Abstract
Aims
The aims of the study were, first, to critically evaluate lipoprotein(a) [Lp(a)] as a cardiovascular risk factor and, second, to advise on screening for elevated plasma Lp(a), on desirable levels, and on therapeutic strategies.
Methods and results
The robust and specific association between elevated Lp(a) levels and increased cardiovascular disease (CVD)/coronary heart disease (CHD) risk, together with recent genetic findings, indicates that elevated Lp(a), like elevated LDL-cholesterol, is causally related to premature CVD/CHD. The association is continuous without a threshold or dependence on LDL- or non-HDL-cholesterol levels. Mechanistically, elevated Lp(a) levels may either induce a prothrombotic/anti-fibrinolytic effect as apolipoprotein(a) resembles both plasminogen and plasmin but has no fibrinolytic activity, or may accelerate atherosclerosis because, like LDL, the Lp(a) particle is cholesterol-rich, or both. We advise that Lp(a) be measured once, using an isoform-insensitive assay, in subjects at intermediate or high CVD/CHD risk with premature CVD, familial hypercholesterolaemia, a family history of premature CVD and/or elevated Lp(a), recurrent CVD despite statin treatment, ≥3% 10-year risk of fatal CVD according to European guidelines, and/or ≥10% 10-year risk of fatal + non-fatal CHD according to US guidelines. As a secondary priority after LDL-cholesterol reduction, we recommend a desirable level for Lp(a) <80th percentile (less than ∼50 mg/dL). Treatment should primarily be niacin 1–3 g/day, as a meta-analysis of randomized, controlled intervention trials demonstrates reduced CVD by niacin treatment. In extreme cases, LDL-apheresis is efficacious in removing Lp(a).
Conclusion
We recommend screening for elevated Lp(a) in those at intermediate or high CVD/CHD risk, a desirable level <50 mg/dL as a function of global cardiovascular risk, and use of niacin for Lp(a) and CVD/CHD risk reduction.
Keywords: Lipids, Hyperlipidemia, Prevention, Myocardial infarction, Stroke
Introduction
Lipoprotein(a) [Lp(a)] has been considered a cardiovascular risk factor for many years.1 Owing to incomplete scientific evidence, screening for and treatment of high Lp(a) levels have to date been performed principally by lipid specialists. However, during the last few years, major advances have been achieved in understanding the causal role of elevated Lp(a) in premature cardiovascular disease (CVD).2–4 These new findings have prompted the present critical appraisal of the evidence base in the form of a Consensus Paper.
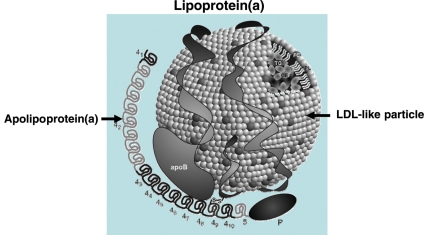
Lipoprotein(a) is a plasma lipoprotein consisting of a cholesterol-rich LDL particle with one molecule of apolipoprotein B100 and an additional protein, apolipoprotein(a), attached via a disulfide bond (Figure 1).1 Elevated Lp(a) levels can potentially increase the risk of CVD (i) via prothrombotic/anti-fibrinolytic effects as apolipoprotein(a) possesses structural homology with plasminogen and plasmin but has no fibrinolytic activity and (ii) via accelerated atherogenesis as a result of intimal deposition of Lp(a) cholesterol, or both.
Figure 1.
Lipoprotein(a) consists of an LDL-like particle to which apolipoprotein(a) is covalently linked. The LDL-like moiety is composed of a central core of cholesteryl esters (CE) and triglycerides (TG) surrounded by phospholipids (PL), free cholesterol (FC), and a single molecule of apolipoprotein B (apoB). Apolipoprotein(a) contains 10 different types of plasminogen kringle 4-like repeats as well as regions homologous to the kringle 5 and protease (P) regions of plasminogen. The kringle 4 type 2 domain (42) is present in multiply repeated copies from 2 to >40 that differ in number between apolipoprotein(a) isoforms.1 Apolipoprotein(a) is linked to apolipoprotein B100 by a single disulfide bond involving an unpaired cysteine residue in kringle 4 type 9. Modified from Koschinsky and Marcovina.18
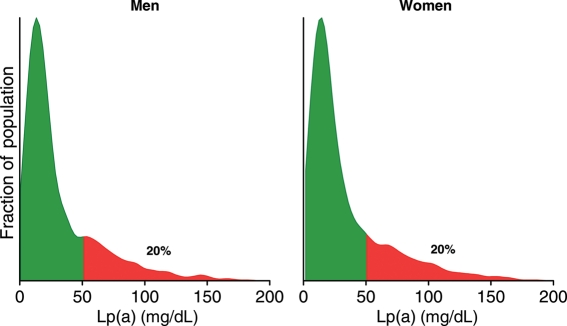
Typical distributions of Lp(a) in Caucasians are shown in Figure 2: plasma levels of Lp(a) are similar in men and women and are skewed in the population with a tail towards the highest levels. Levels are lowest in non-Hispanic Caucasians (e.g. median: 12 mg/dL; inter-quartile range: 5–32),5 Chinese (11, 4–22), and Japanese (13, 5–26), slightly higher in Hispanics (19, 8–43), and even higher levels in Blacks (39, 19–69).1,5 It is significant that Lp(a) has been surrounded by controversy among clinicians for more than 20 years, and the various conceptions and misconceptions are summarized and explained in Supplementary material online, Table S1.
Figure 2.
Typical distributions of lipoprotein(a) levels in the general population. These graphs are based on non-fasting fresh serum samples from ∼3000 men and 3000 women from the Copenhagen General Population Study collected from 2003 through 2004.2 Green colour indicates levels below the 80th percentile, whereas red colour indicates levels above the 80th percentile.
The aim of the present Consensus Paper is to critically evaluate the evidence base supporting the contention that Lp(a) constitutes a significant cardiovascular risk factor. On the basis of the evidence, the Consensus Panel provides recommendations on whom to screen, desirable levels, and finally, how to treat elevated Lp(a) levels. This is the first Consensus Statement on diagnosis, desirable levels, and treatment of elevated Lp(a).
Epidemiology
Findings from earlier prospective studies suggest that the relation between Lp(a) concentration and the risk of CVD may involve a threshold and that the association may be more marked among individuals with elevated LDL cholesterol.6–8 Individual studies, however, are rarely sufficiently powered to assess the shape of the relation or to make precise estimates of relative risk within subgroups of the study populations such as among individuals with high rather than low LDL cholesterol levels.
An early meta-analysis of 18 prospective studies of general populations that was published before 2000, which reported on a pooled analysis of 4000 coronary heart disease (CHD) cases, suggested that the combined relative risk of CHD for individuals in the top vs. bottom thirds of baseline Lp(a) concentrations was 1.7 (95% CI: 1.4–1.9).9 An updated meta-analysis of 31 prospective studies, involving a total of 9870 CHD cases, suggested that the corresponding combined risk was more modest (relative risk: 1.5; 1.3–1.8).10 Subgroups defined by other characteristics pre-specified for investigation, notably study size, sample storage characteristics, and Lp(a) assay isoform sensitivity, were not significantly different.
Although the evidence from literature-based meta-analyses of prospective studies suggests the potential importance of Lp(a) in CHD, it does not provide sufficient detail to allow the assessment of the relevance of this lipoprotein to CVD prevention and treatment. For example, it is not possible to determine, from a literature-based meta-analysis, whether Lp(a) is associated with CHD throughout the concentration range (similar to blood pressure and LDL cholesterol) or whether Lp(a) is particularly atherothrombogenic in specific subgroups of individuals (such as in those with high LDL cholesterol level). Re-analysis of individual participant data from a comprehensive set of prospective epidemiological studies (i.e. individual participant data meta-analysis) can help overcome several of the limitations of individual studies or literature-based meta-analyses of individual studies. However, because some of the earlier studies had problems with the correct measurement of Lp(a) in, for example, frozen samples, the risk estimates reported below should be considered minimal estimates.
The largest epidemiological study to date on Lp(a) assessed individual records of 126 634 participants in 36 prospective studies.3 Lipoprotein(a) concentration was weakly correlated with several known risk factors: positively with total and non-HDL cholesterol, apolipoprotein B100, and inversely with loge triglycerides. Lipoprotein(a) levels were 12% (95% CI: 8–16%) higher in women and 11% (4–17%) lower in people with diabetes. The regression dilution ratio of loge Lp(a), adjusted for age and sex, was 0.87 which was considerably higher than that for total cholesterol (0.65), thereby suggesting that Lp(a) levels are remarkably stable over time.
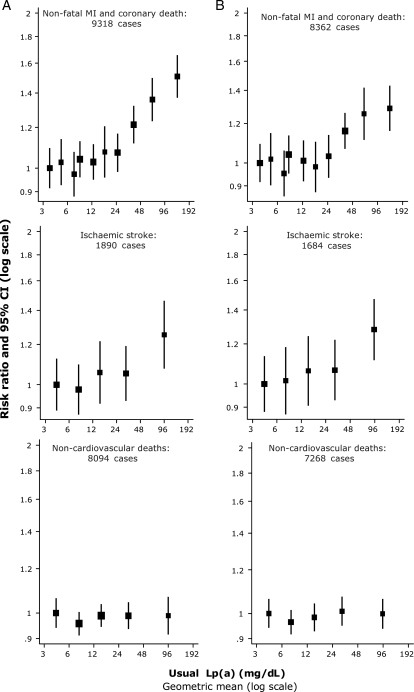
Associations of Lp(a) with CHD risk were broadly continuous in shape and curvilinear, with no evidence of a threshold (Figure 3).3 Assuming a log-linear association, the relative risk for CHD per 3.5-fold (1 SD) higher Lp(a) level, adjusted for age and sex only, was 1.16 and 1.13 (95% CI: 1.09–1.18) following further adjustment for systolic blood pressure, smoking, history of diabetes, and total cholesterol, suggesting that any association is only minimally confounded by conventional risk factors (Figure 4). Importantly, Lp(a) levels may vary up to a 1000-fold between individuals (Figure 2).1 The relative risk for CHD did not vary significantly by sex, non-HDL- or HDL cholesterol, triglycerides, blood pressure, diabetes, or body mass index; in accordance, a recent prospective study found that the Lp(a)/CHD risk association did not depend on levels of other CVD risk factors, including LDL cholesterol levels.11 There was no convincing evidence of major variations in relative risk in studies using isoform-sensitive vs. -insensitive assays.3
Figure 3.
Risk ratios of coronary heart disease, ischaemic stroke and non-vascular death by quantiles of usual lipoprotein(a) levels. CI, confidence interval. Sizes of data markers are proportional to the inverse of the variance of the risk ratios. (A) Adjustment for age and sex only. (B) Further adjustment for systolic blood pressure, smoking status, history of diabetes, body mass index, and total cholesterol. MI, myocardial infarction. Modified from The Emerging Risk Factors Collaboration.3
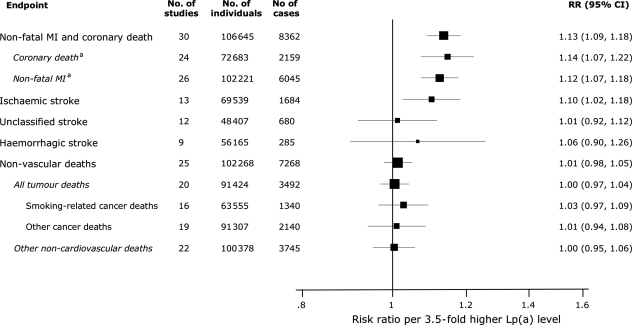
Figure 4.
Risk ratios for various vascular and non-vascular endpoints per 3.5-fold (i.e. 1 SD) higher than usual lipoprotein(a) levels adjusted for cardiovascular risk factors. MI, myocardial infarction. aSubtotals do not add to the total number of coronary heart disease outcomes because some studies did not subdivide outcomes into coronary death and non-fatal MI. RR, relative risk; CI, confidence interval. Modified from The Emerging Risk Factors Collaboration.3
In analyses adjusted for age and sex only, the association of elevated Lp(a) levels with increased risk of ischaemic stroke was less pronounced than that for CHD (Figure 3).3 However, the relatively weak association with ischaemic stroke may be due to heterogeneity of stroke aetiologies, that is, the association in atherothrombotic stroke could be diluted by weaker or no association with other stroke subtypes. Assuming a log-linear association with risk, the age-and-sex-only-adjusted relative risk for ischaemic stroke was 1.11 per 3.5-fold higher than usual Lp(a) levels and was 1.10 (95% CI: 1.02–1.18) following further adjustment for traditional risk factors (Figure 4). The adjusted relative risks per 3.5-fold higher than usual Lp(a) levels was 1.06 (0.90–1.26) for haemorrhagic stroke, 1.01 (0.98–1.05) for nonvascular mortality, 1.00 (0.97–1.04) for all cancer deaths, 1.03 (0.97–1.09) for smoking-related cancer deaths, and 1.00 (0.95–1.06) for non-vascular deaths other than cancer.
In summary, elevated Lp(a) levels associate robustly and specifically with increased CVD risk. The association is continuous in shape without a threshold and does not depend on high levels of LDL or non-HDL cholesterol, or on the levels or presence of other cardiovascular risk factors.
Genetics
Plasma levels of Lp(a) are to a large extent genetically determined via variation in the apolipoprotein(a) gene.1 This makes the apolipoprotein(a) gene ideal for use in a Mendelian randomization study,12 examining whether lifelong, genetically elevated levels of plasma Lp(a) cause CVD. By analogy, familial hypercholesterolaemia with mutations in the LDL receptor or apolipoprotein B genes have lifelong, genetically elevated LDL cholesterol levels and premature CVD,13,14 a fact that has helped establish that elevated LDL cholesterol levels constitute a direct cause of atherosclerosis and CVD.
A Mendelian randomization study needs three pieces of data to help provide evidence for a causal link between elevated plasma Lp(a) levels and CVD.12 First, elevated plasma Lp(a) levels should be associated with increased CVD risk, as demonstrated in the previous section on Lp(a) epidemiology. Secondly, genetic variation should exist in human populations that can explain a large fraction of the variation in plasma Lp(a) levels: such genetic variation has been known for many years, most importantly the kringle IV type 2 size polymorphism (Figure 1, 42), resulting in a variable number from 2 to >40 number of a 5.6 kb repeat associated inversely with plasma Lp(a) levels. Thus, the fewer the repeats in the apolipoprotein(a) gene, the higher the plasma levels of Lp(a), which has also been demonstrated in the past.1 Thirdly, such genetic variation should be linked directly with CVD risk: previous smaller case–control studies (n < 2400) have demonstrated an association of kringle IV type 2 genotype [or the associated apolipoprotein(a) isoform size] with risk of CVD, as reviewed previously.1,2
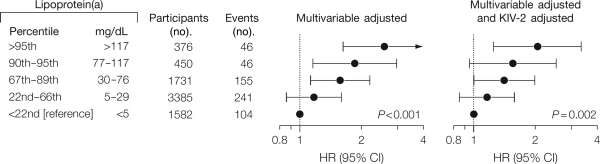
On the basis of the Copenhagen City Heart Study (CCHS), the Copenhagen General Population Study (CGPS), and the Copenhagen Ischemic Heart Disease Study (CIHDS) with 40 000 individuals genotyped for the kringle IV type 2 size polymorphism in the apolipoprotein(a) gene, a large Mendelian randomization study was published in 2009.2 In the CCHS, multifactorially adjusted hazard ratios for myocardial infarction for elevated lipoprotein(a) levels were 1.2 (95% CI: 0.9–1.6) for the 22nd–66th percentile, 1.6 (1.1–2.2) for the 67th–89th percentile, 1.9 (1.2–3.0) for the 90th–95th percentile, and 2.6 (1.6–4.1) for levels >95th percentile, respectively, vs. levels <22nd percentile (trend P< 0.001; Figure 5).2,11
Figure 5.
Risk of myocardial infarction by levels of lipoprotein(a) in the general population. Hazard ratios (HRs) are adjusted for cardiovascular risk factors (multivariable) or for these factors as well as kringle IV type 2 (KIV-2) genotype. P-values are test for trend of hazard ratios where lipoprotein(a) groups with increasing levels were coded 1, 2, 3, 4, and 5. Values are from the 1991–94 examination of the Copenhagen City Heart Study with up to 16 years of follow-up (n= 7524). CI, confidence interval. Modified from Kamstrup et al.2
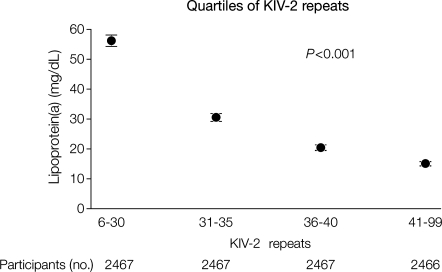
The number of kringle IV type 2 repeats (sum of repeats on both alleles) ranged from 6 to 99 and explained 21 and 27% of all variation in plasma lipoprotein(a) levels in the CCHS and the CGPS, respectively.2 Mean lipoprotein(a) levels were 56, 31, 20, and 15 mg/dL for the first, second, third, and fourth quartiles of kringle IV type 2 repeats in the CCHS, respectively (trend P< 0.001; Figure 6); corresponding values in the CGPS were 60, 34, 22, and 19 mg/dL (trend P< 0.001).
Figure 6.
Mean lipoprotein(a) levels in the Copenhagen City Heart Study as a function of quartiles of apolipoprotein(a) KIV-2 repeats. P-value is for the Cuzick non-parametric test for trend of mean lipoprotein(a) levels. Participants in the 1991–94 or 2001–03 examination were included (n= 9867). KIV-2, kringle IV type 2. Error bars indicate 95% confidence intervals. Modified from Kamstrup et al.2
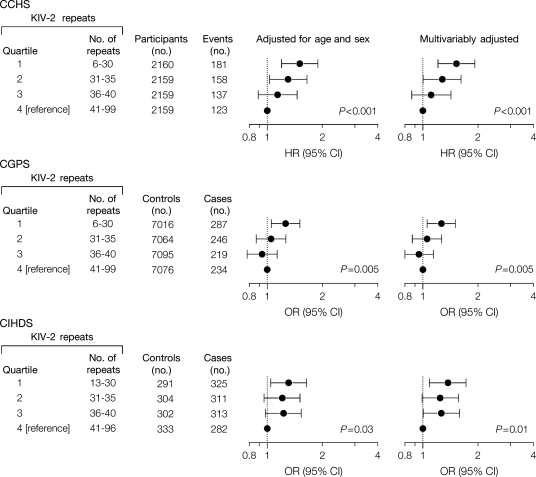
In the CCHS, multifactorially adjusted hazard ratios for myocardial infarction were 1.5 (1.2–1.9), 1.3 (1.0–1.6), and 1.1 (0.9–1.4) for individuals in the first, second, and third vs. fourth quartile of kringle IV type 2 repeats, respectively (trend P< 0.001; Figure 7).2 Corresponding odds ratios were 1.3 (1.1–1.5), 1.1 (0.9–1.3), and 0.9 (0.8–1.1) in the CGPS (trend P= 0.005), and 1.4 (1.1–1.7), 1.2 (1.0–1.6), and 1.3 (1.0–1.6) in the CIHDS (trend P= 0.01). The significant similar trend tests in three separate studies all point to increased CVD risk when the number of kringles is low and thus plasma levels of Lp(a) elevated.2
Figure 7.
Risk of myocardial infarction by quartiles of apolipoprotein(a) KIV-2 repeats in the Copenhagen City Heart Study (CCHS), the Copenhagen General Population Study (CGPS), and the Copenhagen Ischemic Heart Disease Study (CIHDS). P-values are test for trend of risk estimates [hazard ratios (HRs) or odds ratios (ORs)] where kringle IV type 2 (KIV-2) groups with decreasing numbers of KIV-2 repeats were coded 1, 2, 3, and 4. CI, confidence interval (shown as error bars). Modified from Kamstrup et al.2
Later in 2009, a further key paper on this topic including 8000 CHD cases and 8000 controls was published.4 On the basis of genotyping for 49 000 single-nucleotide polymorphisms (SNPs) in 2100 candidate genes for CVD, 2 SNPs in linkage disequilibrium with the kringle IV type 2 size polymorphism in the apolipoprotein(a) gene showed the highest of any association with CHD. These two SNPs combined were found in one in six people and together explained 36% of the variation in plasma Lp(a) levels. The odds ratios for CHD were 1.51 (95% CI: 1.38–1.66) for one variant and 2.57 (1.80–3.67) for two or more variants. Consistent with these observations, a 2007 study tested 12 000 putative functional SNPs in many different genes and found that only one, in the apolipoprotein(a) gene, was consistently associated with severe coronary atherosclerosis.15 Elevated Lp(a) is also an independent CVD risk factor in patients with familial hypercholesterolaemia.16
Taken together, the robust and specific epidemiological association between elevated plasma Lp(a) levels and increased risk of CVD,3 together with the two recent Mendelian randomization studies,2,4 support the contention that elevated Lp(a) levels, like elevated LDL, is causally related to premature development of atherosclerosis and CVD (Table 1).
Table 1.
Comparison of evidence supporting the contention that elevated low-density lipoprotein cholesterol and elevated lipoprotein(a) each cause cardiovascular disease
| Elevated LDL cholesterol | Elevated Lp(a) | |
|---|---|---|
| Human epidemiology | Direct association in numerous studies | Direct association in numerous studies |
| Human genetic studies | Direct association in numerous studies, e.g. familial hypercholesterolaemia | Direct association in numerous studies, e.g. for kringle IV type 2 polymorphism |
| Mechanistic studies | Mechanism clearly demonstrated: LDL accumulate in intima and cause atherosclerosis | Mechanism similar to that for LDL cholesterol and/or prothrombotic/anti-fibrinolytic effects |
| Animal models | Proatherogenic effect in numerous studies | Proatherogenic effect in numerous studies |
| Human intervention trials | Statin trials gave final proof of causality | Niacin trials are favourable |
| Interpretation in 2010 | Causality | Probably causal |
Metabolism
It is believed that plasma concentrations of Lp(a) are determined chiefly by rates of hepatic synthesis of apolipoprotein(a): although the site of formation of Lp(a) has not been definitively identified, evidence suggests that apolipoprotein(a) adducts extracellularly and covalently to apolipoprotein B100-containing lipoproteins, predominantly LDL.17,18 Apolipoprotein(a) genotype, which determines both the synthetic rate and size of the apolipoprotein(a) moiety of Lp(a), alone accounts for 90% of plasma concentrations of Lp(a).17,19,20 As hepatic secretion rates are lower for large apolipoprotein(a) isoforms, and as most individuals are heterozygous for two different isoforms, the smallest isoform typically predominates in plasma. Lipoprotein(a) is thought to be catabolized primarily by hepatic and renal pathways, but these metabolic routes do not appear to govern plasma Lp(a) levels.
Pathophysiological mechanisms underlying the atherothrombotic potential of lipoprotein(a)
After transfer from plasma into the arterial intima, Lp(a) may be more avidly retained than LDL as it binds to the extracellular matrix not only through apolipoprotein(a), but also via its apolipoprotein B component,21 thereby contributing cholesterol to the expanding atherosclerotic plaque. In vitro, Lp(a) binds to several extracellular matrix proteins including fibrin22 and defensins, a family of 29–35 amino acid peptides that are released by neutrophils during inflammation and severe infection.23 It is likely that defensins, like lipoprotein lipase, provide a bridge between Lp(a) and the extracellular matrix.
Transgenic mice expressing a mutant form of apolipoprotein(a) with greatly reduced ability to bind to fibrin exhibited 20% less atherosclerotic lesion area and less accumulation in the arterial wall compared with transgenic mice expressing wild-type Lp(a).24 In addition, Lp(a) seems to be retained at sites of mechanical injury;21 fibrin deposition occurs preferentially at such sites.
Through its apolipoprotein(a) moiety, Lp(a) also interacts with the β2-integrin Mac-1, thereby promoting the adhesion of monocytes and their transendothelial migration.25 In atherosclerotic coronary arteries, Lp(a) was found to localize in close proximity to Mac-1 on infiltrating mononuclear cells.
Lipoprotein(a) has also been shown to bind pro-inflammatory-oxidized phospholipids26 and is a preferential carrier of oxidized phospholipids in human plasma. Lipoprotein(a) also contains lipoprotein-associated phospholipase A2 (equally referred to as Paf-acetylhydrolase), which may cleave oxidized fatty acids at the sn-2 position in oxidized phospholipids to yield short chain fatty acids and lysolecithin.27
Apolipoprotein(a), a homologue of the fibrinolytic proenzyme plasminogen, impairs fibrinolysis.28 Indeed, Lp(a)/apolipoprotein(a) can competitively inhibit tissue-type plasminogen activator-mediated plasminogen activation on fibrin surfaces, although the mechanism of inhibition by apolipoprotein(a) remains controversial. Essential to fibrin clot lysis are a number of plasmin-dependent, positive feedback reactions that enhance the efficiency of plasminogen activation, including the plasmin-mediated conversion of Glu-plasminogen to Lys-plasminogen. It has been observed that the apolipoprotein(a) component of Lp(a) inhibits the key positive feedback step involving conversion of plasmin-mediated Glu-plasminogen to Lys-plasminogen.29 Lipoprotein(a) may also enhance coagulation by inhibiting the function of tissue factor pathway inhibitor.30
Finally, small isoforms of apolipoprotein(a) have been observed to possess elevated potency in inhibiting fibrinolysis and thereby promoting thrombosis.31 Indeed, a recent meta-analysis demonstrated a two-fold increase in the risk of CHD and ischaemic stroke in subjects with small apolipoprotein(a) phenotypes.32 Furthermore, prospective findings in the Bruneck study have revealed a significant association specifically between small apolipoprotein(a) phenotypes and advanced atherosclerotic disease involving a component of plaque thrombosis.33 These data suggest that the determination of apolipoprotein(a) phenotype/genotype may provide clinicians with additional information by which to evaluate Lp(a)/apolipoprotein(a)-associated atherothrombotic risk.
In summary, elevated Lp(a) levels may promote atherosclerosis via Lp(a)-derived cholesterol entrapment in the intima, via inflammatory cell recruitment, and/or via the binding of pro-inflammatory-oxidized phospholipids. The prothrombotic, anti-fibrinolytic actions of apolipoprotein(a) are expressed on the one hand as inhibition of fibrinolysis with enhancement of clot stabilization and on the other as enhanced coagulation via the inhibition of tissue factor pathway inhibitor.
Measurement
Several types of Lp(a) assays are currently available, some commercially; prominent among them are sandwich enzyme-linked immunosorbent assays (ELISAs), non-competitive ELISAs, latex immunoassays, immunonephelometric assays, and immunoturbidometric and fluorescence assays.19 In order for clinical laboratories to provide clinicians with Lp(a) values which allow correct cardiovascular risk evaluation when Lp(a) is included in the estimate, the following elements in standardization between Lp(a) assays are critical.
Inclusion of antibodies in assay kits whose immunoreactivity with Lp(a) is apolipoprotein(a) isoform-insensitive and fully characterized, and for which there is minor variation between batches over time. In this regard, immunosorbent assays are of considerable interest, as they allow the use of polyclonal antibodies [anti-apolipoprotein(a) capture; anti-apolipoprotein B100] and are isoform-insensitive.19,28
Availability of a robust secondary reference Lp(a) preparation at an international level, which has received approval by organizations such as the International Federation of Clinical Chemistry and the World Health Organisation.
The widespread use of methodologies which are robust, highly reproducible with inter- and intra-coefficient of variations <10%, economically priced, and accurate.
A common resolve, based on the uniformity and widespread availability of an approved and standardized Lp(a) assay, to express Lp(a) concentrations as total Lp(a) protein; point (ii) above is critical to this goal,
Standardization of procedures for blood collection, plasma, or serum isolation with a preferential use of fresh samples.34
Ranges and percentiles for Lp(a) protein levels established for individual ethnicities given present knowledge of race-dependent variation in Lp(a),1,5 ultimately leading to race-specific estimates of risk thresholds.
These factors will contribute significantly not only to the reliable diagnosis and classification of subjects presenting with high atherothrombotic risk due to elevation of Lp(a), but also to the success of multicentre clinical trials designed to evaluate pharmacotherapeutic agents targeted to concomitantly reduce elevated Lp(a) levels and CVD risk.
Whom to screen
We suggest that Lp(a) should be measured once in all subjects at intermediate or high risk of CVD/CHD who present with:
premature CVD,
familial hypercholesterolaemia,
a family history of premature CVD and/or elevated Lp(a),
recurrent CVD despite statin treatment,
≥3% 10-year risk of fatal CVD according to the European guidelines,35 and
≥10% 10-year risk of fatal and/or non-fatal CHD according to the US guidelines36
Repeat measurement is only necessary if treatment for high Lp(a) levels is initiated in order to evaluate therapeutic response.
Desirable levels
Scientific and clinical evidence favouring an interpretation of causality between elevated levels of a lipoprotein and increased CVD risk constitutes a pre-requisite for selection of desirable levels. For an interpretation of causality, five types of evidence should each favour causality and all three types of human evidence (epidemiology, genetics, and intervention trials) must concur (Table 1).37 For elevated LDL cholesterol levels, all five criteria are well documented and the consensus is causality. On the basis of the same criteria, elevated Lp(a) levels probably are also causally related to increased CVD risk (Table 1).
Recommendations proposing desirable levels should preferably be based on evidence from a meta-analysis of randomized, controlled intervention trials documenting benefit of treatment (level Ia evidence). Desirable levels for LDL cholesterol levels are based on such evidence from statin trials (Table 2).35,36 For Lp(a), the evidence is less clear; however, a meta-analysis of randomized, controlled intervention trials documenting benefit of niacin (=nicotinic acid) treatment has recently been published38 (see below).
Table 2.
Desirable levels for low-density lipoprotein cholesterol and lipoprotein(a) levels in the fasting or non-fasting state
| Patients with CVD and/or diabetes | Other patients and individuals | Highest level of evidence for treatment | |
|---|---|---|---|
| LDL cholesterol | <2 mmol/La (<77 mg/dL) | <3 mmol/La (<116 mg/dL) | Ia: meta-analysis of randomized, controlled trials of statin treatment |
| Lp(a) | <80th percentile (<∼50 mg/dLb) | <80th percentile (<∼50 mg/dLb) | Ia: meta-analysis of randomised, controlled trials of niacin treatmentc |
For reduction of plasma Lp(a) as a secondary priority after reduction in LDL and total cholesterol levels,35,36 we recommend a desirable level below the 80th percentile (less than ∼50 mg/dL; Table 2). As for LDL reduction,35,36 treatment of high Lp(a) levels in persons without CVD/CHD or diabetes is recommended for those with intermediate or high absolute risk of CVD/CHD (see Graham et al.35 and Grundy et al.36 and Figure 3 in Kamstrup et al.11).
If statin treatment in a person with Lp(a) >50 mg/dl, but without CVD or diabetes, reduces absolute risk for fatal CVD to <3% or for fatal and/or non-fatal CHD to <10%, there might be no need for further treatment with niacin; however, in those with premature CVD, familial hypercholesterolaemia, a family history of premature CVD and elevated Lp(a), or recurrent CVD despite statin treatment, niacin may still be justified despite aggressive LDL cholesterol reduction with a statin.
Treatment
Studies using niacin alone or in combination with, for example, statins have shown cardiovascular benefit;38–43 niacin reduces Lp(a) levels by up to 30–40% in a dose-dependent manner and in addition exerts other potential beneficial effects by reducing LDL cholesterol, total cholesterol, triglycerides, and remnant cholesterol and by raising HDL cholesterol.44 In a meta-analysis including 11 randomized controlled trials with 2682 patients in the active group and 3934 in the control group, niacin 1–3 g/day reduced major coronary events by 25% (95% CI: 13–35%), stroke by 26% (8–41%), and any cardiovascular event by 27% (15–37%).38
However, there have been no randomized, controlled intervention trials with selective reduction in plasma lipoprotein(a) levels aimed to reduce CVD: we urgently need trials demonstrating that selective reduction of Lp(a) in those with Lp(a) above the 80th percentile will benefit clinically with reduced CVD. Until such trials are published, reduction in Lp(a) should mainly be achieved using niacin, as the use of niacin for CVD risk reduction as described above is evidence-based. However, in addition to lowering Lp(a), niacin lowers LDL cholesterol, total cholesterol, triglycerides, and remnant cholesterol and concomitantly increases HDL cholesterol levels. Therefore, the favourable effects of niacin on CVD cannot be ascribed solely to Lp(a) reduction. Nevertheless, these studies clearly demonstrate that the use of niacin for reduction in Lp(a) to the suggested desirable levels (Table 2) is safe and in all likelihood beneficial.
Compared with LDL, Lp(a) is relatively refractory to both lifestyle and drug intervention.20,45 The data on the effects of statins and fibrates on Lp(a) are limited and highly variable.45,46 Overall, statins have, however, been shown to consistently and modestly decrease elevated Lp(a) in patients with heterozygous familial hypercholesterolaemia. Other agents reported to decrease Lp(a) to a minor degree (<10%) include aspirin, l-carnitine, ascorbic acid combined with l-lysine, calcium antagonists, angiotensin-converting enzyme inhibitors, androgens, oestrogen, and its replacements (e.g. tibolone), anti-estrogens (e.g. tamoxifen), and thyroxine replacement in hypothyroid subjects.20,45,47
Larger studies of longer duration of Lp(a) lowering against background statin therapy in high-risk individuals including diabetics are needed. In the FATS angiographic trial,48 aggressive lowering of LDL and apolipoprotein B abrogated the risk due to Lp(a) in patients with established coronary disease. The ongoing AIM-HIGH (http://clinicaltrials.gov/ct2/show/NCT00120289) and HPS2-THRIVE (http://www.controlled-trials.com/ISRCTN29503772) trials will further evaluate this notion, although the niacin employed in these trials is not selective for Lp(a) lowering as noted above.
It is clear that more detailed studies of the metabolism of Lp(a) are required to aid in the design and development of selective and potent therapies for lowering Lp(a). Given the critical role of Lp(a) synthesis in determining the plasma concentration of Lp(a), targeting either the synthesis of apolipoprotein(a) and/or the formation of Lp(a) would appear worthwhile.18,20 Antisense oligonucleotide and thyroid hormone analogue therapies directed at apolipoprotein(a) synthesis may hold particular promise for the future.49,50
Finally, in young or middle-aged patients with evidence of progressive coronary disease and markedly elevated plasma Lp(a), serious consideration should be given to instituting LDL apheresis which removes Lp(a) efficaciously;51 however, this form of treatment is prohibitively expensive and impractical for most patients and most clinical centres.
Future needs in basic and clinical research on lipoprotein(a) and apolipoprotein(a)
The Consensus Panel is convinced that further international effort is required in different ethnicities to assess the atherothrombotic risk due to the Lp(a) particle on the one hand and to apolipoprotein(a) on the other. The potential contribution of Lp(a)-associated phospholipase A2, and equally of Lp(a)-associated oxidized phospholipids, to the pathophysiological mechanisms underlying such elevated risk remains indeterminate. Both cutting-edge basic research, rigorously designed prospective studies and intervention trials of selective Lp(a) lowering agents are required to attain these goals. Furthermore, it is entirely appropriate that Lp(a), as a causal, independent risk factor, should be integrated into existing treatment algorithms. Finally, randomized, controlled intervention trials with selective reduction in plasma lipoprotein(a) levels to reduce CVD in both primary and secondary prevention settings are urgently needed in order to define more precisely who to treat and to what targets.
Author contribution
European Atherosclerosis Society (EAS) Consensus Panel:
Writing committee. B.G.N., M.J.C., K.R., J.B., F.A., G.F.W., H.G.
Co-chairs. M.J.C. and H.G. Members. P.A. (Bichat University Hospital, Paris, France), F.A. (Catholic University Medical School, Rome, Italy), J.B. (University of Gothenburg, Sweden), A.C. (University of Milan, Italy), M.J.C. (INSERM, Paris, France), O.S.D. (Hopital de Jolimont, Belgium), E.F. (New York University, New York, USA), H.G. (Columbia University, New York, USA), P.T.K. (Wihuri Research Institute, Helsinki, Finland), J.A.K. (University of Amsterdam, The Netherlands), P.L. (INSERM, Paris, France), L.M. (Universitat Rovira and Virgili, Reus, Spain), B.G.N. (University of Copenhagen, Denmark), K.R. (St George's University of London, London, UK), Z.R. (University Hospital Center Zagreb, Croatia), M.-R.T. (Biomedicum, Helsinki, Finland), L.T. (Hacettepe University, Ankara, Turkey), A.T.-H. (University of Copenhagen, Denmark), G.F.W. (University of Western Australia, Perth, Australia).
The EAS Consensus Panel met twice in Paris organized and chaired by M.J.C. and H.G. The first meeting critically reviewed the literature, whereas the second meeting scrutinized the first draft of the consensus paper. B.G.N., K.R., J.B., F.A., G.F.W., H.G., and M.J.C. each drafted sections and/or outline for the first version, whereas the complete draft was written up by B.G.N. and M.J.C. All committee members agreed to conception and design, contributed to interpretation of available data, all suggested revisions for this document, and all members approved the final document before submission.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work including Consensus Panel meetings were supported by unrestricted educational grants to EAS from Merck, Kowa, Roche, and AstraZeneca. These companies were not present at the Consensus Panel meetings, had no role in the design or content of the Consensus Statement, and had no right to approve or disapprove of the final document. Funding to pay the Open Access publication charges for this article was provided by funding from the European Atherosclerosis Society.
Conflict of interest
Several of the Consensus Panel members have received lecture honoraria, consultancy fees, and/or research funding from Pfizer (B.G.N., M.J.C., K.R., H.G., J.B., F.A., G.W., L.T., Z.R., O.S.D., P.T.K.), Astra Zeneca (B.G.N., M.J.C., K.R., H.G., J.B., F.A., G.W., L.T., Z.R., O.S.D., E.F., L.M., P.T.K.), Merck (M.J.C., K.R., H.G., J.B., F.A., G.W., L.T., Z.R., O.S.D., E.F., M.-R.T., L.M., P.T.K.), Abbott (B.G.N., K.R., H.G., G.W., L.T.), Boehringer Ingelheim (B.G.N., F.A., M.-R.T.), sanofi-aventis (B.G.N., K.R., J.B., G.W., L.T., O.S.D., M.-R.T.), Karo Bio (B.G.N.), Bayer (F.A.), Daiichi-Sankyo (F.A., K.R., L.T.), Bristol–Meyers Squibb (F.A., K.R., L.T.), Lilly (F.A., K.R., M.-R.T.), Solvay (K.R., L.T., Z.R., O.D.S.), Novartis (K.R., L.T., M.-R.T., L.M., M.J.C.), Menarini (K.R., L.T.), Takeda (E.F.), and Kowa (L.M., M.J.C.).
Supplementary Material
Acknowledgements
We thank Jane Stock for assistance with co-ordination of the writing process.
References
- 1.Utermann G. Lipoprotein(a) In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. pp. 2753–2787. [Google Scholar]
- 2.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 3.Erqou S, Kaptoge S, Perry PL, Di AE, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de FU, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 5.Matthews KA, Sowers MF, Derby CA, Stein E, Miracle-McMahill H, Crawford SL, Pasternak RC. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women's Health Across the Nation (SWAN) Am Heart J. 2005;149:1066–1073. doi: 10.1016/j.ahj.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Cantin B, Gagnon F, Moorjani S, Despres JP, Lamarche B, Lupien PJ, Dagenais GR. Is lipoprotein(a) an independent risk factor for ischemic heart disease in men? The Quebec Cardiovascular Study. J Am Coll Cardiol. 1998;31:519–525. doi: 10.1016/s0735-1097(97)00528-7. [DOI] [PubMed] [Google Scholar]
- 7.Luc G, Bard JM, Arveiler D, Ferrieres J, Evans A, Amouyel P, Fruchart JC, Ducimetiere P. Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis. 2002;163:377–384. doi: 10.1016/s0021-9150(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 8.Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 2006;296:1363–1370. doi: 10.1001/jama.296.11.1363. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 2000;102:1082–1085. doi: 10.1161/01.cir.102.10.1082. [DOI] [PubMed] [Google Scholar]
- 10.Bennet A, Di AE, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, Rumley A, Lowe GD, Danesh J, Gudnason V. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168:598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 11.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 12.Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. pp. 2863–2913. [Google Scholar]
- 14.Tybjaerg-Hansen A, Steffensen R, Meinertz H, Schnohr P, Nordestgaard BG. Association of mutations in the apolipoprotein B gene with hypercholesterolemia and the risk of ischemic heart disease. N Engl J Med. 1998;338:1577–1584. doi: 10.1056/NEJM199805283382203. [DOI] [PubMed] [Google Scholar]
- 15.Luke MM, Kane JP, Liu DM, Rowland CM, Shiffman D, Cassano J, Catanese JJ, Pullinger CR, Leong DU, Arellano AR, Tong CH, Movsesyan I, Naya-Vigne J, Noordhof C, Feric NT, Malloy MJ, Topol EJ, Koschinsky ML, Devlin JJ, Ellis SG. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler Thromb Vasc Biol. 2007;27:2030–2036. doi: 10.1161/ATVBAHA.107.141291. [DOI] [PubMed] [Google Scholar]
- 16.Holmes DT, Schick BA, Humphries KH, Frohlich J. Lipoprotein(a) is an independent risk factor for cardiovascular disease in heterozygous familial hypercholesterolemia. Clin Chem. 2005;51:2067–2073. doi: 10.1373/clinchem.2005.055228. [DOI] [PubMed] [Google Scholar]
- 17.Rader DJ, Cain W, Ikewaki K, Talley G, Zech LA, Usher D, Brewer HB., Jr. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J Clin Invest. 1994;93:2758–2763. doi: 10.1172/JCI117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koschinsky ML, Marcovina SM. Structure-function relationships in apolipoprotein(a): insights into lipoprotein(a) assembly and pathogenicity. Curr Opin Lipidol. 2004;15:167–174. doi: 10.1097/00041433-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein(a) and Cardiovascular Disease: recent advances and future directions. Clin Chem. 2003;49:1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 20.Koschinsky M, Marcovina SM. Lipoprotein(a) In: Ballantyne C, editor. Clinical Lipidology: A Companion to Braunwauld's Heart Disease. Philadelphia: Saunders Elsevier; 2009. pp. 130–143. [Google Scholar]
- 21.Nielsen LB. Atherogenecity of lipoprotein(a) and oxidized low density lipoprotein: insight from in vivo studies of arterial wall influx, degradation and efflux. Atherosclerosis. 1999;143:229–243. doi: 10.1016/s0021-9150(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 22.Lundstam U, Hurt-Camejo E, Olsson G, Sartipy P, Camejo G, Wiklund O. Proteoglycans contribution to association of Lp(a) and LDL with smooth muscle cell extracellular matrix. Arterioscler Thromb Vasc Biol. 1999;19:1162–1167. doi: 10.1161/01.atv.19.5.1162. [DOI] [PubMed] [Google Scholar]
- 23.Bdeir K, Cane W, Canziani G, Chaiken I, Weisel J, Koschinsky ML, Lawn RM, Bannerman PG, Sachais BS, Kuo A, Hancock MA, Tomaszewski J, Raghunath PN, Ganz T, Higazi AA, Cines DB. Defensin promotes the binding of lipoprotein(a) to vascular matrix. Blood. 1999;94:2007–2019. [PubMed] [Google Scholar]
- 24.Boonmark NW, Lou XJ, Yang ZJ, Schwartz K, Zhang JL, Rubin EM, Lawn RM. Modification of apolipoprotein(a) lysine binding site reduces atherosclerosis in transgenic mice. J Clin Invest. 1997;100:558–564. doi: 10.1172/JCI119565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotiriou SN, Orlova VV, Al-Fakhri N, Ihanus E, Economopoulou M, Isermann B, Bdeir K, Nawroth PP, Preissner KT, Gahmberg CG, Koschinsky ML, Chavakis T. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J. 2006;20:559–561. doi: 10.1096/fj.05-4857fje. [DOI] [PubMed] [Google Scholar]
- 26.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 27.Tsimikas S, Tsironis LD, Tselepis AD. New insights into the role of lipoprotein(a)-associated lipoprotein-associated phospholipase A2 in atherosclerosis and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2094–2099. doi: 10.1161/01.ATV.0000280571.28102.d4. [DOI] [PubMed] [Google Scholar]
- 28.Rouy D, Grailhe P, Nigon F, Chapman J, Angles-Cano E. Lipoprotein(a) impairs generation of plasmin by fibrin-bound tissue-type plasminogen activator. In vitro studies in a plasma milieu. Arterioscler Thromb. 1991;11:629–638. doi: 10.1161/01.atv.11.3.629. [DOI] [PubMed] [Google Scholar]
- 29.Feric NT, Boffa MB, Johnston SM, Koschinsky ML. Apolipoprotein(a) inhibits the conversion of Glu-plasminogen to Lys-plasminogen: a novel mechanism for lipoprotein(a)-mediated inhibition of plasminogen activation. J Thromb Haemost. 2008;6:2113–2120. doi: 10.1111/j.1538-7836.2008.03183.x. [DOI] [PubMed] [Google Scholar]
- 30.Pan S, Kleppe LS, Witt TA, Mueske CS, Simari RD. The effect of vascular smooth muscle cell-targeted expression of tissue factor pathway inhibitor in a murine model of arterial thrombosis. Thromb Haemost. 2004;92:495–502. doi: 10.1160/TH04-01-0006. [DOI] [PubMed] [Google Scholar]
- 31.Hervio L, Chapman MJ, Thillet J, Loyau S, Angles-Cano E. Does apolipoprotein(a) heterogeneity influence lipoprotein(a) effects on fibrinolysis? Blood. 1993;82:392–397. [PubMed] [Google Scholar]
- 32.Erqou S, Thompson A, Di AE, Saleheen D, Kaptoge S, Marcovina S, Danesh J. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55:2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 33.Kronenberg F, Kronenberg MF, Kiechl S, Trenkwalder E, Santer P, Oberhollenzer F, Egger G, Utermann G, Willeit J. Role of lipoprotein(a) and apolipoprotein(a) phenotype in atherogenesis: prospective results from the Bruneck study. Circulation. 1999;100:1154–1160. doi: 10.1161/01.cir.100.11.1154. [DOI] [PubMed] [Google Scholar]
- 34.von Eckardstein A, Schulte H, Cullen P, Assmann G. Lipoprotein(a) further increases the risk of coronary events in men with high global cardiovascular risk. J Am Coll Cardiol. 2001;37:434–439. doi: 10.1016/s0735-1097(00)01126-8. [DOI] [PubMed] [Google Scholar]
- 35.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De BG, Ebrahim S, Gjelsvik B, Herrmann-Lingen C, Hoes A, Humphries S, Knapton M, Perk J, Priori SG, Pyorala K, Reiner Z, Ruilope L, Sans-Menendez S, Scholte op RW, Weissberg P, Wood D, Yarnell J, Zamorano JL, Walma E, Fitzgerald T, Cooney MT, Dudina A, Vahanian A, Camm J, De CR, Dean V, Dickstein K, Funck-Brentano C, Filippatos G, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Hellemans I, Altiner A, Bonora E, Durrington PN, Fagard R, Giampaoli S, Hemingway H, Hakansson J, Kjeldsen SE, Larsen ML, Mancia G, Manolis AJ, Orth-Gomer K, Pedersen T, Rayner M, Ryden L, Sammut M, Schneiderman N, Stalenhoef AF, Tokgozoglu L, Wiklund O, Zampelas A. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 36.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol. 2004;24:e149–e161. doi: 10.1161/01.ATV.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 37.Nordestgaard BG. Does elevated C-reactive protein cause human atherothrombosis? Novel insights from genetics, intervention trials, and elsewhere. Curr Opin Lipidol. 2009;20:393–401. doi: 10.1097/MOL.0b013e3283307bfe. [DOI] [PubMed] [Google Scholar]
- 38.Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210:353–361. doi: 10.1016/j.atherosclerosis.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Carlson LA, Rosenhamer G. Reduction in mortality in the Stockholm Ischemic Heart Disease Secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Med Scand. 1988;233:405–418. doi: 10.1111/j.0954-6820.1988.tb15891.x. [DOI] [PubMed] [Google Scholar]
- 40.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 41.Cashin-Hemphill L, Mack WJ, Pogoda JM, Sanmarco ME, Azen SP, Blankenhorn DH. Beneficial effects of colestipol-niacin on coronary atherosclerosis. A 4-year follow-up. JAMA. 1990;264:3013–3017. [PubMed] [Google Scholar]
- 42.Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 43.Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, Weissman NJ, Turco M. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 44.Chapman MJ, Redfern JS, McGovern ME, Giral P. Niacin and fibrates in atherogenic dyslipidemia: pharmacotherapy to reduce cardiovascular risk. Pharmacol Ther. 2010;126:314–345. doi: 10.1016/j.pharmthera.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Tziomalos K, Athyros VG, Wierzbicki AS, Mikhailidis DP. Lipoprotein a: where are we now? Curr Opin Cardiol. 2009;24:351–357. doi: 10.1097/HCO.0b013e32832ac21a. [DOI] [PubMed] [Google Scholar]
- 46.Gonbert S, Malinsky S, Sposito AC, Laouenan H, Doucet C, Chapman MJ, Thillet J. Atorvastatin lowers lipoprotein(a) but not apolipoprotein(a) fragment levels in hypercholesterolemic subjects at high cardiovascular risk. Atherosclerosis. 2002;164:305–311. doi: 10.1016/s0021-9150(02)00072-2. [DOI] [PubMed] [Google Scholar]
- 47.Suk DJ, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52:124–131. doi: 10.1016/j.jacc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a) JAMA. 1995;274:1771–1774. [PubMed] [Google Scholar]
- 49.Merki E, Graham MJ, Mullick AE, Miller ER, Crooke RM, Pitas RE, Witztum JL, Tsimikas S. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 2008;118:743–753. doi: 10.1161/CIRCULATIONAHA.108.786822. [DOI] [PubMed] [Google Scholar]
- 50.Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362:906–916. doi: 10.1056/NEJMoa0905633. [DOI] [PubMed] [Google Scholar]
- 51.Thompson GR. Recommendations for the use of LDL apheresis. Atherosclerosis. 2008;198:247–255. doi: 10.1016/j.atherosclerosis.2008.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.