Abstract
Purpose of review
Nutritional supplementation is paramount to the care of severely injured patients. Despite its widespread use in trauma, many areas of clinical nutrition remain controversial and not well defined. The benefit of early enteral nutrition in the care of injured patients has been well established, with further benefit derived by the administration of immune-enhancing formulas supplemented with glutamine, arginine, nucleotides, and omega-3-fatty acids. A new paradigm of pharmaconutrition has been developed that separates the administration of immunomodulatory nutrients from that of nutritional support. The optimal utilization and benefit of pharmaconutrients, however, remains unclear, as does the need for full caloric provision in the early postinjury phase.
Recent findings
Nutrition studies with the greatest reduction in morbidity and mortality are those utilizing specific nutrients. The use of pharmaconutrients to modulate the inflammatory and immune response associated with critical illness seems to provide benefit to critically ill and injured patients. Additionally, studies at least suggest that trauma patients derive comparable if not additional benefit from hypocaloric feeding during the acute phase of injury.
Summary
Building upon previous well performed studies in trauma patients, the current focus of nutritional investigations center on the use of pharmaconutrients to modulate the inflammatory response and the use of hypocaloric feeds. These practices will be reviewed and evidence presented for their use in critically ill and injured patients.
Keywords: antioxidants, glutamine, hypocaloric feeding, pharmaconutrition
Introduction
Early enteral nutrition has clearly been shown to improve outcomes in severely injured trauma patients [1,2]. Further benefit has now been demonstrated with the use of immunonutrition, standard enteral formulas supplemented with glutamine, arginine, omega-3 poly-unsaturated fatty acids (PUFAs), and nucleotides, in most but not all studies [3–5]. Because of inconsistent results, three meta-analyses have been conducted [6–8]. In the review by Heyland et al. in 2001 [8], the authors included 22 studies and over 2000 patients, the majority of which were conducted in surgical patients and those with malignancies. Eighteen studies reported infectious complications. When these were aggregated, immune-enhancing enteral nutrition resulted in a significant reduction in infectious complications [relative risk (RR), 0.66; 95% confidence interval (CI), 0.54–0.80]. Additionally, when the mortality results were aggregated, there was no advantage associated with immune-enhancing diets (RR, 1.10; 95% CI, 0.93–1.31). There was significant heterogeneity across the studies and the treatment effect appears to depend on the type of immune-enhancing formula, the patient population, and the study quality. On the basis of careful review of these studies, we believe that immune-enhancing formulas should be employed in high-risk trauma patients including those with major torso trauma and hemodynamic instability. Neither the precise nutrient combinations nor amounts required to achieve benefit, however, have been well studied. Additionally, the use of total parenteral nutrition should be reserved for those patients unable to tolerate enteral nutrition. Unlike the immunologic benefits derived from the early use of enteral nutrition, there are no benefits from the early use of total parenteral nutrition and it should therefore be utilized only as a nutritional supplement.
Pharmaconutrition
In the most recent meta-analysis on immunonutrition in critically ill patients, Marik and Zaloga [9•] analyzed studies by the type of formula administered, a somewhat different concept from pharmaconutrition but important as previous meta-analyses have grouped all studies, regardless of the type of supplemental nutrient. The study had significant flaws, mainly related to the small sample size when studies were broken down by patient population and formula type, raising more questions than answered. Half of the 24 studies analyzed involved trauma or burn patients. There was no mortality benefit when all formula types in this patient population were grouped together. Glutamine decreased infections in burn patients and there was a suggestion that arginine combined with fish oils negated the beneficial effect of fish oils. Again, this type of subanalysis is limited by small sample size but reinforces the conclusions of previous studies, mainly that heterogeneous patient populations and formula type make any type of meaningful conclusion difficult. Attempts to sort out the individual effects of nutrients, separate from their nutritional contribution, will be presented below using the concept of pharmaconutrition.
The realization that the greatest benefit in clinical outcomes was from studies utilizing specific nutrients led to the development of pharmaconutrition. This allows the detachment of nutritional support from the provision of key nutrients that may modulate the inflammatory and immune response associated with critical illness (www.criticalcarenutrition.com, an excellent resource with updates on nutrition practice, evidence-based guidelines and protocols for critically ill patients). Trauma, in particular, is characterized by the localized and systemic production and release of multiple proinflammatory mediators as well as a parallel release of anti-inflammatory mediators, which may be responsible for posttraumatic immunosuppression and the increased susceptibility to infections, sepsis, and multiple organ failure seen in these patients [10]. The trauma population may therefore be particularly susceptible to the modulatory and protective effects afforded by pharmaconutrition. In addition, Beale et al. [11•] recently demonstrated in critically ill septic patients the safety and efficacy of early enteral pharmaconutrition in promoting significantly faster recovery of organ function.
Glutamine
Glutamine is a conditionally essential amino acid that can influence genes important in metabolism, signal transduction, and inflammation. It has been shown to induce heat-shock protein when administered parenterally and peroxisome proliferator-activated receptor gamma (PPARγ) when administered enterally, both being associated with protective effects [12•,13•].
It is actively absorbed across the intestinal epithelium, metabolized in the small bowel and serves as an energy source for the enterocyte. In stress conditions, the demand for glutamine is increased and thus pharmacological supplementation may be necessary. Novak et al. [14] performed a systematic review of studies evaluating the effect of glutamine supplementation in elective surgical and critically ill patients. When these were aggregated, glutamine supplementation resulted in a significant reduction in infectious complications and length of stay was decreased. Glutamine enrichment was also associated with a relative risk of mortality of 0.78 (95% CI, 0.58–1.04). Acknowledging the considerable heterogeneity in the systematic review, the authors concluded that in surgical patients, glutamine was associated with decreased infectious complications and lengths of stay. In critically ill patients, they noted a significant decrease in complications and mortality. The authors concluded that the greatest effects were seen in patients receiving high-dose, parenteral glutamine supplementation. The majority of the studies, however, used parenteral rather than enteral nutrition. Jones and Heyland [15••] have subsequently recommended parenteral supplementation when parenteral nutrition is being delivered but enteral administration when enteral nutrition is being utilized. As specific studies in trauma and burn patients have demonstrated benefit with enteral supplementation, it is our practice to provide enteral glutamine supplementation whenever possible [16,17]. In fact, recent data had shown that its use during active-shock resuscitation is well tolerated and may promote tolerance [18•].
Arginine
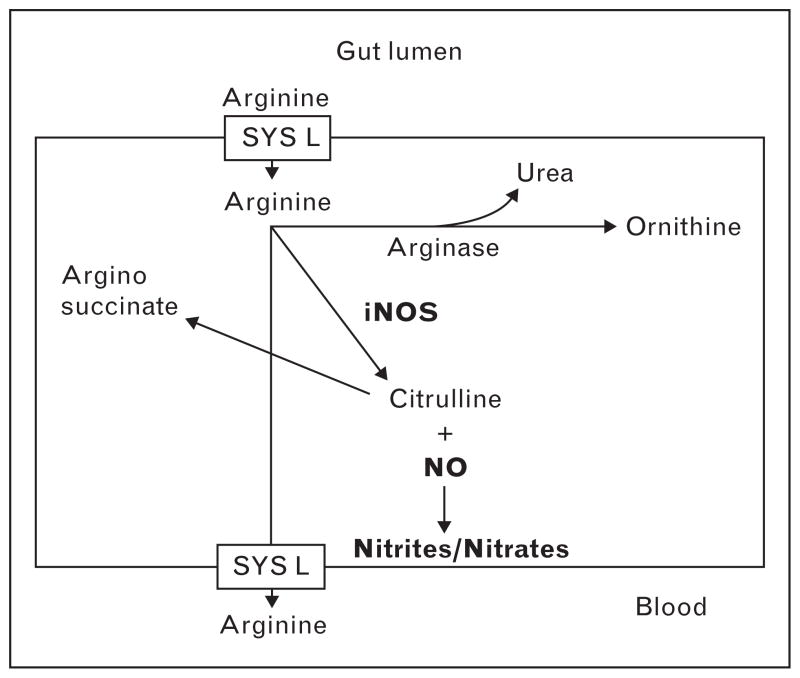
Arginine is a semiessential amino acid important in T-cell functioning, collagen synthesis, and the production of growth hormone, prolactin, somatostatin, insulin, and glucagon. Additionally, it is the chief precursor of nitric oxide [19,20]. It is this association with nitric oxide that has led to speculation that arginine may enhance the systemic inflammatory response syndrome and therefore be potentially harmful [21]. During sepsis, levels of inducible nitric oxide synthase (iNOS) are increased. Arginine is a substrate for iNOS and in its presence combines with molecular oxygen to produce citrulline and nitric oxide (Fig. 1). In septic conditions, nitric oxide has potential adverse effects including vasodilation, cardiac dysfunction, and direct cytotoxic injury [22]. Two studies [23,24] have documented increased mortality in critically ill septic patients receiving an immune-enhancing diet, and arginine has been implicated as the causative agent. However, there has been limited testing of arginine as an isolated pharmaconutrient. There are two studies [25,26] from the 1980s (in gastrointestinal cancer patients and in normal human volunteers), which focused on the effects of arginine on systemic immunity and demonstrated increased CD4 helper T cells and lymphocyte blastogenesis. Ochoa et al. [27] and Tsuei et al. [28] have studied arginine metabolism in trauma patients and demonstrated increased expression of arginase-1, which shunts arginine away from the iNOS pathway (Fig. 1). Though Jacob et al. [29] reported increased nitrate and nitrite levels in septic medical patients, they were unable to detect these products of iNOS in trauma patients. Even when patients became septic, levels of nitrate/nitrite were only minimally elevated. Thus, increased arginine at the systemic level does not appear to be problematic for trauma patients. However, laboratory studies have shown increased gut injury and inflammation when arginine is delivered to the hypoperfused gut during shock [30].
Figure 1. Metabolic fate of arginine.
Arginine is involved in two divergent metabolic pathways. In the first, arginase converts arginine into ornithine and urea. Arginase II, a form of arginase, is involved in the synthesis of polyamines, important in wound healing. Alternatively, arginine can serve as a substrate for nitric oxide synthase, which has vasodilatory and potentially proinflammatory effects. iNOS, inducible nitric oxide synthase.
Nucleotides
Nucleotides are low molecular weight intracellular compounds found in human milk (and as a supplement in infant formula) and diet. They serve as active precursors of DNA, RNA, ATP and coenzymes and play a role in growth and differentiation of the gut [31]. In stress states, they may be necessary to maintain rapid cell proliferation and responsiveness. In these instances, most tissues increase de-novo synthesis of nucleotides; however, lymphocytes, macrophages, and enterocytes rely on increased salvage from the extracellular pool that may be depleted during stress [32]. It is their role in enterocyte function and immunomodulation that led to inclusion of nucleotides in some immune enhancing diets [33]. There have been no trials examining the effect of nucleotides as isolated agents.
Omega-3 fatty acids
Standard enteral formulas often contain a high proportion of omega-6 fatty acids, whereas formulas supplemented with omega-3 fatty acids (or fish oils) are now available. The active components of omega-3 fatty acids are eicosapentaenoic (EPA) and docosahexaenoic acid (DHA). The anti-inflammatory properties of omega-3 fatty acids have been attributed to displacement of arachidonic acid from cellular membranes, antagonizing proinflammatory eicosanoids (such as prostaglandin E2 and leukotriene B4) while promoting production of less-inflammatory eicosanoids (such as thromboxane and prostaglandin E3), and inhibition of proinflammatory mediators, including iNOS [34•]. There have been three prospective randomized trials [35–37] of enteral supplementation with omega-3 fatty acids in critically ill (primarily medical) patients and all have shown benefit. Unfortunately, all these studies included additional supplements, making assignment of benefit to the omega-3 fatty acids difficult, and utilized a high-fat diet as the control. Nonetheless, results are promising and omega-3 fatty acid supplementation should be considered for use in patients with acute respiratory distress syndrome.
Antioxidants and micronutrients
There have also been a number of recent investigations evaluating the use of antioxidants. Part of the difficulty in interpreting these studies is that the types, routes, and doses of antioxidants differ between studies and patient populations. As oxidant stress is being increasingly appreciated as an important component of shock and critical illness, interest in antioxidants has escalated. Micronutrients considered to have antioxidant properties include those with endogenous defense mechanisms such as superoxide dismutase, catalase, and glutathione (including their cofactors, selenium, and zinc) as well as vitamins (particularly vitamin A, C, and E) [38]. Antioxidants generally have shown a reduction in the incidence of organ dysfunction, including in critically ill surgical patients [39]. However, Berger et al. [40•] recently reported that the use of antioxidants in critically ill patients had no influence on infectious complications and no reduction in early organ dysfunction. They did find a significant reduction in the inflammatory response in trauma and cardiac surgery patients, suggesting that conditions inciting an intense inflammatory response, such as that evoked by trauma may show benefit from antioxidant therapy. Indeed, an earlier study [41] of zinc, copper, and selenium supplementation in burn patients showed a decrease in infectious complications, primarily pulmonary. Additionally, although most studies have shown improvement in infectious complications and organ dysfunction, mortality benefit has been harder to demonstrate [42•]. However, Collier et al. [43•] were able to demonstrate a 28% RR adjustment in severely injured trauma patients [average injury severity score (ISS) = 21] who received high-dose antioxidants compared with historical controls. Additionally, a meta-analysis [44] reviewing the use of all antioxidants in critically ill patients found a decrease in mortality (but no impact on infectious complications). This and other studies [45•] suggest that selenium is the responsible agent. Selenium is an essential nutrient that is a cofactor in glutathione’s enzymatic function and possesses immunomodulatory properties [46]. It has been studied primarily in patients with septic shock. Levels are low with critical illness and augmentation enhances endogenous antioxidants. Not all studies have shown benefit with the discrepancies attributed to dosing and timing of administration [42•,47•]. If the fine balance between selenium’s antioxidant and prooxidant effects are upset, increased oxidative stress may actually ensue. As is common for many of these nutrient studies, the optimal dose and timing are not known and not well studied. Because of this and the fact that many of the positive antioxidant studies have included the administration of glutamine, Heyland et al. [48•] are currently conducting a large multicenter, randomized trial with a factorial 2 × 2 design in mechanically ventilated patients with clinical evidence of hypo-perfusion and organ dysfunction. Patients will receive glutamine, antioxidants, or both with mortality as the primary outcome though infectious complications and organ dysfunction are also being studied. Results of the phase I dose-escalating study failed to show any adverse effect on organ function from the nutrients but did show a reduction in markers of oxidative stress, greater preservation of glutathione levels, and an improvement in mitochondrial function. Unfortunately, the number of trauma patients enrolled in this study will likely be small as the entry criteria require evidence of organ dysfunction and hypoperfusion within 24 h of admission. Nonetheless, this important study should provide definitive data on the efficacy of glutamine and antioxidant supplementation in critically ill patients and help to clarify the timing, dosing, and combination benefit lacking in so many of the previous trials.
Hypocaloric feeding
The concept of hypocaloric nutrition began as a feeding strategy for critically ill obese patients but is now being considered for all critically ill patients [49]. It avoids the consequences of overfeeding such as hypercapnia, hyper-glycemia, uremia, and hypertriglyceridemia yet prevents malnutrition by the provision of adequate protein supplementation. There is no standardized definition of hypocaloric feeding but it usually involves meeting energy needs with less than 100% of the recommended nonprotein calories (typically ≤20 kcal/kg) but normal amounts of protein calories (1.5 to 1.75 g of protein/kg). Dickerson et al. [50] found a shorter ICU length of stay and a decreased duration of antibiotic use in obese surgical patients with no impairment in nitrogen balance or pre-albumin levels. There have been several studies in medical ICU patients which have compared outcomes with caloric intake and have shown benefit to patients receiving ‘suboptimal’ caloric intake. In a prospective observational study [51] aimed at examining the success in achieving recommended caloric requirements, medical ICU patients receiving approximately half of the intended calories had the best outcomes with more patients discharged alive and ventilator free. The use of gastric feeds in critically ill patients at least raises the possibility of aspiration as the cause of poorer outcome in patients receiving full caloric supplementation. To support this speculation, when early versus late enteral feeds in ventilated patients were compared, a lower incidence of ventilator-associated pneumonia was demonstrated in patients receiving late feeds [52]. The early feed group received bolus gastric feeds and had a high (49%) incidence of ventilator-associated pneumonia, at least suggesting that aspiration of tube feeds was responsible for the high pneumonia rate. A study [53•] performed in critically ill mechanically ventilated trauma patients is also consistent with aspiration as a contributing factor to pneumonia rates. Patients were randomized to a diet supplemented with glutamine, high fiber, peptides, or synbiotics via a gastric tube and begun no later than 24 h after injury. There was no difference in mortality or infectious complications except for pneumonia rates. Patients receiving the synbiotics had a significantly lower incidence of pneumonia (16% in synbiotic versus 40% in other groups combined), but interestingly much higher gastric residual volumes (1150 ml in synbiotics versus 410 ml in the glutamine group). Whether aspiration of feeds supplemented with synbiotics lowers pneumonia rates can only be speculated. Finally, there has been only one study [54] performed in trauma patients comparing outcomes with quantity of enteral nutrition. This published abstract was a retrospective study of 121 patients divided into quartiles based on daily caloric intake. Patients receiving the greatest caloric intake during the first 7 days after injury had poorer outcomes (increased hospital length of stay and increased ventilator days) than those patients receiving between 25 and 60% less calories per day. Though this study had significant flaws, it suggested the need to further study hypocaloric feeding of critically injured patients during the acute metabolic phase of injury.
Conclusion
By examining immunomodulating agents for their pharmaconutrient potential, a better understanding of their mechanism of action, optimal dosing and timing, and target patient population is being increasingly recognized. Most current studies, however, suffer from combination therapy and mixed patient populations. Recent data suggest that in trauma patients early enteral nutrition is beneficial and enhanced by supplementation with glutamine and antioxidants. Success with hypocaloric feeding in the obese patient has prompted speculation that lower calories with adequate protein supplementation in critically injured patients during the acute metabolic phase of injury, but no longer than 7–10 days postinjury, may be beneficial.
In summary, acutely injured trauma patients benefit from the early administration of enteral nutrition with added benefit from pharmaconutrients. Data reviewing the new paradigm of pharmaconutrition has been presented and suggestions for their use in trauma patients are included. The rationale and potential benefit of hypocaloric feeding in critically injured patients is presented.
Acknowledgments
The present study was funded in part by NIH grants number P50-GM38529 and RO1-GM077282.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 723).
- 1.Moore FA, Moore EE, Jones TN. TEN versus TPN following major abdominal trauma-reduced septic morbidity. J Trauma. 1989;29:916–923. doi: 10.1097/00005373-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on septic morbidity following blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA, Moore EE, Kudsk KA, et al. Clinical benefits of an immune-enhancing diet for early postinjury enteral feeding. J Trauma. 1994;37:607–615. doi: 10.1097/00005373-199410000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Kudsk KA, Minard G, Croce MA, et al. A randomized trial of isonitrogenous enteral diets after severe trauma: an immune-enhancing diet reduces septic complications. Ann Surg. 1996;224:531–540. doi: 10.1097/00000658-199610000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendez C, Jurkovich GJ, Garcia I, et al. Effects of an immune-enhancing diet in critically injured patients. J Trauma. 1997;42:933–940. doi: 10.1097/00005373-199705000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Heys SD, Walker LG, Smith I, Eremin O. Enteral nutrition supplementation with key nutrients in patients with critical illness and cancer. Ann Surg. 1999;229:467–477. doi: 10.1097/00000658-199904000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beale RJ, Bryg DJ, Bihari DJ. Immunonutrition in the critically ill: a systematic review of clinical outcome. Crit Care Med. 1999;27:2799–2805. doi: 10.1097/00003246-199912000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Heyland DK, Novak F, Drover JW, et al. Should immunonutrition become routine in the critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–953. doi: 10.1001/jama.286.8.944. [DOI] [PubMed] [Google Scholar]
- 9•.Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systemic review and analysis of the literature. Intensive Care Med. 2008 doi: 10.1007/s00134-008-1213-6. [Epub ahead of print]. In this meta-analysis, the authors attempt to analyze the benefits of immunonutrition in critically ill patients by formula type. Although this type of analysis is needed, the small number of studies in certain subgroups limits the significance of their findings. [DOI] [PubMed] [Google Scholar]
- 10.Moore FA, Sauaia A, Moore EE, Haenel JB, et al. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–512. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 11•.Beale RJ, Sherry T, Lei K, et al. Early enteral supplementation with key pharmaconutrients improves Sequential Organ Failure Assessment score in critically ill patients with sepsis: outcome of a randomized, controlled, double-blind trial. Crit Care Med. 2008;36:131–144. doi: 10.1097/01.CCM.0000297954.45251.A9. A prospective randomized controlled trial that demonstrated significantly faster recovery of organ function, as measured by Sequential Organ Failure Assessment scores, in medical patients with sepsis who received enteral supplementation with pharmaconutrients. [DOI] [PubMed] [Google Scholar]
- 12•.Singleton KD, Wischmeyer PE. Glutamine’s protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1839–R1845. doi: 10.1152/ajpregu.00755.2006. A rodent model of sepsis that confirms glutamine’s beneficial effect on survival, injury, and inflammation are dependent upon heat shock protein 70 expression. [DOI] [PubMed] [Google Scholar]
- 13•.Ban K, Kozar RA. Enteral glutamine: a novel mediator of PPAR gamma in the postischemic gut. J Leukoc Biol. 2008;84:595–599. doi: 10.1189/jlb.1107764. A review of laboratory studies in a rodent model demonstrating the protective effects of enteral glutamine by PPAR in the postischemic gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novak F, Heyland DK, Avenell A, et al. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med. 2002;30:2022–2029. doi: 10.1097/00003246-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 15••.Jones NE, Heyland DK. Pharmaconutrition: a new emerging paradigm. Curr Opin Gastroenterol. 2008;24:215–222. doi: 10.1097/MOG.0b013e3282f4cdd8. This study provides an excellent overview of the evidence in support of a new paradigm of pharmaconutrition to explain the inconsistent results of immunonutrition. [DOI] [PubMed] [Google Scholar]
- 16.Houdijk AP, Rijnsburger ER, Jansen J, et al. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998;352:1–5. doi: 10.1016/S0140-6736(98)02007-8. [DOI] [PubMed] [Google Scholar]
- 17.Garrell D, Patenaude J, Nedelec B, et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Crit Care Med. 2003;31:2444–2449. doi: 10.1097/01.CCM.0000084848.63691.1E. [DOI] [PubMed] [Google Scholar]
- 18•.McQuiggan M, Kozar R, Sailors RM, et al. Enteral glutamine during active shock resuscitation is safe and enhances tolerance of enteral feeding. JPEN J Parenter Enteral Nutr. 2008;32:28–35. doi: 10.1177/014860710803200128. This study is a prospective randomized pilot study that examined the safety and tolerance of enteral glutamine administered to critically injured patients in shock. [DOI] [PubMed] [Google Scholar]
- 19.Barbul A, Lazarou SA, Efron DT. Arginine enhances wound healing and lymphocyte immune response in humans. Surgery. 1990;108:331–336. [PubMed] [Google Scholar]
- 20.Salzman AL. Nitric oxide in the gut. New Horiz. 1995;3:33–45. [PubMed] [Google Scholar]
- 21.Bower RH, Cerra FB, Bershadsky B, et al. Early administration of a formula (Impact) supplemented with arginine, nucleotides, and fish oil in intensive care patients: results of a multicenter, prospective, randomized, clinical trial. Crit Care Med. 2001;23:436–449. doi: 10.1097/00003246-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Todd SR, Kozar RA, Moore FA. Nutrition support in adult trauma patients. Nutr Clin Pract. 2006;21:421–429. doi: 10.1177/0115426506021005421. [DOI] [PubMed] [Google Scholar]
- 23.Heyland DK, Samis A. Does immunonutrition in patients with sepsis do more harm than good? Intensive Care Med. 2003;29:669–671. doi: 10.1007/s00134-003-1710-6. [DOI] [PubMed] [Google Scholar]
- 24.Bruzzone R, Radrizzani D. Early enteral immunonutrition in patients with severe sepsis. Results of an interim analysis of a randomized multicenter clinical trial. Intensive Care Med. 2003;29:834–840. doi: 10.1007/s00134-003-1711-5. [DOI] [PubMed] [Google Scholar]
- 25.Daly JM, Reynolds J, Thom A, et al. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988;208:512–523. doi: 10.1097/00000658-198810000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbul A, Sisto DA, Wasserkrug HL, Efron G. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery. 1981;90:244–251. [PubMed] [Google Scholar]
- 27.Ochoa JB, Bernard AC, O’Brien WE, et al. Arginase 1 expression and activity in human mononuclear cells after injury. Ann Surg. 2001;233:393–399. doi: 10.1097/00000658-200103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuei BJ, Bernard AC, Barksdale AR, et al. Supplemental enteral arginine is metabolized to ornithine in injured patients. J Surg Res. 2004;123:17–24. doi: 10.1016/j.jss.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Jacob TD, Ochoa JB, Udekwu AO, et al. Nitric oxide production is inhibited in trauma patients. J Trauma. 1993;35:590–597. doi: 10.1097/00005373-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Sato N, Moore FA, Kone BC, et al. Differential induction of peroxisome proliferator activated receptor gamma by luminal arginine glutamine and iNOS by luminal arginine in the rodent postischemic small bowel. Am J Physiol. 2006;290:616–623. doi: 10.1152/ajpgi.00248.2005. [DOI] [PubMed] [Google Scholar]
- 31.Han-Markey T. Nutrient metabolism in children. In: Rolandelli RH, editor. Clinical nutrition enteral and tube feeding. 4. Philadelphia, Pennsylvania: Elsevier Inc; 2005. p. 672. [Google Scholar]
- 32.Van Buren CT, Kulkarni A, Fanslow WC, Rudolph FB. Dietary nucleotides, a requirement for helper/inducer T lymphocytes. Transplantation. 1985;40:694–697. doi: 10.1097/00007890-198512000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Kulkarni AD, Rudolph FB, Van Buren CT. The role of dietary sources of nucleotides in immune function: review. J Nutr. 1994;124 (8 Suppl):1442S–1446S. doi: 10.1093/jn/124.suppl_8.1442S. [DOI] [PubMed] [Google Scholar]
- 34•.Aldridge C, Razzak A, Babcock TA, et al. Lipopolysaccharide-stimulated RAW 264.7 macrophage inducible nitric oxide synthase and nitric oxide production is decreased by an omega-3 fatty acid lipid emulsion. J Surg Res. 2008;149:296–302. doi: 10.1016/j.jss.2007.12.758. This laboratory study demonstrated a reduction in nitric oxide synthase by omega-3 fatty acids following stimulation of macrophages by lipopolysaccharide. A good review of the known molecular mechanisms contributing to the beneficial effect of omega-3 fatty acids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 36.Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 37.Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respirator distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Heyland DK, Dhaliwal R, Day AG, et al. REducing Deaths due to OXidative Stress (The REDOX Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically-ill patients. Proc Nutr Soc. 2006;65:250–261. doi: 10.1079/pns2006505. [DOI] [PubMed] [Google Scholar]
- 39.Nathens AB, Neff MJ, Jurkovich GJ, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Berger MM, Soquel L, Shenkin A, et al. Influence of early antioxidant supplementations on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care. 2008;14:R101. doi: 10.1186/cc6981. A prospective randomized trial of patients in organ failure who received parenteral antioxidants for 5 days, with an initial double-loading dose. The lack of improvement in organ failure may be related to timing and dosing of the antioxidants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger MM, Spertini F, Shenkin A, et al. Trace element supplementation modulates pulmonary infection rates after major burns: a double-blind, placebo-controlled trial. Am J Clin Nutr. 1998;68:365–371. doi: 10.1093/ajcn/68.2.365. [DOI] [PubMed] [Google Scholar]
- 42•.Forceville X, Laviolle B, Annane D, et al. Effects of high-doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study. Crit Care. 2007;11:R73. doi: 10.1186/cc5960. A prospective randomized phase II trial in severe septic shock patients receiving a 9-day infusion of selenium. No toxicity as well as no improvement in clinical outcomes was demonstrated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Collier BR, Giladi A, Dossett LA, et al. Impact of high-dose antioxidants on outcomes in acutely injured patients. JPEN J Parenter Enteral Nutr. 2008;32:384–388. doi: 10.1177/0148607108319808. This retrospective cohort study demonstrated a significant reduction in mortality in acutely injured patients receiving high-dose antioxidants (vitamin C and E and selenium) [DOI] [PubMed] [Google Scholar]
- 44.Heyland DK, Dhaliwal R, Suchner U, et al. Antioxidant nutrients: a systematic review of vitamins and trace elements in the critically ill patient. Intensive Care Med. 2005;31:327–337. doi: 10.1007/s00134-004-2522-z. [DOI] [PubMed] [Google Scholar]
- 45•.Angstwurm MW, Engelmann L, Zimmermann T, et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118–126. doi: 10.1097/01.CCM.0000251124.83436.0E. This prospective trial administered a 14-day course of parenteral selenium to patients with severe systemic response syndrome, sepsis, and severe sepsis. The intent to treat group had no significant reduction in mortality, the primary endpoint. After over 20% of patients were excluded, coagulopathic patients with severe sepsis and patients with high APACHE III scores (≥102) demonstrated a reduced mortality. [DOI] [PubMed] [Google Scholar]
- 46.Raymond MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 47•.Heyland DK. Selenium supplementation in critically ill patients: can too much of a good thing be a bad thing? Crit Care. 2007;11:153. doi: 10.1186/cc5975. This commentary explains how an upset in the fine balance between proinflammation and anti-inflammation by the antioxidant, selenium, can be detrimental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Heyland DK, Dhaliwalm R, Day A, et al. Optimizing the dose of glutamine dipeptides and antioxidants in critically ill patients: a phase I dose-finding study. J Parenter Enteral Nutr. 2007;31:109–118. doi: 10.1177/0148607107031002109. Results of a phase I dose-escalating clinical trial of glutamine, selenium, or both in mechanically ventilated patients with clinical evidence of hypoperfusion. The doses utilized were well tolerated and provided the framework for the ongoing phase III trial examining mortality benefit. [DOI] [PubMed] [Google Scholar]
- 49.Miller JP, Choban PS. Feeding the critically ill obese patient: the role of hypocaloric nutrition support. Respir Care Clin N Am. 2006;12:593–601. doi: 10.1016/j.rcc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Dickerson RN, Boschert KJ, Kudsk KA, Brown RO. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition. 2002;18:241–246. doi: 10.1016/s0899-9007(01)00793-6. [DOI] [PubMed] [Google Scholar]
- 51.Krishnan JA, Parce PB, Martinez A, et al. Caloric intake in the medical ICU patients. Chest. 2003;124:297–305. doi: 10.1378/chest.124.1.297. [DOI] [PubMed] [Google Scholar]
- 52.Ibrahim EH, Mehringer L, Prentice D, et al. Early vs late enteral feeding of mechanically ventilated patients: results of a clinical trial. JPEN J Parenter Enteral Nutr. 2002;26:174–181. doi: 10.1177/0148607102026003174. [DOI] [PubMed] [Google Scholar]
- 53•.Spindler-Vesel A, Bengmark S, Vouk I, et al. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr. 2007;31:119–126. doi: 10.1177/0148607107031002119. A prospective study in trauma patients receiving supplemental synbiotics, prebiotics, glutamine or peptides. No difference in ventilator days, ICU length of stay, or multiple organ failure scores were demonstrated. A reduction in infections was seen in the symbiotic group but only when all other groups were combined. The small sample size of the study limits meaningful conclusions. [DOI] [PubMed] [Google Scholar]
- 54.Ash JL, Gervasio JM, Zaloga GP, Rodman GH. Does the quantity of enteral nutrition affect outcomes in critically ill trauma patients? JPEN J Parenter Enteral Nutr. 2005;29:S10. [Google Scholar]