Abstract
Scope
Resveratrol (3,5,4′-trihydroxystilbene) is a phytoalexin shown to possess a multitude of health-promoting properties in pre-clinical studies. However, the poor in vivo bioavailability of resveratrol due to its rapid metabolism is being considered as a major obstacle in translating its effects in humans. In this study, we examined the hypothesis that piperine will enhance the pharmacokinetic parameters of resveratrol via inhibiting its glucuronidation, thereby slowing its elimination.
Methods and results
Employing a standardized LC/MS assay, we determined the effect of piperine co-administration with resveratrol on serum levels resveratrol and resveratrol-3-O-β-d-glucuronide in C57BL mice. Mice were administered resveratrol (100 mg/kg; oral gavage) or resveratrol (100 mg/kg; oral gavage) + piperine (10 mg/kg; oral gavage), and the serum levels of resveratrol and resveratrol-3-O-β-d-glucuronide were analyzed at different times. We found that the degree of exposure (i.e. AUC) to resveratrol was enhanced to 229% and the maximum serum concentration (Cmax) was increased to 1544% with the addition of piperine.
Conclusion
Our study demonstrated that piperine significantly improves the in vivo bioavailability of resveratrol. However, further detailed research is needed to study the mechanism of improved bioavailability of resveratrol via its combination with piperine as well as its effect on resveratrol metabolism.
Keywords: Absorption, Bioavailability, Pharmacokinetics, Piperine, Resveratrol
1 Introduction
Resveratrol (3,5,4′-trihydroxystilbene; Fig. 1) is a naturally occurring phytoalexin (chemicals produced naturally by plants when under attack by pathogens) found in grapes, red wine, berries, peanuts and many other plants and has been shown to possess a multitude of health-promoting properties [1–5]. The phenomenon of “French Paradox”, the observation that French people suffer a relatively low incidence of coronary heart disease (despite having a diet relatively rich in saturated fats), has been attributed to the consumption of red wine that contains resveratrol [6]. Historically, the medicinal use of resveratrol containing herbs has been known for a long time. For example, the root of the Polygonum cuspidatum that contains resveratrol has been used in traditional Japanese, Chinese, and Korean medicine to prevent or remedy dermatitis, favus, hyperlipidemia, and bacterial infections [7, 8]. In addition, dietary supplements containing resveratrol often use P. cuspidatum standardized to resveratrol. These dietary supplements are being widely marketed and used for a variety of health-related conditions. Thus, resveratrol is probably the most common phytoalexin that has been used in traditional medicine as well as being investigated for its possible use in modern health care.
Figure 1.
Structures of resveratrol, resveratrol-3-O-β-d-glucuronide, piperine, and internal standard naproxen.
Naturally occurring resveratrol is found in cis- and trans-chemical configurations; however, trans-configuration is the major configuration and also represents the most thoroughly studied chemical form. In mice, resveratrol has been shown to be well tolerated at doses as high as 5000 mg/kg/day, for 28 consecutive days; however, high doses were associated with mortality due to the impaction of resveratrol in the gastrointestinal tract [9]. In healthy human volunteers, resveratrol has been administered at doses as high as 5 g as a single dose or at 5 g daily for up to 28 days in two different studies [10, 11]. Although peak serum resveratrol concentrations from a single 5-g dose is below the resveratrol concentrations used and found effective in in vitro studies, this dose was shown to be well tolerated with no serious adverse events. In healthy human volunteers, 5 g of resveratrol with a meal achieved a peak plasma concentration of 539 ng/mL occurring 1.5 h post-dosing [10]. Interestingly, peak levels of two monoglucuronides and resveratrol-3-sulfate were three- to eightfold higher than resveratrol. Pharmacokinetic studies in animals and humans have identified two attributes of resveratrol. First, the majority of trans-resveratrol metabolites were found to be derived by glucuronidation or sulfation, with at least seven different identified forms [12]. Second, the metabolism of resveratrol has been shown to be very rapid with the majority of biotransformation occurring within the first hour following oral administration. Resveratrol metabolites have very short half-life and are subjected to rapid urinary elimination. A few clinical studies have suggested that the time to maximum serum concentration (Tmax) of resveratrol could be impacted and delayed by certain types of foods [13, 14]. However, combining resveratrol with these foods did not significantly increase the absorption of resveratrol. The rapid metabolism and poor pharmacokinetic parameters of resveratrol has represented to be major obstacles in translating its observed therapeutic effect in pre-clinical studies to controlled clinical settings in humans [7].
Some studies have shown that piperine, an alkaloid derived from black pepper (Piper spp.), inhibits glucuronidation [15–17]. In this study, we examined the hypothesis that piperine will enhance the in vivo bioavailability of resveratrol via inhibiting its glucuronidation, thereby slowing its metabolism.
2 Materials and methods
2.1 Chemicals and reagents
Trans-Resveratrol (purity >95%) and piperine (purity >96.6%) were provided by Sabinsa (East Windsor, NJ, USA). Resveratrol-3-O-β-d-glucuronide (purity 98%) was obtained from Toronto Research Chemicals (North York, ON, Canada). Naproxen (purity >98.5%) meeting United States Pharmacopeia (USP) testing specifications was used as an internal standard and purchased from Sigma (St. Louis, MO, USA). Chemical structures of resveratrol, resveratrol-3-O-β-d-glucuronide, piperine, and naproxen is shown in Fig. 1. Liquid chromatography-mass spectrometry (LC-MS) grade solvents (water; CH3CN, ACN; CH3OH, methanol; (CH3)2CHOH, isopropanol; acetic acid, CH3COOH; ammonium hydroxide, NH4OH; and ammonium bicarbonate, NH4HCO3) were obtained from Fisher Scientific (Pittsburgh, PA, USA). Blank plasma for preparation of the calibration curve and method development testing was obtained from the University of Wisconsin Hospital and Clinics.
2.2 Sample preparation
Standards were initially dissolved at >1 mg/mL in 100% ethanol. Because of the photolabile nature of resveratrol and resveratrol glucuronide, all samples were protected from light as much as possible during preparation and processing. For preparation of calibration curve, the internal standard and the standards were further diluted in 65% methanol before being added to samples. Seven point calibration curves (10, 33.3, 100.0, 333, 1000, 3333, and 10 000 ng/mL resveratrol, 33.3, 100, 333, 1000, 3333, 10 000, and 33 333 ng/mL for resveratrol glucuronide) were prepared and processed in parallel with serum samples.
2.3 Instrumentation and LC/MS conditions
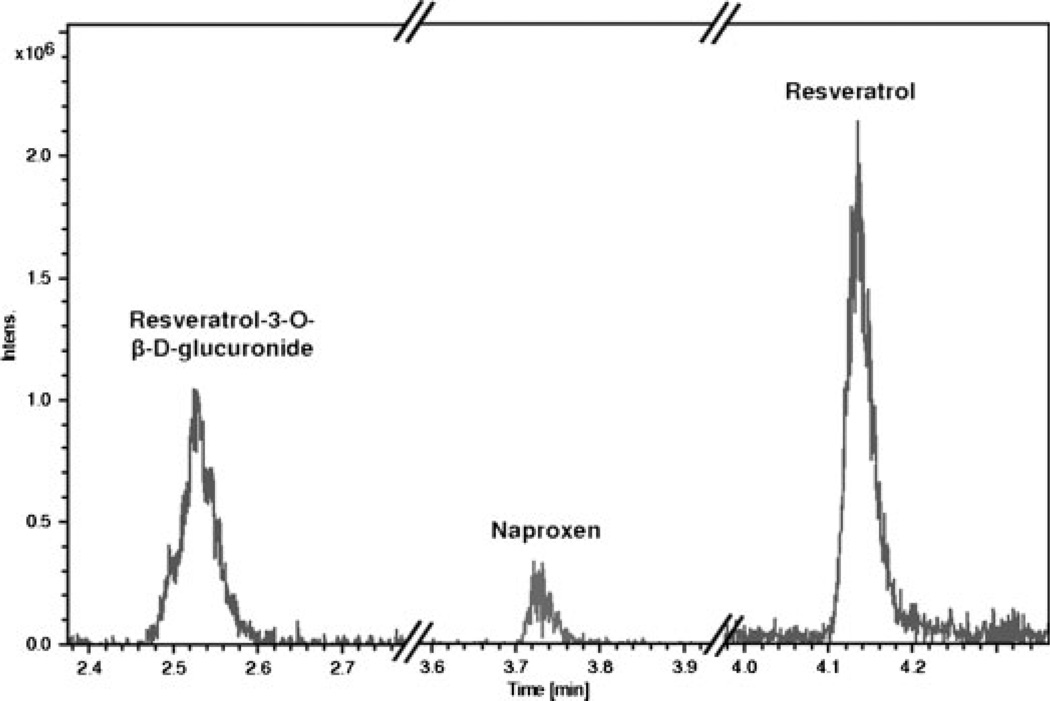
Samples were analyzed by LC separation on a BEH-C18 column (Waters, Milford, MA, USA) with water/1 mM ammonium bicarbonate pH 7.5/2% isopropanol as solvent A and ACN/2% isopropanol as solvent B. Sample (10 µL) was injected onto the column maintained at 35°C that had been equilibrated at 5% B. After a 0.5-min hold at 5% B compounds were eluted with a linear gradient to 55% B in 6.5 min. The column was then washed by ramping to 95% B in 0.5 min, holding at 95% B for 1.5 min, and then the column was re-equilibrated at 5% B for 1.5 min before the next injection. Compounds eluting from the column were analyzed in negative ion mode with an Amazon ion-trap mass spectrometer (Bruker Daltonics, Billerica, MA, USA) following post-column addition of ammonium hydroxide to 0.1%. Between samples, a blank injection of 35% ACN was included to ensure that sample carry-over did not affect results. A typical elution profile is shown in the partial chromatogram in Fig. 2. Peaks were ~0.1 min wide (6 s), reflecting the high-resolution LC provided by the BEH column.
Figure 2.
Elution profiles in a typical chromatogram for the three analytes. Extracted ion chromatograms for resveratrol 3-O-β-d-glucuronide (403.1), a naproxen fragment (170.1 m/z), and resveratrol (227.1 m/z) are shown for a calibration point.
2.4 Animals and treatments
Forty-eight C57BL/6J mice (10–12 wk old) were purchased from the National Cancer Institute at Frederick (Frederick, MD, USA). The mice were housed in plastic cages under standard conditions and received a standard chow and water ad libitum. The mice were maintained on a 12 h/12 h light/dark cycle. Twenty-four mice (non-fasting) received a single dose of resveratrol (100 mg/kg via oral gavage; in 100 µL DMSO) while the other 24 mice (non-fasting) received combination resveratrol (100 mg/kg via oral gavage; in 50 µL DMSO) plus piperine (10 mg/kg via oral gavage; in 50 µL DMSO). All mice were weighed prior to experiment to ensure for accurate dosing of study agent(s). All animal experiments were performed according to the policies and guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin.
2.5 Serum preparation
After treatment with the test agents, at 0.25, 0.5, 1, 2, 4, 6, 12, and 24 h, blood samples were collected from the mandibular vein following the administration of isoflurane as anesthetic. Three mice per time point per treatment group were used for obtaining blood. Blood samples were stored at room temperature for 30 min and were centrifuged at 4000 × g at 4°C for 15 min for isolating serum [18]. Serum samples were transferred to 1.5-mL tubes and stored at −80°C until LC/MS analysis. Serum samples and calibrants (25 µL) were deproteinized by the addition of 1.75 µL of glacial acetic acid and 30 µL of methanol followed by a 10-min vortex then incubation >1 h at −20°C. The mixture was subjected to centrifugation at 21 000 × g and the supernatant (~30 µL) was diluted with 150 µL water and subjected to solid-phase extraction (SPE) enrichment on an Oasis hydrophilic/lipophilic balanced (HLB) matrix (Waters) in a 96-well plate format. Briefly, each well of the plate was conditioned with 1 mL of methanol, then 0.5 mL of 2 M acetic acid in water. Following conditioning, the sample was loaded. The plate was then washed with 0.5 mL of 2 M acetic acid followed by 0.5 mL of 35% methanol/2 M acetic acid. The analytes were recovered by eluting with 0.5 mL methanol with 1 M acetic acid. The samples were dried under nitrogen and suspended in 30 µL of 35% ACN/65% water by vortexing 10 min.
2.6 LC/MS data analysis
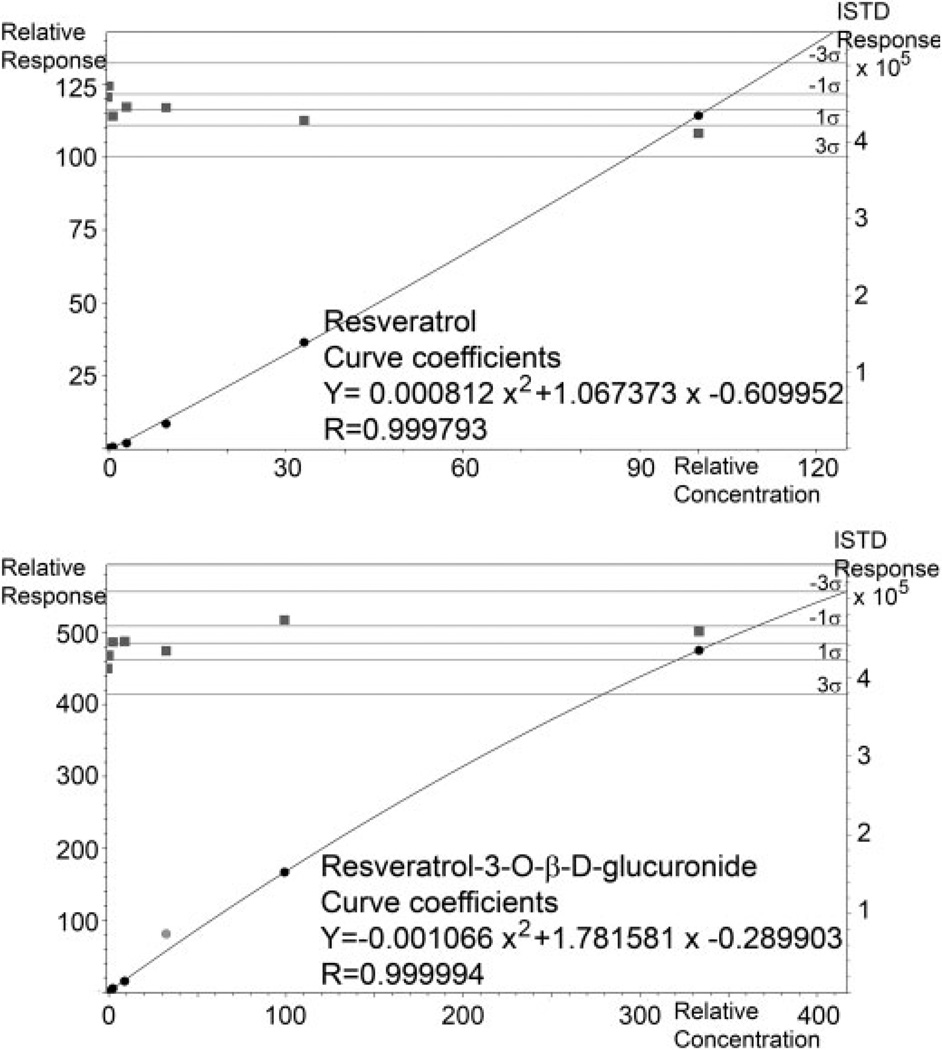
Data were analyzed with the QuantAnalysis software (Bruker Daltonics, Billerica, MA, USA). Extracted ion chromatograms for the analytes and internal standard were created. Quadratic modeling of the analyte area under the curve relative to the internal standard area was used to determine concentrations of resveratrol and resveratrol-3-O-β-d-glucuronide in animal samples (Fig. 3). Quadratic curve fitting of the area under the curve for extracted ion chromatograms of the resveratrol and resveratrol-3-O-β-d-glucuronide relative to the naproxen internal standard was used to determine analyte levels in samples. Analyte peaks typically had >30 data points/peak. Limits of detection were defined by analyte signals with a signal-to-noise ratio of 3. The limit of quantitation was defined as an analyte signal with a signal-to-noise ratio of 10. The percent recovery from plasma samples was calculated to be 67% for resveratrol-3-O-β-d-glucuronide and 78% for resveratrol.
Figure 3.
Calibration curves for resveratrol (top) and resveratrol-3-O-β-d-glucuronide (bottom). Blank plasma samples were spiked with resveratrol, resveratrol-3-O-β-d-glucuronide, and naproxen. The samples were purified using Oasis HLB SPE and analyzed by LC/MS. Quadratic curve fitting (black line) of the area under the curve for extracted ion chromatograms of the resveratrol and resveratrol-glucuronide relative to the naproxen internal standard was used to determine analyte levels in animal samples. Internal standard areas for calibrants fell within three standard deviations of the average (grey points and lines).
2.7 Pharmacokinetic parameters
Mean plasma concentrations of resveratrol and resveratrol-3-O-β-d-glucuronide were plotted versus time. The pharmacokinetic parameters were determined by non-compartmental methods. Area under the concentration-curve AUC0−inf was estimated from the time of dosing and extrapolated to infinity. Analysis was performed using PKSolver 2.0 [19].
3 Results
3.1 Method development and validation
As a first step, we first developed and standardized the LC/MS assay to analyze resveratrol and resveratrol-3-O-β-d-glucuronide in plasma. For this purpose, human plasma samples were used. A typical chromatogram, containing naproxen spiked as an internal standard, is shown in Fig. 2. The retention times of resveratrol-3-O-β-d-glucuronide, naproxen, and resveratrol were 2.53, 3.73, and 4.14 min, respectively (Fig. 2).
We first analyzed five samples to test the small volume preparation method because previous resveratrol sample analysis typically utilized 500 µL of starting material that was not feasible for animal studies in mice. We were able to adapt the SPE method for a small starting volume (25 µL). Calibration curves of resveratrol and resveratrol-3-O-β-d-glucuronide were constructed by plotting the relative peak area ratio (analyte/internal standard) against the concentration of resveratrol or resveratrol-3-O-β-d-glucuronide. The calibration curve for resveratrol had an R value of 0.999793 (Fig. 3). In our assay condition, the limit of detection for resveratrol was found to be 21 ng/mL and the limit of quantitation was 63 ng/mL. The calibration curve for resveratrol glucuronide had an R value of 0.999994 (excluding the 3300 ng/mL calibration point). The limit of detection for resveratrol-3-O-β-d-glucuronide was 10 ng/mL and the limit of quantitation was 30 ng/mL. Thus, at the 24-h time point, resveratrol and resveratrol-3-O-β-d-glucuronide were below the limit of quantification. Two samples analyzing resveratrol in the combination resveratrol and piperine group were above the highest calibration point. The inter-day variation was 12.7% for resveratrol-3-O-β-d-glucuronide and 15.6% for resveratrol. The intra-day (i.e. repeatability) variation was 3.3% for the resveratrol-3-O-β-d-glucuronide and 1.1% for resveratrol.
3.2 Pharmacokinetics of resveratrol
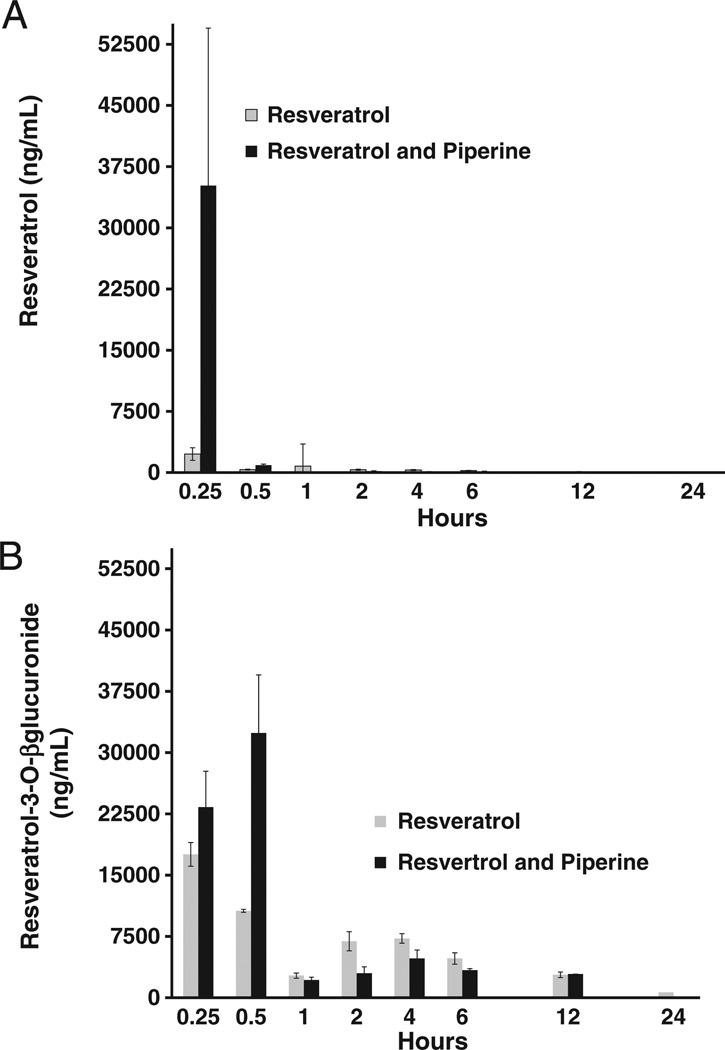
A method was developed and used successfully to support the pharmacokinetic study of resveratrol in mice after a single oral dose of resveratrol or combination of resveratrol and piperine. The mean serum concentrations of resveratrol and resveratrol-3-O-β-d-glucuronide following resveratrol or resveratrol and piperine combination is shown in Fig. 4 and Tables 1 and 2. Table 3 summarizes the main pharmacokinetic parameters of resveratrol and resveratrol-3-O-β-d-glucuronide in mice following administration of resveratrol or combination of resveratrol and piperine and was calculated by non-compartmental analysis. Mice administered 100 mg/kg of resveratrol achieved a maximum serum concentration of 2277 ng/µL at 0.25 h. The resveratrol metabolite resveratrol-3-O-β-d-glucuronide was calculated to achieve a maximum serum concentration of 17 544 ng/µL at 0.25 h. The t1/2 for resveratrol and resveratrol-3-O-β-d-glucuronide were 5.40 and 6.46 h, respectively. The second cohort of mice, which received a combination of resveratrol (100 mg/kg) and piperine (10 mg/kg), achieved a maximum serum concentration of resveratrol of 35 169 ng/L at 0.25 h. The t1/2 was calculated using the equation t1/2 = 0.693/Ke (i.e. Ke = elimination rate constant). The AUC0–inf of resveratrol and resveratrol-3-O-β-d-glucuronide in cohort receiving resveratrol only were 5046 and 89 794 ng/mL h, respectively. The AUC0–inf of resveratrol and resveratrol-3-O-β-d-glucuronide in the cohort receiving combination of resveratrol and piperine were 11 584 and 73 446 ng/mL h, respectively. Thus, our data suggested that the degree of exposure to resveratrol was enhanced to 229% with the addition of piperine and the degree of exposure to the principal metabolite resveratrol-3-O-β-d-glucuronide was decreased to 81%. The Cmax of resveratrol was increased by 1544% with combination resveratrol and piperine. The Tmax of resveratrol-3-O-β-d-glucuronide was 0.5 h with combination of resveratrol and piperine while the resveratrol only cohort had a Tmax of 0.25 h.
Figure 4.
Mean concentration-time profile for resveratrol (A) and resveratrol-3-O-β-d-glucuronide (B). Mice were administered resveratrol (100 mg/kg) or combination of resveratrol (100 mg/kg) and piperine (10 mg/kg). Error bars represent SE. For a detail of exact value, please see Tables 1 and 2.
Table 1.
Average serum levels of resveratrol in C57Bl/6 mice following administration of resveratrol (100 mg/kg) or combination resveratrol (100 mg/kg) plus piperine (10 mg/kg)
| Hours | Serum resveratrol (ng/mL) |
|||
|---|---|---|---|---|
| Resveratrol (100 mg/kg) |
Resveratrol and piperine (100 mg/kg and 10 mg/kg) |
|||
| Mean | SE | Mean | SE | |
| 0.25 | 2277.3 | 776.4 | 35 169.1 | 19 316.9 |
| 0.5 | 374.9 | 50.8 | 910.9 | 126.3 |
| 1 | 801.74 | 312.7 | 96.4 | 7.0 |
| 2 | 353.1 | 68.8 | 181.1 | 33.0 |
| 4 | 321.2 | 63.1 | 117.2 | 5.6 |
| 6 | 243.3 | 23.8 | 144.8 | 22.9 |
| 12 | 112.8 | 14.1 | 83.9 | 5.4 |
| 24 | 41.7 | 12.1 | 41.4 | 12.0 |
Table 2.
Average serum levels of resveratrol-O-β-d-glucuronide in C57Bl/6 mice following administration of resveratrol (100 mg/kg) or combination resveratrol (100 mg/kg) plus piperine (10 mg/kg)
| Hours | Serum resveratrol-O-β-d-glucuronide (ng/mL) |
|||
|---|---|---|---|---|
| Resveratrol (100 mg/kg) |
Resveratrol and piperine (100 mg/kg and 10 mg/kg) |
|||
| Mean | SE | Mean | SE | |
| 0.25 | 17544.3 | 1436.1 | 23 324.9 | 4387.8 |
| 0.5 | 10 639.2 | 181.1 | 32 400.5 | 7093.9 |
| 1 | 2705.7 | 320.3 | 2175.2 | 348.6 |
| 2 | 6914.4 | 1158.9 | 2997.6 | 787.2 |
| 4 | 7256.9 | 585.2 | 4798.5 | 1045.6 |
| 6 | 4806.0 | 692.5 | 3377.1 | 184.2 |
| 12 | 2803.5 | 1415.3 | 2887.7 | 654.5 |
| 24 | 656.2 | 339.6 | 23.4 | 2.2 |
Table 3.
Pharmacokinetic parameters of resveratrol and resveratrol-3-O-β-d-glucuronide following administration of resveratrol (100 mg/kg) or combination resveratrol (100 mg/kg) plus piperine (10 mg/kg)
| Pharmacokinetic parametersa) |
||||
|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | Half-life (h) | AUC0–inf (ng/mLh) | |
| Resveratrol cohort | ||||
| Resveratrol | 2277.27 | 0.25 | 5.40 | 5046.3 |
| Resveratrol-3-O-β-d-glucuronide | 17 544.26 | 0.25 | 6.46 | 89 793.70 |
| Resveratrol and piperine cohort | ||||
| Resveratrol | 35 169.07 | 0.25 | 4.84 | 11 583.70 |
| Resveratrol-3-O-β-d-glucuronide | 32 400.54 | 0.50 | 3.03 | 73 446.60 |
Data were calculated using non-compartmental analysis.
4 Discussion
The major finding of this study is that piperine significantly enhances the serum bioavailability of resveratrol in mice. Because the rapid metabolism seems to be a limiting factor in translating resveratrol’s promising health-promoting effects in humans, in this study we investigated our recently propagated strategy to enhance the pharmacokinetic parameters of resveratrol by partially inhibiting its metabolism via combining it with piperine [7].
Studies in healthy human volunteers have shown that resveratrol is rapidly absorbed but quickly metabolized, usually within 30–60 min following oral or intravenous administration in humans [10, 20]. Similarly, in mice, oral resveratrol (240 mg/kg body weight) has been reported to achieve a Cmax of ~32 µM (n = 3) in 10 min and a AUC of 863 nm/mL min [21]. In our study, we observed oral resveratrol (100 mg/kg body weight) to achieve a Cmax of ~12 µM (n = 3) at 15 min and an AUC of 368 nm/mL min. When taking into account the difference in dose (240 mg/kg versus 100 mg/kg) and time point (10 versus 15 min), our data appear to be consistent with the study by Sale et al., described above [21].
A few studies have shown that piperine inhibits glucuronidation of certain polyphenolic antioxidants, viz. curcumin and epigallocatechin-3-gallate [15–17, 22–24]. Resveratrol has been shown to be extensively metabolized through glucuronidation and sulfation. In this study, we found that piperine appreciably modulates the pharmacokinetics of resveratrol by increasing the Cmax and the AUC. We have found that piperine increase Cmax of resveratrol by 1544%, Cmax of resveratrol-O-β-d-glucuronide by 184%, and an increase in the Tmax from 0.25 h to 0.5 h. The delay in the glucuronidation of resveratrol to resveratrol-O-β-glucuronide is suggestive that piperine inhibited UGT1A1, which has been shown to be responsible for the formation of 3-O-β-glucuronide [25]. In future studies, we plan to evaluate the effect on additional metabolites to determine the impact of piperine on other UGT enzymes such as UGT1A9, which is responsible for the formation of resveratrol-4-O-β-glucuronide. The observed increase in the absorption of resveratrol (i.e. 1544%) by the addition of piperine further suggests toward the possibility of inhibition of glucuronidation in the GI tract. To further support our hypothesis of piperine having a local GI effect, we did not observe an increase in the half-life of resveratrol suggesting the systemic absorption of piperine may not be a critical attribute. It is possible that piperine also modulates the intestinal membrane dynamics by influencing brush border membrane fluidity [26]. Interestingly, in our study, we observed the presence of a second peak in serum with the resveratrol metabolite, which may be explained by enterohepatic recirculation of resveratrol, which has been observed in rats and humans [20, 27]. Furthermore, piperine is known to decrease gastrointestinal emptying and increase transit time, which could be responsible for its observed response [28]. Piperine has been shown to be metabolized by oxidation of the piperidine ring to form a 3,4-dihydroxyphenyl motif, which may allow for glucuronidation and elimination. Interestingly, resveratrol has been shown to inhibit phase I and phase II enzymes and may actually alter the pharmacokinetics of piperine [29, 30]; however, further studies are needed to support this possibility.
In conclusion, our study represents the first evaluation of piperine modulating the pharmacokinetic parameters of resveratrol. Our data indicate that piperine increases the Cmax and degree of exposure (i.e. AUC) to resveratrol, which appears to be through the inhibition of glucuronidation of resveratrol by piperine. While mechanistic details of this effect remain to be elucidated, our study could have direct application to human studies evaluating the pharmacokinetic profile of resveratrol. For instance, clinical studies have used doses as high as 5 g daily, which is the equivalent of ten capsules (500 mg each). This represents a significant shortcoming in the long-term translational development of resveratrol as described above. One study in particular has shown that 1 g of resveratrol, which is the equivalent of two capsules, was administered daily for 28 days and was well tolerated [29]. All documented adverse events were grade 1 or 2 Common Toxicity Criteria, with many being very mild and transient. Given our results, the strategy of piperine co-administration may allow for a significant decrease in the dose of resveratrol (and capsules) in clinical settings. As a result, we are currently in the process of enrolling subjects into a phase I clinical trial in healthy volunteers to study the effect of piperine on the bioavailability of resveratrol in a clinical setting. Finally, piperine has been shown to be well tolerated in animals and humans; however, it is possible that through the inhibition of glucuronidation, there could the potential to increase the degree of exposure (i.e. AUC) to pharmaceuticals metabolized by glucuronidation. To minimize the systemic effects of piperine, our future work is focused on optimizing the dose of piperine to ensure local gastrointestinal effects as a strategy to prevent adverse drug interactions.
Acknowledgments
The authors thank Sabinsa, Inc. and Dr. Muhammad Majeed for generously providing resveratrol and piperine for these studies. This work was partially supported by funding from the National Institutes of Health (1R21 CA149560, R01CA114060, and 1R01AR059130).
Footnotes
The authors have declared no conflict of interest.
References
- 1.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 2.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 3.Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann. NY Acad. Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 4.Reagan-Shaw S, Mukhtar H, Ahmad N. Resveratrol imparts photoprotection of normal cells and enhances the efficacy of radiation therapy in cancer cells. Photochem. Photobiol. 2008;84:415–421. doi: 10.1111/j.1751-1097.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- 5.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front. Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 6.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 7.Ndiaye M, Kumar R, Ahmad N. Resveratrol in cancer management: where are we and where we go from here? Ann. NY Acad. Sci. 2011;1215:144–149. doi: 10.1111/j.1749-6632.2010.05851.x. [DOI] [PubMed] [Google Scholar]
- 8.Nonomura S, Kanagawa H, Makimoto A. Chemical constituents of polygonaceous plants I. Studies on the components of ko-jo-kon (Polygonum cuspidatum) Yakugaku Zasshi. 1963;83:988–990. [PubMed] [Google Scholar]
- 9.Horn TL, Cwik MJ, Morrissey RL, Kapetanovic I, et al. Oncogenicity evaluation of resveratrol in p53(+/−) (p53 knockout) mice. Food Chem. Toxicol. 2007;45:55–63. doi: 10.1016/j.fct.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boocock DJ, Faust GE, Patel KR, Schinas AM, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 11.Brown VA, Patel KR, Viskaduraki M, Crowell JA, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkon A, Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides – two novel resveratrol metabolites in human plasma. Mol. Nutr. Food Res. 2008;52:549–557. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- 13.Almeida L, Vaz-da-Silva M, Falcao A, Soares E, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009;53:S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 14.Vaz-da-Silva M, Loureiro AI, Falcao A, Nunes T, et al. Effect of food on the pharmacokinetic profile of transresveratrol. Int. J. Clin. Pharmacol. Ther. 2008;46:564–570. doi: 10.5414/cpp46564. [DOI] [PubMed] [Google Scholar]
- 15.Shoba G, Joy D, Joseph T, Majeed M, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 16.Reen RK, Jamwal DS, Taneja SC, Koul JL, et al. Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine. Biochem. Pharmacol. 1993;46:229–238. doi: 10.1016/0006-2952(93)90408-o. [DOI] [PubMed] [Google Scholar]
- 17.Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS. Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J. Nutr. 2004;134:1948–1952. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JJ, Syed DN, Suh Y, Heren CR, et al. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev. Res. 2010;3:1112–1123. doi: 10.1158/1940-6207.CAPR-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010;99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 21.Sale S, Verschoyle RD, Boocock D, Jones DJ, et al. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br. J. Cancer. 2004;90:736–744. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. J. Pharmacol. Exp. Ther. 1985;232:258–262. [PubMed] [Google Scholar]
- 23.Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007;47:735–748. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemoprevention: a translational perspective. Phytomedicine. 2010;17:3–13. doi: 10.1016/j.phymed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brill SS, Furimsky AM, Ho MN, Furniss MJ, et al. Glucuronidation of trans-resveratrol by human liver and intestinal microsomes and UGT isoforms. J. Pharm. Pharmacol. 2006;58:469–479. doi: 10.1211/jpp.58.4.0006. [DOI] [PubMed] [Google Scholar]
- 26.Alcala J, Lieska N, Maisel H. Protein composition of bovine lens cortical fiber cell membranes. Exp. Eye Res. 1975;21:581–595. doi: 10.1016/0014-4835(75)90040-8. [DOI] [PubMed] [Google Scholar]
- 27.Marier JF, Vachon P, Gritsas A, Zhang J, et al. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 28.Bajad S, Bedi KL, Singla AK, Johri RK. Piperine inhibits gastric emptying and gastrointestinal transit in rats and mice. Planta Med. 2001;67:176–179. doi: 10.1055/s-2001-11505. [DOI] [PubMed] [Google Scholar]
- 29.Chow HH, Garland LL, Hsu CH, Vining DR, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010;3:1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajad S, Coumar M, Khajuria R, Suri OP, Bedi KL. Characterization of a new rat urinary metabolite of piperine by LC/NMR/MS studies. Eur. J. Pharm. Sci. 2003;19:413–421. doi: 10.1016/s0928-0987(03)00143-x. [DOI] [PubMed] [Google Scholar]