Abstract
Background
Matrix metalloproteinases (MMPs) are a group of endopeptidases induced under inflammatory conditions in the intestine which possess the capacity to degrade components of the extracellular matrix. We have previously demonstrated that MMP-2 expression correlates with increased inducible nitric oxide synthase (iNOS) production in the stomach and that iNOS is upregulated in the postischemic gut by the luminal nutrient arginine and repressed by luminal glutamine. We therefore hypothesized that arginine would enhance expression of MMP-2 in the postischemic gut.
Methods
Jejunal sacs were created in rats at laparotomy and filled with either 60 mM glutamine, arginine, or magnesium sulfate (osmotic control) followed by 60 minutes of superior mesenteric artery occlusion (SMAO) and 6 hours of reperfusion and compared with shams. Jejunum was harvested, and membrane type-1 matrix metalloproteinase (MT1-MMP), MMP-2, and iNOS protein expression was determined by Western analysis and MMP-9 production by gelatin zymography.
Results
MMP-2, MT1-MMP, MMP-9, and iNOS were all increased after SMAO compared with shams. Arginine maintained while glutamine inhibited the increase in iNOS, MT1-MMP, and MMP-2 expression in the postischemic gut. Pretreatment of the arginine group with a selective iNOS inhibitor blunted the induction of MMP-2 in the postischemic gut. There was no differential modulation of MMP-9 by the luminal nutrients.
Conclusions
The arginine-induced upregulation of iNOS may contribute to increased activity of MT1-MMP and MMP-2. The mechanism for this differential regulation by arginine warrants further investigation.
Keywords: metalloproteinase, ischemia reperfusion, intestine, nitric oxide synthase, arginine, trauma
Matrix metalloproteinases (MMPs) are calcium-dependent zinc endopeptidases with the capacity to degrade components of the extracellular matrix. They are subclassified into families according to their substrate specificity and include the collagenases, gelatinases, stromelysins, and the membrane-associated MMPs. The gelatinases, composed of MMP-2 and MMP-9, are the enzymes of primary interest as their activity is directed predominantly against type IV collagen that forms the basement membrane of the intestinal epithelia and vascular structures. Leukocytes, in particular macrophages, are major sources of gelatinase production.1–6 However, endothelial and smooth muscle cells have been reported to produce gelatinases also.7–12
Matrix metalloproteinases are tightly regulated not only at the level of transcription and translation, but also by posttranslational modification. Pro-forms must be cleaved to active forms to obtain full enzymatic capabilities. Mechanisms of activation through binding by membrane type-1 matrix metalloproteinases (MT1-MMP) have been illustrated.13 Additionally, multiple inhibitors of the metalloproteinase have been described including α2-macroglobulin and the tissue inhibitor of metalloproteinases (TIMPs). To date, the TIMP family is comprisod of 4 members (TIMP-1 through TIMP-4). The TIMPs bind with high affinity in a 1:1 molar ratio to the MMPs and inhibit their activity. In normal physiology, MMPs contribute to tissue remodeling during development and to the repair process after tissue injury. However, overactivity of metalloproteinases has been associated with a variety of disease states, including rheumatoid arthritis,14 inflammatory bowel disease,15,16 and sepsis. Moreover, we have previously demonstrated that enhanced MMP expression contributes to endotoxin-induced gastric mucosal injury in the rat that appears to correlate or be associated with an upregulation of inducible nitric oxide synthase (iNOS).17,18
Immune enhancing diets, when added to standard enteral diets, enhance immune responsiveness independent of their presumed nutritional effects. Prospective randomized clinical trials using immunonutrition have indeed demonstrated a reduction in infectious complications in most but not all studies.19 However, their use in critically ill septic patients is questioned, as mortality may be increased due to upregulation of iNOS in this patient population. In this setting arginine, a substrate for iNOS, may be potentially harmful in septic patients although this has not been well investigated.20
The mechanisms by which each of the immune enhancing nutrients exert their effects, particularly under conditions of gut hypoperfusion are unclear. Glutamine is the preferred fuel for the enterocyte and enhances production of protective anti-inflammatory mediators such as heat shock proteins,21,22 or as we have demonstrated through activation of peroxisome proliferator activating receptor (PPAR)γ.23 While glutamine is protective to the postischemic gut, we have also shown that administration of arginine under these same conditions is harmful through increased iNOS expression. However, the effects of luminal nutrients on MMP production in the postischemic gut are unknown. Because MMP-2 production appears to correlate with iNOS expression, we hypothesized that arginine would enhance MMP-2 production in the postischemic gut, an effect that would be reversed by iNOS inhibition.
Materials and Methods
Rodent Model and Study Design
All procedures performed were protocols approved by the University of Texas Houston Medical School Animal Welfare Committee. Male Sprague Dawley rats, each weighing 250–300 g, were fasted with free access to water overnight before laparotomy. Operative procedures were performed using sterile techniques under general anesthesia with isoflurane-inhaled anesthesia.
An upper midline laparotomy was performed and the jejunum identified 5 cm distal to the ligament of Treitz. An 8-cm intestinal sac was created in each animal by occluding the lumen of the bowel with 3-0 silk ligatures, as previously described.24 Sacs were filled with either 30 mM magnesium sulfate (nonabsorbable osmotic control), 60 mM glutamine, or 60 mM arginine as previously reported.25 Sixty millimeters approximates the amount of glutamine and arginine found in commercially available immune enhancing enteral diets. Superior mesenteric artery occlusion (SMAO) was carried out for 60 minutes, based on our previous studies that demonstrated consistent differences in mucosal injury between groups with no measurable mortality.26 After removal of the clamp, the incision was closed and rats were allowed to awaken and then observed for 6 hours. We previously demonstrated that mesenteric ischemia–reperfusion (IR) results in maximal activation of NFKκB, an upstream pro-inflammatory transcription factor activated by mesenteric IR, in the postischemic small bowel at 6 hours.27 Sham animals underwent the identical procedure but without placement of the clamp on the superior mesenteric artery. One group of animals underwent SMAO alone with no enteral sac, as an additional control. After the 6-hour reperfusion period, rats were killed and the jejunum removed for measurement of MMP and iNOS. In addition, because arginine enhanced iNOS and MMP-2 production (see the Results section), a separate groups of rats were given either the selective iNOS inhibitor N-[3(animomethyl)benzyl]-acetamidine (1400w, 20 mg/kg) or vehicle (saline) directly into the enteral sac to further evaluate the role of iNOS in the induction of metalloproteinases.17,28
MMP and iNOS Western Immunoblot Analysis
Nuclear and cytoplasmic extracts from full thickness jejunum were prepared.29 Protein expression by Western immunoblot was performed as previously described.30 In brief, jejunal cytoplasmic extracts were loaded in each well, and the proteins separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes, treated with 5% nonfat milk in Tris-buffered saline solution (20 mmol/L Tris-HCl, 130 mmol/L NaCl [pH 7.6] + 0.1% Tween 20), and incubated at 4°C overnight with a polyclonal anti-iNOS antibody (BD Biosciences Pharmingen, San Jose, CA), anti-MMP-2 antibody (1:200 dilution; Oncogene, San Diego, CA), or anti–MT1-MMP (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were washed with Tris-buffered saline solution and incubated with horseradish peroxidase-conjugated secondary antibody to identify iNOS according to enhanced chemiluminescent Western blotting detection system (Amersham Life Science, Piscataway, NJ). Autoradiograms were then analyzed using image-analysis software (Optimal 6.1) and expressed in arbitrary units.
Gelatin Zymography
Protein extracts (1 μg protein/sample) were mixed with 2× running buffer (0.125 M Tris-HCl, pH 6.8; 4% [w/v] SDS; 20% [v/v] glycerol; 0.04% [w/v] bromphenol blue) and separated on a 10% polyacrylamide gel containing 1 mg/mL gelatin. The gels were washed twice in 2.5% (v/v) aqueous Triton X-100, removing the SDS and allowing the gelatinases to renature within the gel. The gels were incubated overnight in standard activity buffer (0.05%Tris-HCl pH 8.8, 5 mM CaCl2, 0.02% NaN3) at 37° C. After incubation, the gels were fixed in fixing/destaining solution [methanol: acetic acid: water (4.5:1:4.5)] for 20 minutes and subsequently stained with Coomassie blue (0.01% [w/v] in fixing/destaining solution) for 2 hours. After destaining, the gels were scanned using an HP 500 digital scanner and the images were analyzed using Optimus 6.1 software. Areas of increased gelatinolytic activity were visualized as areas of lucency.17
Statistical Analysis
Data are expressed as mean ± SEM (n ≥ 6/group). Data were analyzed by 1-way ANOVA. Individual group means were compared using Tukey’s multiple group comparison test. P-values < .05 were considered significant.
Results
Arginine Enhances Jejunal iNOS
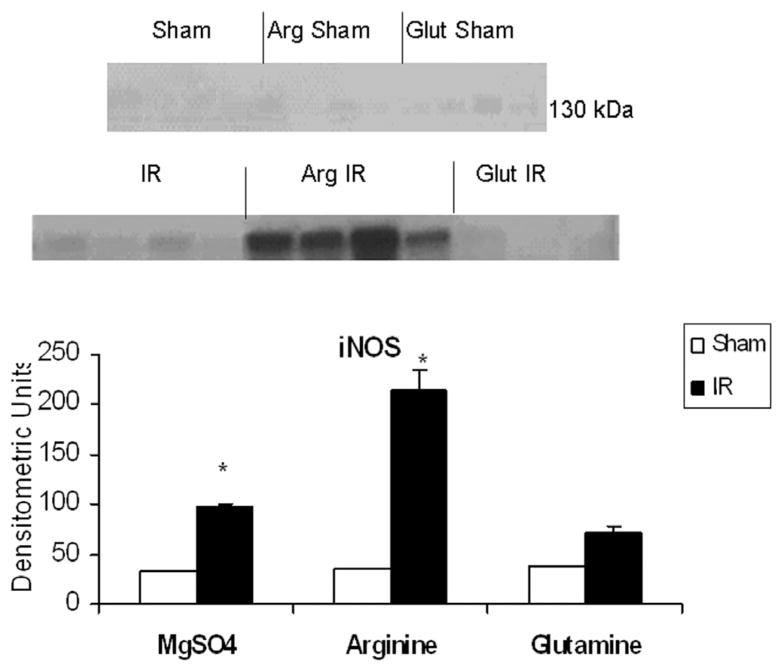
As shown in Figure 1, iNOS protein immunoreactivity was increased in the postischemic gut (0.06 ± 0.02) compared with controls (0.017 ± 0.00). The addition of luminal arginine further augmented iNOS production after ischemia/reperfusion (0.19 ± 0.05), while glutamine (0.02 ± 0.01) was comparable with controls.
Figure 1.
Effect of ischemia–reperfusion (IR) and luminal nutrients in jejunal production of inducible nitric oxide synthase (iNOS). Jejunal gut sacs were created and filled with 60 mM of arginine (Arg), glutamine (Glut), or magnesium sulfate as an iso-osmotic control and subjected to IR as described in the Materials and Methods section. Representative immunoblot and densitometric analysis demonstrates significantly increased iNOS production in the jejunal gut sacs after IR in the magnesium sulfate and Arg groups as compared with sham controls. There was no significant increase in iNOS production in the Glut group. *P < .05 vs corresponding sham (ANOVA)
Arginine Enhances Jejunal MMP Production
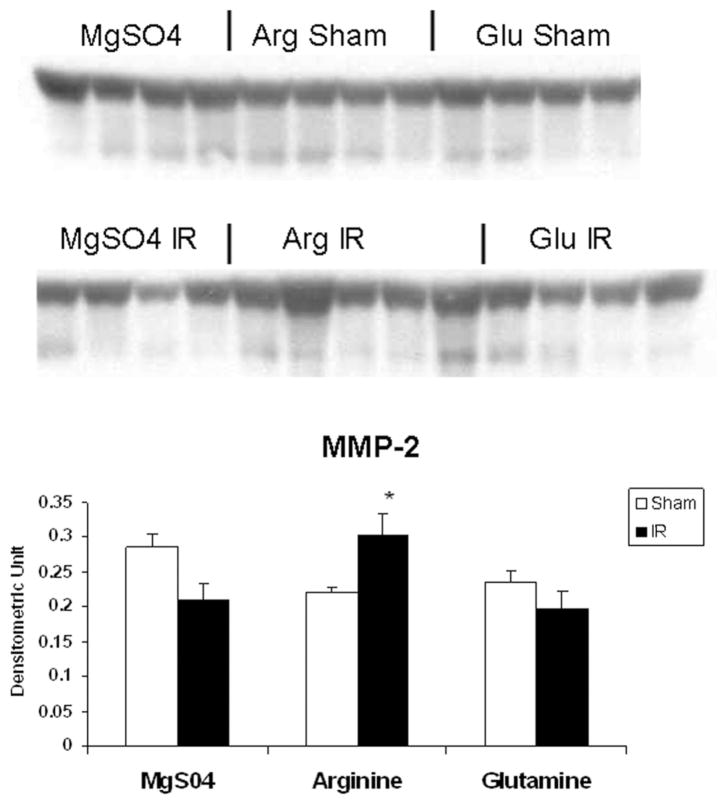
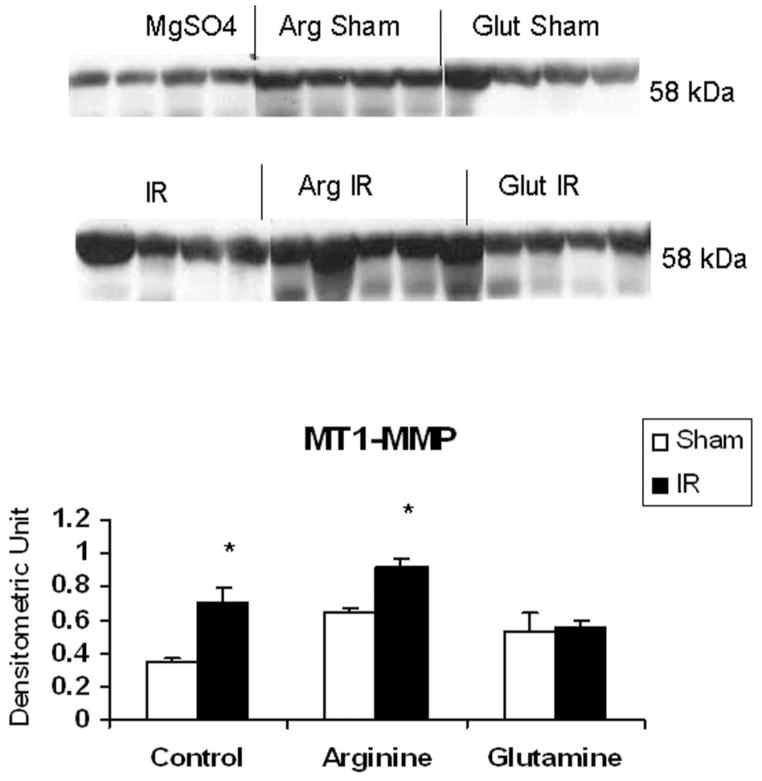
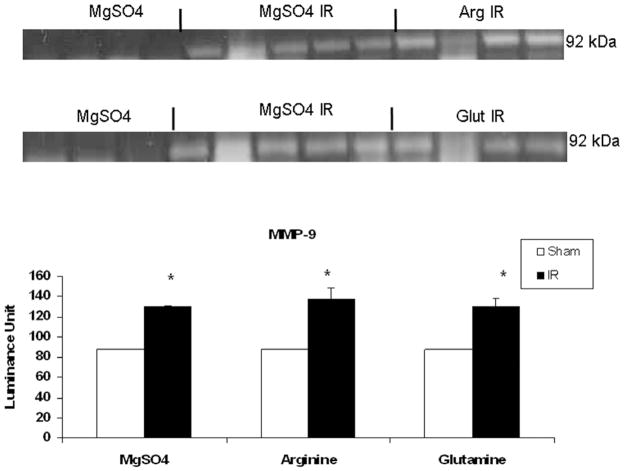
As shown in Figure 2, MMP-2 production in IR controls (0.21 ± 0.02) and IR glutamine (0.19 ± 0.02) were comparable to shams (0.28 ± 0.01), while MMP-2 was significantly increased by arginine (0.32 ± 0.02) in the postischemic gut. In contrast, MT1-MMP production was enhanced by IR in controls (0.70 ± 0.09) when compared with shams (0.34 ± 0.03), an effect accentuated by luminal arginine (0.91 ± 0.04) but not glutamine (0.55 ± 0.04; Figure 3). Of interest, MMP-9 was significantly induced by IR in all treatment groups but was not modulated by luminal nutrients (Figure 4).
Figure 2.
Effect of ischemia–reperfusion (IR) and luminal nutrients on jejunal production of matrix metalloproteinase-2 (MMP-2). Jejunal gut sacs were created and filled with 60 mM of arginine (Arg), glutamine (Glu), or magnesium sulfate as an osmotic control and subjected to IR as described in the Materials and Methods section. Representative immunoblot and densitometric analysis demonstrates significantly increased MMP-2 production in the Arg IR group as compared with sham controls. *P < .05 vs arginine sham (ANOVA).
Figure 3.
Effect of ischemia–reperfusion (IR) and luminal nutrients on jejunal production of membrane type-1 matrix metalloproteinase (MT1-MMP). Jejunal gut sacs were created and filled with 60 mM of arginine (Arg), glutamine (Glut), or magnesium sulfate as an osmotic control and subjected to IR as described in the Materials and Methods section. Representative immunoblot and densitometric analysis demonstrates significantly increased MT1-MMP production after IR in magnesium sulfate as well as the Arg group as compared with sham controls, while Glut attenuated the increase in MT1-MMP after IR. *P < .05 vs sham (ANOVA).
Figure 4.
Effect of ischemia–reperfusion (IR) and luminal nutrients on jejunal production of matrix metalloproteinase-9 (MMP-9). Jejunal gut sacs were created and filled with 60 mM of arginine (Arg), glutamine (Glut), or magnesium sulfate as an osmotic control and subjected to IR as described in the Materials and Methods section. MMP-9 production was assessed by gelatin zymography. Areas of increased MMP-9 activity are detected as areas of increased lucency in the gel at 92 kDa. Representative zymography demonstrates increased MMP-9 activity after IR, which was not modulated by luminal nutrients. The increase in activity was significant when compared with magnesium sulfate controls. There was no increase in MMP-9 activity upon the addition of luminal nutrients in the absence of IR (data not shown). *P < .05 vs magnesium sulfate (ANOVA).
iNOS Inhibition Prevents Arginine Induced Enhancement of Jejunal MMP Production
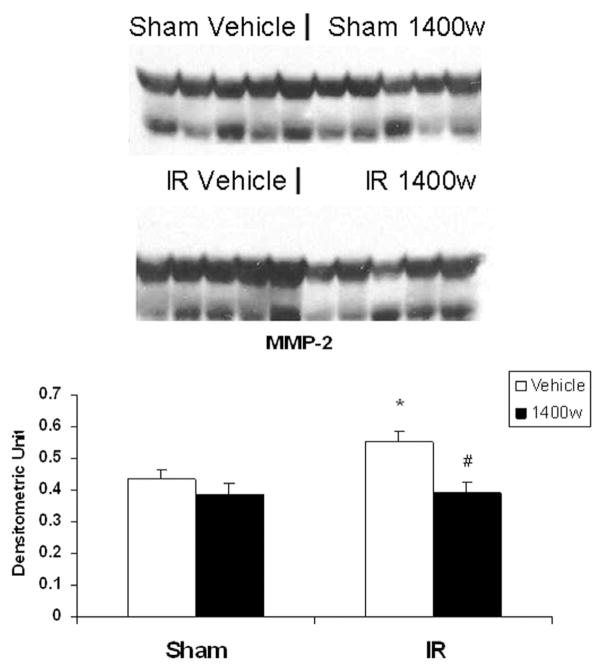
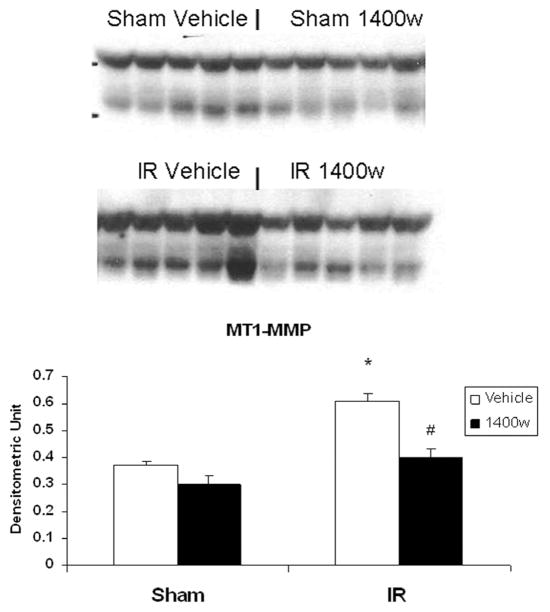
As shown in Figure 5, the selective iNOS inhibitor 1400w abrogated the induction of MMP-2 by arginine in the postischemic gut (0.55 ± 0.03 IR vs 0.39 ± 0.03 IR 1400w). Similarly, the induction of MT1-MMP protein production demonstrated in the IR arginine group (0.60 ± 0.03) was significantly inhibited by the addition of 1400w (0.40 ± 0.02; Figure 6).
Figure 5.
Effect of the selective inducible nitric oxide synthase (iNOS) inhibitor 1400w on matrix metalloproteinase-2 (MMP-2) production in arginine filled gut sacs after ischemia–reperfusion (IR). Jejunal gut sacs were created and filled with arginine (Arg) and 1400w or vehicle as described in the Materials and Methods section. MMP-2 production was assessed by Western immunoblot. MMP-2 was significantly increased in the Arg gut sacs after IR. This effect was inhibited by the addition of the selective iNOS inhibitor 1400w. *P < .05 vs sham vehicle; #P < .05 vs IR vehicle (ANOVA).
Figure 6.
Effect of the selective inducible nitric oxide synthase (iNOS) inhibitor 1400w on membrane type-1 matrix metalloproteinase (MT1-MMP) production in arginine filled gut sacs after ischemia–reperfusion (IR). Jejunal gut sacs were created and filled with arginine and 1400w or vehicle as described in the Materials and Methods section. MT1-MMP production was assessed by Western immunoblot. MT1-MMP was significantly increased in the Arg gut sacs after IR. This effect was inhibited by the addition of the selective iNOS inhibitor 1400w. *P < .05 vs sham vehicle; #P < .05 vs IR vehicle (ANOVA).
Discussion
We have been interested in the differential role of luminal nutrients on gut function and found a correlation to the degree of metabolic stress imposed by the nutrients under conditions of metabolic stress.24,26 Glutamine is absorbed across the enterocyte by either carrier mediated secondary active transport or by facilitative (passive) transport. The majority of glutamine undergoes oxidative metabolism to glutamate and CO2 by glutaminase. Glutamate can then enter the Kreb’s cycle and generate ATP (adenosine 5′-triphosphate) for use by the enterocyte. Arginine can also be absorbed by carrier mediated secondary active transport, but the majority of absorption uses Na-independent carriers. Unlike glutamine, arginine is not oxidatively metabolized and, therefore, cannot generate energy (ATP) for the gut. Arginine is transported out by means of the basolateral membrane and broken down into either ornithine or urea by arginase to assist in wound healing or by nitric oxide synthase to citrulline and nitric oxide. Our data obtained in a rodent model of gut IR suggests the iNOS pathway predominates in the postischemic gut in the presence of arginine and is associated with gut injury and inflammation.22
This is the first study to demonstrate modulation of MMP production in the jejunum by luminal nutrients. Luminal arginine, but not glutamine, enhanced MMP production in the jejunum of the postischemic gut. Furthermore, selective iNOS inhibition prevented the increase in MMP-2 production, suggesting that locally produced nitric oxide from jejunal iNOS modulates MMP production in this setting. These data are consistent with our previous findings of increased MMP-2 in the gastric mucosa in the setting of increased iNOS production and suggest that the pathways for regulating MMPs are similar in both sites.31 Lipopolysaccharide (LPS) induced the transcription as well as translation of MMP-2 along with an associated increase in the MMP-2 activator protein, MT1-MMP.18,31 MT1-MMP cleaves pro-MMP-2 to active MMP-2 by forming a tri-molecular complex on the cell surface with pro-MMP-2 and TIMP-2. Regulation of MMPs can occur at the level of transcription, translation, activation, or modulated by tissue specific inhibitors. The mechanism by which MMP-2 is upregulated in this model is the focus of ongoing investigation.
Although we did not specifically examine gut injury and inflammation in this study, arginine has clearly been shown to induce the pro-inflammatory transcription factor AP-1 and its downstream pro-inflammatory enzyme iNOS, in the postischemic gut. MMP-2 has been shown to promote wound healing in models of indomethacin-induced gastric ulceration.32 However, in the current model of gut ischemia/reperfusion, MMP-2 appears to play a deleterious role in inflammation and injury by means of its association with iNOS production. Future studies to determine the effects of MMP-2 inhibition on the postischemic gut after arginine administration are warranted.
In normal physiology, MMP-2 and MMP-9 produced by connective tissue are thought to contribute to tissue remodeling during development and during the repair process after tissue injury. Leukocytes, in particular macrophages, are major sources of gelatinase production.1–6 However, endothelial and smooth muscle cells have been reported to produce gelatinases also.7–12 While MMP-9 was detected in the postischemic gut, in contrast to MMP-2, this gelatinase was not modulated by luminal nutrients. MMP-9 production is necessary for neutrophil migration across the basement membrane and is commonly induced in other models of gut inflammation such as colitis. MMP-9 is secreted by macrophages and is also located in the cytoplasmic granules of neutrophils. Additionally, inflammatory cytokines stimulate the production of MMP-9 from lymphocytes and stromal cells. While the source of MMP-9 was not evaluated in this study, it is possible that local tissue injury from IR led to the infiltration of inflammatory cells and increased production of MMP-9. The lack of MMP-9 modulation by luminal nutrients is supported by our prior findings in an LPS model of gastric injury. In that model, we demonstrated an increase in MMP-2 production which correlated with increased iNOS production but no change in the expression of MMP-9.18,31
In conclusion, arginine, but not glutamine, induced upregulation of iNOS and may contribute to increased expression of MT1-MMP and MMP-2 in the postischemic gut. The mechanism for this differential regulation by arginine warrants further investigation.
Acknowledgments
The authors thank Raymond Kwan for his technical assistance.
Footnotes
No authors have a conflict of interest.
Financial disclosure: Supported by NIGMS Grants GM38529, RO1 GM077282, and KO8 GM 070812.
References
- 1.Watanabe H, Nakanishi I, Yamashita K, Hayakawa T, Okada Y. Matrix metalloproteinase-9 (92 kDa gelatinase/type iv collagenase) from U937 monoblastoid cells: correlation with cellular invasion. J Cell Sci. 1993;104(pt 4):991–999. doi: 10.1242/jcs.104.4.991. [DOI] [PubMed] [Google Scholar]
- 2.Wahl SM, Allen JB, Weeks BS, Wong HL, Klotman PE. Transforming growth factor beta enhances integrin expression and type IV collagenase secretion in human monocytes. Proc Natl Acad Sci U S A. 1993;90:4577–4581. doi: 10.1073/pnas.90.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keck T, Balcom JH, IV, Fernandez-del Castillo C, Antoniu BA, Warshaw AL. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology. 2002;122:188–201. doi: 10.1053/gast.2002.30348. [DOI] [PubMed] [Google Scholar]
- 4.Min D, Moore AG, Bain MA, Breit SN, Lyons JG. Activation of macrophage promatrix metalloproteinase-9 by lipopolysaccharide-associated proteinases. J Immunol. 2002;168:2449–2455. doi: 10.4049/jimmunol.168.5.2449. [DOI] [PubMed] [Google Scholar]
- 5.Menshikov MY, Elizarova EP, Kudryashova E, et al. Plasmin-independent gelatinase B (matrix metalloproteinase-9) release by monocytes under the influence of urokinase. Biochemistry (Mosc) 2001;66:954–959. doi: 10.1023/a:1012305206245. [DOI] [PubMed] [Google Scholar]
- 6.Pugin J, Widmer MC, Kossodo S, Liang CM, Preas HL, II, Suffredini AF. Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol. 1999;20:458–464. doi: 10.1165/ajrcmb.20.3.3311. [DOI] [PubMed] [Google Scholar]
- 7.Pan MR, Chuang LY, Hung WC. Non-steroidal anti-inflammatory drugs inhibit matrix metalloproteinase-2 expression via repression of transcription in lung cancer cells. FEBS Lett. 2001;508:365–368. doi: 10.1016/s0014-5793(01)03118-0. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y, Kawahara F, Noguchi M, et al. Activation of matrix metalloproteinase-2 in human breast cancer cells overexpressing cyclooxygenase-1 or -2. FEBS Lett. 1999;460:145–148. doi: 10.1016/s0014-5793(99)01328-9. [DOI] [PubMed] [Google Scholar]
- 9.Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of Mt1-MMP. J Cell Sci. 2001;114:131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hozumi A, Nishimura Y, Nishiuma T, Kotani Y, Yokoyama M. Induction of MMP-9 in normal human bronchial epithelial cells by TNF-alpha via Nf-Kappa B-mediated pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1444–L1452. doi: 10.1152/ajplung.2001.281.6.L1444. [DOI] [PubMed] [Google Scholar]
- 11.Soini Y, Satta J, Maatta M, Autio-Harmainen H. Expression of MMP2, MMP9, MT1-MMP, TIMP1, and TIMP2 mRNA in valvular lesions of the heart. J Pathol. 2001;194:225–231. doi: 10.1002/path.850. [DOI] [PubMed] [Google Scholar]
- 12.Porter KE, Turner NA. Statins for the prevention of vein graft stenosis: a role for inhibition of matrix metalloproteinase-9. Biochem Soc Trans. 2002;30:120–126. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 13.Nagase H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 1998;8:179–186. doi: 10.1038/cr.1998.18. [DOI] [PubMed] [Google Scholar]
- 14.Hirai Y, Migita K, Honda S, et al. Effects of nitric oxide on matrix metalloproteinase-2 production by rheumatoid synovial cells. Life Sci. 2001;68:913–920. doi: 10.1016/s0024-3205(00)00998-x. [DOI] [PubMed] [Google Scholar]
- 15.Baugh MD, Perry MJ, Hollander AP, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–822. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 16.Pirila E, Ramamurthy NS, Sorsa T, Salo T, Hietanen J, Maisi P. Gelatinase A (MMP-2), collagenase-2 (MMP-8), and laminin-5 gamma2-chain expression in murine inflammatory bowel disease (ulcerative colitis) Dig Dis Sci. 2003;48:93–98. doi: 10.1023/a:1021790532723. [DOI] [PubMed] [Google Scholar]
- 17.Robinson EK, West SD, Garay A, Mercer DW. Rat gastric gelatinase induction during endotoxemia. Dig Dis Sci. 2006;51:548–559. doi: 10.1007/s10620-006-3169-0. [DOI] [PubMed] [Google Scholar]
- 18.Robinson EK, Seaworth CM, Suliburk JW, Adams SD, Kao LS, Mercer DW. Effect of NOS inhibition on rat gastric matrix metalloproteinase production during endotoxemia. Shock. 2006;25:507–514. doi: 10.1097/01.shk.0000209543.83929.bd. [DOI] [PubMed] [Google Scholar]
- 19.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–953. doi: 10.1001/jama.286.8.944. [DOI] [PubMed] [Google Scholar]
- 20.Heyland DK, Samis A. Does immunonutrition in patients with sepsis do more harm than good? Intensive Care Med. 2003;29:669–671. doi: 10.1007/s00134-003-1710-6. [DOI] [PubMed] [Google Scholar]
- 21.Singleton KD, Wischmeyer PE. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1839–R1845. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- 22.Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol. 1997;272:G879–G884. doi: 10.1152/ajpgi.1997.272.4.G879. [DOI] [PubMed] [Google Scholar]
- 23.Sato N, Moore FA, Kone BC, et al. Differential induction of PPAR-gamma by luminal glutamine and iNOS by luminal arginine in the rodent postischemic small bowel. Am J Physiol Gastrointest Liver Physiol. 2006;290:G616–G623. doi: 10.1152/ajpgi.00248.2005. [DOI] [PubMed] [Google Scholar]
- 24.Kozar RA, Schultz SG, Bick RJ, Poindexter BJ, DeSoignie R, Moore FA. Enteral glutamine but not alanine maintains small bowel barrier function after ischemia/reperfusion injury in rats. Shock. 2004;21:433–437. doi: 10.1097/00024382-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kozar RA, Verner-Cole E, Schultz SG, et al. The immune-enhancing enteral agents arginine and glutamine differentially modulate gut barrier function following mesenteric ischemia/reperfusion. J Trauma. 2004;57:1150–1156. doi: 10.1097/01.ta.0000151273.01810.e9. [DOI] [PubMed] [Google Scholar]
- 26.Kozar RA, Schultz SG, Hassoun HT, et al. The type of sodium-coupled solute modulates small bowel mucosal injury, transport function, and ATP after ischemia/reperfusion injury in rats. Gastroenterology. 2002;123:810–816. doi: 10.1053/gast.2002.35389. [DOI] [PubMed] [Google Scholar]
- 27.Hassoun HT, Zou L, Moore FA, Kozar RA, Weisbrodt NW, Kone BC. Alpha-melanocyte-stimulating hormone protects against mesenteric ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1059–G1068. doi: 10.1152/ajpgi.00073.2001. [DOI] [PubMed] [Google Scholar]
- 28.Sato N, Moore FA, Kone BC, et al. Differential induction of PPAR-gamma by luminal glutamine and iNOS by luminal arginine in the rodent post ischemic small bowel. Am J Physiol Gastrointest Liver Physiol. 2006;290:G616–G623. doi: 10.1152/ajpgi.00248.2005. [DOI] [PubMed] [Google Scholar]
- 29.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques. 1994;16:405. [PubMed] [Google Scholar]
- 30.Sato N, Kozar RA, Zou L, et al. Peroxisome proliferator-activated receptor gamma mediates protection against cyclooxygenase-2-induced gut dysfunction in a rodent model of mesenteric ischemia/reperfusion. Shock. 2005;24:462–469. doi: 10.1097/01.shk.0000183483.76972.ae. [DOI] [PubMed] [Google Scholar]
- 31.Robinson EK, West SD, Garay A, Mercer DW. Rat gastric gelatinase induction during endotoxemia. Dig Dis Sci. 2006;51:548–559. doi: 10.1007/s10620-006-3169-0. [DOI] [PubMed] [Google Scholar]
- 32.Lempinen M, Inkinen K, Wolff H, Ahonen J. Matrix metalloproteinases 2 and 9 in indomethacin-induced rat gastric ulcer. Eur Surg Res. 2000;32:169–176. doi: 10.1159/000008759. [DOI] [PubMed] [Google Scholar]