophobic region of the unspliced protein (67). Furthermore, translational pausing within the C terminus of the XBP1u protein ensures ER membrane targeting. This facilitates rapid splicing by activated IRE1α when the UPR is induced (68). Both preexisting XBP1u mRNA and mRNA that is transcribed during the UPR are believed to be spliced via this membrane-targeting mechanism (67).
The UPR requires translational reprogramming of the stressed cells. The global inhibition of protein synthesis via phosphorylation of eIF2α (eIF2α-P) during the early response (first 3 h) represses translation of most mRNAs (48, 50). However, some mRNAs that encode stress response proteins are translated more efficiently when eIF2α is phosphorylated (51). These include the mRNAs for the transcription factor ATF4, a master transcriptional regulator of the stress response (57), and the phosphatase subunit GADD34 (29, 44). After 6 to 12 h, the increased GADD34 promotes dephosphorylation of eIF2α, which allows translational recovery (16, 42, 43). Overall, positive and negative regulation of mRNA translation by eIF2α-P during the UPR controls the cellular stress response (34).
Regulation of mRNA degradation is important during stress. One study found an increase in the stability of mRNAs whose translation was inhibited during the UPR (23). A similar finding was made in hypoxic cells, in which stress-induced mRNAs were stabilized in a process that required eIF2α phosphorylation (10). We also showed that the mRNA for the Cat-1 cationic amino acid transporter was stabilized during amino acid starvation (66). However, recent studies did not support a single mechanism that regulates mRNA turnover during stress (46, 47). These findings prompted us to examine the relationship between XBP1 mRNA levels and translational control during the UPR.
In this study, we tested the hypothesis that regulation of XBP1 mRNA stability is part of the integrated stress response and contributes to XBP1 accumulation during the UPR. We investigated whether the stability of this mRNA changes during stress. Interestingly, we observed a stabilization of the XBP1s mRNA during early, but not late, ER stress. This stabilization required both cytoplasmic splicing of the XBP1 mRNA and phosphorylation of eIF2α. The stabilization of XBP1s mRNA was dependent on the reduced translation of this message during early ER stress. Because we only observed stress-induced stabilization when the XBP1s mRNA was expressed from a cDNA encoding the unspliced form, we propose that cytoplasmic splicing “marks” the XBP1s mRNA for stabilization during the translational repression of the UPR. These findings suggest that the XBP1s mRNA is stabilized during early stress when translation is inhibited so that it can be translated after the inhibition is relieved as part of the stress response program. Because XBP1s induces transcription of its own gene and genes involved in protein folding and degradation, we propose that control of XBP1s mRNA stability is a molecular switch that regulates protein quality control during the UPR.
MATERIALS AND METHODS
Cell culture and transfections.
Mouse embyronic fibroblasts (MEFs) were cultured as described previously (5). MEF lines included the wild type (S/S), the S51A mutant of eIF2α (A/A; gift of R. Kaufman), GADD34−/− (gift of D. Ron), XBP1−/− (gift of R. Wek), and IRE1α−/− (gift of F. Papa). Thapsigargin (Tg) treatment was performed as described previously (31). Plasmid DNAs were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. 1-NM-PP1 {4-amino-1-tert-butyl-3-(1′-naphthylmethyl)pyrazolo[3,4-d]pyrimidine} (5 μM) and cordycepin (10 μg/ml) were obtained from Calbiochem and Sigma, respectively. UPF1 was depleted in A/A cells by transfection with UPF1 SMARTpool small interfering RNA (siRNA) (75 nM final concentration; Dharmacon), and mRNA half-lives were evaluated 48 h after transfection. Control siRNA was obtained from Ambion.
To measure mRNA half-life, cells were treated with actinomycin D (ActD; 10 μg/ml; Sigma) for the indicated times. Total RNA was prepared with TRIzol (Invitrogen), and mRNA levels were quantified by reverse transcription-quantitative PCR (RT-qPCR) using the primers listed in Table S1 of the supplemental material. Values were normalized to 18S rRNA signals, and half-lives were calculated by nonlinear curve fitting of single exponentials. For analysis of mRNA from the constructs shown in Fig. 3A, below, mRNAs were reverse transcribed using a primer specific for vector sequences in the 3′-untranslated region (UTR).
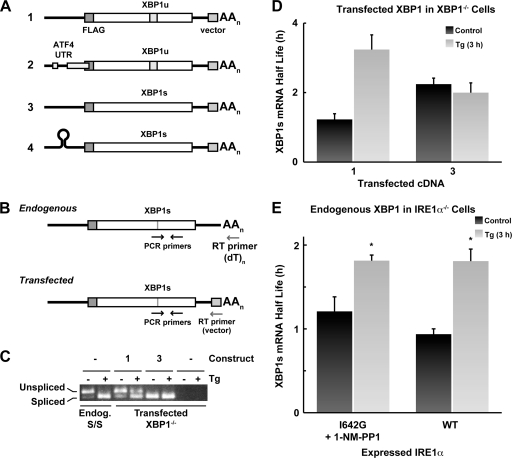
Fig 3.
Splicing of XBP1 mRNA is required for increased stability during the UPR. (A) Expression plasmids used to analyze the role of mRNA splicing in the stabilization of XBP1s mRNA during the UPR. (B) Scheme for selective analysis of endogenous and exogenous XBP1s mRNAs by RT-PCR. To detect exogenous mRNAs, mRNAs were reverse transcribed using a primer complementary to vector sequences in the 3′-UTR. (C) XBP1−/− cells transfected with the indicated plasmids were cultured with Tg for 3 h. Splicing of endogenous mRNA from S/S cells and transgenic mRNAs from XBP1−/− cells were analyzed by RT-PCR and agarose gel electrophoresis. The specificity of the method for detecting exogenous mRNA was demonstrated by the lack of bands in untransfected cells. (D) XBP1−/− MEFs were transfected with the indicated constructs and treated with Tg for 3 h, and the half-life of XBP1s mRNA was measured as described for Fig. 1. (E) IRE1α−/− MEFs were transfected with either I642G or WT IRE1α expression plasmids. The former cells were cultured with 1-NM-PP1 overnight before the experiment. Cells were treated with Tg for 3 h, and the half-life of XBP1s mRNA was assessed as described for Fig. 1. *, response of Tg-treated cells was significantly different from the control value (P < 0.05).
Validation of PCR primers for the detection of XBP1 mRNA species.
In this study, we used specific primer pairs to detect either XBP1s, XBP1u, or total XBP1 mRNAs by RT-PCR. To demonstrate the specificity of these primers, we performed PCR analysis using XBP1s and XBP1u cDNAs as templates. Figure S1A in the supplemental material shows that the XBP1u-specific and XBP1s-specific primers amplified the appropriate cDNAs. In addition, the primers for total XBP1, which are complementary to a region in the 3′-UTR, amplified both cDNAs. The specificities of the primers were further confirmed by RT-PCR on RNA from IREα−/− and S/S cells (see Fig. S1B in the supplemental material). IREα−/− cells express only XBP1u mRNA; as expected, a PCR product was seen with the XBP1u but not the XBP1s primers. Similarly, the XBP1u primers gave a strong signal in control S/S cells but not in Tg-treated S/S cells, whereas the XBP1s primers gave the opposite results.
Plasmids.
Mammalian expression vectors encoding FLAG-tagged mouse XBP1u or XBP1s cDNA (see Fig. 3A, constructs 1 and 3, below) were generated by RT-PCR amplification of mouse total RNA from either control or Tg-treated cells using the primers 5′-CCGAAGCTTATGGATTACAAGGACGACGACGATAAG GTGGTGGTGGCAGCGGCGCCGAGCGCG-3′ and 5′-AAAGCGGCCGCTCTGTGTTGCTTTTTTTTTAATTGCAAGGG-3′. The amplification added a FLAG epitope to the N terminus of the XBP1 open reading frame (ORF). cDNAs were inserted into the HindIII and NotI sites of pcDNA3.1/Hygro(+) (Invitrogen), followed by sequencing to identify XBP1u and XBP1s clones. Construct 2, which contains the 5′-UTR of the ATF4 mRNA, was generated by PCR amplification of this region from an ATF4-LUC plasmid (obtained from R. C. Wek) using 5′-GAGGCCTAGGCTTTTGCAAAAAGCTTATTTCTGCTTGCTGTCTGCCGGTTTAAGTTG and 5′-TTTAAGCTTATGCAGTTGCTCTCCAGCGGTTCCATCTTC as primers (57). This construct carried a small segment of the LUC ORF. This was added to construct 1. Construct 4, which encodes a hairpin in the mRNA 5′-UTR, was created by annealing the 5′-phosphorylated primers 5′-AGCTTCAAGCTTATCGATTTCGAACCCGGGGTAGA and 5′-CCGGTCTACCCCGGGTTCGAAATCGATAAGCTTGA, followed by ligation into the HindIII site of construct 3. Mammalian expression vectors for wild-type (WT) IRE1α and the IRE1α I642G mutant were obtained from R. Kaufman (University of Michigan) and F. Papa (University of California, San Francisco), respectively.
Poly(A) tail length assay.
The lengths of mRNA poly(A) tails were measured using the poly(A) tail length assay kit (Affymetrix) according to the manufacturer's protocol. Briefly, 1.4 μg of total RNA was G-I tailed and reverse transcribed using a tail-specific primer. Samples were amplified by PCR using an XBP1-specific forward primer and either an XBP1-specific reverse primer or the universal reverse primer provided with the kit (see Table S1 in the supplemental material). PCR products were analyzed on a 2.5% agarose gel and visualized by ethidium bromide staining.
Other methods.
The incorporation of [35S]Met/Cys into protein was measured as described previously (5). Western blotting was performed as described previously (5) using antibodies to mouse XBP1 (Santa Cruz Biotechnology), mouse eIF2α (Quality Controlled Biochemicals), mouse tubulin (Sigma), rabbit eIF2α-P (Invitrogen), and FLAG (Sigma-Aldrich). Antibodies against UPF1 were a kind gift of N. Gehring, Heidelberg, Germany. Polyribosome analysis was performed on cell lysates by sucrose gradient sedimentation as described previously (31), and mRNAs were quantified by RT-qPCR as described above.
RESULTS
Phosphorylation of eIF2α is required for increased XBP1s mRNA stability during the UPR.
We previously showed that the accumulation of XBP1s protein during the UPR is partially dependent on eIF2α-P (19). In this study, we examined the mechanism for this effect, focusing on the stability of XBP1s mRNA as a possible regulatory site. There are no studies on the regulation of XBP1s mRNA translation and turnover during the UPR, and a genome-wide screen by the Gorospe group did not identify XBP1 as an mRNA with regulated turnover (23).
We used WT MEFs (S/S) or MEFs with the S51A mutation in eIF2α, which cannot be phosphorylated (A/A), and induced the UPR by Tg treatment, which lowers ER calcium by inhibiting transport from the cytoplasm (56). As previously reported (19), XBP1s protein levels showed a large gradual increase between 3 and 9 h in Tg-treated S/S cells (Fig. 1A). In contrast, the increase was greatly attenuated in A/A cells. In order to address the mechanism of the differential response of S/S and A/A cells, we examined the levels of XBP1s mRNA in the same experiment (Fig. 1B). XBP1s mRNA accumulated to levels 30-fold higher after 3 h of Tg treatment, with an additional 1.5-fold increase between 3 and 9 h. There was a striking difference in A/A cells, where the increase was only 5-fold. The difference in accumulation was not due to differences in the extent of UPR-induced cytoplasmic splicing of the XBP1 mRNA; nearly all (90%) of the XBP1 mRNA was spliced after 3 h of Tg treatment in both cell lines (Fig. 1C). Note that low levels of XBP1s mRNA are expressed in control cells, even though the UPR is not induced.
Fig 1.
XBP1s mRNA is stabilized after 3 h of the UPR in a process that requires translational repression. (A) S/S or A/A cells were incubated with Tg for the indicated times and analyzed for the indicated proteins by Western blotting. (B) S/S and A/A cells were incubated with Tg and/or Hip for the indicated times, and XBP1s mRNA levels were assessed by RT-qPCR. In the right-most sample, A/A cells were incubated for 6 h with Tg and Hip and then for 3 h with Tg only. (C) S/S and A/A cells were incubated with Tg for the indicated times, and the levels of XBP1u and XBP1s mRNAs were assessed by RT-qPCR. The graph shows the amount of XBP1s mRNA as a percentage of the total. (D) S/S and A/A cells were treated with Tg for 3 h and then treated with ActD and Tg for the indicated times, and XBP1s mRNA was quantified by RT-qPCR. Values were normalized to 18S rRNA and expressed as percentages of the levels before the addition of ActD.
These data suggest that control of transcription and mRNA turnover may account for the differential regulation of XBP1 gene expression in S/S and A/A cells. XBP1 gene transcription is regulated by XBP1 itself and the transcription factor ATF6 (69), which is activated during the UPR. The contribution of eIF2α phosphorylation to ATF6 activation during the UPR has not been extensively studied. However, it has been reported by the Mori and Wek groups that accumulation of the active ATF6 protein decreases in late stress in PERK-deficient cells, suggesting an involvement of PERK/eIF2α phosphorylation signaling in ATF6 activation (1, 55). Although transcriptional control accounts for some of the observed differences in XBP1 mRNA accumulation in S/S and A/A cells, we focused on the regulation of XBP1s mRNA turnover during the UPR as an additional mechanism of gene regulation. The half-lives of the XBP1s mRNA in S/S and A/A cells were determined using ActD, an inhibitor of transcription. The rate of mRNA loss during ActD treatment is a measure of the turnover rate. The half-life of XBP1s mRNA in S/S-treated cells increased from 2.8 ± 0.5 to 6.6 ± 1.8 h (means ± standard errors) after 3 h of Tg treatment (Fig. 1D). Because nearly all of the XBP1 mRNA is spliced after 3 h of Tg treatment, the conversion of XBP1u to XBP1s mRNA does not contribute to the accumulation of XBP1s mRNA during the measurement of the mRNA half-life. The half-life of total XBP1 mRNA (measured with qPCR primers that can detect both XBP1s and XBP1u mRNAs) was 2.2 ± 0.2 in controls and 4.8 ± 0.3 after 3 h of Tg treatment. Because the XBP1u mRNA has a short half-life that does not change during treatment with Tg (see Table S2 in the supplemental material), our data support the notion that the increased half-life of the total XBP1 mRNA at 3 h of Tg treatment was mainly due to the XBP1s species. In contrast, the half-life of XBP1s mRNA in A/A-treated cells decreased from 2.2 ± 0.4 to 1.2 ± 0.1 h. These data suggest that enhanced stability of XBP1s mRNA in S/S cells, but not A/A cells, contributes to the differential accumulation of XBP1s mRNA in S/S and A/A cells during the UPR. These data also suggest that phosphorylation of eIF2α is required for stabilization of the XBP1s mRNA during the early UPR. Similar data were obtained when the UPR was induced with tunicamycin, an inhibitor of protein glycosylation (data not shown).
Inhibition of translation initiation during ER stress stabilizes the XBP1s mRNA.
The effect of eIF2α-P on XBP1s mRNA stability could be the result of signaling pathways that are triggered by eIF2α-P or could be a direct effect of the inhibition of protein synthesis caused by eIF2α-P. We favor the latter possibility, because XBP1s mRNA stability increased in S/S cells after 30 min of Tg treatment (data not shown). To test this hypothesis, we used A/A cells, in which protein synthesis is not inhibited during the UPR. If the inhibition of protein synthesis increased the half-life of XBP1s mRNA, we reasoned that its stability would increase when Tg-treated A/A cells were incubated with hippuristanol (Hip), which inhibits the initiation of protein synthesis. In agreement with our hypothesis, the half-life of XBP1s mRNA in Tg-treated A/A cells increased from 1.2 ± 0.1 to 9 ± 2 h in the presence of Hip (Fig. 2A and B). The effect of Hip was reversible, because the XBP1s half-life was 1.7 ± 0.1 h when Hip was removed after 3 h followed by 4 h of incubation in Tg and ActD for evaluation of half-life.
Fig 2.
Inhibition of protein synthesis in A/A cells stabilizes XBP1s mRNA. (A) A/A cells were incubated with Tg and Hip as indicated. After 3 h, ActD was added, and the XBP1s mRNA half-life was measured as described for Fig. 1. (B) S/S and A/A cells were treated as described for Fig. 1B, and XBP1s protein was quantified by Western blotting. Tubulin was measured as a loading control.
Additional support for our hypothesis was obtained by measuring the levels of XBP1s mRNA and protein. Incubation of A/A cells with Hip and Tg gave greater mRNA levels than Tg alone, even though Hip had no effect by itself (Fig. 1B). In addition, the XBP1s mRNA level fell when Hip was removed and cells were incubated with Tg alone. In order to further support the functional significance of this regulation of XBP1 gene expression, we determined the levels of XBP1 protein in A/A cells. Accumulation of XBP1s protein was lower in A/A cells than in S/S cells treated with Tg for similar times (Fig. 1A and 2B). As expected, because it inhibits protein synthesis, Hip treatment diminished XBP1s protein levels in A/A cells (Fig. 2B). However, when Hip was removed at 6 h and Tg treatment continued for an additional 3 h, the level of XBP1s protein was higher than in A/A cells incubated with Tg for 9 h. This is consistent with Hip-induced accumulation of XBP1s mRNA (Fig. 1B) followed by translation after the Hip was removed (Fig. 2B). The functional importance of increased XBP1s levels in A/A cells was demonstrated by examining the target gene ERDJ4 (25). A/A cells treated with Tg and Hip for 6 h followed by 3 h in Tg alone resulted in a 3.7-fold increase in ERDJ4 mRNA compared to A/A cells treated with Tg alone (data not shown). These data suggest that inhibition of protein synthesis during the UPR plays a central role in the increased XBP1s mRNA stability, which leads to amplification of the XBP1s-mediated transcription program.
Cytoplasmic splicing of XBP1u is required for the increased XBP1s mRNA stability during the UPR.
In order to analyze the element(s) of the XBP1s mRNA that contributes to increased stability during the UPR, we used expression vectors for XBP1u and XBP1s cDNAs (Fig. 3A, constructs 1 and 3). These were transfected into MEFs deficient in XBP1 (XBP1−/−), and the expressed mRNAs were analyzed for their turnover during the UPR. The XBP1u mRNA was efficiently spliced in XBP1−/− cells treated with Tg, as determined by RT-PCR (Fig. 3C). The half-lives of XBP1s mRNA expressed from the transfected XBP1u or XBP1s expression vectors were evaluated in XBP1−/− cells treated with Tg for 3 h. Interestingly, Tg treatment of XBP1u-transfected cells caused an increase of the half-life XBP1s mRNA from 1.6 to 3.2 h (Fig. 3D). In contrast, no change in the half-life of the exogenously expressed XBP1s mRNA was observed. These data suggest that the increased stability of XBP1s mRNA during the UPR requires a “tagging” that occurs during cytoplasmic splicing.
To further support the requirement for both protein synthesis inhibition and cytoplasmic mRNA splicing for increased XBP1s mRNA stability, we used a system where XBP1 mRNA splicing was induced in the absence of stress (12). The IRE1α protein initiates XBP1u mRNA splicing by cleavage of the mRNA. Its RNase domain is activated by the UPR-induced stimulation of the IRE1α protein kinase and autophosphorylation (28). We used a mutant IRE1α (I642G) in which the RNase activity can be activated by the kinase inhibitor 1-NM-PP1 in the absence of stress (12). We hypothesized that IRE1α−/− MEFs transfected with IRE1α I642G and treated with 1-NM-PP1 should splice the endogenous XBP1u mRNA to XBP1s without increasing its stability; in addition, Tg treatment should stabilize the XBP1s mRNA. As shown in Fig. 3E, the half-life of XBP1s mRNA in IRE1α-1642G-expressing cells treated with 1-NM-PP1 was 1.2 h, which increased to 1.8 h when Tg was added. Furthermore, the half-life of the XBP1s mRNA in IRE1α−/− cells transfected with the WT IRE1α was 1.1 h, and 1.9 h after treatment with Tg for 3 h (Fig. 3E). These data support our hypothesis that both mRNA splicing and Tg-induced inhibition of protein synthesis are required for increased stability of the XBP1s mRNA during early ER stress.
Modulation of poly(A) tail length is not a critical factor for increased XBP1s mRNA stability during the UPR.
The poly(A) tail lengths of mRNAs contribute to their stability (62). Poly(A) tail shortening initiates the major mRNA decay pathways, and it is usually the rate-limiting step in mRNA degradation (45). We hypothesized that the XBP1s mRNA poly(A) tails would be longer after 3 h of stress, at a time when mRNA stability is increased. To test this hypothesis, we used the poly(A) tail length (PAT) assay (Fig. 4A) in S/S and A/A cells. In this assay, isolated mRNAs were tailed and reverse transcribed. They were then analyzed using two sets of primers: (i) a 5′ primer specific for XBP1 and a 3′ primer complementary to the junction of the poly(A) and the added tail, and (ii) the same 5′ primer and a 3′ primer specific for the 3′ end of the XBP1 3′-UTR. The length of the poly(A) tail was estimated from the size differences of the products. Because of stress-induced splicing, this assay mostly detects XBP1u mRNA in untreated cells and XBP1s mRNA in Tg-treated cells.
Fig 4.
mRNA poly(A) tail length does not affect the stabilization of XBP1s mRNA during the UPR. (A) Method for detecting mRNA poly(A) tail length. poly(G · I) tails were added to mRNAs followed by reverse transcription with a tail-specific primer. PCR was performed using a common upstream primer (3) and a downstream primer complementary to the added tail (1) or the 3′-UTR (2). The differences in the sizes of the products were due to the poly(A) tail. (B) S/S and A/A MEFs were cultured with Tg for the indicated times and analyzed as described for panel A. (C, D, and E) S/S cells were cultured with cordycepin for 2 h, followed by cordycepin and Tg for 3 h as indicated. (C) XBP1 mRNA splicing was assessed by RT-PCR; (D) poly(A) tail length was assessed; (E) XBP1s mRNA half-life was measured as described for Fig. 1.
The PAT assay (Fig. 4B) showed that the length of poly(A) tails on XBP1 mRNA in S/S cells transiently increased during the UPR. Poly(A) tails were longer after 3 h of stress, and this increase was no longer evident at 7 h. In contrast, the poly(A) tail length was not affected by Tg treatment of A/A cells. Therefore, the accumulation of XBP1s mRNA with longer poly(A) tails correlates with the increased mRNA stability during the early UPR. To determine if the longer poly(A) tail length is required for increased XBP1s mRNA stability in S/S cells during the UPR, we examined the effect of cordycepin, an inhibitor of polyadenylation. Cordycepin treatment of cells did not significantly affect UPR-induced XBP1 mRNA splicing (Fig. 4C), but it did reduce poly(A) tail length in both control and stressed cells (Fig. 4D). We measured the effect of cordycepin on the half-life of XBP1s mRNA in S/S cells (Fig. 4E). The half-life in cordycepin-treated cells was 3.9 ± 0.1 h. This value increased to 6.6 ± 1.5 h in cells treated with cordycepin and Tg, which is similar to the value seen with Tg alone (7.0 ± 0.9 h). These data suggest that XBP1s mRNA is stabilized in S/S cells during the early UPR, independently of mechanisms that involve deadenylation.
Translational recovery during the UPR leads to decreased stability of the XBP1s mRNA.
It is well established that the translational repression during the UPR is followed by partial recovery via induction of the PP1 phosphatase subunit GADD34 and subsequent dephosphorylation of eIF2α (Fig. 1A and 5A) (43). Because the increase in XBP1s mRNA stability was dependent on inhibition of protein synthesis, we tested the stability of XBP1s mRNA during the translational recovery period (7 to 9 h of Tg treatment). The half-life of the XBP1s mRNA after 7 h of Tg treatment in S/S cells was shorter than in untreated cells (Fig. 5B). Decreased XBP1s mRNA stability was also observed in Tg-treated A/A cells after 7 h of stress. Furthermore, translational recovery following Hip treatment of A/A cells caused destabilization of the XBP1s mRNA (Fig. 2A). These data support our hypothesis that eIF2α-P-mediated inhibition of protein synthesis during the UPR is required for increased XBP1s mRNA stability. To further support this conclusion, we tested XBP1s mRNA stability in GADD34−/− MEFs (43), which sustain both eIF2α-P and decreased translation rates during the late UPR (7 to 9 h). In agreement with our hypothesis, the half-life of the XBP1s mRNA was longer than 10 h in GADD34−/− MEFs treated with Tg for 3 and 7 h (Fig. 5C). The very long half-life of XBP1s mRNA in GADD34−/− MEFs can be explained by the severe inhibition of protein synthesis that results in the absence of XBP1s protein in these cells (data not shown). Our combined data suggest that during the UPR, translational repression stabilizes the XBP1s mRNA and translational recovery destabilizes it, as a means of controlling the level of the stress response.
Fig 5.
Dephosphorylation of eIF2α during prolonged stress results in loss of XBP1s mRNA stabilization. (A) S/S and A/A cells were treated with Tg for the indicated times, and the incorporation of [35S]Met/Cys was measured. (B) S/S cells were cultured with Tg for 7 h, and the half-life of XBP1s mRNA was assessed as described for Fig. 1. *, result for Tg-treated cells was significantly different from the control value (P < 0.05). (C) GADD34−/− MEFs were cultured with Tg for the indicated times, and the XBP1s mRNA half-life was assessed as described for Fig. 1.
Translational repression of the XBP1s mRNA is required for increased stability during the early UPR.
Our finding that XBP1s mRNA stabilization during the UPR requires inhibition of protein synthesis raises the question of whether this effect is due to global inhibition of protein synthesis or specific inhibition of XBP1s mRNA translation. As a first step in answering this question, we determined the distribution of XBP1s mRNA on polyribosomes of S/S cells during the UPR, as a measurement of its ability to be translated. mRNAs are found in heavier polyribosomes when they are efficiently translated and in lighter polyribosomes or free ribonucleoprotein when they are translationally repressed (37). The XBP1s mRNA was efficiently translated in control cells, with 67% of the mRNA present on polyribosomes (Fig. 6A). Tg treatment caused a shift of the XBP1s mRNA species out of polyribosomes, with 24% remaining at 1 h (Fig. 6A). The translational recovery during late stress (Fig. 5A) was evident, with 87% of the XBP1s mRNA in polyribosomes after 9 h of treatment.
Fig 6.
Decreased translational efficiency is required for stabilization of XBP1s mRNA during ER stress. (A) S/S cells were treated with Tg for the indicated times, and the distribution of XBP1s mRNA in polyribosomes was analyzed. (B) S/S cells were transfected with the indicated constructs from Fig. 3A and incubated with or without Tg for 3 h, and the splicing of XBP1 mRNA was assessed by RT-PCR and gel electrophoresis. (C) S/S cells were transfected with the indicated constructs (Fig. 3A), and the expression of Flag-XBP1s was detected by Western blotting using anti-FLAG antibody. (D) S/S cells were transfected and cultured as for panel B. (Left) The half-lives of the expressed XBP1s mRNAs were determined as described for Fig. 1. (Right) The distribution of XBP1s mRNA on polyribosome profiles was analyzed and used to calculate the percentage of the total signal in the polyribosome fraction. *, result for Tg-treated cells was significantly different from the control value (P < 0.05); **, result for control cells transfected with construct 2 was significantly different from cells with control cells with construct 1 (P < 0.05). A representative experiment for the distribution of XBP1s mRNA on polyribosome profiles is shown.
In order to directly assess if the stabilization of XBP1s mRNA during the UPR requires translational inhibition of its own mRNA, we examined constructs that encode XBP1 mRNAs with 5′-UTRs that affect the translational efficiency (Fig. 3A). To detect these XBP1s mRNAs by RT-qPCR in S/S cells that express endogenous XBP1, they were selectively reverse transcribed using a primer complementary to vector sequences in the 3′-UTR (Fig. 3B). Transgenic XBP1s mRNA levels were then measured by qPCR using primers specific for XBP1s. In addition, the encoded XBP1 protein contained a FLAG epitope.
To determine if the increased stability of XBP1s mRNA during the UPR requires the specific inhibition of this mRNA's translation, we compared the half-lives of XBP1s mRNA from constructs 1 and 2 (Fig. 3A). Both constructs encode XBP1u; construct 2 also contains the 5′-UTR of the ATF4 mRNA. ATF4 mRNA was chosen because it is poorly translated in control cells and efficiently translated during the early UPR (32, 59). RT-PCR analysis showed the expression of XBP1u mRNAs from these constructs in control cells and the UPR-induced accumulation of XBP1s mRNA after 3 h of Tg treatment (Fig. 6B).
As expected, the half-life of XBP1s mRNA expressed from construct 1 increased from 2.3 to 5.3 h after 3 h of Tg treatment (Fig. 6D). In contrast, XBP1s mRNA from construct 2 had a long half-life under control conditions and decreased stability during the UPR (5.2 versus 2.8 h). Moreover, the half-lives of these mRNAs correlated with their translational efficiencies. Polyribosome analysis showed that mRNA from construct 1 was efficiently translated in control cells and poorly translated after 3 h of Tg treatment, similar to endogenous XBP1s mRNA (Fig. 6D). The opposite was seen with construct 2. It is noteworthy that the stability of the mRNA from construct 2 decreased during the UPR when its translational efficiency increased. Because this occurred at a time when global translation was inhibited, it is likely that the translational efficiency of XBP1s mRNA is a key factor in regulating its stability during the UPR.
To confirm the importance of cytoplasmic splicing in the regulation of XBP1s stability in S/S cells, we compared the FLAG-XBP1 mRNAs from constructs 1 and 3, which encode XBP1u and -s, respectively (Fig. 3A). As noted above, the half-life of XBP1s mRNA from construct 1 increased after 3 h of Tg treatment (Fig. 6D), and this increased stability was correlated with a decrease in polyribosome association. In contrast, the half-life of mRNA expressed as XBP1s from construct 3 had similar half-lives with and without Tg treatment (2.9 versus 2.2 h). Despite the absence of a change in half-life, the translational efficiency of this mRNA was reduced by Tg treatment, supporting our hypothesis that the stabilization of XBP1s mRNA during the UPR requires both cytoplasmic splicing of XBP1u and translational inhibition.
Finally, we compared mRNAs from constructs 3 and 4 (Fig. 3A) to test if XBP1s mRNA is stabilized by decreased translation efficiency in the absence of stress. These constructs encode XBP1s mRNA. In addition, construct 4 contains a hairpin in the 5′-UTR, which should inhibit translation initiation (9). This was confirmed by low expression of FLAG-XBP1s protein (Fig. 6C) and low association with polyribosomes (Fig. 6D). The half-lives of the FLAG-XBP1s mRNAs from constructs 3 and 4 were similar in unstressed cells (2.9 versus 2.7 h). This demonstrated that decreased translation efficiency by itself does not impart increased stability to the XBP1s mRNA from construct 4 that has not been subjected to cytoplasmic splicing. Our combined data support the conclusion that the UPR increases the stability of XBP1s mRNA via a mechanism that requires cytoplasmic splicing and inhibition of its own translation.
To determine if other stress-related mRNAs are stabilized during the UPR by a mechanism similar to the one we have described for XBP1s, we analyzed the mRNAs for ATF4 and CHOP. Expression of these leucine zipper transcription factors is induced during the UPR (42). In S/S cells, the half-life for ATF4 mRNA increased from 2.3 h in control S/S cells to 4.6 h after 3 h of Tg treatment (see Fig. S1 in the supplemental material). Similarly, the half-life of CHOP mRNA increased from 1.5 h in control S/S cells to 5.1 h after 3 h of stress. Polyribosome analysis indicated that the translational efficiency of ATF4 mRNA increased markedly after 3 h of stress, whereas the CHOP mRNA was inefficiently translated in controls and after 3 h of Tg treatment. In agreement with previous studies (44), we found an increased association of CHOP mRNA with the polyribosome fraction after 6 to 9 h of Tg treatment (data not shown). Thus, even though the ATF4 and CHOP mRNAs showed increased stability during the early UPR, this change was not accompanied by the reduced translational efficiency we reported for XBP1s mRNA. Therefore, our data suggest that there is not a common mechanism for regulation of mRNA turnover of UPR-induced mRNAs.
Molecular memory of the XBP1s mRNA regulates turnover independently of the NMD pathway.
Our hypothesis that regulation of XBP1s mRNA stability requires “marks” left on the mRNA by splicing is reminiscent of the non-sense-mediated mRNA decay (NMD) pathway (7, 10). NMD is a mechanism for degradation of mRNAs that contain premature termination codons (PTCs). This process involves marks left on mRNAs at the exon junctions which target these mRNAs for degradation if they are upstream of PTCs during the pioneer round of translation (20). Previous studies have shown that cellular stress (nutrient deprivation and hypoxia) stabilizes NMD-targeted mRNAs (10). Several stress-induced mRNAs have been described as NMD targets (10), and a recent report elegantly showed that stress-induced eIF2α phosphorylation inhibits NMD (58). Based on these findings, we tested if the increased stability of XBP1s mRNA during the UPR was due to inhibition of NMD. This was accomplished by examining the effect of depleting the RNA helicase UPF1/Rent1 with an siRNA which inhibited NMD (39). The experiment was carried out in A/A cells, because NMD is not inhibited by ER stress in these cells (58).
We first showed that the specific siRNA depleted UPF1 protein in A/A cells (Fig. 7A) and that it did not affect the UPR-induced splicing of XBP1 mRNA (Fig. 7B). Next, we showed that depletion of UPF1 did not affect the half-life of XBP1s mRNA in control or Tg-treated A/A cells (Fig. 7C). In contrast, DNAJB2 mRNA, a target of the NMD pathway (10), was stabilized in UPF1-depleted A/A cells (Fig. 7C). These data suggest that the XBP1s mRNA is not targeted by the NMD pathway.
Fig 7.
NMD-independent marking of the XBP1s mRNA during the UPR. (A to C) A/A cells were transfected with control siRNA or siRNA to UPF1. After 48 h, UPF1 protein was measured by Western blotting (A), XBP1s splicing was measured by RT-PCR (B), and the half-lives of XBP1s and DNABJ2 mRNAs were measured as described for Fig. 1. (D) S/S cells were treated with Tg and Hip as indicated, and the XBP1s mRNA half-life was evaluated after 8 h as described for Fig. 1.
We examined the effect of translational inhibition late in the UPR as a test of our hypothesis that XBP1 mRNA splicing marks the mRNA in a way that enables its stabilization. As described above, we found that XBP1s mRNA is less stable during late stress when translation is efficient than in early stress when translation is inhibited (Fig. 6). We reasoned that if the mark on the XBP1s mRNA persists throughout the UPR, inhibition of translation during late stress should cause mRNA stabilization. To test this hypothesis, we measured the half-life of the XBP1s mRNA in late stress (8 h of Tg treatment) in the presence and absence of Hip (Fig. 7D). Inhibition of XBP1s mRNA translation in late stress dramatically stabilized the XBP1s mRNA, increasing the half-life from 1.35 ± 0.3 to >10 h (Fig. 7D). These data suggest that “molecular memory” of cytoplasmic splicing controls XBP1s mRNA turnover during the UPR.
DISCUSSION
The transcriptional and translational reprogramming of cells exposed to diverse stress conditions is a crucial part of the adaptive response to stress (13, 18). A common feature of the response to many stressors is the phosphorylation of eIF2α. This inhibits global protein synthesis and also reprograms cells to express proteins, such as ATF4, that induce the transcription program (49, 60). As the response progresses, dephosphorylation of eIF2α allows translation of the stress-induced mRNAs and execution of the adaptive response.
The XBP1u mRNA contains a 26-nucleotide intron that is spliced out in the cytoplasm via a sophisticated mechanism that has been described recently (11). Splicing occurs very early in the stress response (by 30 min, 60% of the XBP1 mRNA is spliced). However, the XBP1s mRNA cannot be translated early in the stress response due to the eIF2α-P-mediated inhibition of translation. We show here that spliced and inefficiently translated XBP1s mRNA is protected from degradation early in the stress response. The accumulated XBP1s mRNA becomes available for translation during late stress, when eIF2α is dephosphorylated and translation increases (42).
We present here a new paradigm of regulation of gene expression during stress that involves increased stability of the XBP1 mRNA via the cooperation of cytoplasmic splicing and inhibition of its translation (Fig. 8). The requirement for splicing is established by the fact that exogenously expressed XBP1u mRNA produced an XBP1s mRNA that was stabilized during the early UPR. In contrast, exogenously expressed XBP1s mRNA was not stabilized. The importance of translational inhibition of the XBP1s mRNA was shown using construct 2, which contains the 5′-UTR of the ATF4 mRNA. In unstressed cells, this mRNA was translated poorly and it was stabilized, whereas in stressed cells, the mRNA was translated efficiently and it was not stabilized. Finally, inclusion of a hairpin in the 5′-UTR of XBP1s mRNA inhibited the translation but did not affect mRNA stability in unstressed cells, establishing a requirement for both translational inhibition and cytoplasmic splicing. These data support a model where cytoplasmic splicing “marks” the XBP1s mRNA for stabilization via a mechanism that involves its inefficient translation.
Fig 8.
Model for regulation of XBP1s mRNA stability during the UPR. In unstressed cells, basal IRE1α activity produces small amounts of XBP1s mRNA that are degraded rapidly. Induction of the UPR activates IRE1α, which induces XBP1 mRNA splicing and deposition of protein factors on the mRNA. Phosphorylation of eIF2α by PERK inhibits XBP1s mRNA translation. The combination of cytoplasmic splicing and translational inhibition leads to the reduced turnover of XBP1s mRNA. As the UPR progresses, GADD34 induces dephosphorylation of eIF2α and translational recovery. XBP1s protein is synthesized from the accumulated mRNA, which stimulates the transcription of XBP1 and its target genes. The loss of translational inhibition leads to rapid turnover of the XBP1s mRNA, limiting the accumulation of this protein.
The mechanism by which cytoplasmic splicing of the XBP1 mRNA leads to its stabilization is unknown. XBP1 is the only mRNA known to be spliced in the cytoplasm. Splicing begins with mRNA cleavage by the IRE1α nuclease, with subsequent ligation of the cleaved fragments by unknown ligases (14). We propose (Fig. 8) that factors (likely to be proteins) associate with the mRNA during splicing and mark the mRNA for stabilization by translational repression during the early UPR. The association of these proteins must be part of a stable ribonucleoprotein complex, because the stabilization persists for several hours during the early UPR. In our model, recovery of mRNA translation during the late UPR destabilizes the mRNA. It is possible that this occurs via disruption of macromolecular interactions within the XBP1s mRNA by translating ribosomes.
Our model requires that splicing create a mark on the XBP1s mRNA that is stable over the life of the mRNA. This idea is supported by our findings that the mRNA was stabilized during the early UPR after splicing had occurred and that the XBP1 mRNA with the 5′-UTR of the ATF4 mRNA (construct 2) was stabilized in unstressed cells when it was poorly translated. Further evidence for this idea was obtained with our finding that XBP1s mRNA is stabilized by translational repression during the late UPR (Fig. 7). Because the mRNA is normally unstable at this time, this experiment suggests that the “mark” placed by splicing can cause mRNA stabilization by recurrence of translational repression.
Marks on mRNAs associated with their turnover have been extensively studied for NMD of mRNAs containing premature termination codons (41). The postsplicing mRNP complex which is formed near exon-exon junctions in the nucleus has been suggested to mark these mRNAs for nuclear export and decay (21), although it is still unclear how NMD complexes are placed on mRNAs with premature termination codons (40). In the case of regulation of XBP1s mRNA stability, we speculate that a similar complex may be formed following cytoplasmic splicing of the XBP1u mRNA, leading to regulation of its half-life. We have shown here that the XBP1s mRNA is not a target of NMD (Fig. 7). Future studies will determine if regulation of XBP1s mRNA stability shares elements with the NMD pathway. However, we should mention that inhibition of XBP1s mRNA translation is required for its stabilization during ER stress. This is in contrast to NMD target mRNAs that are stabilized during ER stress in a manner independent of their own translational efficiency (10). Although eIF2α phosphorylation is required for inhibition of NMD during ER stress, it is likely to involve a mechanism different than inhibition of NMD target mRNA translation (10).
Most cases of regulated mRNA turnover involve the binding of trans-acting factors to sequences in the 3′-UTR of mRNAs (53) which either promote or prevent degradation (3). The 3′-UTR may play a role in the regulation of XBP1 mRNA turnover. However, it is not sufficient for this regulation, because exogenously expressed XBP1s, which contains the 3′-UTR but not the 5′-UTR, was not stabilized during the early UPR (Fig. 6, compare constructs 1 and 3). In contrast, exogenously expressed XBP1u mRNA, which contained the 3′-UTR, was stabilized (Fig. 6, construct 1). If the 3′-UTR is involved, it must interact with other factors, including the “mark” that is added to the mRNA during cytoplasmic splicing. This intramolecular interaction may lead to mRNA stabilization early in the UPR. Efficient translation of the XBP1s mRNA during the late UPR may disrupt the intramolecular interactions, causing mRNA destabilization.
Our studies showed that the mechanism for increased XBP1s mRNA stability during the early UPR does not involve regulation of deadenylation. In eukaryotic cells, the poly(A) tail acts as a stabilizing element of cytoplasmic mRNAs (63), with degradation starting with poly(A) shortening (45, 61). However, shortening the poly(A) tail of the XBP1s mRNA with an inhibitor of polyadenylation did not affect its stability during the early UPR (Fig. 4). Our data suggest that the regulation of XBP1s mRNA stability is independent of poly(A) length and support the idea that this mechanism is specific for this mRNA.
It is well established that the XBP1s mRNA resides in the cytoplasm, in contrast to the unspliced XBP1 mRNA, which associates with the ER membrane (67). Our findings of increased stability at a time of translational repression during the UPR suggest that the XBP1s mRNA may be protected from degradation by accumulating in stress granules (SGs). SGs are dynamic cytoplasmic structures that can harbor translationally repressed mRNAs during stress (24). However, inhibition of stress granule formation by preventing expression of the key TIAR protein had no effect on regulation of XBP1s mRNA stability during the UPR (data not shown). Therefore, the mechanism of regulation of XBP1s mRNA stability during the UPR cannot be explained by simple sequestration in SGs.
The regulation of XBP1s mRNA stability is part of a positive feedback loop that regulates XBP1s protein accumulation during the UPR. An immediate response to the UPR is the cytoplasmic splicing of the XBP1u mRNA. Transcription of the XBP1 gene also increases during the UPR via the binding of XBP1s and ATF6 to an ER response element in the XBP1 gene promoter (65). The combined transcriptional activation and cytoplasmic mRNA splicing contribute to a significant accumulation of the XBP1s protein during stress (69). We showed that the increased stability of the XBP1s mRNA during the early UPR is required for maximum induction of XBP1 gene expression and transcription of its target genes (Fig. 2). This positive feedback leads to increased XBP1 protein levels during the UPR, thus further enhancing transcription of XBP1s target genes involved in protein quality control (65).
A novel observation of this report is that inhibition of protein synthesis via PERK-eIF2α signaling is required for maximal induction of XBP1s protein levels (reviewed in reference 52). PERK-eIF2α signaling during the UPR is known to induce both protective (33) and apoptotic (35, 38) transcription programs. We have shown here that the cytoprotective XBP1-induced transcription program during the UPR is also downstream of the eIF2α-P-mediated inhibition of protein synthesis. Our findings further highlight the importance of translational control during the UPR in cell fate decisions.
Finally, although we have studied the regulation of XBP1s mRNA stability during chemical induction of the UPR (with thapsigargin and tunicamycin), our findings may have more significant implications in many other cellular programs where XBP1s protein levels play important roles. For example, XBP1 levels are important in innate immunity as they regulate inflammatory gene expression (36), in hepatic lipogenesis via induction of transcription of lipogenic genes (26), and in glucose homeostasis via its actions independent of transcription (71). It should also be mentioned that increased levels of XBP1 have been associated with diseases such as inflammatory bowel disease (22) and atherosclerosis (70). Our studies introduce a novel mechanism for the regulation of XBP1 protein levels via regulation of stability of the mRNA. We also introduce a new paradigm in stress-induced gene expression that combines the cytoplasmic processes of spicing and translation inhibition. It will be interesting to determine if this mechanism of regulation of XBP1 levels is altered in inflammatory and obesity disease models.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants DK060596 and DK053307 (to M.H.) and DK42304, HL52173, and HL057346 (to R.J.K.) from the National Institutes of Health.
We thank Michael Harris, Case Western Reserve University, for assistance with the studies evaluating the half-lives of the mRNAs and David Ron, University of Cambridge, for GADD34−/− MEFs.
ADDENDUM
While the manuscript was under review, a report by the Gardner group (58) included XBP1 in the results of a microarray analysis of a genome-wide screen for NMD target mRNAs. The data showed that emetine and tunicamycin treatment of cells caused increased stability of XBP1 mRNA.
Footnotes
Published ahead of print 3 January 2012
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Adachi Y, et al. 2008. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct 33:75–89 [DOI] [PubMed] [Google Scholar]
- 2. Anelli T, Sitia R. 2008. Protein quality control in the early secretory pathway. EMBO J. 27:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barreau C, Paillard L, Osborne HB. 2005. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 33:7138–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded- protein response. Nat. Cell Biol. 2:326–332 [DOI] [PubMed] [Google Scholar]
- 5. Bevilacqua E, et al. 2010. eIF2α phosphorylation tips the balance to apoptosis during osmotic stress. J. Biol. Chem. 285:17098–17111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calfon M, et al. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96 [DOI] [PubMed] [Google Scholar]
- 7. Chiu SY, Lejeune F, Ranganathan AC, Maquat LE. 2004. The pioneer translation initiation complex is functionally distinct from but structurally overlaps with the steady-state translation initiation complex. Genes Dev. 18:745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fribley A, Zhang K, Kaufman RJ. 2009. Regulation of apoptosis by the unfolded protein response. Methods Mol. Biol. 559:191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaccioli F, et al. 2006. Amino acid starvation induces the SNAT2 neutral amino acid transporter by a mechanism that involves eukaryotic initiation factor 2α phosphorylation and cap-independent translation. J. Biol. Chem. 281:17929–17940 [DOI] [PubMed] [Google Scholar]
- 10. Gardner LB. 2008. Hypoxic inhibition of nonsense-mediated RNA decay regulates gene expression and the integrated stress response. Mol. Cell. Biol. 28:3729–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glimcher LH. 2010. XBP1: the last two decades. Ann. Rheum. Dis. 69(Suppl. 1):i67–i71 [DOI] [PubMed] [Google Scholar]
- 12. Han D, et al. 2009. IRE1α kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harding HP, Calfon M, Urano F, Novoa I, Ron D. 2002. Transcriptional and translational control in the Mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 18:575–599 [DOI] [PubMed] [Google Scholar]
- 14. Harding HP, et al. 2008. An intact unfolded protein response in Trpt1 knockout mice reveals phylogenic divergence in pathways for RNA ligation. RNA 14:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harding HP, Zhang Y, Ron D. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274 [DOI] [PubMed] [Google Scholar]
- 16. Harding HP, et al. 2009. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2α) dephosphorylation in mammalian development. Proc. Natl. Acad. Sci. U. S. A. 106:1832–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haze K, Yoshida H, Yanagi H, Yura T, Mori K. 1999. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10:3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holcik M, Sonenberg N. 2005. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 6:318–327 [DOI] [PubMed] [Google Scholar]
- 19. Huang CC, et al. 2010. Temporal regulation of Cat-1 (cationic amino acid transporter-1) gene transcription during endoplasmic reticulum stress. Biochem. J. 429:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishigaki Y, Li X, Serin G, Maquat LE. 2001. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 106:607–617 [DOI] [PubMed] [Google Scholar]
- 21. Isken O, Maquat LE. 2007. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 21:1833–1856 [DOI] [PubMed] [Google Scholar]
- 22. Kaser A, et al. 2008. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134:743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawai T, Fan J, Mazan-Mamczarz K, Gorospe M. 2004. Global mRNA stabilization preferentially linked to translational repression during the endoplasmic reticulum stress response. Mol. Cell. Biol. 24:6773–6787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kedersha N, Anderson P. 2002. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem. Soc. Trans. 30:963–969 [DOI] [PubMed] [Google Scholar]
- 25. Lee AH, Iwakoshi NN, Glimcher LH. 2003. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23:7448–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee AH, Scapa EF, Cohen DE, Glimcher LH. 2008. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320:1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee K, et al. 2002. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16:452–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee KP, et al. 2008. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 132:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee YY, Cevallos RC, Jan E. 2009. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J. Biol. Chem. 284:6661–6673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Korennykh AV, Behrman SL, Walter P. 2010. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl. Acad. Sci. U. S. A. 107:16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, et al. 2008. Differential control of the CCAAT/enhancer-binding protein beta (C/EBPβ) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J. Biol. Chem. 283:22443–22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu PD, Harding HP, Ron D. 2004. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo S, Baumeister P, Yang S, Abcouwer SF, Lee AS. 2003. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J. Biol. Chem. 278:37375–37385 [DOI] [PubMed] [Google Scholar]
- 34. Malhotra JD, Kaufman RJ. 2007. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 18:716–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marciniak SJ, et al. 2004. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18:3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martinon F, Chen X, Lee AH, Glimcher LH. 2010. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11:411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masek T, Valasek L, Pospisek M. 2011. Polysome analysis and RNA purification from sucrose gradients. Methods Mol. Biol. 703:293–309 [DOI] [PubMed] [Google Scholar]
- 38. McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. 2001. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 21:1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. 2004. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat. Genet. 36:1073–1078 [DOI] [PubMed] [Google Scholar]
- 40. Nicholson P, Muhlemann O. 2010. Cutting the nonsense: the degradation of PTC-containing mRNAs. Biochem. Soc. Trans. 38:1615–1620 [DOI] [PubMed] [Google Scholar]
- 41. Nicholson P, et al. 2010. Nonsense-mediated mRNA decay in human cells: mechanistic insights, functions beyond quality control and the double-life of NMD factors. Cell. Mol. Life Sci. 67:677–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Novoa I, Zeng H, Harding HP, Ron D. 2001. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153:1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Novoa I, et al. 2003. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 22:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palam LR, Baird TD, Wek RC. 2011. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem. 286:10939–10949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Parker R, Song H. 2004. The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 11:121–127 [DOI] [PubMed] [Google Scholar]
- 46. Raaben M, Groot Koerkamp MJ, Rottier PJ, de Haan CA. 2007. Mouse hepatitis coronavirus replication induces host translational shutoff and mRNA decay, with concomitant formation of stress granules and processing bodies. Cell. Microbiol. 9:2218–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reinhardt HC, et al. 2010. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Mol. Cell 40:34–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ron D, Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8:519–529 [DOI] [PubMed] [Google Scholar]
- 49. Rutkowski DT, Kaufman RJ. 2003. All roads lead to ATF4. Dev. Cell 4:442–444 [DOI] [PubMed] [Google Scholar]
- 50. Rutkowski DT, Kaufman RJ. 2007. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem. Sci. 32:469–476 [DOI] [PubMed] [Google Scholar]
- 51. Scheuner D, et al. 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7:1165–1176 [DOI] [PubMed] [Google Scholar]
- 52. Sonenberg N, Hinnebusch AG. 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stoecklin G, Anderson P. 2007. In a tight spot: ARE-mRNAs at processing bodies. Genes Dev. 21:627–631 [DOI] [PubMed] [Google Scholar]
- 54. Tabas I, Ron D. 2011. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13:184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Teske BF, et al. 2011. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol. Biol. Cell 22:4390–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. 1990. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. U. S. A. 87:2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vattem KM, Wek RC. 2004. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 101:11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang D, et al. 2011. Inhibition of nonsense-mediated RNA decay by the tumor microenvironment promotes tumorigenesis. Mol. Cell. Biol. 31:3670–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wek RC, Cavener DR. 2007. Translational control and the unfolded protein response. Antioxid. Redox Signal. 9:2357–2371 [DOI] [PubMed] [Google Scholar]
- 60. Wek RC, Jiang HY, Anthony TG. 2006. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 34:7–11 [DOI] [PubMed] [Google Scholar]
- 61. Wiederhold K, Passmore LA. 2010. Cytoplasmic deadenylation: regulation of mRNA fate. Biochem. Soc. Trans. 38:1531–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilusz CJ, Wormington M, Peltz SW. 2001. The cap-to-tail guide to mRNA turnover. Nat. Rev. Mol. Cell Biol. 2:237–246 [DOI] [PubMed] [Google Scholar]
- 63. Wormington M. 1993. Poly(A) and translation: development control. Curr. Opin. Cell Biol. 5:950–954 [DOI] [PubMed] [Google Scholar]
- 64. Xu C, Bailly-Maitre B, Reed JC. 2005. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 115:2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. 2004. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J. Biochem. 136:343–350 [DOI] [PubMed] [Google Scholar]
- 66. Yaman I, et al. 2002. Nutritional control of mRNA stability is mediated by a conserved AU-rich element that binds the cytoplasmic shuttling protein HuR. J. Biol. Chem. 277:41539–41546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yanagitani K, et al. 2009. Cotranslational targeting of XBP1 protein to the membrane promotes cytoplasmic splicing of its own mRNA. Mol. Cell 34:191–200 [DOI] [PubMed] [Google Scholar]
- 68. Yanagitani K, Kimata Y, Kadokura H, Kohno K. 2011. Translational pausing ensures membrane targeting and cytoplasmic splicing of XBP1u mRNA. Science 331:586–589 [DOI] [PubMed] [Google Scholar]
- 69. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891 [DOI] [PubMed] [Google Scholar]
- 70. Zeng L, et al. 2009. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc. Natl. Acad. Sci. U. S. A. 106:8326–8331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhou Y, et al. 2011. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat. Med. 17:356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.