Abstract
Background
Some 40 000 lower limb amputations are performed in Germany each year, 70% of them in diabetics. About 80% of all major amputations may be preventable with the use of new interventional and vascular surgical procedures, particularly on the arteries of the leg and foot. We present the current state of the art in revascularization techniques and evaluate their usefulness for preservation of the lower limb.
Methods
This overview is based on the guidelines for the diagnosis and treatment of peripheral artery disease (PAD) and diabetic foot syndrome (DFS) that have been issued by the American Heart Association (AHA), the American College of Cardiology (ACC), the German Society of Angiology (DGA), the Trans-Atlantic Intersociety Consensus (TASC II), the German Society of Vascular Surgery (DGG), and the German Diabetes Society (DDG). A selective search in PubMed for relevant articles that appeared from 2000 to 2011 was conducted with the search terms “pedal bypass,” “vascular intervention crural pedal,” and “crural-pedal revascularization.”
Results
Most of the data on crural and pedal revascularization are derived from small-scale studies. The few comparative studies of interventional treatments and bypass surgery have not revealed any significant differences in outcome, but all studies of revascularization have shown good success rates for lower limb preservation.
Conclusion
Though the data are still sparse, the high reported rates of limb preservation imply that peripheral revascularization techniques can play a major role in the treatment of chronic critical limb ischemia (CLI). Therefore, these techniques are recommended without exception by the current guidelines.
The greatest challenge in the treatment of patients with peripheral arterial disease (PAD) and diabetic foot syndrome (DFS) is avoiding major amputation. Over 40 000 such amputations of the lower limb are carried out each year in Germany; 70% of the patients are diabetics (1– 3). Impaired arterial perfusion is the principal cause of poor wound healing and refractory rest pain. Arteries of the pelvis, upper leg and proximal lower leg used to be the main target vessels for bypass surgery and catheter interventions, but nowadays arteries right down to the foot can be successfully revascularized. Our intention here is to review the prospects for limb preservation and healing of chronic wounds by means of this approach. The data we present were gleaned from a selective search of publications in the period from 2000 to 2011 and from current guidelines for the treatment of PAD (4– 6, e5) and DFS (7).
Indications
Some 1.8 million people in Germany have symptomatic PAD, and each year 50 000 to 80 000 of them develop chronic critical limb ischemia (CLI) (6). The prevalence of diabetes mellitus in the German population is 7%, and around 90 000 people suffer from DFS with angiopathic component (7).
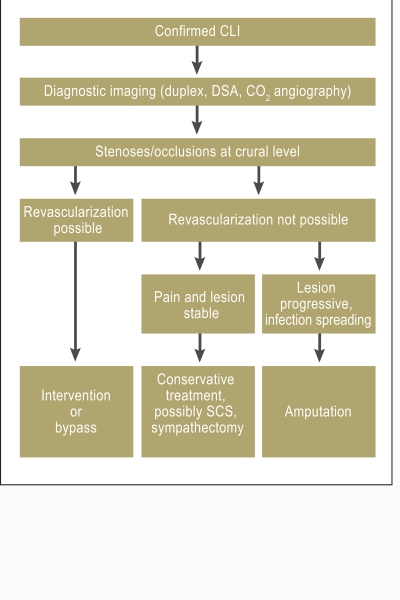
CLI and DFS are the only confirmed indications for crural and pedal revascularization, and the decision to undertake such treatment should be arrived at jointly by angiologists, radiologists, and vascular surgeons. Apart from determination of the appropriate technique for revascularization—surgical or interventional—the timing is crucial: soon after failure of conservative treatment, not when advanced infection and spreading necrosis of the foot already make amputation unavoidable.
In deciding whether revascularization should be achieved by surgical or interventional means, we adhere to the TASC II criteria, in the knowledge that these recommendations were actually formulated for the iliac and femoropopliteal vessels. There are as yet inadequate comparative data for crural and pedal vascular interventions, so no guidelines are available. While at the claudication stage the sole criterion of success is the long-term patency rate after revascularization, the primary treatment goal in CLI is limb preservation.
Depending on the method used, timely revascularization achieves 1-year limb preservation rates of 70% to 90% (8, 9). Statistics on long-term patency and limb preservation following revascularization are of limited relevance, because the mortality of CLI is >20% at 1 year and 55% after 5 years. Only 8% of the patients survive 10 years (5).
Diagnostic imaging
Basic non-invasive angiological investigations (pulse, Doppler blood pressure measurement, oscillography) can confirm the diagnosis of PAD and supply information on location and severity, but alone do not permit a decision regarding the appropriate technique of revascularization in crural and pedal arteries. The essential requirements for the data to be provided by diagnostic imaging are laid down in the standards of TASC II. The relevant aspects are the length and sequence of stenosed segments, differentiation of occlusion from stenosis, and the length of the occluded segment (6).
Duplex sonography is valuable particularly in the diagnostic imaging of the pelvic region and the femoropopliteal arteries. However, its usefulness is restricted by attenuation phenomena in the leg in this predominantly multimorbid group of patients (diabetes mellitus, renal failure) (4).
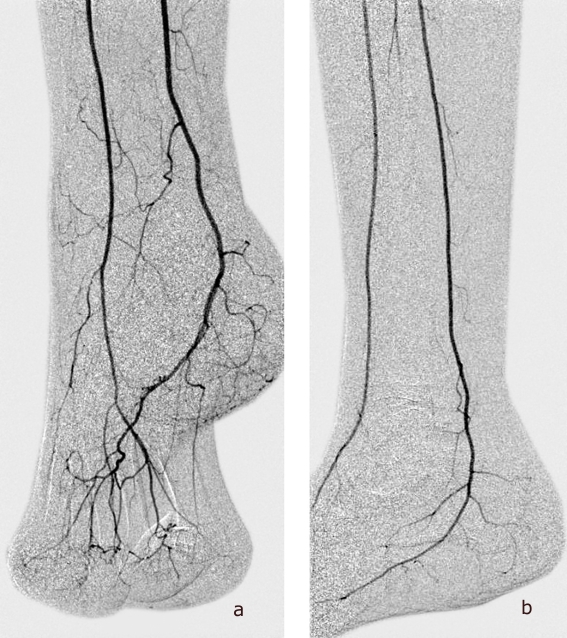
After localization of the site of impaired perfusion by means of clinical examination and non-invasive techniques, particularly in patients with renal failure, primary digital subtraction angiography (DSA) preparatory to intervention can render other diagnostic investigations (CTA, MRA) unnecessary. Precise delineation of all crural and pedal arteries, also in the late phase, is important (Figure 1). Even in patients with pronounced calcifications, the very high spatial and temporal-dynamic resolution permit diagnosis and treatment in one single step. In the case of impaired renal function (glomerular filtration rate [GFR] <60 mL/min/1.73 m2), it may be best to restrict diagnostic DSA to the crural level. Moreover, nephroprotective measures (NaCl 0.9 i.v. 10 mL/kg BW 12 h before and up to 12 h after administration of contrast medium [CM], ACC 2×600 mg i.v. or p.o. on the day before administration of CM and on the day of examination, discontinuation of diuretics and non-steroidal antirheumatics [NSAR] 24 h before examination) are advisable (4) (Table).
Figure 1.
Digital subtraction angiography of the arteries of the foot (a, anteroposterior; b, lateral)
Tabelle. Recommendations for diagnostic imaging in CLI, depending on renal function.
| Stage of renal failure | Diagnostic strategy |
| GFR >60 mL/min/1,73 m² | DSA of pelvic and lower limb arteries with visualization of pedal arteries |
| GFR 30–60 mL/min/1,73 m² | Duplex sonography of pelvic arteries |
| + | |
| DSA of the arteries of the affected leg, including the foot (fine-needle angiography) | |
| 12 h before and after administration of CM: | |
|
|
| GFR <30 ml/min/1,73 m² | Duplex sonography of pelvic arteries |
| + | |
| CO² angiography of the arteries of the affected leg right down to the foot |
CLI, chronic critical limb ischemia; CM, contrast medium; DSA, digital substraction angiography; GFR, glomerular filtration rate; NSAR, non-steroidal antirheumatics
Magnetic resonance angiography (MRA) visualizes the whole vascular tree with clarity comparable to that achieved with DSA. The method is non-invasive and uses gadolinium-containing CM that are non-nephrotoxic, carry a tenfold lower risk of allergy than iodine-containing CM, and also have no impact on thyroid function. Disadvantages of MRA are overestimation of the degree of stenosis, signal extinction by stents, failure to visualize vessel wall calcifications, venous superimposition by AV shunts in the lower leg in the presence of CLI (13, 14), and artifacts caused by turbulent or slow blood flow (6). Venous superimposition can be minimized by dynamic MRA with imaging every 2 s for 30 s. However, it often necessitates administration of a second dose of gadolinium to depict the arteries of the pelvis and upper leg. Resolution, particularly of the arteries of the foot, is reduced. These methodological limitations can be partially overcome by the use of 3-T MR devices and CM that do not permeate the vascular lumen (11).
Recent advances in magnetic resonance imaging (MRI) (e.g., improved data acquisition, new coil technology) permit high-quality visualization of the whole vascular tree even in the presence of marked calcifications (12). An additional significant contraindication to MRI in patients with GFR <30 mL/min/1.73 m2 has emerged with the recognition of nephrogenic systemic fibrosis (NSF) caused by gadolinium in patients with renal failure (German Federal Institute for Drugs and Medical Devices [BFArM]: over 500 cases worldwide up to October 2008) (15). Time-of-flight sequences avoid this problem by enabling visualization of vessels without CM, but they have poor resolution and have not yet been validated for the crural level. Multislice computed tomography angiography (MS-CTA) with multirow CT scanners (≥16 detector rows) is superior to MRI for portrayal of calcifications and in-stent stenoses. The improved image quality comes at the price of higher radiation exposure of the patient than is the case for DSA (10). CO2 angiography, although it is not listed in the current guideline recommendations for diagnosis, has experienced a renaissance since the recognition of NSF in connection with the administration of gadolinium-containing CM in patients with advanced renal failure. It does not attain the image quality of the other imaging procedures, but features none of the risks associated with CM. The combination of duplex sonography at the pelvic level and DSA of the arteries of the leg—alternatively, in the presence of advanced renal failure, CO2 angiography of the crural and pedal arteries—represents a diagnostic algorithm that delivers all the information required to decide on the appropriate treatment while minimizing the risks of CM administration (Table).
Conservative treatment
Conservative treatment has three different functions in patients with CLI.
Preparation for planned crural/pedal bypass surgery
Multimorbid patients must first be rendered operable. To this end, decompensating cardiopulmonary disorders must be recompensated. Local conditions at the operation site often have to be dealt with before bypass surgery can be carried out, for example skin infections in the area of the planned anastomosis or ischemic or lymphogenic edema, which if left untreated predisposes to postoperative disturbance of wound healing.
Postoperative treatment after bypass implantation
The aim of postoperative conservative treatment is prevention of early bypass malfunction. This includes optimal local treatment of the surgical wounds and timely manual lymph drainage to deal promptly with the frequent postoperative lymphedema. Particularly in the case of reduced arterial flow, the perfusion pressure in the vessels is so low that any additional increase in pressure by interstitial edema formation can endanger bypass function. The edema can be treated by compression only if the Doppler pressure reading is clearly above the pressure of 40 to 50 mm Hg caused by a compression bandage.
Of course, depending on the bypass situation, postoperative treatment includes administration of thrombocyte aggregation inhibitors or anticoagulants, with corresponding instruction of the patient.
Measures to deal with the cardiovascular risk factors form part of the conservative treatment in every stage of PAD. Because of the often simultaneous occurrence of coronary heart disease and cerebral arteriovenous disease in the stage of CLI, the same strict treatment goals apply as for cardiology patients (4).
Conservative treatment when intervention and bypass surgery are not possible
In the absence of a suitable peripheral target vessel for intervention or bypass surgery, the prospects for success of conservative treatment alone are at best limited. The first-line option in these circumstances is PGE1 infusion, but cardiac adverse effects mean that this treatment is occasionally not tolerated (4– 6). Intra-arterial infusion at reduced dosage may be helpful in such cases. However, 40% of exclusively conservatively treated patients have to have a major amputation within 6 months (6).
Conservative treatment also includes adequate oxygenation of the blood (compensation of cardiopulmonary diseases, compensation of anemia with hemoglobin under 9 g/dL) and connective tissue massage, which leads to widening of peripheral vessels. Any infection requires systemic antibiotic treatment following swabbing or wound biopsy to determine the bacteria present and produce an antibiogram (4, 16).
Pain treatment to avoid stress-related vasoconstriction is particularly important. Spinal cord stimulation (SCS) is useful: It alleviates pain and has metabolic effects that help to improve the microcirculation (17, 18).
Lumbar sympathectomy is used in patients with endangiitis obliterans in whom there is no potential for revascularization, but is of no therapeutic benefit in diabetics because of the autonomic neuropathy associated with diabetes (19).
Basic treatment measures include foot-down positioning and strict relief of pressure on the affected foot, including the heel. Patients and their relatives must be shown how to inspect the foot every day and avoid pressure points. In addition to the acute treatment, referral of high-risk patients to specialized foot clinics can avoid future amputations (20).
Interventional treatment
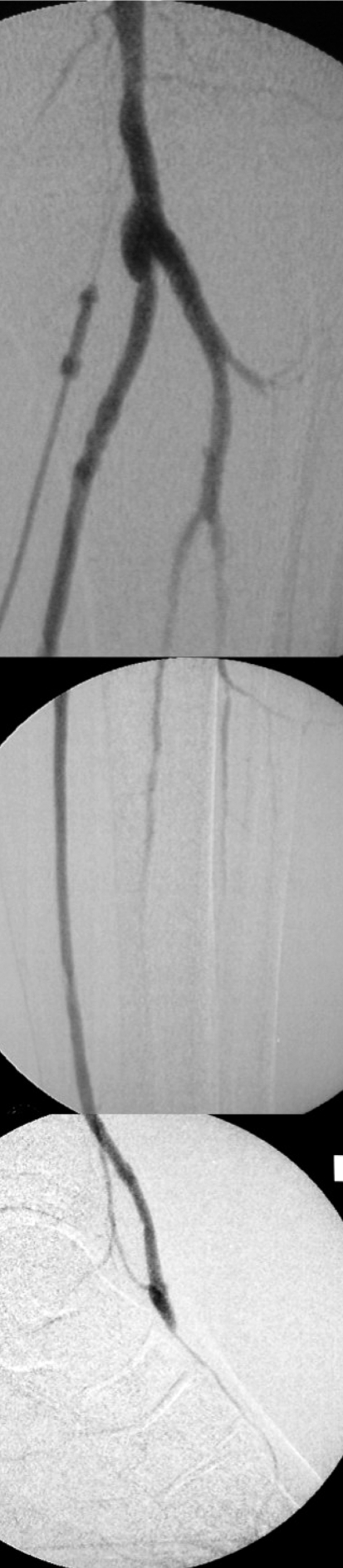
The data on interventional revascularization of crural and/or pedal arteries in CLI are still too sparse to permit formulation of evidence-based guidelines. The indication criteria are oriented on the TASC II recommendations for treatment at the well-investigated femoropopliteal level: the shorter the stenoses/occlusions and the fewer arteries requiring treatment, the better the clinical outcome. The technical success rate of infragenicular interventions is 90% and the clinical success rate 70% to 90% (6, 21– 23) (Figure 2). The restenosis rate remains very high, however; 65% in the first 2 years after treatment. The rate of complications for therapeutic interventions in the leg is 2.4% to 17% (6).
Figure 2.
Crural intervention (a, before; b, after percutaneous transluminal angioplasty)
Various new techniques (long balloons for diffuse long-segment stenoses, drug-eluting stents [DES], bare metal stents [BMS], drug-coated balloons, cutting balloons, laser angioplasty, atherectomy) are being investigated in studies (24, 25, e1– e4), but none can yet be recommended for routine clinical application. Early results indicate, however, that, in the leg, DES achieves better patency rates at one-year follow-up than balloon percutaneous transluminal angioplasty (PTA) or BMS (DES 81% to 85%, balloon PTA 58%, BMS 56%) (e12) (Scheinert D: Achilles study—randomized comparison of sirolimus eluting stents vs PTA for BTK obstructions. Presented at LINC 2011 Annual Meeting, 19 January 2011, Leipzig, Germany).
Surgical treatment
In line with the TASC II criteria for the femoropopliteal level, peripheral bypass surgery is also recommended for revascularization of long-segment occlusions of the crural and pedal circulation (4, 6, 7, e5).
Autologous vein material should be preferred. One-year follow-up rates of 80% to 90% for bypass patency and 90% to 95% for limb preservation can be achieved by this method (6, e7). If no suitable vein is available, thin-walled polytetrafluoroethylene (PTFE) implants can be used; however, the secondary 5-year patency rate for PTFE implants is 35%, compared with 60% for venous bypasses (6).
The implanted bypass should be no longer than necessary. All variants (femoro-crural, popliteo-crural, popliteo/cruro-pedal) are technically feasible (Figure 3). The 5-year rates for bypass patency and limb preservation are approximately 60% and 80% respectively (6, e7). Bypasses can be implanted in all three arteries of the leg, with no differences in prognosis (e7, e8). There is also no significant difference in outcome between diabetics and non-diabetics (e9). The dorsal artery of the foot and the posterior tibial artery as far as the plantar artery are suitable for pedal reconstruction. Imaging of the vessels of the foot reveals amenable pedal arteries in around 90% of patients, particularly in diabetics (e9).
Figure 3.
Digital subtraction angiography of a popliteo-pedal bypass from the popliteal artery (P3) to the dorsal artery of the foot
Postoperative wound healing is problematic in 15% to 25% of cases, and the rate of bypass infections is 1% to 3% (predominantly in artificial implants). The perioperative cardiovascular mortality is 1% to 2%, and 10% of patients have died by a year after operation (6). Cruro-pedal bypasses have to be monitored especially closely in the first year, with clinical examination at 3-month intervals. In our experience additional duplex sonography at each follow-up is beneficial, although the published data are contradictory (e10).
Discussion
Revascularization of crural or pedal arteries should be reserved for patients with CLI (Fontaine stage III and IV or Rutherford stage IV to VI). Infragenicular procedures, whether surgical or interventional, are not indicated in intermittent claudication.
Duplex sonography (performed by an experienced examiner) and MRA have proved their worth in preoperative imaging. However, these procedures do not yield sufficient information in candidates for crural and pedal vascular reconstruction. These patients require visualization of vessels right down to the pedal arcade, so DSA still has a role in diagnosis. The combination of duplex sonography of the pelvic arteries with DSA of the arteries of the leg and foot (CO2 angiography instead of DSA in patients with GFR <30 mL/min/1.73 m2) yields all the information needed for planning crural or pedal surgical revascularization.
The decision whether revascularization should be carried out by interventional means or by open surgery is a matter for an interdisciplinary team. In the absence of adequate comparative data on crural and pedal interventions and bypasses, we are guided by the morphologically defined TASC II criteria for iliac and femoropopliteal procedures (Figure 4). Furthermore, a significant part is played by factors such as the patient’s general health and fitness for anesthesia, the condition of the skin in the area of the planned anastomosis, and the experience of the team members.
Figure 4.
Indications for crural/pedal bypass implantation or intervention. PAD, peripheral arterial disease; DFS, diabetic foot syndrome
Because surgical treatment is more invasive than a catheter intervention and the rates of both patency and limb preservation are comparable, intervention should be preferred, provided the anatomic preconditions are fulfilled (4).
Some new interventional procedures cannot yet be recommended for general use because of low case numbers and short observation periods. In general, one should ensure in all peripheral vascular interventions that a distal segment of uninjured artery is left that can be used for a functioning bypass anastomosis.
Autologous veins should preferably be used for surgical revascularization of crural or pedal arteries. In the absence of suitable veins, composite or sequential bypasses can be created. Finally, an extra-anatomic bypass to the anterior tibial artery can be considered.
In cases of multilevel disease endovascular and surgical procedures can be combined; the flow of blood into or out of a bypass can be improved by interventional means.
If revascularization is no longer possible, infusion of PGE1 may alleviate pain and promote ulcer healing (Figure 5). If all options for revascularization and for conservative treatment have been exhausted, SCS may help to preserve the limb in selected cases.
Figure 5.
Treatment strategy in critical limb ischemia
(SCS, spinal cord stimulation)
No patient with CLI should be subjected to below-knee or above-knee amputation without the feasibility of crural and pedal revascularization being explored. Limb preservation must remain the foremost goal in order to preserve quality of life and avoid dependence on care. Crural and pedal revascularization, whether achieved by interventional means, open surgery, or a combination of both, achieves limb preservation rates of 90% to 95% after 1 year and 80% to 90% after 3 to 5 years.
Key Messages.
Vascular reconstruction—crural or pedal, interventional or surgical—is indicated in peripheral arterial disease stage III or IV and in diabetic foot syndrome. These diseases are responsible for 40 000 major amputations each year in Germany.
The primary aim of treatment for chronic critical limb ischemia is limb preservation. If all options for revascularization are exploited, this goal can be achieved in around 90% of cases.
No amputation may be performed without previous diagnostic imaging of the arterial circulation down to and including the foot, followed by interdisciplinary discussion of the potential for revascularization.
Duplex sonography, digital subtraction angiography, CT angiography, MR angiography, and in the case of advanced renal failure CO2 angiography, singly or in combination, are suitable for diagnostic imaging of crural and pedal arteries.
The long-term outcomes of interventional and operative cruropedal revascularization do not differ significantly. The complication rate for interventional procedures is 2.4% to 17%, while wound healing is problematic after 15% to 25% of bypass operations. If at all possible, the less invasive intervention method should be tried first, always leaving a distal segment of artery that can be used to create a bypass if necessary.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Prof. Zeller acts as consultant to Cordis, Cook, ev3, and Medtronic. He has received lecture fees and reimbursement of training and accommodation costs from ev3, Cordis, Medtronic, Cook, Gore, Biotronik, Abbott, Vascular IDEV, and Straub Medical.
Dr. Eder has received reimbursement of costs for congress attendance and accommodation from Gore and Bard.
Dr. Euringer has received reimbursement of costs for congress attendance and accommodation from Terumo, Nicolei, and Vaskutek.
The remaining authors declare that no conflict of interest exists.
References
- 1.Catalano M. Epidemiology of critical limb ischaemia: North Italian data. Eur J Med. 1993;2:11–14. [PubMed] [Google Scholar]
- 2.The Vascular Surgical Society of Great Britain and Ireland. Critical limb ischaemia management and outcome. Report of a national survey. Eur J Vasc Endovasc Surg. 1995;10:108–113. doi: 10.1016/s1078-5884(05)80206-0. [DOI] [PubMed] [Google Scholar]
- 3.Hauner H. Epidemiologie und Kostenaspekte des Diabetes in Deutschland. Dtsch Med Wochenschr. 2005;130(Suppl 2):64–65. doi: 10.1055/s-2005-870872. [DOI] [PubMed] [Google Scholar]
- 4.Diagnostik und Therapie der peripheren arteriellen Verschlusskrankheit (PAVK) AWMF-Leitlinien-Register 065-003. 2009. [PubMed] [Google Scholar]
- 5.Hirsch AT, Haskal Z, Hertzer N, et al. Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus and Vascular Disease Foundation. Circulation. 2006;113:463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 6.Norgren L, Hiatt W, Dormandy J, Nehler M, Harris K, Fowkes F. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(1):1–75. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Nationale Versorgungsleitlinie Typ 2-Diabetes. AWMF-Leitlinien-Register nvl-001c , 02/2010. Präventions- und Behandlungsstrategien für Fußkomplikationen. [Google Scholar]
- 8.Holstein PE, Sorensen S. Limb salvage experience in a multidisciplinary diabetic foot unit. Diabetes Care. 1999;22(2):B97–B103. [PubMed] [Google Scholar]
- 9.The Vascular Surgical Society of Great Britain and Ireland Critical limb ischaemia Management and outcome. Report of a national survey. Eur J Vasc Endovasc Surg. 1995;10:108–13. doi: 10.1016/s1078-5884(05)80206-0. [DOI] [PubMed] [Google Scholar]
- 10.Schernthaner R, Stadler A, Lomoschitz F, et al. Multidetector CT angiography in the assessment of peripheral arterial occlusive disease: accuracy in detecting the severity, number and length of stenosis. Eur Radiol. 2008;18:665–671. doi: 10.1007/s00330-007-0822-8. [DOI] [PubMed] [Google Scholar]
- 11.Haneder S, Attenberger UI, Riffel P, Henzler T, Schoenberg SO, Michaely HJ. Magnetic resonance angiography (MRA) of the calf station at 30 T: intraindividual comparison of non-enhanced ECG-gated flow-dependent MRA, continuous table movement MRA and time-resolved MRA. Eur Radiol. 2011;21:1452–1461. doi: 10.1007/s00330-011-2063-0. [DOI] [PubMed] [Google Scholar]
- 12.Gutzeit A, Sutter R, Froehlich JM, et al. ECG-Triggered Non-Contrast-Enhanced MR Angiography (TRANCE) versus Digital Subtraction Angiography (DSA) in patients with peripheral arterial occlusive disease of the lower extremities. Eur Radiol. 2011;21:1979–1987. doi: 10.1007/s00330-011-2132-4. [DOI] [PubMed] [Google Scholar]
- 13.Lakshminarayan R, Simpson JO, Ettles DF. Magnetic resonance angiography: current status in the planning and follow-up of endovascular treatment in lower-limb arterial disease. Cardiovasc Intervent Radiol. 2009;32:397–405. doi: 10.1007/s00270-008-9467-5. [DOI] [PubMed] [Google Scholar]
- 14.Meyer BC, Werncke T, Foert E, et al. Do the cardiovascular risk profile and the degree of arterial wall calcification influence the performance of MDCT angiography of lower extremity arteries? Eur Radiol. 2010;20:497–505. doi: 10.1007/s00330-009-1555-7. [DOI] [PubMed] [Google Scholar]
- 15.Breitschaft A, Stahlmann R. Nephrogene systemische Fibrose. Med Monatsschr Pharm. 2009;32:377–382. [PubMed] [Google Scholar]
- 16.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 17.Slovut DP, Sullivan TM. Critical limb ischemia: medical and surgical management. Vasc Med. 2008;13:281–291. doi: 10.1177/1358863X08091485. [DOI] [PubMed] [Google Scholar]
- 18.Ubbink DT, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2003;3 doi: 10.1002/14651858.CD004001. CD004001. [DOI] [PubMed] [Google Scholar]
- 19.Deutsche Gesellschaft für Gefäßchirurgie und Gefäßmedizin. AWMF-Leitlinienregister Nr. 004/013. 2008. Leitlinie zur Endarteriitis obliterans. [Google Scholar]
- 20.Mc Cabe, Stevenson RCF, Dolan AM. Evaluation of a diabetic foot screening and protection programme. Diabet Med. 1998;15:80–84. doi: 10.1002/(SICI)1096-9136(199801)15:1<80::AID-DIA517>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Faglia E, Mantero M, Caminiti M, et al. Extensive use of peripheral angioplasty, particularly infrapopliteal, in the treatment of ischaemic diabetic foot ulcers: clinical results of a multicentric study of 221 consecutive diabetic subjects. J Intern Med. 2002;252:225–232. doi: 10.1046/j.1365-2796.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- 22.Dorros G, Jaff MR, Dorros AM, et al. Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia: five-year follow-up. Circulation. 2001;104:2057–2062. doi: 10.1161/hc4201.097943. [DOI] [PubMed] [Google Scholar]
- 23.Tefera G, Turnipseed W, Tanke T. Limb salvage angioplasty in poor surgical candidates. Vasc Endovascular Surg. 2003;37:99–104. doi: 10.1177/153857440303700203. [DOI] [PubMed] [Google Scholar]
- 24.Rand T, Basile A, Cejna M, et al. PTA versus carbofilm-coated stents in infrapopliteal arteries: Pilot study. Cardiovasc Intervent Radiol. 2006;29:29–38. doi: 10.1007/s00270-005-0276-9. [DOI] [PubMed] [Google Scholar]
- 25.Rastan A, Tepe G, Krankenberg H, Zahorsky R, Schwarzwälder U, Noory E, et al. Sirolismus-eluting stents versus bare-metal stents for treatment of focal lesions in infrapopliteal arteries: A double blind multi-center randomized clinical trial. Eur Heart J. 2011;32:2274–2281. doi: 10.1093/eurheartj/ehr144. [DOI] [PubMed] [Google Scholar]
- e1.Bosiers M, Deloose K, Verbist J, Peters P. Nitinol stenting for treatment of “below-the-knee” critical limb ischemia: 1-year angiographic outcome after Xpert stent implantation. J Cardiovasc Surg. 2007;48:455–461. [PubMed] [Google Scholar]
- e2.Scheinert D, Ulrich M, Scheinert S, et al. Comparison of sirolimus-eluting vs. bare-metal stents for the treatment of infrapopliteal obstructions. Eurointervention. 2006;2:169–174. [PubMed] [Google Scholar]
- e3.Siablis D, Karnabatidis D, Katsanos K, et al. Sirolimus-eluting versus bare stents for after suboptimal infrapopliteal angioplasty for critical limb ischemia: enduring 1-year angiographic and clinical benefit. J Endovasc Ther. 2007;14:241–250. doi: 10.1177/152660280701400217. [DOI] [PubMed] [Google Scholar]
- e4.Zeller T, Sixt S, Schwarzwälder U, et al. Long-term results after directional atherectomy of infrapopliteal arteries with use of the Silverhawk device. J Endovasc Ther. 2007;14:232–240. doi: 10.1177/152660280701400216. [DOI] [PubMed] [Google Scholar]
- e5.Zehle A. Deutsche Gesellschaft für Gefäßchirurgie. Leitlinien zu Diagnostik und Therapie in der Gefäßchirurgie. Heidelberg: Springer; 2010. Unterschenkelarterienverschlüsse (S2) Leitlinie zur Diagnostik und Therapie von chronischen Stenosen und Verschlüssen der A. tibialis anterior, posterior und der A. peronea; pp. 111–120. [Google Scholar]
- e6.Holstein P, Ellitsgaard N, Olsen BB, Ellitsgaard V. Decreasing incidence of major amputations in people with diabetes. Diabetologia. 2000;43:844–847. doi: 10.1007/s001250051459. [DOI] [PubMed] [Google Scholar]
- e7.Albers M, Romiti M, Brochado-Neto FC, De Luccia N, Pereira CA. Meta-analysis of popliteal-to-distal vein bypass grafts for critical ischemia. Vasc Surg. 2006;43:498–503. doi: 10.1016/j.jvs.2005.11.025. [DOI] [PubMed] [Google Scholar]
- e8.Pomposelli FB, Kansal N, Hamdan AD, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg. 2003;37:307–315. doi: 10.1067/mva.2003.125. [DOI] [PubMed] [Google Scholar]
- e9.Wölfle KD, Bruijnen H, Loeprecht H, et al. Graft patency and clinical outcome of femorodistal arterial reconstruction in diabetic and non-diabetic patients: results of a multicentre comparative analysis. Eur J Vasc Endovasc Surg. 2003;25:229–234. doi: 10.1053/ejvs.2002.1849. [DOI] [PubMed] [Google Scholar]
- e10.Visser K, Idu MM, Buth J, Engel GL, Hunink MG. Duplex scan surveillance during the first year after infrainguinal autologous vein bypass grafting surgery: costs and clinical outcomes compared with other surveillance programs. J Vasc Surg. 2001;33:123–130. doi: 10.1067/mva.2001.109745. [DOI] [PubMed] [Google Scholar]
- e11.Charbonnel B, Erdmann E, Ferrannini E, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary: The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- e12.Deloose K, Bosiers M, Peeters P. One year outcome after primary stenting of infrapopliteal lesions with the Chromis Deep stent in the management of critical limb ischaemia. EuroIntervention. 2009;5:318–324. doi: 10.4244/v5i3a50. [DOI] [PubMed] [Google Scholar]