Abstract
Brain changes in response to nerve damage or cochlear trauma can generate pathological neural activity that is believed to be responsible for many types of chronic pain and tinnitus1–3. Several studies have reported that the severity of chronic pain and tinnitus is correlated with the degree of map reorganization in somatosensory and auditory cortex, respectively1,4. Direct electrical or transcranial magnetic stimulation of sensory cortex can temporarily disrupt these phantom sensations5. However, there is as yet no direct evidence for a causal role of plasticity in the generation of pain or tinnitus. Here we report evidence that reversing the brain changes responsible can eliminate the perceptual impairment in an animal model of noise-induced tinnitus. Exposure to intense noise degrades the frequency tuning of auditory cortex neurons and increases cortical synchronization. Repeatedly pairing tones with brief pulses of vagus nerve stimulation completely eliminated the physiological and behavioural correlates of tinnitus in noise-exposed rats. These improvements persisted for weeks after the end of therapy. This method for restoring neural activity to normal may be applicable to a variety of neurological disorders.
Damage to the peripheral nervous system causes plasticity in multiple regions of the central nervous system. Significant changes have been reported in map organization, spontaneous activity, neural synchronization and stimulus selectivity2. The ideal method of testing whether map plasticity or some other form of plasticity is directly responsible for chronic pain and tinnitus would be to reverse the plasticity and evaluate the perceptual consequence.
Recent attempts to use sensory exposure or discrimination training to reverse the map changes in individuals with tinnitus or chronic pain have provided some temporary relief6,7. Although the clinical benefits were limited, these studies provide some support for the hypothesis that neural plasticity could be used to treat these conditions. It is possible that a long-lasting reversal of the pathological plasticity in these patients would provide significant relief.
Studies in animals have shown that repeatedly pairing sensory stimuli with electrical stimulation of the cholinergic nucleus basalis generates powerful and long-lasting changes in cortical organization8. In principle, this method could be used to reverse the effect of pathological plastic changes that are associated with tinnitus and chronic pain1–3,6. However, nucleus basalis stimulation is highly invasive and, thus, not practical for clinical use. We have developed a less invasive method for generating targeted neural plasticity by pairing vagus nerve stimulation (VNS) with sensory inputs, and have demonstrated a potential clinical application.
VNS triggers the release of neuromodulators known to promote plastic changes. The efficacy of VNS in enhancing plasticity seems to lie in the synergistic action of multiple neuromodulators acting in the cerebral cortex and other brain regions9. VNS improves learning and memory of associated events in rats and humans using identical VNS parameters10.
Our study tests the hypothesis that the pairing of VNS with tones could be used to drive neural plasticity that would reverse the behavioural correlate of tinnitus in noise-exposed rats. The first set of experiments confirms that repeatedly pairing a single tone frequency with VNS is sufficient to generate specific and long-lasting changes in cortical maps. The rationale for our tinnitus therapy is that increasing the number of cortical neurons tuned to frequencies other than the tinnitus frequency ought to reduce the overrepresented tinnitus frequency. The second set of experiments confirms that repeatedly pairing a range of tone frequencies with VNS can be used to reverse the behavioural and neural correlates of tinnitus in noise-exposed rats.
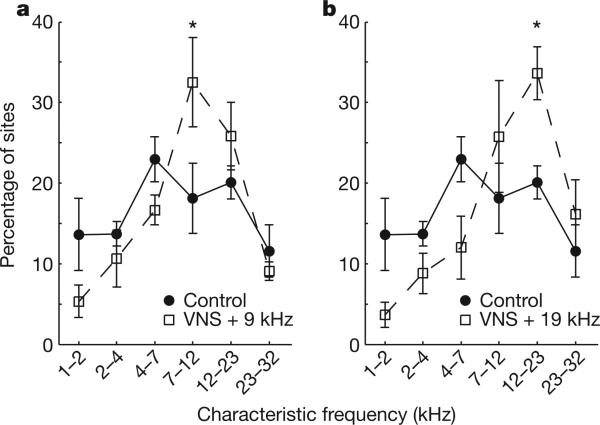
In our first set of experiments, we sought to evaluate whether pairing VNS with tones can generate precise, long-lasting and large-scale changes in the frequency representation in the cortex, as we found for nucleus basalis stimulation. We paired VNS with a 9-kHz, 60-dB SPL tone (n = 8 rats) or a 19-kHz, 50-dB SPL tone (n = 5 rats) for 20 days (SPL, sound pressure level), 300 times per day in normal-hearing rats with cuff electrodes implanted on the left cervical vagus nerve (Methods). The VNS–tone pairing procedure was identical to earlier tone pairing procedures with nucleus basalis, ventral tegmentum or locus coeruleus stimulation that generate long-lasting map plasticity8,11,12. VNS parameters (30 Hz, 0.8 mA) were similar to the parameters used in previous rat and human VNS studies, except that the duration of stimulation and the widths of individual pulses were reduced by 60-fold and fivefold, respectively (Methods and Supplementary Fig. 2). The 0.5 s of VNS used in this study was sufficient to reduce the amplitude of the cortical electroencephalogram briefly (Supplementary Fig. 3 and supplemental data). Twenty-four hours after the last VNS–tone pairing session, we used standard microelectrode mapping techniques to document frequency map plasticity. VNS–tone pairing caused a 70–79% increase in the number of primary auditory cortex (A1) sites with a characteristic frequency near the paired tone frequency (Fig. 1). This result confirms our hypothesis that VNS–tone pairing can be used to direct map plasticity lasting more than 24 h.
Figure 1. VNS–tone pairing causes map plasticity.
Repeatedly pairing VNS with a tone increases the number of A1 recordings sites tuned to the paired frequency. a, VNS was paired with a 9-kHz tone 6,000 times over 20 days in eight rats. b, VNS was paired with a 19-kHz tone in five rats. This group heard 4-kHz tones equally often but without VNS pairing. Asterisks indicate significant (P < 0.05) increases in the fraction of A1 sites with characteristic frequencies near the paired tone. Error bars, s.e.m. This result in normal-hearing rats suggested that VNS–tone pairing might be used to reverse the map distortions induced by exposure to intense noise.
Pairing VNS with sensory stimuli is a potentially attractive method of modifying neural circuits without significant side effects. VNS is well tolerated in the 50,000 patients who currently receive VNS therapy for epilepsy or depression13. By pairing tones with brief trains of VNS, we have been able to alter cortical frequency maps significantly in rats using only 1% of the VNS that is delivered clinically (that is, 30 s every 5 min, 24 h per day) for epilepsy treatment in humans.
Having demonstrated that VNS can be used to generate specific and long-lasting map plasticity, in our second set of studies we sought to evaluate whether VNS-directed plasticity could be adapted to renormalize pathological plasticity and eliminate tinnitus. Exposure to intense, high-frequency noise is known to generate an overrepresentation of mid-frequency tones, degrade frequency selectivity and increase excitability and synchronization of auditory neurons14–16. We induced noise trauma by exposing rats to 1 h of 115-dB SPL, octave-band noise centred at 16 kHz (ref. 17; Methods). Auditory brainstem responses were used to confirm the effects of the noise exposure on hearing threshold, including temporary deafness for frequencies above 8 kHz and a long-lasting increase of auditory brainstem response thresholds and latency18 (Supplementary Figs 4 and 5). After noise exposure, twice as many A1 recording sites were tuned to frequencies between 2 and 4 kHz in comparison with naive controls (35 ± 7% versus 14 ± 2%, P < 0.05), and very few neurons responded to frequencies above 23 kHz (1.7 ± 1% versus 11.5 ± 3%, P < 0.01). The average frequency bandwidth of A1 neurons increased by 21% (1.75 ± 0.04 versus 1.47 ± 0.03 octaves at 10 dB above threshold, P < 0.00001), and the average number of spikes evoked by a tone within each site's receptive field increased by 30% (4.3 ± 0.1 versus 3.3 ± 0.1, P < 0.00001). The average spontaneous rate increased by 23% (17.7 ± 0.6 versus 14.3 ± 0.4 Hz, P < 0.00001). The degree of synchronization during silence measured using the correlation coefficient between multiunit activity recorded at nearby sites was significantly increased (1.7 ± 0.01 versus 0.19 ± 0.01 synchronous spikes per second of silence, P < 0.05; Methods). These changes in frequency tuning and synchronization are similar to the physiological changes observed after noise exposure that have been proposed to be directly responsible for tinnitus2,19. Earlier studies using several different methods have documented that noise exposure can generate behavioural correlates of tinnitus near the low-frequency edge of the noise trauma17,20–22. However, few studies have directly compared neurophysiology and behavioural observations from the same animals20,23. It was therefore of great interest to us to relate noise-induced plasticity to perceptual disturbances.
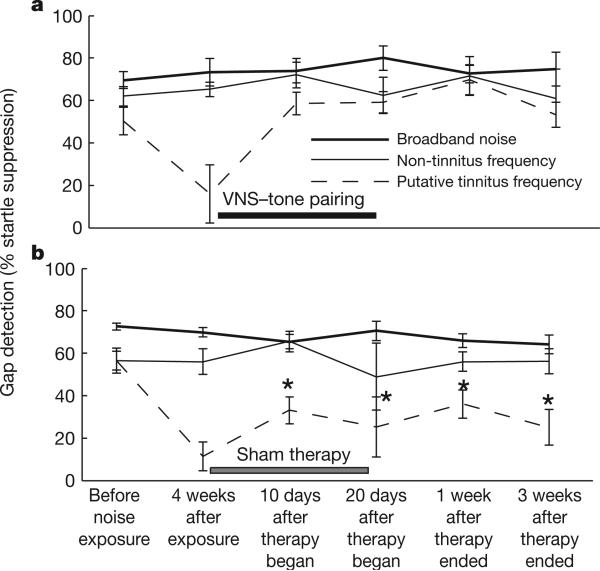
Each of the eighteen noise-exposed rats used in this study was significantly impaired in its ability to detect a gap in narrowband noise centred on 8 or 10 kHz, but showed no impairment when the gap occurred in narrowband noise centred on 2 or 4 kHz or in broadband noise (Fig. 2, 4 weeks after exposure). Several studies have concluded that a frequency-specific impairment in gap detection is a likely sign that noise-exposed rats experience a mid-frequency tinnitus percept which fills the silent gaps17,23 (Methods and Supplementary Figs 6–9). Although it is not possible to evaluate the subjective experience of rats definitively, the gap impairment has been taken as a possible behavioural correlate of tinnitus.
Figure 2. VNS/multiple tone pairing eliminates the behavioural correlate of tinnitus.
Four weeks after noise exposure, each of the rats in both groups was unable to detect a gap in one or more of the narrowband noises tested (P > 0.05; Supplementary Fig. 8b). The frequency with the greatest impairment four weeks after noise exposure is the putative tinnitus frequency for each rat. For both groups, gap detection at the putative tinnitus frequency was significantly impaired in comparison to broadband noise (P < 0.05). The gap detection at the non-tinnitus frequency is based on gap detection in 16-kHz narrowband noise. a, Gap detection at the putative tinnitus frequency (dotted line) improved significantly after ten days of VNS–tone pairing, and the improvement persisted at least until the acute physiology experiment (n = 5 rats). b, The sham group (n = 9 rats) continued to be impaired. Two sham rats did not contribute data at the non-tinnitus frequency because they showed gap impairments at 16 kHz (as well as 8 and 10 kHz) four weeks after noise exposure. Black and grey horizontal bars represent duration of VNS and sham therapy, respectively. Asterisks represent significant differences (P < 0.05) in gap detection at the putative tinnitus frequency between VNS therapy and sham therapy rats. Error bars, s.e.m.
Map distortion and tuning curve broadening (but not changes in spontaneous activity or synchronization) were significantly correlated with the degree of gap impairment in untreated noise-exposed rats (R > 0.7 (Pearson correlation coefficient), P < 0.05, n = 8 sham rats; Figs 3a, b and 4a–d and Supplementary Fig. 13). These correlations must be interpreted with caution because any variability in the initial cochlear trauma could generate a correlation between neural and behavioural changes even in the absence of a causal relationship. Though still not definitive, the best test for a causal relationship would be to reverse specifically the plasticity generated by noise exposure and document the reversal of the gap detection impairment.
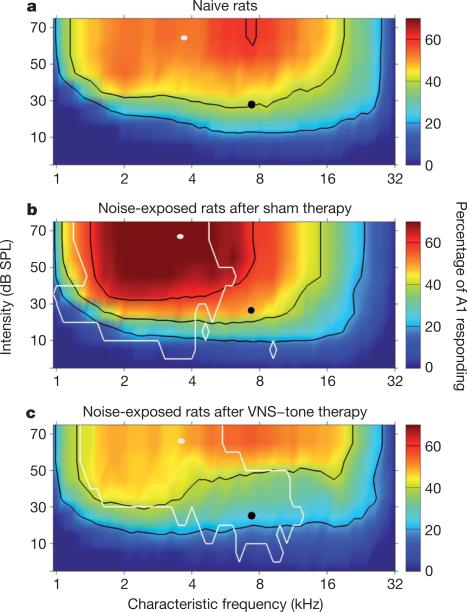
Figure 3. VNS/multiple tone pairing reverses map distortion.
The increased response of A1 neurons to tones following noise exposure is reversed by VNS/multiple tone pairing. a, Colour indicates the percentage of A1 neurons in naive rats that respond to a tone of any frequency and intensity combination. b, Percentage of A1 neurons that respond to each tone in noise-exposed rats that received sham therapy. c, Percentage of A1 neurons that respond to each tone in noise-exposed rats that received the VNS/multiple tone therapy. Black contour linesindicate 20, 40, and 60% responses. The white linesin b surround theregions of tones that are significantly increased (P < 0.01) in comparison with naive rats. The white lines in c indicate significant decreases (P < 0.01) in comparison with noise-exposed sham therapy rats. The filled white circles indicate the tone for which the increase in the number of cortical neurons was greatest, which is used to quantify the degree of map distortion in Fig. 4a, b. The filled black circles indicate the tone for which the proportional increase was greatest.
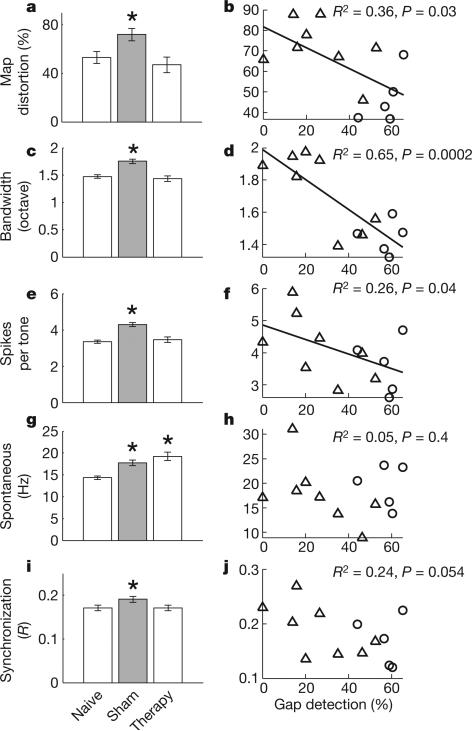
Figure 4. Neurophysiological properties of naive, sham and therapy rats.
a, c, e, g, i, Noise exposure caused a significant map distortion (a), decreased frequency selectivity (c), increased the tone-evoked response (e), increased the spontaneous rate (g) and increased the degree of cortical synchronization (i). VNS/multiple tone pairing returned each of these parameters, except spontaneous activity, to normal levels. b,d,f, Map organization (b), frequency selectivity (d) and tone-evoked response strength (f) were all correlated with the degree of gap impairment in individual rats. h, j, Spontaneous activity (h) and synchronization (j) were not significantly correlated with gap impairment. Each rat's gap detection ability was quantified as the average gap detection at the putative tinnitus frequency of each rat, averaged across the four time points collected after the beginning of therapy (Fig. 2). Error bars, s.e.m. Asterisks represent significant differences compared with naive rats (P values as indicated). Triangles and circles represent rats from the sham and therapy groups, respectively.
We speculated that pairing VNS with randomly interleaved pure tones that span the rat hearing range, but exclude the overrepresented frequencies, could decrease the cortical representation of the excluded frequencies24. We also expected that pairing multiple tone frequencies with VNS (‘VNS/multiple tone’ pairing) would increase frequency selectivity and decrease synchronization as in our earlier nucleus basalis stimulation experiments25. We quantified behavioural and physiological correlates of tinnitus in noise-exposed rats and then tested whether pairing VNS with multiple tone frequencies could reverse the pathological plasticity and eliminate the perceptual disturbance in these rats.
VNS was repeatedly paired with multiple pure tones 300 times per day for 18 days in seven noise-exposed rats with impaired gap detection for mid-frequency sounds (Methods). Because we found that gap impairment occurred at 8–10 kHz, we selected the frequency of each randomly interleaved tone to be 1.3, 2.2, 3.7, 17.8 or 29.9 kHz. This pairing procedure was chosen because previous studies suggest it would reduce the cortical response to mid-frequency tones, increase frequency selectivity and decrease cortical synchronization2,25. After ten days of therapy, each of the seven rats showed a significant startle reduction in cued trials relative to uncued trials for every frequency tested (P < 0.05; Fig. 2a and Supplementary Fig. 9a). Thus, pairing of VNS with multiple tones reversed the behavioural effect of noise exposure, which suggests that the rats’ presumed tinnitus was no longer present. In contrast, rats in the sham therapy group showed a consistent impairment in their ability to detect gaps in the putative tinnitus frequency (Fig. 2b). Each of the nine rats that received sham therapy (tones with no VNS, VNS with no tones or no therapy; n = 4, 2, 3 rats, respectively) did not show a significant startle reduction in cued trials (P > 0.05; Supplementary Fig. 9b) for at least one of the frequencies tested at each time point.
In the rats that received VNS paired with multiple tones, the impairment in gap detection was also eliminated when measured one day, one week and three weeks after the end of the therapy. This impairment was maintained in all three control groups at every time point tested (Supplementary Figs 9 and 10). These results indicate that pairing VNS with multiple tone frequencies is sufficient to eliminate the gap impairment induced by noise exposure (Supplementary Fig. 11). This is the first method reported to generate a long-lasting reversal of a behavioural correlate of chronic tinnitus.
Three weeks after the end of VNS/multiple tone pairing or sham therapy, we evaluated the physiological properties of the auditory cortex of each rat to determine whether the restored behaviour in the treated group was due to renormalization of the auditory cortex. After VNS/multiple tone pairing, most of the A1 properties that were degraded by noise exposure returned to normal levels. For example, the proportion of A1 neurons with characteristic frequencies between 12 and 23 kHz was indistinguishable from that in naive controls after VNS/multiple tone treatment (naive, 20 ± 2%; sham, 15 ± 5%; therapy, 30 ± 9%; Supplementary Figs 12 and 13a). The proportion of A1 neurons responding to 4-kHz, 70-dB SPL tones significantly increased relative to naive controls in sham rats and returned to normal levels in rats that had received the therapy three weeks earlier (naive, 45.4 ± 5.0%; sham, 74.1 ± 7.6%; therapy, 49.1 ± 6.6%; Figs 3 (white circles) and 4a). The degree of low-frequency map distortion was positively correlated with the degree of gap impairment observed in individual rats (Fig. 4b and Supplementary Fig. 13b). The percentage of cortex responding to 8-kHz, 30-dB SPL tones (Fig. 3, black circles) was also well correlated with the gap detection impairment (R2 = 0.51, P = 0.006). These results support the earlier hypothesis that changes in cortical maps are causally related to tinnitus4,26.
VNS/multiple tone pairing reversed the increase in the width of frequency tuning of A1 multiunit activity (that is, decreased frequency selectivity) observed in noise-exposed rats (Fig. 4c). The bandwidth (measured at 10, 20, 30 or 40 dB above threshold) averaged across all A1 sites was highly correlated with the degree of gap impairment (Fig. 4d and Supplementary Fig. 14), thus supporting the earlier hypothesis that decreased frequency selectivity is causally related to tinnitus27.
VNS/multiple tone pairing reversed the increase in cortical excitability observed in noise-exposed rats (Fig. 4e). The average number of spikes evoked by tones within each site's receptive field was weakly correlated with the degree of impairment of gap detection (Fig. 4f), supporting the earlier hypothesis that tinnitus is related to increased excitability of cortical neurons28,29.
Finally, VNS/multiple tone pairing also reversed the increase in cortical synchronization observed in noise-exposed rats, but did not reverse the increase in cortical spontaneous activity observed in noise-exposed rats (Fig. 4g, i). There was a trend for the degree of synchronization to be correlated with the degree of gap impairment, but no correlation between the rate of spontaneous activity and the degree of gap impairment (Fig. 4h, j). Our observation that noise-induced increases in spontaneous activity and synchronization are not significantly correlated with behavioural correlates of tinnitus in individual rats is consistent with earlier reports19,23. However, given the potential for small changes in anaesthesia level to influence spontaneous activity and synchronization in the cortex, it remains a possibility that these factors contribute to tinnitus.
Hearing loss, hyperacusis and tinnitus often result from noise exposure and could contribute to the gap impairments observed in this study. Our results confirm that exposure to intense, high-frequency noise causes pathological plasticity that is well correlated with the inability to detect a gap in a mid-frequency, 65-dB SPL tone. Correlations alone do not suggest that these changes cause tinnitus because another confounding factor (such as variability in the degree of cochlear trauma) could cause both variables to be correlated without a causal connection. By randomizing the treatment of rats with identical noise exposure, we were able to eliminate the potential confound caused by variability in the response to noise exposure. Thus, our observation that pairing multiple tone frequencies with VNS can reverse both the neural and behavioural correlates of tinnitus provides good evidence that abnormal activity in the central auditory system is responsible for the subjective experience of tinnitus. In addition, neural correlates of hearing loss (tone thresholds) and hyperacusis (rate level functions) were not correlated with gap impairment in the rats tested (Supplementary Information). Thus, it is reasonable to conclude that the gap impairments observed in this study are primarily related to tinnitus.
VNS-directed plasticity represents a potentially powerful approach to treating tinnitus. Unlike pharmaceutical approaches, this method provides the possibility of generating long-lasting and stimulus-specific changes to neural circuits with minimal side effects. Our control experiments demonstrate that VNS-directed plasticity is driven by the repeated association of VNS with tones, and not by VNS alone. Additional studies are needed to determine whether the pairing of other sensory events with brief periods of VNS could be used to reverse the pathological plasticity associated with other common neurological conditions, such as chronic pain and amblyopia.
METHODS SUMMARY
The VNS–tone pairing protocols, noise exposure procedure, gap detection testing, neurophysiology techniques and analysis are described in Methods. The noise exposure procedure, gap detection testing, and neurophysiology techniques were identical to those in earlier reports8,17,25.
Supplementary Material
Acknowledgements
We would like to thank A. Kuzu, J. Omana, D. Vuppala, H. Rasul, M. Fink, E. Hanacik, R. Miller and C. Walker for help with rat behavioural training. We would also like to thank J. Eggermont, A. Møller, C. Bauer, J. Fritz, H. Reed, C. Engineer, A. Reed, M. Brosch, R. Rennaker, R. Beitel, V. Miller, C. McIntyre, G. White, P. Pandya, R. Tyler and D. deRidder for suggestions about earlier versions of the manuscript. This work was supported by the James S. McDonnell Foundation, the Texas Advanced Research Program, the National Institute for Deafness and other Communication Disorders, and MicroTransponder Inc.
METHODS
VNS surgical protocol
Female Sprague–Dawley rats (250–350 g) were implanted with a platinum–iridium bipolar cuff electrode around the leftcervicalvagus nerve9. As in humans, only the left vagus nerve was stimulated because the right vagus nerve contains efferents that stimulate the sinoatrial node and can cause cardiac complications13. Leads from the electrode were tunnelled subcutaneously to the top of the head. A four-channel connector was used to deliver current to the stimulating electrode and monitor the electroencephalogram (EEG) during daily VNS sessions. Bone screws placed over the vertex and the cerebellum were used to record auditory brainstem responses (ABRs) and EEG. Each rat was given antibiotics to prevent infection and a single dose of atropine and dexamethazone to reduce fluid accumulations in the lungs immediately after completion of the surgery.
VNS stimulation parameters and single-tone pairing procedures
VNS was delivered to unanaesthetized, unrestrained rats in a 25 × 25 × 25 cm3 wire cage, located inside a 50 × 60 × 70 cm3 chamber lined with acoustic insulating foam. A pilot study was conducted to determine the minimal VNS parameters that reliably reduced EEG amplitude during slow-wave sleep (Supplementary Fig. 3). VNS parameters were identical for every rat in this study. Each 100-μs, charge-balanced biphasic pulse was delivered with a current of 0.8 mA. The stimulation was delivered as a train of 15 pulses at 30 Hz (500-ms train duration). Cuff impedances were measured daily (~5 kΩ). The impedance for three rats was unusually high after implantation and these rats were assigned to the tone-alone and no-therapy groups. The impedance was stable across the duration of training for all other rats. The 500-ms pure tones began 150 ms after the onset of the VNS train (Supplementary Figs 1 and 2). For our earlier nucleus basalis stimulation studies, stimulation beginning either 200 ms before tone onset or 50 ms after tone onset generated indistinguishable map plasticity8.
VNS was delivered 300 times per day for 20 days, during a VNS–tone pairing session that lasted 2.5 h (Supplementary Figs 2). To prevent rats from anticipating stimulation timing, there was a 50% chance that VNS would be delivered every 15 s. Twenty-four hours after the last pairing, rats were anaesthetized with pentobarbital and the right auditory cortex was exposed to allow for high-density extracellular microelectrode mapping8.
One group of rats (n = 8) was exposed to a single 9-kHz, 60-dB SPL tone paired with VNS. No sound was presented when VNS was not delivered. A second group (n = 5) was exposed to a 19-kHz, 50-dB SPL tone paired with VNS. During the trials in which no VNS was delivered (50%), a 4-kHz, 50-dB SPL tone was presented. As a result, a 19- or 4-kHz tone was delivered every 15 s. Frequency and intensity calibrations were performed with an ACO Pacific microphone (PS9200-7016) and Tucker-Davis Technologies SIGCAL v4.2 software. The free-field tones were presented from a speaker (Optimus) suspended 20 cm above the wire cage. All paired tones had a 5-ms rise–fall time. The intensity of every tone was selected to be approximately 20 dB SPL above the rat hearing threshold.
Noise exposure and ABRs
Twenty-eight experimental and control rats were barbiturate-anaesthetized and exposed to 16-kHz, 115-dB SPL, octave-band noise for 1 h (refs 17, 20). A single speaker was positioned 5 cm from the left ear. No ear plugs were used to restrict the noise exposure to one ear. Bilateral noise exposure was used because it best approximates the noise exposure that occurs in humans. To confirm cochlear trauma, elevated thresholds were quantified using ABRs in ten ratsunder pentobarbital anaesthesia before noise exposure, immediately after exposure and 11 weeks after noise exposure (when the auditory cortex was mapped). For ABR recordings, the speaker was positioned 10 cm from the left ear and pure tones (10 ms long, 2.5-ms rise–fall time) were delivered at a rate of 20 Hz. Tone frequencies were 4, 10, 16 and 32 kHz in 10-dB steps from 0 to 85 dB SPL. Tones were randomly interleaved with 1,500 repeats for each frequency–intensity combination. The signals were filtered from 100 to 3,000 Hz and recorded using BRAINWARE v8.12 (Tucker-Davis Technologies). Threshold was defined as the lowest 10-dB SPL step at which an ABR could be recognized (Supplementary Fig. 4).
Gap detection testing
The Turner gap detection method was used to assess a behavioural correlate of tinnitus in every noise-exposed rat17 (Supplementary Figs 6–8). This method has previously been cross-validated with a conditioned lever suppression task20 (R = 0.75) and a licking suppression task21. The gap detection method was selected because it avoids the need for food or water deprivation, electric shock or months of behavioural training17. Testing took place in a 20 × 20 × 20 cm3 wire-mesh cage in a 67 × 67 × 67 cm3 chamber lined with 5-cm acoustic foam. The cage was placed on a startle platform (Lafayette Instrument Co.) that used a piezoelectric transducer to generate a continuous record of downward force. Sounds were generated using System 3 hardware and software (Tucker-Davis Technologies) and were delivered by a speaker (Tucker-Davis Technologies FF1) mounted 20 cm above the cage. Rats underwent gap detection testing with different band-pass-filtered (1,000-Hz bandwidth) sounds centred at 2, 4, 8, 10, 16, 20 and 24 kHz at 65 dB SPL (ref. 17). Startle responses were elicited by a 20-ms burst of white noise at 100 dB SPL. In 50% of trials, a 50-ms gap embedded in the continuous sound served as a warning of a subsequent startling noise and allowed rats to reduce the amplitude of the response (Supplementary Fig. 7b). The gap in the narrowband noise began 100 ms before the onset of the broadband startling noise. Rats underwent 30 trials during each session. The order of sessions with different continuous sounds was counterbalanced across rats. The interval between each startle sound was 30–35 s.
In untreated noise-exposed rats, gaps in a specific narrowband sound (usually 8 or 10 kHz) did not serve as an effective warning, presumably because the ongoing tinnitus percept prevented the rats from detecting the silent gap. Thus, the animals were not warned that a loud startling noise was coming and exhibited a strong startle response (Supplementary Figs 7b and 8b). Gap detection was quantified as one minus the ratio of the startle amplitude when the startling noise was preceded by a gap in the 65-dB SPL, continuous narrowband sound to the startle amplitude when the startling noise was not preceded by a warning gap. Supplementary Fig. 8 shows typical data from one noise-exposed rat for a session in which the noise burst was cued with a gap in broadband noise (left) and a session in which a gap in an 8-kHz tone served as the warning cue (right). The warning gap typically reduced the startle amplitude by 60–70% (Supplementary Fig. 8a). In noise-exposed rats, gaps in the narrowband noise centred near the low edge of the trauma noise typically reduced the startle amplitude by less than 20%, which is not a statistically significant reduction (Supplementary Fig. 8b). The same procedure was also administered using gaps in 65-dB SPL broadband noise as warning cues of the startling noise (Supplementary Fig. 7a). The frequency with the greatest impairment four weeks after noise exposure is the putative tinnitus frequency for each rat (Fig. 2).
Thirty-six rats were initially tested using the gap startle task for inclusion in this study. Five rats were excluded from the study because they showed no detectable startle response to the noise burst. Of the 31 remaining rats, three were excluded because their startle responses were unusually variable. Twenty-eight rats received noise exposure. Eighteen of these showed a statistically significant impairment in the detection of gaps in one or both mid-frequency (8- or 10-kHz) narrowband sounds tested, relative to gap detection before noise exposure (P < 0.05). Three rats were excluded from further study because they no longer showed a startle response to the noise burst (that is, could no longer detect the startle stimulus). Seven rats were excluded from further study because they showed no impairment in gap detection (that is, no evidence of tinnitus). Our observation that gap impairments do not always result from noise exposure is consistent with human and animal studies showing that although hearing loss is common in individuals with tinnitus, the majority of individuals with hearing loss do not have tinnitus20,30,31.
Each of the eighteen rats included in this study showed a significant impairment in its ability to detect a gap in narrowband noise centred on 8 kHz (16 of 18) or 10 kHz (12 of 18). None of the 18 rats showed a significant impairment in the ability to detect a gap in low-frequency narrowband noises (2 or 4 kHz) or in broadband noise (Fig. 2 and Supplementary Fig. 11). This result indicates that these rats are able to respond normally to the startling noise burst and that the mechanisms for modulating the startle response using silent gaps remain intact. Our observation that noise-exposed rats can show gap detection impairments centred at a single frequency or across a narrow range of frequencies is consistent with clinical studies showing significant heterogeneity across subjects in the spectral characteristics of the tinnitus percept22,32,33. Despite this heterogeneity, a large fraction of tinnitus patients can match their tinnitus to a pitch and describe their phantom sound as tonal22.
VNS tone delivery to noise exposed rats
Rats were tested for gap impairment four weeks after noise exposure and 10 and 20 days after the beginning of the sham or experimental therapy. In the VNS/multiple tone paired group (n = 5 rats), tones were paired with VNS every 15 s with no VNS–tone pairing 50% of the time. The tone frequencies paired with VNS in the therapy group were designed to reduce the 8–10-kHz region of the frequency map. VNS was repeatedly paired with a 1.3-, 2.2-, 3.7-, 17.8- or 29.9-kHz tone that was randomly selected every trial (300 trials per day). Each tone was presented at ~20 dB above the normal hearing threshold for that frequency. The tone-alone control group was passively exposed to the same tones on the same schedule as used in the paired group. A VNS-alone control group received VNS stimulation on the same schedule as used in the paired group without presentation of tones. The third control group did not receive tones or VNS.
To test whether the tinnitus percept remained suppressed after the end of VNS–tone pairing, rats were also tested on gap detection one and three weeks after the end of therapy. At the end of three weeks (that is, 11 weeks after noise exposure), multiunit responses were recorded from auditory cortex neurons from the therapy, sham and naive control rats using dense microelectrode mapping techniques. Physiological and behavioural results from the tone-alone, VNS-alone and notherapy groups were statistically indistinguishable (Supplementary Fig. 10 and physiological data not shown). Data from the three groups are combined and referred to as sham controls in the main text.
Neurophysiology
In this study, we recorded from a total of 1,492 sites in 21 rats (n = 8 naive controls, n = 5 VNS therapy and n = 8 sham controls). Nine hundred and sixty-five of those sites were in A1 and were included in the analysis presented in this report. We recorded 220 multiunit responses from A1 sites in noise-exposed rats that received VNS/multiple tone pairing (n = 5). We also recorded 321 A1 sites from noise-exposed rats that did not receive VNS/tone pairing (n = 8). The latter group included noise-exposed rats that received tones with no VNS (n = 3), VNS with no tones (n = 2) or no therapy (n = 3). Because neural and behavioural responses were similar in all three control groups, the results were pooled to form a single data set referred to as the sham therapy group. During the acute electrophysiology recordings, sounds were delivered in a foam-lined, double-walled, sound-attenuated chamber using a speaker (Motorola 40-1221) positioned directly opposite the left ear at a distance of 10 cm. Multiunit responses were recorded using Parylene-coated tungsten electrodes that were glued together (250-μm separation, 2 MΩ at 1 kHz; FHC) and lowered approximately 500 μm below the cortical surface. Frequency and intensity calibrations were performed with an ACO Pacific microphone (PS9200-7016) and Tucker-Davis Technologies SIGCAL v4.2 software. Auditory frequency tuning curves were determined at each site by presenting 81 logarithmically spaced frequencies spanning 1 to 32 kHz at 16 intensities from 0 to 75 dB SPL (1,296 total stimuli). The tones (25-ms duration, 5-ms rise–fall time) were randomly interleaved and separated by 500 ms. Tuning curve parameters were determined by an experienced blind observer using custom software written in MATLAB v7.9 (Mathworks) to randomize the order of data from each recording site across all groups. Experimenters were blind to the experimental conditions of each rat during electrophysiology recordings.
Data analysis
Gap discrimination was quantified as the percentage inhibition of the startle response when a gap (warning cue) preceded the starling noise relative to the startle response when no gap was present17. Eight of 36 rats tested failed to generate consistent startle responses and were excluded from the study before noise exposure. Noise exposure eliminated the startle response in three of the remaining 28 rats, and these rats were excluded from the study. Noise exposure failed to generate any impairment in gap detection in seven of the remaining 25 rats, and these rats were also excluded from the study. Eighteen noise-exposed rats were included in the study. Neural responses were collected from thirteen rats (five VNS–tone paired rats and eight sham therapy rats). One rat died before neural responses could be collected. Only behavioural responses (and EEG) were collected from the remaining four rats (two treated rats and two shams) so that the duration of the benefit could be estimated.
Sites were determined to be in A1 on the basis of continuous tonotopy. At each A1 recording site, characteristic frequency, frequency bandwidth, response threshold, spontaneous rate and latency were determined using a standard method in which the experimenter was blind to the experimental group and recording location8. At each pair of simultaneously recorded A1 sites, neural synchrony during silence (300 s) was quantified as the cross-correlation function25. The peak in the cross-correlation function (with or without subtraction of the shift predictor) was also computed and gave similar results to Pearson correlation coefficient (R). Map plasticity was quantified as the percent of A1 neurons with a characteristic frequency in a given range or as the percent of A1 neurons responding toeach frequency–intensity combination using the Voronoi tessellation method of interpolation8,34. Frequency selectivity was quantified as the bandwidth 10, 20, 30 or 40 dB above threshold. Results were similar regardless of the intensity above threshold used. Excitability was quantified as the number of spikes evoked by each tone within each site's receptive field and as the spontaneous activity rate during silence.
All protocols and recording procedures comply with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Texas at Dallas.
Footnotes
Author Contributions N.D.E., J.R.R., J.D.S., S.P.S. and M.S.B. did the behaviour training sessions, noise exposure and auditory brainstem response recordings. N.D.E., J.R.R., J.D.S., W.A.V. and J.A.S. did cortical microelectrode mappings. J.A.S. did the A1 mapping surgeries. S.P.S. and N.D.E. did all the VNS implant surgeries. M.P.K. and N.D.E. designed the experiments, wrote the manuscript and performed data analysis. All authors discussed the paper and commented on the manuscript.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare competing financial interests: details accompany the full-text HTML version of the paper at www.nature.com/nature. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Flor H. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 2.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Møller AR. Tinnitus and pain. Prog. Brain Res. 2007;166:47–53. doi: 10.1016/S0079-6123(07)66004-X. [DOI] [PubMed] [Google Scholar]
- 4.Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc. Natl Acad. Sci. USA. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Ridder D, De Mulder D, Menovsky T, Sunaert S, Kovacs S. Electrical stimulation of auditory and somatosensory cortices for treatment of tinnitus and pain. Prog. Brain Res. 2007;166:377–388. doi: 10.1016/S0079-6123(07)66036-1. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto H, Stracke H, Stoll W, Pantev C. Listening to tailor-made notched music reduces tinnitus loudness and tinnitus-related auditory cortex activity. Proc. Natl Acad. Sci. USA. 2010;107:1207–1210. doi: 10.1073/pnas.0911268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flor H, Denke C, Schaefer M, Grüsser S. Effect of sensory discrimination training on cortical reorganization and phantom limb pain. Lancet. 2001;357:1763–1764. doi: 10.1016/S0140-6736(00)04890-X. [DOI] [PubMed] [Google Scholar]
- 8.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 9.Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J. Pharmacol. Exp. Ther. 2006;318:890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- 10.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nature Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 11.Bao S, Chan VT, Merzenich NM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 12.Bollinger JJ. PhD thesis. Univ. California San Francisco; 2006. Adult Auditory Cortical Plasticity Modulated by Locus Coeruleus Activity. [Google Scholar]
- 13.Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J. Clin. Neurophysiol. 2001;18:415–418. doi: 10.1097/00004691-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Noreña AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J. Neurophysiol. 2003;90:2387–2401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- 15.Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear. Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- 16.Eggermont J. In: Tinnitus: Pathophysiology and Treatment. Langguth B, Hajak G, Kleinjung T, Cacace A, Møller AR, editors. Elsevier; 2007. pp. 19–35. Prog. Brain Res. 166. [Google Scholar]
- 17.Turner JG, et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav. Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 18.Murphy WJ, van Campen LE. Temporary threshold shift in ABRs and DPOAEs following noise exposure in Long–Evans rats. J. Acoust. Soc. Am. 2001;109:2373. [Google Scholar]
- 19.Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J. Neurosci. Res. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J. Assoc. Res. Otolaryngol. 2001;2:54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC). Hear. Res. 2004;190:109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- 22.Moore BC, Sandhya V. The relationship between tinnitus pitch and the edge frequency of the audiogram in individuals with hearing impairment and tonal tinnitus. Hear. Res. 2010;261:51–56. doi: 10.1016/j.heares.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Yang G, et al. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear. Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Noreña AJ, Eggermont JJ. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J. Neurosci. 2005;25:699–705. doi: 10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilgard MP, Vazquez JL, Engineer ND, Pandya PK. Experience dependent plasticity alters cortical synchronization. Hear. Res. 2007;229:171–79. doi: 10.1016/j.heares.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietrich V, Nieschalk M, Stoll W, Rajan R, Pantev C. Cortical reorganization in patients with high frequency cochlear hearing loss. Hear. Res. 2001;158:95–101. doi: 10.1016/s0378-5955(01)00282-9. [DOI] [PubMed] [Google Scholar]
- 27.Dauman R, Cazals Y. Auditory frequency selectivity and tinnitus. Arch. Otorhinolaryngol. 1989;246:252–255. doi: 10.1007/BF00463566. [DOI] [PubMed] [Google Scholar]
- 28.Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear. Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 29.Diesch E, Andermann M, Flor H, Rupp A. Interaction among the components of multiple auditory steady-state responses: enhancement in tinnitus patients, inhibition in controls. Neuroscience. 2010;167:540–553. doi: 10.1016/j.neuroscience.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J. Neurosci. Res. 2007;85:1489–1498. doi: 10.1002/jnr.21259. [DOI] [PubMed] [Google Scholar]
- 31.König O, Schaette R, Kempter R, Gross M. Course of hearing loss and occurrence of tinnitus. Hear. Res. 2006;221:59–64. doi: 10.1016/j.heares.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Burns EM. A comparison of variability among measurements of subjective tinnitus and objective stimuli. Audiology. 1984;23:426–440. doi: 10.3109/00206098409081535. [DOI] [PubMed] [Google Scholar]
- 33.Ochi K, Ohashi T, Kenmochi M. Hearing impairment and tinnitus pitch in patients with unilateral tinnitus: comparison of sudden hearing loss and chronic tinnitus. Laryngoscope. 2003;113:427–431. doi: 10.1097/00005537-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Engineer ND, et al. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J. Neurophysiol. 2004;92:73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.