Abstract
Aims
To establish the safety, tolerability and most promising regimen of darexaban (YM150), a novel, oral, direct factor Xa inhibitor, for prevention of ischaemic events in acute coronary syndrome (ACS).
Methods
In a 26-week, multi-centre, double-blind, randomized, parallel-group study, 1279 patients with recent high-risk non-ST-segment or ST-segment elevation ACS received one of six darexaban regimens: 5 mg b.i.d., 10 mg o.d., 15 mg b.i.d., 30 mg o.d., 30 mg b.i.d., or 60 mg o.d. or placebo, on top of dual antiplatelet treatment. Primary outcome was incidence of major or clinically relevant non-major bleeding events. The main efficacy outcome was a composite of death, stroke, myocardial infarction, systemic thromboembolism, and severe recurrent ischaemia.
Results
Bleeding rates were numerically higher in all darexaban arms vs. placebo (pooled HR: 2.275; 95% CI: 1.13–4.60, P = 0.022). Using placebo as reference (bleeding rate 3.1%), there was a dose–response relationship (P = 0.009) for increased bleeding with increasing darexaban dose (6.2, 6.5, and 9.3% for 10, 30, and 60 mg daily, respectively), which was statistically significant for 30 mg b.i.d. (P = 0.002). There was no decrease (indeed a numerical increase in the 30 and 60 mg dose arms) in efficacy event rates with darexaban, but the study was underpowered for efficacy. Darexaban showed good tolerability without signs of liver toxicity.
Conclusions
Darexaban when added to dual antiplatelet therapy after ACS produces an expected dose-related two- to four-fold increase in bleeding, with no other safety concerns but no signal of efficacy. Establishing the potential of low-dose darexaban in preventing major cardiac events after ACS requires a large phase III trial.
ClinicalTrials.gov Identifier: NCT00994292
Keywords: Anticoagulant, Acute coronary syndrome, Secondary prevention, Darexaban
See page 2486 for the commentary on this article (doi:10.1093/eurheartj/ehr314)
Introduction
The management of acute coronary syndrome (ACS) has improved considerably over the past decades, leading to a substantial decline in morbidity and mortality.1 Guidelines from the European Society of Cardiology (ESC)2,3 and the American College of Cardiology/American Heart Association (ACC/AHA)4–6 recommend the continuation of dual antiplatelet therapy [acetylsalicylic acid (ASA) and clopidogrel] for up to 1 year after an ACS event.
Despite this, the recurrence of ischaemic events after an ACS event remains high, up to 9.1% at 6 months,7 generating interest for improved antithrombotic therapy in addition to the current standard of antiplatelet therapy. Since thrombin plays a pivotal role in the formation and consolidation of thrombi in ACS, there has been interest in thrombin inhibitors, particularly as oral forms have become available. Conventional oral anticoagulation with vitamin K antagonists (VKAs), while effective at preventing ischaemic events,8 is fraught with problems related to the narrow therapeutic range of these agents, the need for careful monitoring, the frequent interactions with drugs and food, and the delay in onset and offset of action.9–12 Use of chronic triple antithrombotic therapy with ASA, thienopyridines, and VKAs beyond the acute phase is associated with a high risk of bleeding.13,14 In this context, more selective oral anticoagulants, direct thrombin inhibitors such as dabigatran15 or factor Xa (FXa) inhibitors such as apixaban16 and rivaroxaban,17 have generated interest.
Darexaban (YM150) and its major metabolite darexaban glucuronide (YM-222714) are direct inhibitors of FXa. Darexaban is rapidly absorbed from the gut and almost completely converted into darexaban glucuronide which reaches maximum plasma levels at 1–1.5 h post-dose and has a terminal half-life of 14–18 h. Darexaban glucuronide is the main active moiety driving the antithrombotic effect and demonstrates a predictable pharmacokinetic (PK)/pharmacodynamic (PD) profile. Darexaban has minimal interaction with food18 and importantly no drug–drug interactions with CYP3A4/P-glycoprotein inhibitors and inducers.19 Darexaban shows no PK or PD interaction with ASA and clopidogrel, although the skin bleeding time increases as expected.20 In addition, no interaction was observed with naproxen.20 The drug is excreted equally through the kidneys and faeces.21 Since direct FXa inhibitors are small molecules that bind to and inhibit FXa, independent of other proteins, they are thought to bind to both clot-bound and free FXa.22 The potential benefit of darexaban has been shown in venous thromboembolic disease,23,24 and has recently been explored in the prevention of stroke in subjects with non-valvular atrial fibrillation in a phase II study (Clinical Trials.gov identifier: NCT00938730).25–27 RUBY-1 (150-CL-201 ClinicalTrials.gov identifier: NCT00994292) is a phase II trial aimed at exploring the potential role of darexaban in patients with recent ACS, as well as defining a dose range in this indication.
Methods
Study objectives
The primary objective of this study was the evaluation of the safety and tolerability of different doses and dose regimens of darexaban on top of standard treatment with ASA with or without clopidogrel, for the secondary prevention of ischaemic vascular events in patients with recent ACS. The secondary objectives were the evaluation of the efficacy of different doses and dose regimens of darexaban on top of standard dual antiplatelet treatment in the secondary prevention of ischaemic vascular events in patients with recent ACS, as well as helping to define the suitable population for further development (i.e. phase III).
Study population
Male or female patients with a diagnosis of non-ST-segment elevation (NSTE)-ACS and high-risk features, or ST-segment elevation (STE)-ACS were eligible for randomization. Acute coronary syndrome was defined according to accepted international guidelines.3,5 If eligible, patients were randomized as early as possible after clinical stabilization, as long as this occurred within 7 days of presentation of the index event. In total, 1264 patients with NSTE-ACS or STE-ACS as the index event were planned for randomization. The complete inclusion and exclusion criteria are detailed in Appendix 2.
Study design and treatments
RUBY-1 was a prospective, randomized, double-blind, multi-centre, multiple-dose, placebo-controlled, parallel-group trial in subjects presenting with ACS. Following presentation with the index ACS event, patients were managed according to local standards of care, which could include primary percutaneous coronary intervention (PCI), thrombolysis, or medical management. All subjects were to receive antiplatelet treatment as per current guidelines.3,5 Acetylsalicylic acid was used at a dose of 75–325 mg daily, as per local practice. The lower dose range of ASA (i.e. 75–81 mg daily) was recommended, or clopidogrel 75 mg daily if ASA was contraindicated or not tolerated, or a combination of ASA 75–325 mg and clopidogrel 75 mg daily (a maintenance dose of clopidogrel of up to 150 mg was allowed up to 7 days after the index event).
Subjects were screened for eligibility following presentation with ACS. Once stabilized, eligible subjects were randomized, via an interactive voice response system, in a double-blind manner, to one of seven parallel study treatment groups within 7 days of presentation with the index event. The study drug was administered for a total duration of 26 weeks, in addition to standard antiplatelet treatment. Six dose groups of darexaban and one placebo control group were evaluated. Randomization was stratified by country and clopidogrel use. The allocation ratio was 1:1:1:1:1:1:2 to the following groups: darexaban 5 mg b.i.d., darexaban 10 mg o.d., darexaban 15 mg b.i.d., darexaban 30 mg o.d., darexaban 30 mg b.i.d., and darexaban 60 mg o.d., or placebo. The selection of this dose range was based on previous experience in healthy volunteers (in whom doses of up to 320 mg for 14 days were well tolerated), as well as on prior experience in venous thromboembolism prophylaxis,23,24 where doses of up to 120 mg daily have been studied and were safe and effective; prior experience in a phase II trial of stroke prevention in atrial fibrillation28 where darexaban was safe; and an indirect comparison with rivaroxaban suggesting that darexaban 60 mg resulted in approximately the same FXa inhibition as rivaroxaban 10 mg.18 In addition, two drug–drug interaction studies suggested no clinically relevant interaction with ASA with or without clopidogrel.20 Given that most ACS patients would be on dual antiplatelet therapy, it appeared prudent to explore the lower end of the dose range in order to study the drug at doses that may minimize the bleeding caused by superimposition of oral anticoagulation, which is why a daily dose of 10 mg was selected as the starting point. Other antithrombotic drugs given for the initial management of the index event during the acute phase had to be discontinued before initiation of study treatment. Other medical treatments, such as beta-blockers and angiotensin II inhibitors, were given as per local practice and guidelines. The study was approved by the Institutional Review Boards/Ethical Review Committees for each participating hospital. All patients provided written informed consent prior to randomization.
Outcome measures
Primary outcomes
The primary outcome was the incidence of major and clinically relevant non-major (CRNM) bleeding events, according to a modified version of the International Society on Thrombosis and Haemostasis (ISTH) definition,29 during the 6 months of the study. All overt bleeding events were adjudicated by the independent adjudication committee (IAC) as either major, CRNM, or minor bleeding events. Bleeds were also adjudicated according to the thrombolysis in myocardial infarction (TIMI) bleeding definition.30 Definitions of bleeding events are detailed in Appendix 3.
Secondary outcomes
Secondary outcomes included TIMI major bleeding events, and, as the main secondary outcome, the composite of all-cause mortality, non-fatal myocardial infarction (MI), non-fatal stroke, and severe recurrent ischaemia. Severe recurrent ischaemia was defined as worsening anginal symptoms lasting at least 10 min and associated with at least two of the following: dynamic ≥0.1 mV ST depression or elevation, hospitalization, or unplanned cardiac catheterization with evidence of significant coronary stenosis. Stent thromboses were also analysed after adjudication as per the academic research consortium criteria.31
Sample size
Assuming an incidence of major and CRNM bleeding events at 6 months of 3% for placebo and 4, 7, and 9% for darexaban 10, 30, and 60 mg per day, respectively, a sample size of 1264 randomized subjects allowed a 91% power to detect a linear trend in the mentioned incidence vs. daily dose, using a two-sided test with 95% confidence level.
Statistical analysis
The primary analysis was performed based upon the modified intention-to-treat data set, defined as all randomized subjects who took at least one dose of study drug. Primary and secondary variables were analysed while patients were on study treatment and 1 day after the discontinuation of treatment. Cumulative risk and 95% confidence intervals (CIs) at 30 days and 6 months were calculated using Kaplan–Meier estimates. These variables were also inferentially analysed using a Cox regression model, using the treatment group and antiplatelet therapy as fixed effects. There was no adjustment for multiple comparisons. The data analysis was generated using SAS software (version 9.1.3; SAS Institute, Inc., Cary, NC, USA).
Results
Patient disposition
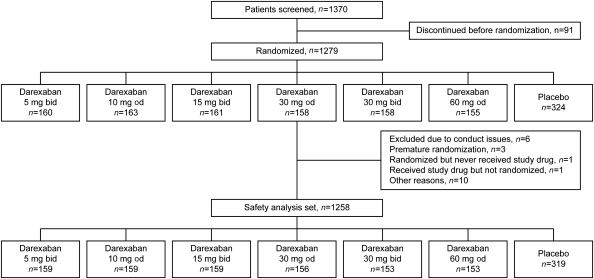
From September 2009 to July 2010, 1279 subjects were enrolled from 152 centres in 24 countries in Europe, the Americas, South Africa, and Asia. The patient disposition is highlighted in Figure 1. Overall, 1258 patients were included in the modified-intention-to-treat data set. Patient demographics and clinical characteristics for the overall study cohort is summarized in Table 1. The patient demographics and clinical characteristics for all treatment groups are summarized in Appendix 4. The majority of patients were enrolled in Eastern European countries. Overall, the treatment groups appeared comparable clinically, with a median age of 56 years old (inter-quartile range: 50–64 years old), the majority of patients enrolled following ST-elevation MI (71.1%) and 74.6% had PCI for treatment of the index event. The average risk, measured by the GRACE (Global Registry of Acute Coronary Events) score32 at presentation was similar at baseline across the various groups, indicating that baseline risks of in-hospital and 6-month death were comparable across the groups. Since 97% of patients were in the ASA and clopidogrel strata, results are reported together for patients on ASA only and dual antiplatelet therapy. Permanent discontinuation rates were 21.3% for placebo and 23.7% for darexaban.
Figure 1.
Study flow. b.i.d., twice daily; o.d., once daily.
Table 1.
Clinical characteristics of the patients (overall study cohort)
| Grand total | |
|---|---|
| n | 1258 |
| Age, mean (SD) | 56.9 (10.40) |
| Female, % | 20.4 |
| Ethnicity, n (%) | |
| Caucasian | 991 (78.8) |
| Black/African American | 8 (0.6) |
| Asian | 220 (17.5) |
| Other | 39 (3.1) |
| Smoker, n (%) | |
| Former | 297 (23.6) |
| Current | 531 (42.2) |
| Never | 430 (34.2) |
| Hypertension, n (%) | 760 (60.4) |
| Dyslipidaemia, n (%) | 627 (49.8) |
| Diabetes mellitus Type 2, n (%) | 277 (22.0) |
| Primary diagnosis for index event, n (%) | |
| STEMI | 894 (71.1) |
| NSTEMI | 364 (28.9) |
| Use of PCI for index event | 938 (74.6) |
| GRACE risk score at presentation (evaluated population) | 132.8 (n = 1243) |
| Hx of prior CHF, n (%) | 30 (2.4) |
| Hx of stroke/TIA, n (%) | 37 (2.9) |
| Hx of prior MI, n (%) | 150 (11.9) |
| Hx of CABG, n (%) | 31 (2.4) |
| Hx of PCI, n (%) | 111 (8.8) |
| Peripheral arterial disease, n (%) | 45 (3.6) |
SD, standard deviation; STEMI, ST-segment elevation; NSTEMI, non-ST-segment; PCI, percutaneous coronary intervention; GRACE, Global Registry of Acute Coronary Events; Hx, history; CHF, chronic heart failure; TIA, transient ischaemic attack; MI, myocardial infarction; CABG, coronary artery bypass graft.
Bleeding
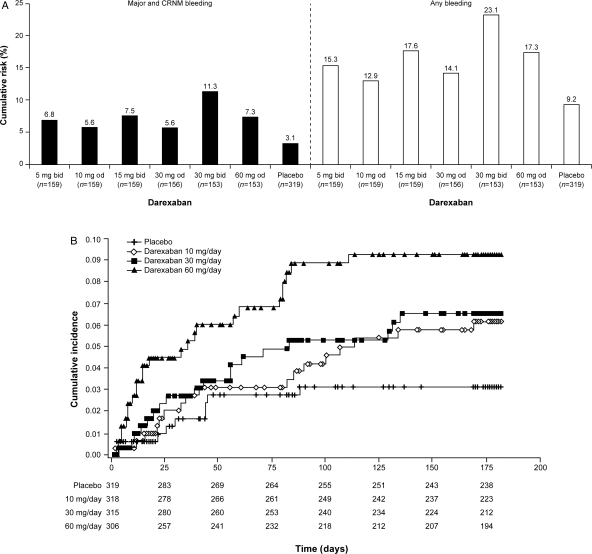
The primary safety outcome of major and/or CRNM bleeds while on study treatment (including 1 day after treatment discontinuation) is shown in Table 2 and Figure 2A and B. This primary analysis shows that the bleeding rate was numerically higher in all darexaban treatment arms than in the placebo arm, with hazard ratios (HRs) ranging from 1.8 to 3.8. Starting with placebo as reference (bleeding rate 3.1%), there was a dose–response relationship (P = 0.009, for trend analysed using the Cox regression model) for increased bleeding rates with increasing darexaban dose, since the cumulative incidence of bleeding was 6.2, 6.5, and 9.3% for patients receiving total daily doses of 10 (n = 318), 30 (n = 315), and 60 mg (n = 306) darexaban, respectively (Figure 2B). This increase was statistically significant for the darexaban 30 mg b.i.d. dose (P = 0.002).
Table 2.
Safety outcomes
| Placebo KM rate (n/N) | Total daily darexaban dose | Once daily dosing |
Twice daily dosing |
||||
|---|---|---|---|---|---|---|---|
| KM rate (n/N) | HR (95% CI) vs. placebo | P-value | KM rate (n/N) | HR (95% CI) vs. placebo | P-value | ||
| Primary safety endpoint—Kaplan–Meier analysis of the cumulative risk of major and clinically relevant non-major bleeding events | |||||||
| 3.1% (9/319) | |||||||
| 10 mg | 5.6% (8/159) | 1.775 (0.68, 4.60) | 0.238 | 6.8% (9/159) | 2.045 (0.81, 5.15) | 0.129 | |
| 30 mg | 5.6% (8/156) | 1.831 (0.71, 4.75) | 0.213 | 7.5% (10/159) | 2.269 (0.92, 5.59) | 0.075 | |
| 60 mg | 7.3% (10/153) | 2.425 (0.98, 5.97) | 0.054 | 11.3% (15/153) | 3.796 (1.66, 8.68) | 0.002 | |
| Darexaban, 5 mg b.i.d. (n = 159) | Darexaban, 10 mg o.d. (n = 159) | Darexaban, 15 mg b.i.d. (n = 159) | Darexaban, 30 mg o.d. (n = 156) | Darexaban, 30 mg b.i.d. (n = 153) | Darexaban, 60 mg o.d. (n = 153) | Placebo (n = 319) | |
| Absolute event rates for individual categories of bleeding events | |||||||
| Major and CRNM | 9 (5.7) | 8 (5.0) | 10 (6.3) | 8 (5.1) | 15 (9.8) | 10 (6.5) | 9 (2.8) |
| Major | 1 (0.6) | 1 (0.6) | 2 (1.3) | 3 (1.9) | 3 (2.0) | 0 (0.0) | 1 (0.3) |
| CRNM | 8 (5.0) | 7 (4.4) | 8 (5.0) | 5 (3.2) | 13 (8.5) | 10 (6.5) | 8 (2.5) |
| Minor | 13 (8.2) | 12 (7.5) | 16 (10.1) | 12 (7.7) | 17 (11.1) | 16 (10.5) | 18 (5.6) |
| Any | 21 (13.2) | 18 (11.3) | 24 (15.1) | 20 (12.8) | 30 (19.6) | 23 (15.0) | 26 (8.2) |
| TIMI bleeding events | |||||||
| Major | 1 (0.6) | 1 (0.6) | 0 (0.0) | 3 (1.9) | 1 (0.7) | 0 (0.0) | 1 (0.3) |
| Minor | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Requiring medical attention | 8 (5.0) | 6 (3.8) | 9 (5.7) | 5 (3.2) | 13 (8.5) | 10 (6.5) | 6 (1.9) |
| Insignificant | 13 (8.2) | 13 (8.2) | 16 (10.1) | 14 (9.0) | 19 (12.4) | 16 (10.5) | 20 (6.3) |
| Any | 21 (13.2) | 18 (11.3) | 24 (15.1) | 20 (12.8) | 30 (19.6) | 23 (15.0) | 26 (8.2) |
All data are n (%); KM, Kaplan–Meier; HR, hazard ratio; CI, confidence interval, b.i.d., twice daily; o.d, once daily; CRNM, clinically relevant non-major; TIMI, thrombolysis in myocardial infarction.
Figure 2.
(A) Kaplan–Meier analysis of the cumulative risk of major and clinically relevant non-major bleeding (black bars), and any bleeding events (white bars) at 6 months (safety analysis set). (B) Kaplan–Meier analysis of the cumulative risk of the primary safety endpoint of major and clinically relevant non-major bleeding events for the three total daily doses of darexaban (10, 30, and 60 mg). Cumulative incidences are calculated using Kaplan–Meier estimates and presented as relative to 1 (e.g. 0.06 represents 6%). CRNM, clinically relevant non-major; b.i.d., twice daily; o.d., once daily.
The rates of bleeding were similar for patients receiving b.i.d. (n = 471) vs. o.d. (n = 468) dosing with darexaban (8.4 vs. 6.1%, respectively, P = 0.310). Pooling together all dose arms of darexaban (in a post hoc analysis), the primary endpoint was more frequent with darexaban than with placebo (HR: 2.275; CI: 1.13–4.60; P = 0.022).
Additional information regarding other bleeding events is included in Table 2, reporting each component of the primary safety outcome, and the additional outcomes of minor bleeding, any bleeding, and bleeding categorized according to the TIMI scheme. There were no cases of fatal bleeding or intracranial haemorrhage in any group. All bleeding events (Figure 2A) followed a pattern similar to that of the primary safety outcome.
Bleeding rates analysed for the entire duration of follow-up (including the 4-week observation period following treatment completion or premature permanent discontinuation) were similar to results observed during the period on treatment (data not shown).
The overall number of subjects with adverse events (AEs; which included bleeding and efficacy outcomes) was similar across the darexaban arms and, consistent with the higher bleeding rates previously described, was slightly higher than with placebo. In addition, AEs leading to discontinuation of the study drug were higher in the darexaban 30 mg o.d., 30 mg b.i.d., and 60 mg o.d. arms. The percentage of subjects with alanine transaminase (ALT) or aspartate transaminase (AST) >3 or >5× the upper limit of normal (ULN) was similar across all treatment arms (Table 3). The percentage of subjects with total bilirubin >2× ULN ranged from 0% with placebo and darexaban 15 mg b.i.d. to 1.4% with darexaban 30 mg b.i.d., with no clear pattern across darexaban doses. Two subjects, both receiving darexaban, had concurrent elevations, one of whom had total bilirubin >3× ULN. Additionally, there was no difference in creatinine elevations between groups (data not shown).
Table 3.
Overview of adverse events, including liver-related clinical laboratory abnormalities during the treatment period
| Darexaban, 5 mg b.i.d. (n = 159) | Darexaban, 10 mg o.d. (n = 159) | Darexaban, 15 mg b.i.d. (n = 159) | Darexaban, 30 mg o.d. (n = 156) | Darexaban 30 mg b.i.d. (n = 153) | Darexaban 60 mg o.d. (n = 153) | Darexaban, all (n = 939) | Placebo (n = 319) | |
|---|---|---|---|---|---|---|---|---|
| Adverse events (%) | 100 (62.9) | 102 (64.2) | 100 (62.9) | 96 (61.5) | 101 (66.0) | 99 (64.7) | 598 (63.7) | 181 (56.7) |
| AEs leading to discontinuation (%) | 16 (10.1) | 25 (15.7) | 21 (13.2) | 27 (17.3) | 29 (19.0) | 25 (16.3) | 95 (10.1) | 31 (9.7) |
| ALT or AST >3× ULN | 5/143 (3.5) | 4/149 (2.7) | 2/148 (1.4) | 1/138 (0.7) | 2/139 (1.4) | 2/137 (1.5) | 16/854 (1.9) | 7/290 (2.4) |
| ALT or AST >5× ULN | 2/149 (1.3) | 2/155 (1.3) | 0 (0.0) | 0 (0.0) | 1/146 (0.7) | 1/144 (0.7) | 6/909 (0.7) | 2/302 (0.7) |
| Total bilirubin >2× ULN | 1/150 (0.7) | 1/151 (0.7) | 0 (0.0) | 1/147 (0.7) | 2/141 (1.4) | 1/141 (0.7) | 6/889 (0.7) | 0 (0.0) |
| Total bilirubin >3× ULN | 0 (0.0) | 1/151 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1/931 (0.1) | 0 (0.0) |
All data are n (%); b.i.d., twice daily; o.d., once daily; AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; ULN, upper limit of normal.
Efficacy
The composite of all-cause mortality, non-fatal MI, non-fatal stroke, and severe recurrent ischaemia at 6 months was the main secondary outcome (Table 4) Event rates at 6 months showed no decrease with darexaban compared with placebo. Although this study was not powered for efficacy, the cumulative incidence of this composite efficacy outcome was numerically higher in the darexaban 30 and 60 mg daily dose arms compared with placebo. At these doses, the increase appeared largely driven by severe recurrent ischaemia. The composite efficacy outcome was infrequent with the lowest doses of darexaban (10 mg daily arms), and numerically lower than placebo. Other secondary efficacy outcomes are overviewed in Table 4: event rates were low and similar between dose groups. Definite or probable stent thromboses (as per ARC criteria) occurred in 1.1% (8/703) of patients receiving darexaban and 0.4% (1/319) of patients receiving placebo.
Table 4.
Efficacy outcomes
| Darexaban, 5 mg b.i.d. (n = 159) | Darexaban, 10 mg o.d. (n = 159) | Darexaban, 15 mg b.i.d. (n = 159) | Darexaban, 30 mg o.d. (n = 156) | Darexaban, 30 mg b.i.d. (n = 153) | Darexaban, 60 mg o.d. (n = 153) | Darexaban, all (n = 939) | Placebo (n = 319) | |
|---|---|---|---|---|---|---|---|---|
| Kaplan–Meier analysis of cumulative risk of composite of all-cause mortality, non-fatal myocardial infarction, non-fatal stroke, and severe recurrent ischaemia | ||||||||
| Cumulative risk | 4.3 | 4.3 | 7.4 | 7.3 | 6.9 | 9.3 | 6.5 | 5.2 |
| 95% CI | 2.0–9.4 | 2.0–9.4 | 4.0–13.3 | 4.0–13.1 | 3.6–12.9 | 5.4–15.8 | 5.0–8.5 | 3.1–8.6 |
| Absolute frequencies and proportions of secondary efficacy endpoints at 6 months | ||||||||
| Non-fatal MI, non-fatal stroke, severe recurrent ischaemia and all deaths, n (%) | 6 (3.8) | 6 (3.8) | 10 (6.3) | 10 (6.4) | 9 (5.9) | 12 (7.8) | 53 (5.6) | 14 (4.4) |
| Non-fatal MI, non-fatal stroke, and all deaths n (%) | 4 (2.5) | 5 (3.1) | 4 (2.5) | 5 (3.2) | 5 (3.3) | 4 (2.6) | 27 (2.9) | 7 (2.2) |
| Deaths, n (%) | 0 (0.0) | 2 (1.3) | 0 (0.0) | 3 (1.9) | 1 (0.7) | 1 (0.7) | 7 (0.7) | 2 (0.6) |
| MI, n (%) | 4 (2.5) | 4 (2.5) | 4 (2.5) | 5 (3.2) | 4 (2.6) | 4 (2.6) | 25 (2.7) | 6 (1.9) |
| Severe recurrent ischaemia, n (%) | 2 (1.3) | 1 (0.6) | 6 (3.8) | 5 (3.2) | 4 (2.6) | 8 (5.2) | 26 (2.8) | 7 (2.2) |
| Stroke, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| TIA, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 1 (0.7) | 2 (0.2) | 0 (0.0) |
| STE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
In each row, only the first event is counted, however, patients may be counted in more than one row. All data are n (%) unless otherwise stated; b.i.d., twice daily; o.d., once daily; CI, confidence interval; MI, myocardial infarction; TIA, transient ischaemic attack; STE, systemic thromboembolic event.
Discussion
This phase II dose-ranging study showed that the frequency of major or CRNM bleeding events during 6 months of double-blind treatment (on top of dual antiplatelet therapy) after ACS was two- to four-fold higher (HRs ranging from 1.8 to 3.8) with the various doses of darexaban than with placebo. There was an increase in bleeding rates with increasing increments in the total daily dose. As with most phase II dose-ranging trials of antithrombotic drugs, RUBY-1 was underpowered to study efficacy. There was no signal for efficacy. There was no significant difference in the composite of non-fatal MI, non-fatal stroke, severe recurrent ischaemia, and death due to any cause during 6 months of double-blind treatment. The incidence of these events was numerically higher than placebo at the higher dose range, and numerically lower at the lower end of the dose range. However, the CIs around these point estimates were wide. Liver safety, as measured by ALT, AST, and bilirubin levels, appeared similar between placebo and the various doses of darexaban.
Classic trials of VKAs added to single antiplatelet therapy with ASA have demonstrated superior efficacy compared with antiplatelet therapy alone in secondary prevention after ACS,33,34 although this was achieved at the expense of an increased risk of major bleeding.8,34 However, VKAs have a narrow therapeutic margin, a slow onset and offset of action, and numerous food and drug interactions, and, therefore, require careful monitoring. New oral anticoagulants targeting thrombin or factor X have been designed to overcome these problems, and darexaban is particularly attractive in this setting given its predictable PK properties18 and minimal potential for drug–drug interactions.19,20 Indeed, there is evidence from several phase II studies15–17,35 that adding an oral anticoagulant to antiplatelet therapy in patients with recent ACS may have some efficacy in the prevention of ischaemic events and death compared with antiplatelet therapy alone, and this concept has led to several phase III trials.36,37
However, even with novel anticoagulants that have a broader therapeutic margin than VKAs, the selection of dose is critical for secondary prevention after ACS, because in this setting (in which most patients have received coronary stents) dual antiplatelet therapy is part of the standard of care and is recommended by guidelines.2,3,5 Indeed, in the present study, 95% of patients were on dual antiplatelet therapy, contrasting with prior studies with VKAs, which were performed before the era of dual antiplatelet therapy after ACS. Therefore, adding an anticoagulant would result in long-term triple antithrombotic therapy, which may result in unacceptable bleeding, and indicates that this strategy would need to show a tangible efficacy benefit, with a reduction in cardiovascular death, MI, and stroke, to offset any bleeding hazard. In fact, a large phase III trial of apixaban in this setting was recently stopped due to increased bleeding which was not offset by increased efficacy,37 and development of another FXa inhibitor in ACS was halted after a phase II study.38 Another phase III trial with rivaroxaban after ACS36 is ongoing and is expected to be reported on later this year. This trial will be key in understanding whether the concept of adding an oral anticoagulant to standard of care dual antiplatelet therapy in order to prevent recurrent events after ACS is valid or whether in the current environment of potent antiplatelet therapy, this can only result in increased bleeding without compensatory reductions in thrombotic/ischaemic endpoints.
Darexaban has been previously studied in human volunteers18–20 and in patients with venous thromboembolism or atrial fibrillation for the prevention of stroke.23–28 It provides reliable inhibition of FXa and shows no unexpected safety concerns. Given the concerns regarding bleeding associated with long-term triple oral antithrombotic therapy, the observation in RUBY-1 that bleeding increases with dose suggests that a phase III trial of darexaban after ACS should use doses at the lower end of the dose range (e.g. 10 mg daily). The lower doses of darexaban are expected to result in an increased risk of bleeding, as shown in this study, but may provide sufficient FXa inhibition to result in an efficacy benefit. Prior studies with other FXa antagonists have previously shown that they may have a flat dose–response, as seen in a phase II dose-ranging trial of fondaparinux after ACS,39 in which the lowest doses appeared to be associated with the lowest rates of bleeding but no benefit in increasing the dose in terms of efficacy. There was no efficacy signal in RUBY-1, even though the trial was underpowered for efficacy. Given the failure of FXa inhibition to add benefit to dual antiplatelet therapy after ACS in the APPRAISE-2 trial, the validity of the concept of combining dual antiplatelet therapy with anticoagulant therapy after ACS remains at best uncertain. The likelihood of the success of a phase III trial with darexaban will need to be placed in the context of the results of ongoing trials like ATLAS ACS-2.17,36
As with any clinical trial, results from this study should be interpreted with consideration of the potential limitations. This study had limited power to detect differences in efficacy between the various dose groups and placebo, as is clear from the wide CIs of the point estimates of the event rates. However, efficacy results from phase II studies in this setting have been documented as unreliable, with encouraging phase II results not confirmed in phase III16,37 and apparently neutral phase II studies being followed by resounding efficacy in properly powered phase III trials.40–42 This study does, however, provide clear information and guidance regarding the tolerability of darexaban, in terms of bleeding, when added as a third antithrombotic agent to treatment with ASA and clopidogrel, as well as the fact that the relative risks of bleeding are consistent regardless of the severity and bleeding scale used. Another limitation of this study is that it is only relevant to treatment when added on top of ASA and clopidogrel. The safety of darexaban when combined with more potent P2Y12 antagonists such as prasugrel43 or ticagrelor42 is unknown. Given the superiority of these agents over clopidogrel after ACS, the potential clinical efficacy benefit of adding darexaban to dual antiplatelet therapy may be reduced and the bleeding risk increased when ASA is combined with either of these new agents. Generalizability of results is always an important consideration in trials, and even though the majority of patients were enrolled in Eastern Europe, our study has broad geographical representation with enrolment in 24 countries over four continents, including India and South Africa. In addition, the trial enrolled a relatively young and low-risk patient population, with a predominance of STEMI. The bleeding rates observed may not necessarily be representative of bleeding rates, which might be expected in a less selected, older patient population, with more comorbidities. The trial has pooled together STE-ACS and NSTE-ACS, which may have introduced further heterogeneity, although current recommendations for secondary prevention with antithrombotics are identical for both conditions,2–5 and many prior trials of antithrombotics in this setting have done so.15–17,35–37,42,43
Conclusion
Darexaban, when added to dual antiplatelet therapy after ACS (including patients who have undergone PCI), produces an expected dose-related two- to four-fold increase in bleeding. While the trial was underpowered to study efficacy, there was no reduction in the composite efficacy outcome, which was numerically higher than placebo at the higher dose range and numerically lower at the lower dose range. Establishing the potential of low-dose darexaban in preventing major cardiac events after ACS would require a large phase III trial to test whether it might achieve clinical efficacy on top of dual antiplatelet therapy without an unacceptable increase in the risk of bleeding.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The study was conducted by the Executive Steering Committee and was funded by Astellas Pharma. The clinical execution and monitoring were performed by ICON plc. All authors had access to the clinical data. The manuscript was written by the authors and provided to the sponsor for comments. Editorial support was funded by Astellas Pharma. Funding to pay the Open Access publication charges for this article was provided by Servier.
Conflict of interest: P.G.S. has received research grants from Servier; fees for consultancy or participation in advisory board meetings from Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo/Eli Lilly alliance, GlaxoSmithKline, Medtronic, Merck Sharpe and Dohme, Pfizer, Roche, sanofi-aventis, Servier, and The Medicines Company; payment for development of educational presentations from AstraZeneca, Boehringer Ingelheim, and The Medicines Company; and has equity ownership in Aterovax. S.R.M. has served as a consultant for Astellas, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Eisai, and sanofi-aventis. J.W.J. has received research grants from and was a speaker on (CME accredited) meetings sponsored by Astellas, AstraZeneca, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cordis, Daiichi Sankyo, Lilly, Genzyme, Medtronic, Merck, Schering-Plough, Pfizer, Orbus Neich, Novartis, Roche, Servier, the Netherlands Heart Foundation, the Inter-University Cardiology Institute of the Netherlands, and the European Community Framework FP7 programme. G.Y.H.L. has served as a consultant for Bayer, Astellas, Merck, AstraZeneca, sanofi-aventis, Bristol-Myers Squibb/Pfizer, and Boehringer Ingelheim, and has been on the Speakers Bureau for Bayer, Bristol-Myers Squibb /Pfizer, Boehringer-Ingelheim, and sanofi-aventis. C.M.G. has received research grant support and consulting monies from Johnson and Johnson, Bayer, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo/Eli Lilly alliance, Merck Sharpe and Dohme, and sanofi-aventis. ICON PLC contracted with Boston Clinical Research Institute LLC for C.M.G.'s adjudication services. F.K. has nothing to disclose. P.K. has nothing to disclose. A.G.-H. is an employee of Astellas Pharma Global Development. R.W.R. is an employee of Astellas Pharma Global Development. C.B.G. has received research grants or contracts from Astellas Pharma US, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, GSK, Medtronic Vascular, Inc., Merck & Co, and sanofi-aventis; fees for consultancy or other services (including CME) from AstraZeneca, Bristol-Myers Squibb, GSK, Hoffman-La Roche, Novartis, and sanofi-aventis; and fees for consultancy and other non-CME services from Boehringer Ingelheim, sanofi-aventis, and Otsuka.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the editorial assistance of Frances Grambling and Tim Blackstock, Medicus International.
Appendix 1
RUBY-1
Executive Steering Committee: Ph Gabriel Steg (Chair), Christopher B Granger, J Wouter Jukema, Gregory YHK Lip, Shamir Mehta, Ronny W Renfurm (non-voting).
Steering Committee: Soon-Kil Kim, Steen Husted, Luc Janssens, Christoph Bode, Frantisek Kovar, Petr Kala, István Édes, Ton Oude Ophuis, Nicolay Gratsiansky, David Brieger, Escobar Franco, Maria Dorobantu, Jayaraman Balchanadar, Gregory Y.H.K. Lip, Aleksandr Parkhomenko, Ernesto Cardona, Andrzej Rynkiewicz, Anthony Dalby, José Nicolau, Enrique Gurfinkel, Jeyaindran Sinnadurai, Andrejs Erglis.
Independent Adjudication Committee: C. Michael Gibson (Chair), Duane S. Pinto, Yuri Pride, J.F. Dashe.
Data Monitoring Committee: Frans van de Werf (Chair), Freek W.A. Verheugt, Nick Freemantle.
Investigators
Argentina (21 patients): Horacio Avaca, Ernesto Duronto, Stella Macin, Carlos Cuneo, Edmundo Falu, Juan Jose Fuselli, Walter Mauricio Zuckerman, Hugo Luquez, Hugo Colombo, Victorino Jose Fuentealba, Guillermo Moises Aziza, Ricardo Leon de la Fuente.
Australia (5): Raibhan Yadav, David Brieger, Harvey Newnham, Geoffrey Toogood.
Belgium (36): Luc Janssens, François Cardinal, Bertin Foading Deffo, Mathias Vrolix Christophe Beauloye Raf Roelandt, Michaël Rosseel, Marcelo Goldstein Emmanuel Haine.
Brazil (33): Harry Correa Filho, Marco Antonio Mattos, João Moraes Jr, Maria Fernanda Garcia, Antonio Carlos Carvalho, Paulo Ernesto Leães, Elizabeth dos Santos, Célia Sampaio.
Canada (60): Jean-Pierre Déry, Denis-Carl Phaneuf, Thao Huynh, Shamir Mehta, Asim Cheema, A. Shekhar Pandey, Martine Montigny, François Deslongchamps, Yves Pesant, Monique Ruel, Serge LePage.
Columbia (12): Tomas Romero, Fernando Manzur, Jaime Rodriguez, Adalberto Quintero, Jorge Guillermo Velazquez.
Czech Republic (161): Petr Kala, Zdenek Coufal, David Horak, Martin Maly, Karel Sochor, Zdenek Klimsa.
Denmark (4): Steen Husted, Peer Grande, Christian Torp-Pedersen, Lars Frost.
France (5): Franck Paganelli, Gabriel Steg, Bernard Charbonnier, George Amat.
Germany (53): Holger Nef, Andreas Zirlik, Michael Haude, Karl-Friedrich Appel, Sebastian Schellong, Arnold Schmidt.
Hungary (120): Béla Merkely, Tamás Forster, István Édes, Géza Lupkovics, Mátyás Sereg, Péter Andréka, András Nagy, Dezső Apró, Kálmán Csapó, József Pátkay, Péter Polgár.
India (150): Raghu Cherukupalli, Sameer Dani, Bhagavatula Sastry, Rajiv Passey, K Latchumanadhas, Keyur Parikh, Tejas Patel, R. Purushotham, Aziz Khan, Narendra Jathappa, T Tippeswamy, Durgaprasad Rajasekhar, Tapan Shah, Kamal Doshi, Jaspal Arneja, Anil Jawaharani.
Latvia (7): Galina Dormiontova, Artis Kalnins, Leva Sime, Andrejs Erglis.
Malaysia (12): Chuey Uan Lee, Kok Han Chee, Jeyaindran Sinnadurai.
Mexico (26): Gilberto Inzunza, Jaime Chavez, Carlos Gutierrez Hernández, Luis Fernando, Flota Cervera, Mariano Ledesma, Jose Luis, Arenas Leon, Ignacio Rodriguez, Briones Virginia, Samaniego Mendez.
Netherlands (51): Pieter Smits, Ad C. Bredero, J.W.M. van Eck, Reginald Groutars, Peter Bendermacher, Rob Breedveld, Timo Lenderink, Ton Oude Ophuis, Pete-Zwart.
Poland (76): Wlodzimierz Musial, Pawel Miekus, Krzysztof Zmudka, Mariusz Kedzierski, Zygfryd Reszka, Andrzej Rynkiewicz, Marek Bronisz, Andrzej Wysokinski, Maciej Zarebinski, Artur Milnerowicz.
Romania (59): Mircea Vladoianu, Andrei Gheorghe Dan, Imre Benedek, Adriana Salajan, Maria Dorobantu, Calin Pop, Octavian Istratoaie, Bogdan Minescu, Florin Ortan.
Russia (87): Olga Barbarash, Victor Mordovin, Emilia Volkova, Boris Sidorenko, Dmitriy Zateyshchikov, Vadim Kuznetsov, Sergey Shalaev, Zaur Shogenov, Grigoriy Arutyunov, Ivan Gordeev, Yury Shvarts, Ivan Maximov, Oleg Khruslalev, Vladimir Yakusevich, Nicolay Gratsiansky, Evgeny Zhilyaev, Alexander Sherenkov, Eduard Paniev, Elena Grunina.
Slovakia (189): Roman Margoczy, Marian Hranai, Karol Micko, Frantisek Kovar, Antonin Hrubon.
South Africa (23): Johannes Breedt, Anthony Dalby, Rajendran Moodley, Dawie Kruger, Christo Van Dyk, Mohamed Sarvan, Thayabran Pillay, Andre Saaiman, Johan Roos, Susan Booysen.
South Korea (56): Soon-Kil Kim, Eak Kyun Shin, Bum-Kee Hong, Wook Bum Pyun, Myung-Ho Jeong, Moo Hyun Kim.
Ukraine (9): Leonid Rudenko, Katerina Amosova, Vira Tseluyko, Oleksandr Karpenko, Ludmila Kononenko, Valeriy Batushkin, Mykola Kopytsa, Elena Koval, Andriy Yagensky, Aleksandr Parkhomenko, Oksana Shershynova, Viktor Shcherbak.
United Kingdom (3): Gregory Y.H.K. Lip, Anthony Scriven, Iain Simpson, Azfar Zaman, Andreas Baumbach.
Appendix 2: Complete inclusion and exclusion criteria
| Inclusion criteria—all needed to be fulfilled for a patient to be eligible for the study |
| 18 years of age or older |
| Diagnosis of STE-ACS or NSTE-ACS as index event |
| Elevated cardiac biomarkers (Troponin T or I, or CK-MB) >2× ULN for CK-MB or >ULN for Troponin |
| For subjects with a diagnosis of NSTE-ACS, at least one of the following additional risk factors for ischaemic events had to be present: |
| ST deviation on ECG at any time between presentation and randomization |
| Age 65 years of age or older |
| Previous ACS <12 months prior to randomization |
| Multi-vessel coronary artery disease |
| Ischaemic stroke or TIA at least 12 months prior to randomization |
| Type 2 diabetes mellitus |
| Peripheral arterial disease |
| Clinically stable (defined as discontinuation of parenteral antithrombotics and not likely to require reinstitution of parenteral antithrombotics at the time of randomization) and receiving current standard oral antiplatelet therapy |
| Able to be randomized within 7 days after presentation. Subjects should be randomized as soon as possible after discontinuation of parenteral antithrombotics |
| Has provided Institutional Review Board-/Independent Ethics Committee-approved written informed consent and privacy language as per national regulations or informed consent has been obtained from the legally authorized representative prior to any study-related procedures |
| Main exclusion criteria |
| Need for ongoing therapy with parenteral or oral anticoagulants, thrombolytics, glycoprotein IIb/IIIa antagonists, or other antiplatelet drugs |
| Patient planned for myocardial revascularization or any other invasive procedure with increased risk for bleeding (i.e. elective surgical procedures) within 60 days |
| Active bleeding or, in the opinion of the investigator, high risk of bleeding during the study |
| Recent stroke or TIA <12 months prior to index event |
| Bleeding diathesis or any other condition or laboratory abnormality with increased tendency for bleeding (e.g. platelet count <100 000/µL) |
| Female of childbearing potential who refuses to use a medically acceptable form of contraception throughout the study |
| Female who is pregnant or lactating |
| Persistent SBP of 160 mmHg or higher and/or DBP of 100 mmHg or higher at baseline with or without medication |
| Hepatic insufficiency or ALT >2.0 times the ULN or total bilirubin >1.5 times the ULN |
| Renal creatinine clearance of <60 mL/min (Cockcroft–Gault equation) |
| Any concurrent illness which may interfere with treatment or evaluation of safety or completion of this study |
| Participation in another clinical trial within 30 days |
| Participation in any darexaban clinical trials |
| Known allergy to the study drug or any of its components |
STE-ACS, ST segment elevation ACS; ACS, acute coronary syndrome; NSTE-ACS, non-ST segment elevation ACS; CK, creatine kinase; ULN, upper limit of normal; ECG, electrocardiogram; TIA, transient ischaemic attack; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALT, alanine transaminase.
Appendix 3: Definitions of bleeding events
| Type of bleeding | Definition |
|---|---|
| Major bleeding | Fatal bleeding, clinically overt bleeding associated with a decrease in the haemoglobin level of >2 g/dL (1.24 mmol/L) compared with the pre-bleeding level, clinically overt bleeding leading to transfusion of two or more units of whole blood or packed cells, and symptomatic bleeding in a critical area or organ, such as retroperitoneal, intracranial, intraocular, intra-spinal, intra-articular, pericardial, or intramuscular bleeding with compartment syndrome |
| CRNM bleeding | Any bleeding event considered as clinically relevant by the IAC that did not meet the criteria of a major bleeding event, e.g. any bleeding event that required medical attention or any bleeding requiring discontinuation of blinded study drug treatment |
| All other bleeding events | Those events not fulfilling the criteria of major or CRNM bleeding events. In addition, transfusions and the reasons behind them were tracked, and for each bleeding episode, an adjusted decrease in haemoglobin was computed by adding the number of units of packed red blood cells transfused to the decrease in haemoglobin |
CRNM, clinically relevant non-major; IAC, independent adjudication committee.
Appendix 4: Clinical characteristics of the patients
| Variable | Darexaban, 5 mg b.i.d. | Darexaban, 10 mg o.d. | Darexaban, 15 mg b.i.d. | Darexaban, 30 mg o.d. | Darexaban, 30 mg b.i.d. | Darexaban, 60 mg o.d. | Placebo |
|---|---|---|---|---|---|---|---|
| n | 159 | 159 | 159 | 156 | 153 | 153 | 319 |
| Age, mean (SD) | 57.4 (10.43) | 57.1 (10.35) | 56.3 (9.58) | 56.1 (10.05) | 57.2 (11.53) | 55.7 (10.39) | 57.5 (10.44) |
| Female, % | 20.8 | 21.4 | 18.2 | 16.0 | 21.6 | 17.0 | 24.1 |
| Ethnicity, n (%) | |||||||
| Caucasian | 124 (78.0) | 123 (77.4) | 124 (78.0) | 126 (80.8) | 117 (76.5) | 120 (78.4) | 257 (80.6) |
| Black/African American | 2 (1.3) | 4 (2.5) | 1 (0.6) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 0 (0.0) |
| Asian | 27 (17.0) | 28 (17.6) | 30 (18.9) | 26 (16.7) | 28 (18.3) | 27 (17.6) | 54 (16.9) |
| Other | 6 (3.8) | 4 (2.5) | 4 (2.5) | 4 (2.6) | 7 (4.6) | 6 (3.9) | 8 (2.5) |
| Enrolment region, n (%) | |||||||
| Europe | 108 (67.9) | 108 (67.9) | 108 (67.9) | 108 (69.2) | 104 (68.0) | 105 (68.6) | 219 (68.7) |
| North America | 9 (5.7) | 7 (4.4) | 7 (4.4) | 8 (5.1) | 7 (4.6) | 7 (4.6) | 15 (4.7) |
| Central/South America | 11 (6.9) | 13 (8.2) | 11 (6.9) | 10 (6.4) | 12 (7.8) | 11 (7.2) | 24 (7.5) |
| Asia/Pacific/South Africa | 31 (19.5) | 31 (19.5) | 33 (20.8) | 30 (19.2) | 30 (19.6) | 30 (19.6) | 61 (19.1) |
| Smoker, n (%) | |||||||
| Former | 42 (26.4) | 34 (21.4) | 31 (19.5) | 33 (21.2) | 33 (21.6) | 41 (26.8) | 83 (26.0) |
| Current | 69 (43.4) | 65 (40.9) | 68 (42.8) | 63 (40.4) | 62 (40.5) | 64 (41.8) | 140 (43.9) |
| Never | 48 (30.2) | 60 (37.7) | 60 (37.7) | 60 (38.5) | 58 (37.9) | 48 (31.4) | 96 (30.1) |
| Hypertension, n (%) | 90 (56.6) | 99 (62.3) | 95 (59.7) | 91 (58.3) | 95 (62.1) | 96 (62.7) | 194 (60.8) |
| Dyslipidaemia, n (%) | 77 (48.4) | 74 (46.5) | 77 (48.4) | 89 (57.1) | 76 (49.7) | 81 (52.9) | 153 (48.0) |
| Diabetes mellitus Type 2, n (%) | 39 (24.5) | 37 (23.3) | 37 (23.3) | 43 (27.6) | 34 (22.2) | 27 (17.6) | 60 (18.8) |
| Primary diagnosis for index event, n (%) | |||||||
| STEMI | 111 (69.8) | 121 (76.1) | 119 (74.8) | 106 (67.9) | 108 (70.6) | 109 (71.2) | 220 (69.0) |
| NSTEMI | 48 (30.2) | 38 (23.9) | 40 (25.2) | 50 (32.1) | 45 (29.4) | 44 (28.8) | 99 (31.0) |
| Use of PCI for index event | 122 (76.7) | 116 (73.0) | 119 (74.8) | 115 (73.7) | 118 (77.1) | 113 (73.9) | 235 (73.7) |
| Standard antiplatelet therapy, n (%) | |||||||
| With clopidogrel | 154 (96.9) | 152 (95.6) | 152 (95.6) | 152 (97.4) | 148 (96.7) | 148 (96.7) | 309 (96.9) |
| Without clopidogrel | 5 (3.1) | 7 (4.4) | 7 (4.4) | 4 (2.6) | 5 (3.3) | 5 (3.3) | 10 (3.1) |
| Time from index event to first dose (mean days) | 4.1 | 4.0 | 4.3 | 3.8 | 4.1 | 4.2 | 4 |
| GRACE risk score at presentation (evaluated population) | 132.6 (n = 158) | 134.7 (n = 156) | 135.1 (n = 156) | 130.6 (n = 154) | 132.3 (n = 152) | 131.2 (n = 153) | 132.8 (n = 314) |
| Hx of prior CHF, n (%) | 4 (2.5) | 3 (1.9) | 6 (3.8) | 2 (1.3) | 3 (2.0) | 4 (2.6) | 8 (2.5) |
| Hx of stroke/TIA, n (%) | 3 (1.9) | 4 (2.5) | 4 (2.5) | 8 (5.1) | 6 (3.9) | 6 (3.9) | 6 (1.9) |
| Hx of prior MI, n (%) | 19 (11.9) | 9 (5.7) | 17 (10.7) | 24 (15.4) | 18 (11.8) | 18 (11.8) | 45 (14.1) |
| Hx of CABG, n (%) | 8 (5.0) | 3 (1.9) | 1 (0.6) | 5 (3.2) | 3 (2.0) | 5 (3.3) | 6 (1.9) |
| Hx of PCI, n (%) | 10 (6.3) | 8 (5.0) | 13 (8.2) | 13 (8.3) | 22 (14.4) | 20 (13.1) | 25 (7.8) |
| Peripheral arterial disease, n (%) | 9 (5.7) | 4 (2.5) | 6 (3.8) | 5 (3.2) | 4 (2.6) | 4 (2.6) | 13 (4.0) |
| Premature permanent study drug discontinuation, n (%) | 30 (18.9) | 31 (19.5) | 32 (20.1) | 44 (28.2) | 50 (32.7) | 36 (23.5) | 68 (21.3) |
| Duration of drug exposure, median weeks (Q1, Q3) | 26.0 (23.9, 26.1) | 26.0 (24.3, 26.1) | 26.0 (19.6, 26.1) | 26.0 (20.6, 26.1) | 25.7 (11.6, 26.1) | 26.0 (17.7, 26.1) | 26.0 (25.0, 26.1) |
| Concomitant medications, n (%) | |||||||
| Beta-blockers | 146 (91.8) | 147 (92.4) | 149 (93.7) | 145 (92.9) | 139 (90.8) | 133 (86.9) | 293 (91.8) |
| ACE-inhibitors | 128 (80.5) | 126 (79.2) | 121 (76.1) | 117 (75.0) | 123 (80.4) | 116 (75.8) | 248 (77.7) |
| Angiotensin receptor blockers | 17 (11.0) | 23 (14.5) | 25 (15.7) | 17 (10.9) | 19 (12.4) | 23 (15.0) | 43 (13.5) |
| Statins | 150 (94.3) | 154 (96.9) | 156 (98.1) | 146 (93.6) | 146 (95.4) | 145 (94.8) | 304 (95.3) |
| Fibrates | 4 (2.5) | 6 (3.8) | 3 (1.9) | 5 (3.2) | 2 (1.3) | 5 (3.3) | 10 (3.1) |
| PPIs | 50 (31.4) | 49 (30.8) | 47 (29.6) | 67 (42.9) | 63 (41.2) | 60 (39.2) | 99 (31.0) |
| Variable | Darexaban, b.i.d. | Darexaban, o.d. | Darexaban, 10 mg doses | Darexaban, 30 mg doses | Darexaban, 60 mg doses | Darexaban, total | Grand total |
| n | 471 | 468 | 318 | 315 | 306 | 939 | 1258 |
| Age, mean (SD) | 57.0 (10.52) | 56.3 (10.26) | 57.2 (10.37) | 56.2 (9.80) | 56.5 (10.98) | 56.6 (10.39) | 56.9 (10.4) |
| Female, % | 20.2 | 18.2 | 21.1 | 17.1 | 19.3 | 19.2 | 20.4 |
| Ethnicity, n (%) | |||||||
| Caucasian | 365 (77.5) | 369 (78.8) | 247 (77.7) | 250 (79.4) | 237 (77.5) | 734 (78.2) | 991 (78.8) |
| Black/African American | 4 (0.8) | 4 (0.9) | 6 (1.9) | 1 (0.3) | 1 (0.3) | 8 (0.9) | 8 (0.6) |
| Asian | 85 (18.0) | 81 (17.3) | 55 (17.3) | 56 (17.8) | 55 (18.0) | 166 (17.7) | 220 (17.5) |
| Other | 17 (3.6) | 14 (3.0) | 10 (3.1) | 8 (2.5) | 13 (4.2) | 31 (3.3) | 39 (3.1) |
| Enrolment region, n (%) | |||||||
| Europe | 320 (67.9) | 321 (68.6) | 216 (67.9) | 216 (68.6) | 209 (68.3) | 641 (68.3) | 860 (68.4) |
| North America | 23 (4.9) | 22 (4.7) | 16 (5.0) | 15 (4.8) | 14 (4.6) | 45 (4.8) | 60 (4.8) |
| Central/South America | 34 (7.2) | 34 (7.3) | 24 (7.5) | 21 (6.7) | 23 (7.5) | 68 (7.2) | 92 (7.3) |
| Asia/Pacific/South Africa | 94 (20.0) | 91 (19.4) | 62 (19.5) | 63 (20.0) | 60 (19.6) | 185 (19.7) | 246 (19.6) |
| Smoker, n (%) | |||||||
| Former | 106 (22.5) | 108 (23.1) | 76 (23.9) | 64 (20.3) | 74 (24.2) | 214 (22.8) | 297 (23.6) |
| Current | 199 (42.3) | 192 (41.0) | 134 (42.1) | 131 (41.6) | 126 (41.2) | 391 (41.6) | 531 (42.2) |
| Never | 166 (35.2) | 168 (35.9) | 108 (34.0) | 120 (38.1) | 106 (34.6) | 334 (35.6) | 430 (34.2) |
| Hypertension, n (%) | 280 (59.4) | 286 (61.1) | 189 (59.4) | 186 (59.0) | 191 (62.4) | 566 (60.3) | 760 (60.4) |
| Dyslipidaemia, n (%) | 230 (48.8) | 244 (52.1) | 151 (47.5) | 166 (52.7) | 157 (51.3) | 474 (50.5) | 627 (49.8) |
| Diabetes mellitus Type 2, n (%) | 110 (23.4) | 107 (22.9) | 76 (23.9) | 80 (25.4) | 61 (19.9) | 217 (23.1) | 277 (22.0) |
| Primary diagnosis for index event, n (%) | |||||||
| STEMI | 338 (71.8) | 336 (71.8) | 232 (73.0) | 225 (71.4) | 217 (70.9) | 674 (71.8) | 894 (71.1) |
| NSTEMI | 133 (28.2) | 132 (28.2) | 86 (27.0) | 90 (28.6) | 89 (29.1) | 265 (28.2) | 364 (28.9) |
| Use of PCI for index event, n (%) | 359 (76.2) | 344 (73.5) | 238 (74.8) | 234 (74.3) | 231 (75.5) | 703 (74.9) | 938 (74.6) |
| Standard antiplatelet therapy, n (%) | |||||||
| With clopidogrel | 454 (96.4) | 452 (96.6) | 306 (96.2) | 304 (96.5) | 296 (96.7) | 906 (96.5) | 1215 (96.6) |
| Without clopidogrel | 17 (3.6) | 16 (3.4) | 12 (3.8) | 11 (3.5) | 10 (3.3) | 33 (3.5) | 43 (3.4) |
| Time from index event to first dose (mean days) | 4.2 | 4.0 | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 |
| GRACE risk score at presentation (evaluated population) | 133.3 (n = 466) | 132.2 (n = 463) | 133.6 (n = 314) | 132.9 (n = 310) | 131.8 (n = 305) | 132.8 (n = 929) | 132.8 (n = 1243) |
| Hx of prior CHF, n (%) | 13 (2.8) | 9 (1.9) | 7 (2.2) | 8 (2.5) | 7 (2.3) | 22 (2.3) | 30 (2.4) |
| Hx of stroke/TIA, n (%) | 13 (2.8) | 18 (3.8) | 7 (2.2) | 12 (3.8) | 12 (3.9) | 31 (3.3) | 37 (2.9) |
| Hx of prior MI, n (%) | 54 (11.5) | 51 (10.9) | 28 (8.8) | 41 (13.0) | 36 (11.8) | 105 (11.2) | 150 (11.9) |
| Hx of CABG, n (%) | 12 (2.5) | 13 (2.8) | 11 (3.5) | 6 (1.9) | 8 (2.6) | 25 (2.7) | 31 (2.5) |
| Hx of PCI, n (%) | 45 (9.6) | 41 (8.8) | 18 (5.7) | 26 (8.3) | 42 (13.7) | 86 (9.2) | 111 (8.8) |
| Peripheral arterial disease, n (%) | 19 (4.0) | 13 (2.8) | 13 (4.1) | 11 (3.5) | 8 (2.6) | 32 (3.4) | 45 (3.6) |
| Premature permanent study drug discontinuation, n (%) | 112 (23.8) | 111 (23.7) | 61 (19.2) | 76 (24.1) | 86 (28.1) | 223 (23.7) | 291 (23.1) |
| Duration of drug exposure, median weeks (Q1,Q3) | 26.0 (19.1, 26.1) | 26.0 (21.9, 26.1) | 26.0 (24.1, 26.1) | 26.0 (19.6, 26.1) | 26.0 (13.1, 26.1) | 26.0 (20.0, 26.1) | 26.0 (22.0, 26.1) |
| Concomitant medications, n (%) | |||||||
| Beta-blockers | 434 (92.1) | 425 (90.8) | 293 (92.1) | 294 (93.3) | 272 (88.9) | 859 (91.5) | 1152 (91.6) |
| ACE-inhibitors | 372 (79.0) | 359 (76.7) | 254 (79.9) | 238 (75.6) | 239 (78.1) | 731 (77.8) | 979 (77.8) |
| Angiotensin receptor blockers | 61 (13.0) | 63 (13.5) | 40 (12.6) | 42 (13.3) | 42 (13.7) | 124 (13.2) | 167 (13.3) |
| Statins | 452 (96.0) | 445 (95.1) | 304 (95.6) | 302 (95.9) | 291 (95.1) | 897 (95.5) | 1201 (95.5) |
| Fibrates | 9 (1.9) | 16 (3.4) | 10 (3.1) | 8 (2.5) | 7 (2.3) | 25 (2.7) | 35 (2.8) |
| PPIs | 160 (34.0) | 176 (37.6) | 99 (31.1) | 114 (36.2) | 123 (40.2) | 336 (35.8) | 435 (34.6) |
SD, standard deviation; STEMI, ST-segment elevation; NSTEMI, non-ST-segment; PCI, percutaneous coronary intervention; GRACE, Global Registry of Acute Coronary Events; Hx, history; CHF, chronic heart failure; TIA, transient ischaemic attack; MI, myocardial infarction; CABG, coronary artery bypass graft; PPI, proton pump inhibitor; Q, quartile; ACE, angiotensin-converting enzyme.
References
- 1.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Jr, Granger CB, Flather MD, Budaj A, Quill A, Gore JM GRACE Investigators. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–1900. doi: 10.1001/jama.297.17.1892. doi:10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 2.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M ESC Committee for Practice Guidelines (CPG) Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. doi:10.1093/eurheartj/ehn526. [DOI] [PubMed] [Google Scholar]
- 3.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Avilés F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. doi:10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 4.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Ornato JP. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. doi:10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction); American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. doi:10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr 2011 WRITING GROUP MEMBERS; ACCF/AHA TASK FORCE MEMBERS. 2011 ACCF/AHA Focused update incorporated into the ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–e579. doi: 10.1161/CIR.0b013e318212bb8b. doi:10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 7.Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA, Jr, Granger CB. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) Br Med J. 2006;333:1091. doi: 10.1136/bmj.38985.646481.55. doi:10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143:241–250. doi: 10.7326/0003-4819-143-4-200508160-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hirsh J. Oral anticoagulant drugs. N Engl J Med. 1991;324:1865–1875. doi: 10.1056/NEJM199106273242606. doi:10.1056/NEJM199106273242606. [DOI] [PubMed] [Google Scholar]
- 10.Bradley BC, Perdue KS, Tisdel KA, Gilligan DM. Frequency of anticoagulation for atrial fibrillation and reasons for its non-use at a veterans affairs medical center. Am J Cardiol. 2000;85:568–572. doi: 10.1016/s0002-9149(99)00813-9. doi:10.1016/S0002-9149(99)00813-9. [DOI] [PubMed] [Google Scholar]
- 11.Ansell J, Hirsh J, Poller L, Bussey H, Jacobson A, Hylek E. The pharmacology and management of the vitamin K antagonists: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:204S–233S. doi: 10.1378/chest.126.3_suppl.204S. doi:10.1378/chest.126.3_suppl.204S. [DOI] [PubMed] [Google Scholar]
- 12.Eikelboom W, Weitz JI. A replacement for warfarin: the search continues. Circulation. 2007;116:131–133. doi: 10.1161/CIRCULATIONAHA.107.712349. doi:10.1161/CIRCULATIONAHA.107.712349. [DOI] [PubMed] [Google Scholar]
- 13.Karjalainen PP, Porela P, Ylitalo A, Vikman S, Nyman K, Vaittinen MA, Airaksinen TJ, Niemelä M, Vahlberg T, Airaksinen KE. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J. 2007;28:726–732. doi: 10.1093/eurheartj/ehl488. doi:10.1093/eurheartj/ehl488. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen R, Hansen ML, Abildstrom SZ, Hvelplund A, Andersson C, Jørgensen C, Madsen JK, Hansen PR, Køber L, Torp-Pedersen C, Gislason GH. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374:1967–1974. doi: 10.1016/S0140-6736(09)61751-7. doi:10.1016/S0140-6736(09)61751-7. [DOI] [PubMed] [Google Scholar]
- 15.Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, Tijssen JG, Van de Werf F, Wallentin L for the RE-DEEM investigators. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. doi: 10.1093/eurheartj/ehr113. Published online ahead of print 22 March 2011. [DOI] [PubMed] [Google Scholar]
- 16.Alexander JH, Becker RC, Bhatt DL, Cools F, Crea F, Dellborg M, Fox KA, Goodman SG, Harrington RA, Huber K, Husted S, Lewis BS, Lopez-Sendon J, Mohan P, Montalescot G, Ruda M, Ruzyllo W, Verheugt F, Wallentin T APPRAISE Steering Committee and Investigators. Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation. 2009;119:2877–2885. doi: 10.1161/CIRCULATIONAHA.108.832139. doi:10.1161/CIRCULATIONAHA.108.832139. [DOI] [PubMed] [Google Scholar]
- 17.Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, Hricak V, Barnathan ES, Bordes P, Witkowski A, Markov V, Oppenheimer L, Gibson CM ATLAS ACS-TIMI 46 study group. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29–38. doi: 10.1016/S0140-6736(09)60738-8. doi:10.1016/S0140-6736(09)60738-8. [DOI] [PubMed] [Google Scholar]
- 18.Groenendaal D, Heeringa M, Kadokura T, Verheggen F, Strabach G, Heinzerling H. YM150, an oral direct inhibitor of factor Xa, demonstrated a predictable and dose-proportional pharmacokinetic/pharmacodynamic profile after single and multiple dosing: results from three studies. Blood. 2010;116:3323. [Google Scholar]
- 19.Heeringa M, Groenendaal D, Strabach G, Garcia-Hernandez A, Kadokura T, Heeringa M, Mol R, Eltink C, Heinzerling H. The pharmacokinetics of YM150, an oral direct factor Xa inhibitor, are not affected to a clinically relevant degree by strong inhibition or induction of CYP3A4 and P-gp. J Thromb Hemost. 2011 9(Suppl. 2):359–360. [Google Scholar]
- 20.Heeringa M, Groenendaal D, Strabach G, Garcia-Hernandez A, Dickinson J, Eltink C, Mol R, Kadokura T, Renfurm R, Heinzerling H. Co-administration of darexaban (YM150), an oral direct factor Xa inhibitor, with acetylsalicylic acid, clopidogrel and naproxen: no evidence of clinically relevant interactions. J Thromb Hemost. 2007;9(Suppl. 2):360. [Google Scholar]
- 21. Astellas Pharma. Data on file.
- 22.Rupprecht HJ, Blank R. Clinical pharmacology of direct and indirect factor Xa inhibitors. Drugs. 2010;70:2153–2170. doi: 10.2165/11538030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson BI, Turpie AGG, Lassen MR, Prins MH, Agnelli G, Kälebo P, Gaillard ML, Meems L ONYX study group. A dose escalation study of YM150, an oral direct Factor Xa inhibitor, in the prevention of venous thromboembolism in elective primary hip replacement surgery. J Thromb Haemost. 2007;5:1660–1665. doi: 10.1111/j.1538-7836.2007.02644.x. doi:10.1111/j.1538-7836.2007.02644.x. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson BI, Turpie AGG, Lassen MR, Prins MH, Agnelli G, Kälebo P, Wetherill G, Wilpshaar JW, Meems L ONYX-2 STUDY GROUP. Prevention of venous thromboembolism with an oral factor Xa inhibitor, YM150, after total hip arthroplasty. A dose finding study (ONYX-2) J Thromb Haemost. 2010;8:714–721. doi: 10.1111/j.1538-7836.2010.03748.x. doi:10.1111/j.1538-7836.2010.03748.x. [DOI] [PubMed] [Google Scholar]
- 25.Lip GYH, Halperin JL, Petersen P, Rodgers GM, Renfurm RW. Safety and tolerability of the oral factor Xa inhibitor, YM150 versus warfarin in 1297 patients with non-valvular atrial fibrillation: a Dose Confirmation Study (OPAL-2) J Thromb Hemost. 2011;9(Suppl. 2):748. [Google Scholar]
- 26.Rodgers GM, Renfurm RW, Halperin JL, Petersen P, Lip GYH. Efficacy of the oral FXa inhibitor, darexaban (YM150) on biomarkers for thrombogenesis in 1297 patients with non-valvular atrial fibrillation: Phase IIb Dose Confirmation Study (OPAL-2) J Thromb Hemost. 2011;9(Suppl. 2):887. [Google Scholar]
- 27.Astellas Pharma Europe BV. A study evaluating safety and tolerability of YM150 compared to warfarin in subjects with atrial fibrillation (OPAL-2) Astellas Pharma Inc. Clinicaltrials.gov identifier: NCT00938730. Available at: http://clinicaltrials.gov/ct2/show/NCT00938730?term=NCT00938730&rank=1. Last accessed 17 July 2011.
- 28.Turpie AGG, Lip GHY, Minematsu K, Goto S, Renfurm RW, Wong KSL. Safety and tolerability of YM150 in subjects with non-valvular atrial fibrillation: a Phase II Study. Eur Heart J. 2010;21(Suppl. 1):173. Presented at the European Society for Cardiology Annual Meeting, 28 August–1 September 2010. [Google Scholar]
- 29.Schulman S, Kearon C Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. doi:10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 30.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Brindis RG, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins-Nakai RL, Ellis SG, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, Mckay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. doi:10.1016/S0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 31.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. doi:10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 32.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA Global Registry of Acute Coronary Events Investigators. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. doi:10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 33.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–974. doi: 10.1056/NEJMoa020496. doi:10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 34.Andreotti F, Testa L, Biondi-Zoccai GG, Crea F. Aspirin plus warfarin compared to aspirin alone after acute coronary syndromes: an updated and comprehensive meta-analysis of 25 307 patients. Eur Heart J. 2006;27:519–526. doi: 10.1093/eurheartj/ehi485. doi:10.1093/eurheartj/ehi485. [DOI] [PubMed] [Google Scholar]
- 35.Wallentin L, Wilcox RG, Weaver WD, Emanuelsson H, Goodvin A, Nystrom P, Bylock A. Oral ximelagatran for secondary prophylaxis after myocardial infarction: the ESTEEM randomised controlled trial. Lancet. 2003;362:789–797. doi: 10.1016/S0140-6736(03)14287-0. doi:10.1016/S0140-6736(03)14287-0. [DOI] [PubMed] [Google Scholar]
- 36.Gibson CM, Mega JL, Burton P, Goto S, Verheugt F, Bode C, Plotnikov A, Sun X, Cook-Bruns N, Braunwald E. Rationale and design of the Anti-Xa therapy to lower cardiovascular events in addition to standard therapy in subjects with acute coronary syndrome-thrombolysis in myocardial infarction 51 (ATLAS-ACS 2 TIMI 51) trial: a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of rivaroxaban in subjects with acute coronary syndrome. Am Heart J. 2011;161:815–821. doi: 10.1016/j.ahj.2011.01.026. doi:10.1016/j.ahj.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 37.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman SG, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vienereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L for the APPRAISE 2 investigators. Apixaban with antiplatelet therapy after acute coronary syndrome. New Engl J Med. Published online ahead of print 24 July 2011. [Google Scholar]
- 38.Takeda Pharmaceutical Company Limited. Takeda announces top line results from Phase 2 Study of investigational compound, TAK-442 for the patients with ACS. Available at http://www.takeda.com/press/article_38715.html. Last accessed 17 July 2011.
- 39.Simoons ML, Bobbink IW, Boland J, Gardien M, Klootwijk P, Lensing AW, Ruzyllo W, Umans VA, Vahanian A, Van De Werf F, Zeymer U PENTUA Investigators. A dose-finding study of fondaparinux in patients with non-ST-segment elevation acute coronary syndromes: the Pentasaccharide in Unstable Angina (PENTUA) Study. J Am Coll Cardiol. 2004;43:2183–2190. doi: 10.1016/j.jacc.2004.02.051. doi:10.1016/j.jacc.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 40.Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, Storey RF DISPERSE-2 Investigators. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol. 2007;50:1844–1851. doi: 10.1016/j.jacc.2007.07.053. doi:10.1016/j.jacc.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 41.Held C, Asenblad N, Bassand JP, Becker RC, Cannon CP, Claeys MJ, Harrington RA, Horrow J, Husted S, James SK, Mahaffey KW, Nicolau JC, Scirica BM, Storey RF, Vintila M, Ycas J, Wallentin L. Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. J Am Coll Cardiol. 2011;57:672–684. doi: 10.1016/j.jacc.2010.10.029. doi:10.1016/j.jacc.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 42.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Freij A, Thorsén M PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. doi:10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 43.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. doi:10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.