Abstract
Chronic activation of the renin-angiotensin system plays a deleterious role in progressive kidney damage, and the renal proximal tubule is known to play an important role in tubulointerstitial fibrosis; however, the underlying molecular mechanism is unclear. Here we report that in the proximal tubule-like LLCPKcl4 cells expressing angiotensin II (Ang II) type 1 receptor, Ang II induced changes in cell morphology and expression of epithelial-to-mesenchymal transition (EMT) markers, which were inhibited by the miotogen-activated protein (MAP) kinase/extracellular signal-regulated kinase (ERK)-activating kinase (MEK) inhibitor PD98059 or the Src kinase inhibitor PP2. Ang II-stimulated phosphorylation of caveolin-1 (Cav) at Y14 and epidermal growth factor receptor (EGFR) at Y845 and induced association of these phosphoproteins in caveolin-enriched lipid rafts, thereby leading to prolonged EGFR-ERK signaling that was inhibited by Nox4 small interfering RNA (siRNA) and Src siRNA. Two different antioxidants not only inhibited phosphorylation of Src at Y416 but also blocked the EGFR-ERK signaling. Moreover, erlotinib (the EGFR tyrosine kinase inhibitor), EGFR siRNA, and Cav siRNA all inhibited both prolonged EGFR-ERK signaling and phenotypic changes induced by Ang II. Thus, this report provides the first evidence that reactive oxygen species (ROS)/Src-dependent activation of persistent Cav-EGFR-ERK signaling mediates renal tubular cell dedifferentiation and identifies a novel molecular mechanism that may be involved in progressive renal injury caused by chronic exposure to Ang II.
INTRODUCTION
Chronic kidney disease (CKD) is considered to be an irreversible process that eventually leads to end-stage renal disease (ESRD). In addition to glomerular injury, it is now generally accepted that progressive injury to the tubulointerstitial compartment is an essential factor in progressive kidney injury. In this regard, the dedifferentiation of epithelial cells, with decreased expression of epithelial markers and the appearance of a mesenchymal phenotype, is thought to play an essential role in mediating the increased deposition of extracellular matrix (ECM) produced by myofibroblasts (24) and possibly providing a source for a minority of the interstitial myofibroblasts through frank epithelial-to-mesenchymal transition (EMT) (18). Among the potential mediators inducing this renal epithelial cell dedifferentiation, the renin-angiotensin system is widely acknowledged to play a central role. The cellular actions of angiotensin II are mediated by two subtypes of seven-transmembrane G protein-coupled receptors (GPCR), AT1 and AT2 (37). Renal cells express primarily the AT1 receptor, which mediates most of the known physiological and pathological effects of Ang II. However, the signaling events downstream of the AT1 activation that mediates renal epithelial cell dedifferentiation are still under investigation.
The epidermal growth factor receptor (EGFR) is a member of the ErbB family of receptor tyrosine kinases; this family includes EGFR (ErbB1/HER1), ErbB2/Neu/HER2, ErbB3/HER3, and ErbB4/HER4 (35). EGFR is widely expressed in the mammalian kidney, including the glomeruli, proximal tubules, and cortical and medullary collecting ducts (3, 15, 16). There is increasing evidence that EGFR transactivation serves as an important signaling response to numerous hormones, growth factors, and cytokines. This transactivation can occur as a response to metalloproteinase-dependent cleavage and release of soluble EGFR ligands from membrane-associated precursors. In addition, non-ligand-mediated transactivation of EGFR may occur in response to cellular stress (17). EGFR has also been implicated in the pathogenesis of progressive renal fibrosis induced by angiotensin II (Ang II) (21), but the detailed molecular mechanisms underlying renal injury following chronic Ang II treatment remain to be clarified. The current study demonstrates that renal proximal tubule epithelial cells undergo EMT in response to chronic Ang II treatment through AT1 receptor-mediated production of reactive oxygen species (ROS) and activation of Src kinase, thereby leading to phosphorylation and association of EGFR and caveolin-1 (Cav) and resulting in prolonged ERK activation.
MATERIALS AND METHODS
Reagents and antibodies.
Antibodies against EGFR, extracellular signal-regulated kinase (ERK), Shc, GRB2, Cav, N-cadherin, phospho-EGFR (Y1173, Y845), phospho-Src (Y416), and phospho-ERK were from Cell Signaling Technology (Beverly, MA). Antibody against β-actin and all secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to phospho-Cav (Y14) and E-cadherin were from BD Bioscience (Franklin Lakes, NJ). Antibodies against Nox2 and Nox4 were from Novus Biological (Littleton, CO). Alexa 594-conjugated donkey anti-rabbit antibody and Alexa 488-conjugated donkey anti-mouse antibody were from Invitrogen Corporation (Carlsbad, CA). OptiPrep was purchased from Accurate Chemical & Scientific Corp. (Westbury, NY). Erlotinib was purchased from LC Laboratories (Woburn, MA). Ang II, 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (tempol), phalloidin-fluorescein isothiocyanate (phalloidin-FITC), 4′,6-diamidino-2-phenylindole (DAPI), Percoll, and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture.
Clone 4 of LLC-PK1 cells (LLCPKcl4), expressing the characteristics of renal proximal tubular epithelial cells, was subcloned from cells of the parental porcine renal proximal tubule LLC-PK1 cell line and stably transfected with AT1 receptor (AT1R/Cl4) or empty vector (Vector/Cl4) and maintained in culture medium as we described previously (5). Cells were made quiescent in serum-free culture medium for 24 h followed by treatment with different reagents for different times as indicated in Results. For induction of EMT in cultured cells, AT1R/Cl4 cells were cultured in Dulbecco's modified Eagle's medium/F-12 (DMEM/F-12) containing 0.5% serum and left untreated or treated with 100 nM Ang II; this induction medium was prepared and applied to the cells daily for up to 4 to 7 days.
Preparation of caveolin-enriched membranes.
Membranes were purified using a detergent-free method as described previously (38). Briefly, AT1/Cl4 cells were scraped into cold buffer A (0.25 M sucrose, 1 mM EDTA, 20 mM Tricine; pH 7.8) and pelleted by centrifugation at 1,400 × g for 5 min in an IEC Centra-4B centrifuge, followed by resuspension in 0.5 ml of buffer A and homogenization using a 1-ml Wheaton Dounce tissue grinder. The suspensions were centrifuged at 1,000 × g for 10 min. The supernatant fraction was transferred into a clean 1.5-ml centrifuge tube and resuspended in 0.5 ml of cold buffer A, followed by homogenization and recentrifugation. The two supernatants were combined and brought to a 2-ml volume with cold buffer A, followed by layering the 2 ml of supernatant onto 23 ml of 30% Percoll in buffer A and centrifuging at 84,000 × g for 30 min. The plasma membrane fractions were collected and adjusted to 2.0 ml with cold buffer A. The isolated plasma membrane fractions were sonicated on ice followed by mixing with 0.16 ml of buffer A and 1.84 ml of buffer C, 50% OptiPrep (Accurate Chemical & Scientific Corp., Westbury, NY) in buffer B (0.25 M sucrose, 6 mM EDTA, 120 mM Tricine; pH 7.8). The mixture was loaded into a centrifuge tube followed by pouring a linear OptiPrep gradient (20% to 10%; prepared by diluting buffer C with buffer A) onto the sample and then centrifuging at 52,000 × g for 90 min to obtain OptiPrep 1. The top 5 ml (containing fractions 1 to 7) of the OptiPrep 1 solution was collected, placed in a fresh centrifuge tube, and mixed with 4 ml of buffer C. The sample was overlaid with 2 ml of 5% OptiPrep and centrifuged at 52,000 × g for another 90 min at 4°C to obtain OptiPrep 2. A distinct opaque band, fraction 3, was present about 4 to 5 mm above the interface. Fraction 3 had previously been demonstrated to be a highly purified preparation of caveolin-enriched membranes (38). In our subsequent experiments, we utilized fraction 3 in AT1/Cl4 cells treated with Ang II, EGF, or vehicle alone to study protein-protein associations by immunoprecipitation (IP) and immunoblotting (IB) analysis.
Transfection of Src, EGFR, Nox4, and Cav siRNA.
Duplexes (21-bp) of EGFR small interfering RNA (siRNA), Cav siRNA, or Silencer Negative Control 1 siRNA (catalogue no. AM4611; Applied Biosystems/Ambion, Austin, TX) or Src or NOX4 siRNA (Thermo Fisher Scientific, Lafayette, CO) were transfected into subconfluent AT1R/Cl4 cells by the Lipofectamine method (Invitrogen Corporation, Carlsbad, CA) as we described previously (4). Three different duplex sequences were examined for knocking down EGFR (for EGFR siRNA1, 5′-CGCUGGAGGAGAAGAAAGUTT-3′; for EGFR siRNA2, 5′-GAAGGAGACGGAAUUCAAATT-3′; and for EGFR siRNA3, 5′-CACCGUGGAGAAGAUCCCUTT-3′) or caveolin-1 (for caveolin-1 siRNA1, 5′-GGAGAUAGACUUGGUCAACTT-3′; for caveolin-1 siRNA2, 5′-AACCAGAAGGGACACACAGTT-3′; and for caveolin-1 siRNA3, 5′-CCAGAAGGGACACAVAUUUTT-3′) (see Fig. 4C and 9A). A pool of the EGFR siRNA1 and EGFR siRNA3 duplex sequences or a pool of caveolin-1 siRNA1 and caveolin-1 siRNA2 duplex sequences was used for later experiments. For inhibition of Nox4 expression, either Thermo Scientific Dharmacon ON-TARGETplus SMARTpool L-010194-00-0005 or siGenome smartPool M-010194-00-0005 siRNAs were used. For Src expression inhibition, either Thermo Scientific Dharmacon ON-TARGETplus SMARTpool L-003175-00-0005 or siGenome smartPool M-003175-03-0005 siRNAs (Thermo Fisher Scientific, Lafayette, CO) were used. For signaling studies, 48 h after transfection, cells were made quiescent in serum-free medium for another 24 h followed by the indicated treatments. For monitoring the progress of EMT, after 48 h of transfection, medium was changed with 0.5% serum and 100 nM Ang II daily for another 3 to 7 days.
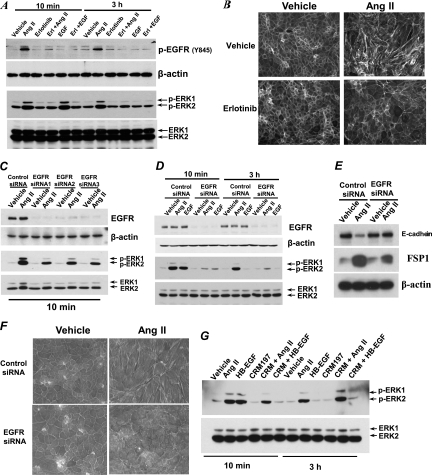
Fig 4.
Chronic Ang II treatment induced EMT in AT1R/Cl4 cells through an EGFR-ERK activation-dependent but HB-EGF-independent pathway. AT1R/Cl4 cells were pretreated with erlotinib at 100 nM for 30 min, followed by exposure to indicated stimuli for 10 min or 3 h. (A) Cell lysates were subjected to immunoblotting with indicated antibodies. (B) Cells were treated as described for Fig. 1A with or without erlotinib (100 nM) for 4 days, and photographs were taken at ×400 magnification following FITC-conjugated phalloidin staining. (C and D) At 48 h after transfection with different EGFR siRNAs or scrambled control siRNA, quiescent AT1R/Cl4 cells were exposed to indicated stimuli for 10 min or 3 h and cell lysates were subjected to immunoblotting analysis with the indicated antibodies. (E and F) At 48 h after siRNA transfection as indicated, AT1R/Cl4 cells were subjected to induction of EMT with Ang II as indicated in Fig. 1 and cell lysates were immunoblotted with indicated antibodies (E) or cell photographs were taken at ×400 magnification following FITC-conjugated phalloidin staining (F). (G) AT1R/Cl4 cells were treated as indicated for either 10 min or 3 h followed by immunoblotting analysis of cell lysates with indicated antibodies.
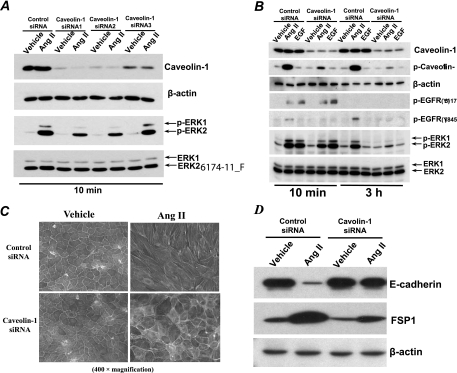
Fig 9.
Ang II induced EGFR-ERK activation and EMT by a Cav-dependent signaling pathway in AT1R/Cl4 cells. (A) At 48 h after transfection with different Cav siRNAs or scrambled control siRNA, quiescent cells lysates were subjected to immunoblotting with indicated antibodies following exposure to indicated stimuli for 10 min. (B to D) At 48 h after siRNA transfection as indicated, quiescent AT1R/Cl4 cell lysates were subjected to immunoblotting analysis with indicated antibodies (B) or the cells were subjected to induction of EMT with Ang II as indicated in Fig. 1, cell photographs were taken at ×400 magnification following FITC-conjugated phalloidin staining (C), and cell lysates were subjected to immunoblotting analysis with the indicated antibodies (D).
Immunoprecipitation and immunoblotting.
These procedures were performed as we previously described (5, 6). Briefly, cells were made quiescent in serum-free medium for 24 h, treated with the indicated reagents, and lysed with lysis buffer (0.5% Nonidet P-40, 50 mm NaCl, 10 mmTris-HCl [pH 7.4], 2 mm EDTA, 2 mm EGTA, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 100 μm Na3VO4, 100 mm NaF, 30 mm sodium pyrophosphate, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin). After centrifugation of the cell lysates at 10,000 × g for 15 min at 4°C, equal amounts of protein were subjected to immunoprecipitation with the indicated antibodies as described previously (7). Immunoprecipitation samples were resuspended and boiled in sample buffer before separation using 7% to 15% SDS-PAGE and immunoblotted onto Immobilon-P transfer membranes (Millipore, Bedford, MA). After blocking with 3% bovine serum albumin in 150 mm NaCl–50 mm Tris-HCl (pH 7.4) (TBS) for 1 h at room temperature, blots were probed with the indicated primary antibodies. The blots were washed 3 times at room temperature with 0.05% Tween 20–TBS, incubated with the appropriate secondary antibody conjugated with horseradish peroxidase, and detected with enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom).
Measurement of intracellular ROS generation.
AT1R/Cl4 cells were cultured in a 24-well plate and made quiescent in serum-free culture medium for 24 h, followed by washing once with HEPES buffered salt solution (HBSS; pH = 7.4) (0.5 ml/well) containing 25 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 25 mM NaHCO3, and 5.5 mM glucose. The cells then were then left untreated or treated with apocynin for 30 min before addition of 10−7 M Ang II and 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) (100 uM) for 2 h, and the fluorescence intensity was measured by using a fluorescence multiwell plate reader with excitation and emission wavelengths of 485 nm and 530 nm.
Immunofluorescence staining.
Cells cultured in a 16-well Lab-Tek chamber slide system (Nalge Nunc International, Rochester, NY) were fixed with 4% paraformaldehyde–phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100–PBS, and washed three times with PBS. After incubation with phalloidin-FITC (50 μg/ml) at room temperature for 40 min, the cells were washed three times with PBS and covered with coverslips. In additional experiments, cells were incubated with rabbit anti-phospho-EGFR (Y845; 1:50) and mouse anti-phospho-Cav (Y14; 1:50) antibodies for 1 h at room temperature following fixation and permeability experiments and then incubated with Alexa 594-conjugated donkey anti-rabbit antibody or Alexa 488-conjugated donkey anti-mouse antibody for 1 h. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were captured using a Nikon TE300 fluorescence microscope and a Spot-Cam digital camera (Diagnostic Instruments).
RESULTS
Chronic Ang II treatment induced AT1R/Cl4 cells to undergo EMT by a Src- and MEK-dependent signaling pathway.
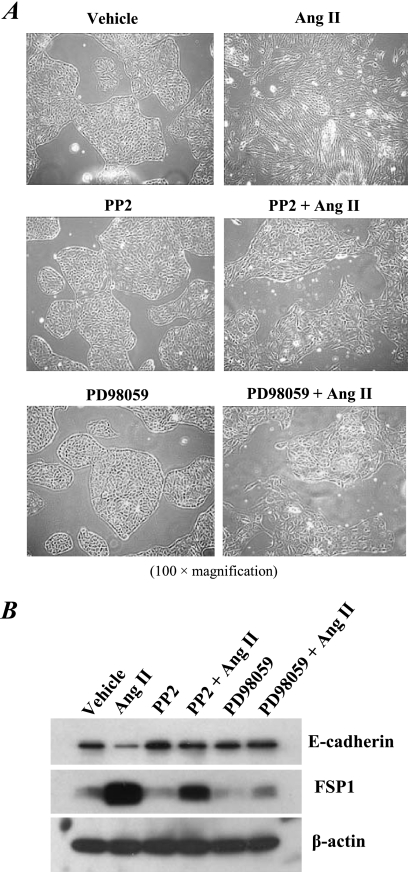
In vivo, the proximal tubule expresses high levels of AT1 receptors (25); we therefore utilized stable transfectants of LLCPKcl4, the renal proximal tubular epithelial cell line, that expressed functional Ang II type 1 receptors (AT1R/Cl4 cells) (5). We have previously reported that AT1R/Cl4 cells responded to acute Ang II treatment by increasing EGFR phosphorylation due in part to release of heparin-binding EGF-like growth factor (HB-EGF) within 10 min (5). Dysregulated or sustained activation of the renin-angiotensin system is known to play a detrimental role in progressive kidney damage. Accordingly, the present study examined chronic effects of Ang II in renal proximal tubular epithelial cells. When we extended the exposure of AT1R/Cl4 cells to Ang II for 4 days, we observed striking morphological changes from an epithelial to a fibroblast-like morphology (Fig. 1A, top panels). The Ang II-induced morphological changes were largely prevented by either the Src kinase inhibitor, PP2 (Fig. 1A, middle panels), or the ERK-activating kinase (MEK) inhibitor, PD98059 (Fig. 1A, bottom panels). Immunoblotting analysis revealed decreased expression of the adherent junction protein, E-cadherin, and increased expression of the fibroblast specific protein, FSP-1, in the Ang II-treated AT1R/Cl4 cells; these Ang II-induced alterations in gene expression were largely prevented by the presence of either the Src kinase inhibitor, PP2, or the MEK inhibitor, PD98059 (Fig. 1B). These data suggest that chronic Ang II exposure causes AT1R/Cl4 cells to undergo EMT through a Src- and MEK-dependent mechanism.
Fig 1.
Chronic Ang II treatment induced AT1R/Cl4 cells to undergo EMT by a Src- and MEK-dependent signaling pathway. AT1R/Cl4 cells were cultured in 6-well plates and made quiescent by serum deprivation for 24 h and then maintained in DMEM/F12 medium containing 0.5% serum and Ang II (10−7 M) with or without the Src kinase inhibitor PP2 (5 μM) or the MEK inhibitor PD98059 (5 μM) for 4 days; during this period, such an EMT-inducing medium with or without inhibitors was prepared and changed daily for the cells. (A) Photographs were taken at ×100 magnification. (B) Cell lysates were subjected to immunoblotting with an antibody against E-cadherin or FSP1, followed by stripping and reprobing with an antibody to β-actin, respectively. (All of the data shown in these studies are representative of at least three separate experiments with similar results).
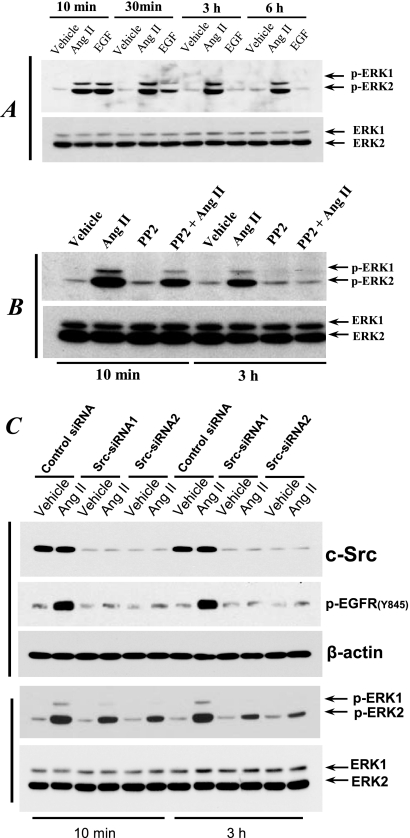
Ang II treatment induced persistent EGFR phosphorylation at tyrosine 845 (Y845) but not tyrosine 1173 (Y1173) in AT1R/Cl4 cells by a Src-dependent mechanism.
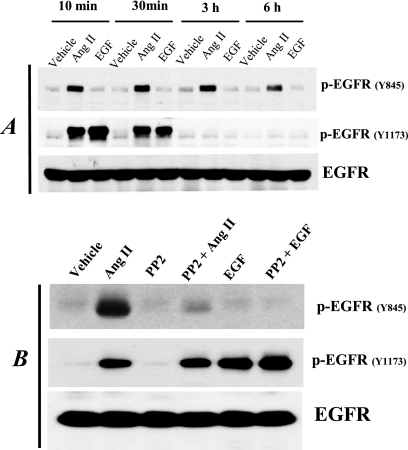
In our previous study, using immunoprecipitation (IP) with anti-phosphotyrosine antibodies (anti-PY) and immunoblotting (IB) with an EGFR antibody (anti-EGFR), we found that Ang II induced EGFR tyrosine phosphorylation in AT1R/Cl4 cells within 5 min; however, we did not determine the specific tyrosine residues of EGFR that were phosphorylated in response to Ang II treatment (5). In the present study, using phosphotyrosine residue-specific antibodies, we found that although Ang II induced EGFR phosphorylation at both tyrosine 845 (Y845) and tyrosine 1173 (Y1173) in AT1R/Cl4 cells within 10 min, Ang II-stimulated EGFR Y1173 phosphorylation peaked within 10 min, decreased within 0.5 h, and returned to basal level within 3 h whereas Ang II-stimulated EGFR Y845 phosphorylation remained elevated even after 6 h (Fig. 2A). In contrast, administration of EGF did not induce EGFR phosphorylation at Y845 but did stimulate EGFR phosphorylation at the autophosphorylation site, Y1173, with a rapid peak and a return to basal levels within 3 h (Fig. 2A).
Fig 2.
Ang II treatment induced persistent EGFR phosphorylation at tyrosine 845 (Y845) but not tyrosine 1173 (Y1173) in AT1R/Cl4 cells by an Src-dependent mechanism. (A) AT1R/Cl4 cells were rendered quiescent and exposed to Ang II (10−7 M), EGF (30 nM), or vehicle alone for 10 min, 30 min, 3 h, or 6 h. (B) Quiescent AT1R/Cl4 cells were pretreated with the Src kinase inhibitor PP2 (5 μM) or with vehicle alone for 30 min prior to exposure to Ang II (10−7 M), EGF (30 nM), or vehicle alone for 10 min. Cell lysates were subjected to immunoblotting with antibodies against Y845- or Y1173-phosphorylated EGFR, followed by stripping and reprobing of the blots with an antibody recognizing total EGFR, as indicated.
EGFR can be directly phosphorylated at Y845 by Src kinase activity (2, 22, 26). We therefore pretreated AT1R/Cl4 cells with the Src kinase inhibitor, PP2, before exposing the cells to Ang II. As indicated in Fig. 2B, PP2 pretreatment markedly inhibited Ang II-induced EGFR Y845 phosphorylation but did not inhibit EGFR Y1173 phosphorylation induced by either Ang II or EGF. These data suggest that EGFR Y845 phosphorylation is mediated by Src kinase activity but that EGFR Y1173 phosphorylation is mediated by autophosphorylation through the intrinsic tyrosine kinase activity of EGFR in response to Ang II, which induces rapid HB-EGF shedding to act as a ligand for EGFR (5).
Src kinase mediates prolonged ERK1/2 activation in AT1R/Cl4 cells in response to Ang II.
Previous studies have suggested that activation of the MEK-ERK pathway is a mediator of EMT (14, 41) and that this pathway is a classic downstream effecter of EGFR activation (17, 27). Our observation that the MEK inhibitor, PD98059, inhibited the morphological changes and EMT marker expression alterations suggested the involvement of the MEK-ERK pathway in the EMT process in AT1R/Cl4 cells in response to chronic Ang II treatment (Fig. 1). We examined the phosphorylation of ERK1/2 at different times after administration of Ang II to AT1R/Cl4 cells and found that Ang II treatment induced persistent phosphorylation of ERK1/2. In contrast, administration of EGF led to transient ERK1/2 activation, which was diminished within 0.5 h and returned to basal levels by 3 h (Fig. 3A).
Fig 3.
Ang II treatment induced Src-mediated prolonged ERK activation in AT1R/Cl4 cells. (A) Quiescent AT1R/Cl4 cells were treated as described for Fig. 2A. (B) Quiescent AT1R/Cl4 cells were pretreated with PP2 as indicated in Fig. 2B and treated with Ang II (10−7 M) or vehicle alone for 10 min or 3 h. Cell lysates were subjected to immunoblotting with indicated antibodies. At 48 h after transfection with Src siRNA or scrambled control siRNA, quiescent AT1R/Cl4 cells were exposed to Ang II (10−7 M) or vehicle alone for 10 min or 3 h. (C) Cell lysates were subjected to immunoblotting with indicated antibodies.
pp60c-src (Src) is the prototype of a family of nine cytosolic nonreceptor tyrosine kinases that function as cotransducers of transmembrane signals emanating from a variety of growth factor/hormone receptors (11), including AT1 receptors in vascular smooth muscle (34) and in kidney cells (40). We found that pretreatment of the cells with PP2 partially inhibited the early phase (10 min of treatment) of ERK1/2 activation but completely blocked the late phase (3 h of treatment) of ERK1/2 activation induced by Ang II (Fig. 3B). Knocking down the Src gene expression by its specific siRNA sequences markedly blunted the Ang II treatment-induced persistent EGFR Y845 and ERK1/2 phosphorylation (Fig. 3C), suggesting that Ang II treatment induced persistent EGFR-ERK1/2 activation by a Src kinase-dependent mechanism.
Chronic Ang II treatment induced EMT in AT1R/Cl4 cells through an EGFR-ERK activation-dependent but HB-EGF-independent pathway.
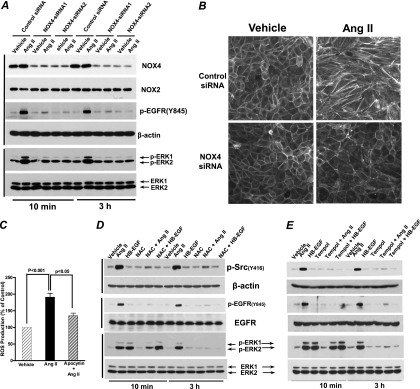
To determine whether EGFR transactivation is essential in the prolonged ERK1/2 activation and epithelial cell dedifferentiation in response to chronic Ang II treatment, we pretreated the cells with erlotinib, the specific EGFR tyrosine kinase inhibitor, and found that pretreatment of the cells with erlotinib not only inhibited prolonged phospho-EGFR and phospho-ERK signaling but also eliminated the morphological changes (Fig. 4A and B). Furthermore, we knocked down EGFR expression with different EGFR sequence-specific siRNAs (Fig. 4C and D) and found that downregulation of EGFR expression inhibited the prolonged ERK1/2 activation (Fig. 4D) and reversed the alterations in E-cadherin and FSP-1 expression in response to Ang II (Fig. 4E) and prevented the morphological changes (Fig. 4F). These data confirmed that EGFR is located upstream of the ERK1/2 activation in response to Ang II treatment in AT1R/Cl4 cells.
Because our previous studies had indicated that the immediate activation of ERK1/2 in response to Ang II is partially mediated by EGFR transactivation due to release of soluble HB-EGF (5), we further determined whether prolonged ERK1/2 activation by Ang II was also mediated by release of soluble HB-EGF. We therefore preincubated AT1R/Cl4 cells with CRM197, a nontoxic and catalytically inactive (Glu-52) mutant of diphtheria toxin that binds to the extracellular HB-EGF domain and inhibits the mitogenic activity of HB-EGF. As shown Fig. 4G, CRM197 almost completely inhibited the transient ERK1/2 activation induced by exogenously administered HB-EGF but only partially inhibited the early phase (10 min of treatment) of ERK1/2 activation induced by Ang II and had no effect on the late phase (3 h after treatment) of Ang II-mediated ERK1/2 activation. These results suggest that the Ang II-induced early phase of ERK1/2 activation is partially mediated by HB-EGF but that the late phase of ERK1/2 activation is independent of HB-EGF.
Antioxidants blocked Ang II-induced Src activation and prolonged EGFR-ERK signaling.
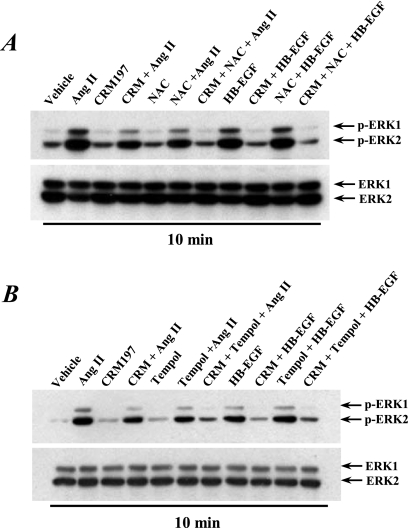
NADPH oxidase-dependent reactive oxygen species (ROS) are important mediators of Ang II signaling and known to activate Src (12, 13, 33). Although multiple isoforms are potentially present in the kidney, the constitutively active isoform Nox4 is predominantly expressed in epithelial cells (8). In AT1R/Cl4 cells, we utilized Nox4 sequence-specific siRNAs to knock down Nox4 gene expression without affecting Nox2 expression (Fig. 5A) and found that downregulation of Nox4 expression markedly inhibited prolonged EGFR-ERK activation (Fig. 5A) and reversed fibroblast cell morphological changes in response to Ang II treatment (Fig. 5B). In addition, Ang II increases in ROS production were inhibited by apocynin, the NAD(P)H oxidase inhibitor (Fig. 5C). Phosphorylation of Src at tyrosine 416 (Y614) is a well-established readout of Src kinase activity (20), and immunoblotting with an antibody that recognizes Y416-phosphorylated Src indicated that Ang II activated Src in AT1R/Cl4 cells within 10 min and remained activated at 3 h after addition of the stimuli (Fig. 5D and E). Furthermore, pretreatment of the cells with an antioxidant (n-acetylcysteine [NAC] or tempol) blocked both Src Y416 phosphorylation and EGFR Y845 phosphorylation (Fig. 5D and E), consistent with ROS activation of Src, which then phosphorylates EGFR at Y845 (2, 22, 26). HB-EGF did transiently activate ERK1/2 phosphorylation but did not induce Src phosphorylation of EGFR Y845 phosphorylation. Although HB-EGF-induced ERK1/2 activation was not altered in the presence of the antioxidant, both NAC and tempol partially inhibited the Ang II-induced early phase of ERK1/2 phosphorylation and almost completely inhibited the late phase of ERK1/2 activation in response to Ang II treatment (Fig. 5D and E). Interestingly, the Ang II-induced early phase of ERK phosphorylation was only partially inhibited by CRM197, NAC, or tempol alone but was almost completely blocked by pretreatment of the cells with the combination of CRM197 with either NAC or tempol (Fig. 6). These results suggest that the early phase of Ang II-induced ERK activation is mediated by both HB-EGF release and ROS-dependent Src activation, whereas the late phase of Ang II-induced ERK activation is mediated solely by continued ROS production and Src activation, although both the early and late phases of ERK activation involve transactivation of EGFR, albeit through different mechanisms.
Fig 5.
Antioxidants blocked Ang II-induced Src activation and prolonged EGFR-ERK signaling in AT1R/Cl4 cells. (A and B) Nox4 siRNA- or scrambled control siRNA-transfected AT1R/Cl4 cells were treated as indicated, and the cell lysates were subjected to immunoblotting with indicated antibodies (A) or cell photographs were taken at ×400 magnification after FITC-conjugated phalloidin staining (B). (C) ROS production was measured by the fluorescence intensity of 2′,7′-dichlorodihydrofluorescin (DCFH) in Ang II-treated AT1R/Cl4 cells with or without a NADPH oxidase inhibitor, apocynin pretreatment. (D and E) AT1R/Cl4 cell lysates were immunoblotted with indicated antibodies after the cells were exposed to indicated stimuli following pretreatment with or without an antioxidant, N-acetylcysteine (NAC; 5 mM) (D) or tempol (3 mM) (E).
Fig 6.
The early phase of ERK activation in response to Ang II treatment was mediated through both ROS production and HB-EGF release in AT1R/Cl4 cells. Quiescent AT1R/Cl4 cells were pretreated with or without CRM197 (10 μg/ml) and with or without NAC (5 mM) (A) or tempol (3 mM) (B), followed by treatment with Ang II (10−7 M), HB-EGF (15 ng/ml), or vehicle alone for 10 min. Cell lysates were immunoblotted with anti-phospho-ERK1/2, followed by stripping and reprobing with an antibody to total ERK1/2.
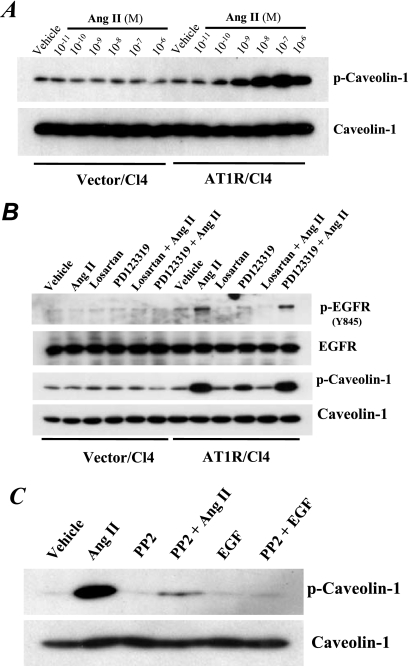
Ang II-induced Src-dependent phosphorylation and association of Cav and EGFR in AT1R/Cl4 cells.
Activated Src kinase is also known to directly phosphorylate Cav at tyrosine 14 (Y14) (23). Administration of Ang II induced Cav Y14 phosphorylation within 10 min in a concentration-dependent manner in AT1R/Cl4 cells but not in the empty vector-transfected LLCPKcl4 (Vector/Cl4) cells (Fig. 7A). Ang II-induced EGFR Y845-phosphorylation and Cav Y14 phophorylation were both blocked by losartan (10−6 M), the AT1R antagonist, but not by PD123319 (10−6 M), the AT2R antagonist (Fig. 7B). Moreover, PP2, the Src kinase inhibitor, inhibited Ang II-induced Cav phosphorylation (Fig. 7C).
Fig 7.
Ang II-induced phosphorylation of both EGFR at Y845 and caveolin-1 at Y14 was mediated by AT1 receptors in an Src-dependent manner. (A) Phosphorylation of caveolin-1 (Cav) at Y14 was induced by Ang II in a concentration-dependent manner in AT1R/Cl4 cells but not in empty vector-transfected LLCPKcl4 cells (Vector/Cl4). (B) A selective AT1R antagonist, losartan (10−6 M), but not the selective AT2R antagonist, PD123319 (10−6 M), inhibited Ang II-induced phosphorylation of EGFR at Y845 and Cav at Y14. (C) An Src kinase inhibitor, PP2 (5 μM), inhibited phosphorylation of Cav at Y14.
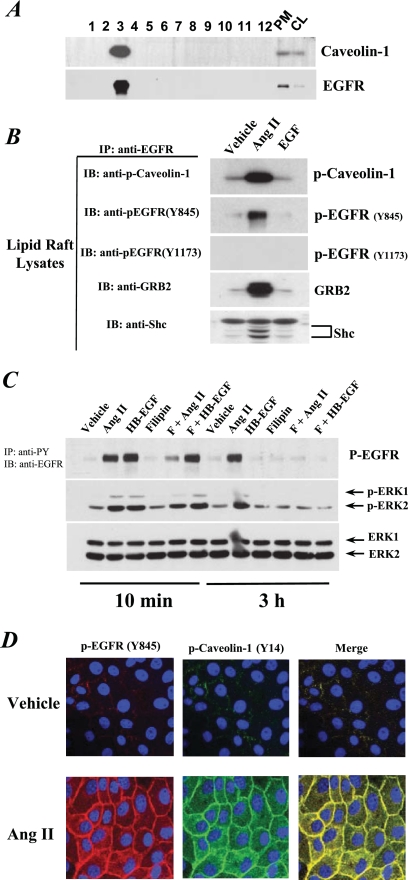
Immunoblotting indicated that Cav was found at a high concentration in fraction 3 of Ang II-treated cell subcellular components separated on an OptiPrep density gradient. EGFR from these cells was also concentrated in fraction 3 (Fig. 8A). Treatment with Ang II led to EGFR association with Y14-phosphorylated Cav and increased EGFR phosphorylation at Y845 but not at Y1173 in this Cav-enriched fraction, while EGF treatment had no such effect (Fig. 8B). In addition, Ang II treatment also induced EGFR association with its downstream signaling adaptors, SHC and GRB2, in fraction 3 (Fig. 8B). When we pretreated the cells with filipin III, which selectively binds cholesterol and inhibits the formation of caveolae and lipid rafts, we found that filipin III treatment partially inhibited Ang II-induced early-phase EGFR and ERK1/2 phosphorylation and nearly completely blocked late-phase EGFR-ERK1/2 signaling; in contrast, filipin III did not affect HB-EGF-mediated EGFR and ERK1/2 phosphorylation (Fig. 8C). Immunofluorescent staining of AT1/Cl4 cells revealed colocalization of Y14-phosphorylated Cav and Y845-phosphorylated EGFR in response to Ang II treatment (Fig. 8D). Of note, the majority of the phospho-Cav and phospho-EGFR remained at the cell membrane.
Fig 8.
Ang II treatment-induced phosphorylation of Cav and EGFR and their association in lipid rafts. (A) Lipid raft fractions were prepared as described in Materials and Methods followed by immunoblotting analysis with an antibody to Cav or EGFR along with plasma membrane (10 μg) and cell lysates (10 μg) as controls. (B) AT1R/Cl4 cells were exposed to different stimuli for 10 min, and then the lipid rafts were isolated and subjected to immunoprecipitation (IP) with an anti-EGFR antibody and immunoblotting (IB) with indicated antibodies. (C) AT1R/Cl4 cell lysates were prepared after treatment with the indicated stimuli and were subjected to immunoprecipitation (IP) with anti-phosphotyrosine antibodies (anti-PY), followed by immunoblotting (IB) with an antibody against EGFR (top panel). Another aliquot of cell lysates was directly immunoblotted with indicated antibodies. (D) Quiescent AT1R/Cl4 cells cultured in a 16-well Lab-Tek chamber slide system were exposed to Ang II (10−7 M) or vehicle alone for 3 h. Y845-phosphorylated EGFR (red) and Y14-phosphorylated Cav (green) were visualized and photographed by fluorescence microscopy at ×400 magnification. Nuclei were visualized with DAPI (blue).
Cav siRNA inhibited Ang II-elicited signaling activity and EMT in AT1/Cl4 cells.
siRNA-mediated downregulation of Cav gene expression inhibited Ang II-induced ERK1/2 activation (Fig. 9A). Furthermore, siRNA silencing of Cav expression blocked Ang II-induced Cav Y14 and EGFR Y845 phosphorylation, partially inhibited the Ang II-induced early phase of ERK1/2 activation, and almost completely blocked Ang II-induced prolonged ERK1/2 activation (Fig. 9B). Cav siRNA did not affect EGFR phosphorylation at Y1173 in response to either Ang II or EGF (Fig. 9B). Cav knockdown also inhibited Ang II-induced changes in cell morphology (Fig. 9C) and alterations in E-cadherin and FSP-1 expression (Fig. 9D). These data indicate that Cav expression and phosphorylation at Y14 are essential for prolonged activation of the EGFR-ERK signaling pathway that mediates EMT in response to chronic Ang II exposure.
DISCUSSION
The present study demonstrates an important role for prolonged activation of EGFR-ERK signaling pathway in epithelial cell dedifferentiation in response to chronic Ang II treatment. It also demonstrates that Ang II-activated persistent EGFR signaling in renal proximal tubule epithelial cells results primarily from non-ligand-mediated receptor transactivation mediated by ROS-dependent Src activation, leading to phosphorylation of both EGFR and Cav and their association in lipid rafts. This persistently activated EGFR serves as a scaffold for SHC/GRB2-mediated ERK activation, thereby serving as an important mediator of subsequent EMT.
We have previously demonstrated that short-term administration of Ang II transactivates EGFR in part by release of HB-EGF following binding to AT1 receptors in renal proximal tubule epithelial cells (5). Following ligand-mediated EGFR activation, the ligand-receptor complex normally undergoes endocytosis by clathrin-coated pits, followed by degradation through the endosomal/lysomal pathway, thereby downregulating sensitivity to EGFR activation (9, 39). The results of the current studies indicate that in renal epithelial cells, persistent Ang II exposure also transactivates EGFR by a non-ligand-dependent pathway in which the receptors associate with phospho-Cav and consequently continue to signal. This persistent activation is largely due to Src-mediated EGFR tyrosine phosphorylation at Y845 rather than persistent tyrosine phosphorylation at Y1173, the tyrosine residue that is phosphorylated by autophosphorylation following ligand-mediated activation. We have also found that this EGFR phosphorylation requires persistent Ang II-mediated Src activation, since removal of Ang II from the culture medium or addition of PP2, the Src kinase inhibitor, 3 h after the initiation of Ang II exposure quickly inhibited EGFR tyrosine phosphorylation at Y845 and downstream ERK activation (data not shown).
Ang II is known to increase reactive oxygen species (ROS) in renal proximal tubule epithelial cells due to NADPH oxidase activation, and ROS can activate Src kinases (12, 13, 33). The results of our studies indicate that inhibition of ROS production or Src activity not only prevents Ang II-induced EGFR phosphorylation upon Y845 and ERK activation but also inhibits epithelial cell EMT in response to chronic Ang II treatment.
Cav was originally described as an integral component of caveolae (32); however, more recent studies have indicated the presence of Cav in membrane-associated noncaveolar lipid rafts as well (1, 28, 30, 31). Previous in vitro studies have shown that EGFR associates with phospho-Cav (10, 19) and thereby excludes the receptors from clathrin-coated pits and vesicles. However, there is controversy in the literature about whether the EGFR-Cav association leads to receptor activation or inactivation. Although some studies indicate that when EGFRs are sorted to caveolae they become inactivated (29, 30), our studies, as well as those of others (36), demonstrate that interaction of EGFR and Cav in membrane-associated caveolae/lipid rafts leads to persistent EGFR-dependent signaling in renal proximal tubule epithelial cells.
In this study, Ang II but neither EGF nor HB-EGF treatment induced EMT. Our results suggest that Ang II induces production of ROS, which mediates phosphorylation of caveolin-1 at Y14 and EGFR at Y845 and their association in cell membrane lipid rafts, thereby preventing phosphorylated EGFR endocytosis by clathrin-coated pits and leading to persistent EGFR activation. However, binding of natural EGFR ligands, such as EGF or HB-EGF, to EGFR is normally followed by endocytosis of the ligand-receptor complex into clathrin-coated pits and degradation through the endosomal/lysomal pathway, thereby downregulating sensitivity to EGFR activation (30, 31). Therefore, the EGFR ligands, EGF and HB-EGF, induced transient EGFR activation that was not in itself capable of inducing cells to undergo EMT.
In summary, the results of the current studies demonstrate for the first time an important role for transactivation of EGFR in the mediation of EMT following chronic exposure to Ang II. Furthermore, we have delineated a novel mechanism whereby Ang II causes chronic transactivation of EGFR through ROS-dependent Src kinase activation, which phosphorylates both caveolin-1 at Y14 and EGFR at Y845, resulting in association of EGFR with phospho-caveolin-1 and the adaptor proteins SHC and GRB2 and leading to sustained EGFR-ERK signaling and phenotypic changes (dedifferentiation/EMT) of renal proximal tubule epithelial cells (Fig. 10). Given the increasing evidence that reactive oxygen species are important mediators of progressive kidney damage in response to a wide range of inciting factors, including diabetes, toxins, ischemia, and inflammation, these new findings suggest that persistent EGFR activation might be a central component of progressive renal injury.
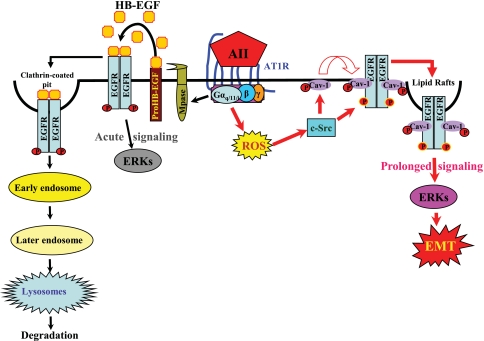
Fig 10.
A novel mechanism linking ROS/Src/caveolin-mediated activation of the EGFR-ERK signaling pathway to chronic Ang II exposure-induced EMT in cultured renal tubular epithelial cells. Our studies utilizing the renal proximal tubular epithelial cells expressing functioning AT1 receptors suggest that binding of Ang II to the seven-transmembrane G-protein-coupled AT1 receptor activates metalloproteinase (Mpase)-dependent release of soluble HB-EGF, a natural EGFR ligand that binds and transactivates EGFR, as we have recently documented (5), resulting in acute signaling leading to ERK activation; such ligand-activated ERK signaling diminishes within 1 h (Fig. 5, 6, and 8C). The transient nature of this EGFR-ERK signaling is presumably due to the quick endocytosis of the ligand (HB-EGF)-activated EGFR through clathrin-coated pits, followed by degradation through the endosomal/lysosomal pathway (9, 39). Meanwhile, Ang II activation of AT1 receptors also leads to transactivation of EGFR through ROS-dependent Src kinase activation, which phosphorylates both caveolin-1 (Cav) at Y14 and EGFR at Y845, thus inducing association of EGFR with Cav and the adaptor proteins SHC and GRB2, resulting in prolonged EGFR-ERK signaling. In the case of chronic Ang II exposure, such prolonged signaling activity persists in the epithelial cells, leading to alterations in gene expression and consequently eliciting a phenotypic change to EMT.
ACKNOWLEDGMENTS
This work was supported by funds from a Department of Veterans Affairs Merit Award and National Institutes of Health grants DK38226, DK51265, DK62794, and DK79341 to R.C.H. and funds from a Vanderbilt Diabetes Research and Training Center Pilot and Feasibility grant 2P60DK020593, American Heart Association Scientist Development grant 0630274N, and National Institutes of Health R01 grant DK83575 to J.-K.C.
Footnotes
Published ahead of print 3 January 2012
REFERENCES
- 1. Anderson RG. 1998. The caveolae membrane system. Annu. Rev. Biochem. 67:199–225 [DOI] [PubMed] [Google Scholar]
- 2. Biscardi JS, et al. 1999. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 274:8335–8343 [DOI] [PubMed] [Google Scholar]
- 3. Breyer MD, Redha R, Breyer JA. 1990. Segmental distribution of epidermal growth factor binding sites in rabbit nephron. Am. J. Physiol. 259:F553–F558 [DOI] [PubMed] [Google Scholar]
- 4. Chen J, et al. 2007. Mitogenic activity and signaling mechanism of 2-(14,15-epoxyeicosatrienoyl)glycerol, a novel cytochrome p450 arachidonate metabolite. Mol. Cell. Biol. 27:3023–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Chen JK, Neilson EG, Harris RC. 2006. Role of EGF receptor activation in angiotensin II-induced renal epithelial cell hypertrophy. J. Am. Soc. Nephrol. 17:1615–1623 [DOI] [PubMed] [Google Scholar]
- 6. Chen JK, Chen J, Neilson EG, Harris RC. 2005. Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J. Am. Soc. Nephrol. 16:1384–1391 [DOI] [PubMed] [Google Scholar]
- 7. Chen JK, Falck JR, Reddy KM, Capdevila J, Harris RC. 1998. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J. Biol. Chem. 273:29254–29261 [DOI] [PubMed] [Google Scholar]
- 8. Clavreul N, Sansilvestri-Morel P, Magard D, Verbeuren TJ, Rupin A. 2011. (Pro)renin promotes fibrosis gene expression in HEK cells through a Nox4-dependent mechanism. Am. J. Physiol. Renal Physiol. 300:F1310–F1318 [DOI] [PubMed] [Google Scholar]
- 9. Dikic I. 2003. Mechanisms controlling EGF receptor endocytosis and degradation. Biochem. Soc. Trans. 31:1178–1181 [DOI] [PubMed] [Google Scholar]
- 10. Dittmann K, Mayer C, Kehlbach R, Rodemann HP. 2008. The radioprotector Bowman-Birk proteinase inhibitor stimulates DNA repair via epidermal growth factor receptor phosphorylation and nuclear transport. Radiother. Oncol. 86:375–382 [DOI] [PubMed] [Google Scholar]
- 11. Erpel T, Courtneidge SA. 1995. Src family protein tyrosine kinases and cellular signal transduction pathways. Curr. Opin. Cell Biol. 7:176–182 [DOI] [PubMed] [Google Scholar]
- 12. Feliers D, Gorin Y, Ghosh-Choudhury G, Abboud HE, Kasinath BS. 2006. Angiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen species. Am. J. Physiol. Renal Physiol. 290:F927–F936 [DOI] [PubMed] [Google Scholar]
- 13. Gill PS, Wilcox CS. 2006. NADPH oxidases in the kidney. Antioxid. Redox Signal. 8:1597–1607 [DOI] [PubMed] [Google Scholar]
- 14. Grände M, et al. 2002. Transforming growth factor-beta and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thyrocytes. J. Cell Sci. 115:4227–4236 [DOI] [PubMed] [Google Scholar]
- 15. Harris DCH, Chan L, Schrier RW. 1988. Remnant kidney hypermetabolism and progression of chronic renal failure. Am. J. Physiol. 254:F267–F276 [DOI] [PubMed] [Google Scholar]
- 16. Harris RC. 1989. Response of rat inner medullary collecting duct to epidermal growth factor. Am. J. Physiol. 256(Pt. 2):F1117–F1124 [DOI] [PubMed] [Google Scholar]
- 17. Harris RC, Chung E, Coffey RJ. 2003. EGF receptor ligands. Exp. Cell Res. 284:2–13 [DOI] [PubMed] [Google Scholar]
- 18. Iwano M, et al. 2002. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110:341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khan EM, et al. 2006. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J. Biol. Chem. 281:14486–14493 [DOI] [PubMed] [Google Scholar]
- 20. Kmiecik TE, Johnson PJ, Shalloway D. 1988. Regulation by the autophosphorylation site in overexpressed pp60c-src. Mol. Cell. Biol. 8:4541–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lautrette A, et al. 2005. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat. Med. 11:867–874 [DOI] [PubMed] [Google Scholar]
- 22. Leu TH, Maa MC. 2003. Functional implication of the interaction between EGF receptor and c-Src. Front. Biosci. 8:s28–s38 [DOI] [PubMed] [Google Scholar]
- 23. Li SL, Cougnon N, Bresson-Bepoldin L, Zhao SJ, Schlegel W. 1996. c-fos mRNA and FOS protein expression is induced by Ca2+ influx in GH3B6 pituitary cells. J. Mol. Endocrinol. 16:229–238 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y. 2004. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15:1–12 [DOI] [PubMed] [Google Scholar]
- 25. Mujais SK, Kauffman S, Katz AI. 1986. Angiotensin II binding sites in individual segments of the rat nephron. J. Clin. Invest. 77:315–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nair VD, Sealfon SC. 2003. Agonist-specific transactivation of phosphoinositide 3-kinase signaling pathway mediated by the dopamine D2 receptor. J. Biol. Chem. 278:47053–47061 [DOI] [PubMed] [Google Scholar]
- 27. Navolanic PM, Steelman LS, McCubrey JA. 2003. EGFR family signaling and its association with breast cancer development and resistance to chemotherapy. Int. J. Oncol. 22:237–252 [PubMed] [Google Scholar]
- 28. Oakley FD, Smith RL, Engelhardt JF. 2009. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J. Biol. Chem. 284:33255–33264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Orth JD, McNiven MA. 2006. Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res. 66:11094–11096 [DOI] [PubMed] [Google Scholar]
- 30. Pike LJ. 2005. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim. Biophys. Acta 1746:260–273 [DOI] [PubMed] [Google Scholar]
- 31. Pike LJ, Han X, Chung KN, Gross RW. 2002. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41:2075–2088 [DOI] [PubMed] [Google Scholar]
- 32. Rothberg KG, et al. 1992. Caveolin, a protein component of caveolae membrane coats. Cell 68:673–682 [DOI] [PubMed] [Google Scholar]
- 33. Sachse A, Wolf G. 2007. Angiotensin II-induced reactive oxygen species and the kidney. J. Am. Soc. Nephrol. 18:2439–2446 [DOI] [PubMed] [Google Scholar]
- 34. Schieffer B, Drexler H, Ling BN, Marrero MB. 1997. G protein-coupled receptors control vascular smooth muscle cell proliferation via pp60c-src and p21ras. Am. J. Physiol. 272:C2019–C2030 [DOI] [PubMed] [Google Scholar]
- 35. Schlessinger J. 2002. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110:669–672 [DOI] [PubMed] [Google Scholar]
- 36. Shah BH, Catt KJ. 2003. A central role of EGF receptor transactivation in angiotensin II-induced cardiac hypertrophy. Trends Pharmacol. Sci. 24:239–244 [DOI] [PubMed] [Google Scholar]
- 37. Siragy HM. 2004. AT1 and AT2 receptor in the kidney: role in health and disease. Semin. Nephrol. 24:93–100 [DOI] [PubMed] [Google Scholar]
- 38. Smart EJ, Ying YS, Mineo C, Anderson RG. 1995. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 92:10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sorkin A. 2001. Internalization of the epidermal growth factor receptor: role in signalling. Biochem. Soc. Trans. 29(Pt. 4):480–484 [DOI] [PubMed] [Google Scholar]
- 40. Tsuganezawa H, Preisig PA, Alpern RJ. 1998. Dominant negative c-Src inhibits angiotensin II induced activation of NHE3 in OKP cells. Kidney Int. 54:394–398 [DOI] [PubMed] [Google Scholar]
- 41. Xie L, et al. 2004. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia 6:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]