Abstract
Summary
Clinicians can diagnose high urine calcium by asking patients to collect urine for 24 h or to provide a random urine specimen. In this study, random urine calcium levels were not as accurate as those from the 24-h collection. Clinicians should only use 24-h collections to diagnose high urine calcium.
Introduction
Clinicians diagnose hypercalciuria using a 24-h urine calcium (24HUC) or a spot urine-calcium-to-creatinine ratio (SUCCR) specimen. The SUCCR is reportedly interchangeable with the 24HUC. However, studies to date show mixed results when comparing SUCCR and 24HUC values. We systematically compared fasting and postprandial SUCCR measurements to 24HUC measurements using Bland–Altman analysis.
Methods
Twenty-one postmenopausal women aged 58± 7 years came to the research ward for three 24-h inpatient stays. At each study visit, research nurses collected fasting morning (n=62) and postprandial (n=62) spot urine specimens along with carefully timed and complete 24-h urine specimens (n=63) from each woman.
Results
Hypercalciuria was present in 13 24HUC samples (21%) using an upper limit of 250 mg/24-h. The fasting SUCCR underestimated the 24HUC (Bland–Altman bias −71 mg/24-h), with a sensitivity and specificity for diagnosing hypercalciuria of 0% and 98%, respectively. The postprandial SUCCR overestimated the 24HUC (Bland–Altman bias +61 mg/24-h), with a sensitivity and specificity of 77% and 61%, respectively. The average of fasting and postprandial SUCCR measurements had a lower Bland–Altman bias of −3 mg/24-h but demonstrated a sensitivity and specificity of only 42% and 78%, respectively.
Conclusions
The SUCCR is not interchangeable with the 24HUC. The fasting SUCCR systematically underestimates, and the postprandial SUCCR systematically overestimates, 24HUC. The average SUCCR demonstrates low sensitivity and specificity for hypercalciuria. Clinicians must use the 24HUC to diagnose hypercalciuria in postmenopausal women.
Keywords: Calcium, Diagnosis, Hypercalciuria, Nephrolithiasis, Osteoporosis, Urine
Introduction
Hypercalciuria can cause nephrolithiasis and osteoporosis [1], and its diagnosis influences management of these conditions [2]. Clinicians can diagnose hypercalciuria by measuring 24-h urine calcium (24HUC) levels, an approach considered as the gold standard [3, 4]. However, outpatients find this collection difficult and often provide incomplete specimens, leading to invalid test results. In place of the onerous 24HUC, clinicians can instead request a spot urine calcium-to-creatinine ratio (SUCCR) to diagnose hypercalciuria. The SUCCR is defined as the ratio of calcium in milligrams per deciliter to creatinine in milligrams per deciliter [5]. Authors [4–7] recommend multiplying the SUCCR by 1,000 to determine 24HUC levels and report that the SUCCR is interchangeable with the 24HUC.
However, studies to date (Table 1, [3–11]) show mixed results when assessing whether the SUCCR is truly interchangeable with 24HUC values. While many studies reported a high correlation between SUCCR and 24HUC values, few studies employed Bland–Altman plotting and analysis of data. The Bland–Altman test [12], first reported in 1986, is a statistical method to confirm agreement between tests and uncover the bias of a new test compared to the gold standard test. It is imperative to use such an analysis before suggesting the replacement of one test over another because correlation coefficients only measure the strength of relationship, and not the agreement, between two tests [12]. Despite the lack of Bland–Altman analyses, many experts recommend using the SUCCR to diagnose hypercalciuria [13–15].
Table 1.
Summary of studies assessing the validity of the spot urine calcium-to-creatinine ratio to diagnose hypercalciuria
| Study | Patient population | Methods | Conclusions |
|---|---|---|---|
| Nordin et al. 1959 [6] |
|
|
|
| Gokce et al. 1991 [5] |
|
|
|
| Isaacson et al. 1963 [9] |
|
|
|
| Wills et al. 1962 [10] |
|
|
|
| Ghazali et al. 1974 [8] |
|
|
|
| Strohmaier et al. 1997 [3] |
|
|
|
| Ogawa et al. 2003 [7] |
|
|
|
| Koyun et al. 2006 [11] |
|
|
|
| Topal et al. 2008 [4] |
|
|
|
Two recent studies used the Bland–Altman method to compare paired 24HUC and SUCCR specimens. One study [16] investigated outpatient stone formers and found moderate correlation (r=0.67) but lack of agreement (Bland–Altman bias=−67 mg/24-h) between 24HUC and early morning, presumably fasting, SUCCR measurements. We likewise reported a reasonable correlation, but poor agreement, between the 24HUC and fasting SUCCR measurements (r=0.73, Bland–Altman bias=−83 mg/24-h) in healthy, inpatient, postmenopausal women [17].
We undertook the present study to evaluate whether postprandial SUCCR measurements, when multiplied by 1,000, could be used interchangeably with 24HUC values. As a result of intestinal calcium absorption, both urine and serum calcium levels increase after a meal. We therefore hypothesized that the postprandial SUCCR, or an average of the fasting and postprandial SUCCR, would reliably estimate 24HUC and diagnose hypercalciuria. Additionally, we performed the current study in order to validate formulas previously derived to correct fasting SUCCR measurements and predict 24HUC values [17].
Methods
Patients
We conducted this study as part of a protocol to investigate whether the proton pump inhibitor omeprazole decreases intestinal calcium absorption [18]. We recruited 21 post-menopausal women to complete three 24-h visits at the University of Wisconsin (UW) Clinical and Translational Research Core (CTRC). During each woman’s three inpatient stays, spaced approximately 1 month apart, we collected 24-h, fasting and postprandial spot urine specimens. Eligible women were at least 5 years past menopause and did not take proton pump inhibitors, antacids, or medication known to interfere with calcium absorption such as steroids, anticonvulsants, or antibiotics. We also excluded women taking medications known to interact with omeprazole and those with chronic kidney disease stage 4 or 5, defined as estimated GFR <30 mL/min.
Protocol
Women came to the UW CTRC for three 24-h inpatient stays, each approximately 1 month apart. Between the second and third inpatient stay, women took 40 mg of omeprazole daily, approximately 30 min prior to breakfast. Women fasted from midnight the night before each inpatient stay, voided at home upon awakening, and reported to the research unit at approximately 0700 hours. Upon arrival, subjects provided a second void fasting spot urine specimen which was used to determine the fasting SUCCR. Subsequently, highly trained research nurses supervised the collection of 24-h urine specimens from each subject. During the 24-h collection, nurses instructed subjects to void into a plastic hat within 30 min of consuming lunch to provide a specimen for postprandial SUCCR values. Nurses removed a 5-mL aliquot for the postprandial spot urine specimen and mixed the remaining postprandial void with the 24-h collection. Nurses collected urine in plastic, preservative-free, acid-free jugs and refrigerated all specimens until analysis [19]. Because nurses supervised carefully timed 24-h collections and the utility of urine creatinine measurement to verify a complete 24-h urine collection has been criticized [20–23], we did not measure 24-h urine creatinine.
We recorded subjects’ demographic variables including age, height, weight, and race and measured serum calcium, creatinine, parathyroid hormone, 1,25(OH)2D and 25(OH) D at each research visit [18]. We replicated each subject’s inpatient diets using 7-day food diaries; thus, any calcium and vitamin D ingested during the inpatient stays matched participants’ normal outpatient intake. Subjects received oral and intravenous stable calcium isotope tracers with breakfast; the total dose of calcium administered as tracers was tiny (<11 mg per stay) and therefore did not contribute significantly to each subject’s calcium intake [18].
Laboratory
The Wisconsin State Lab of Hygiene measured calcium concentrations in 24-h urine samples using magnetic-sector (high-resolution) inductively coupled plasma mass spectrometry as described elsewhere [18]. All 24-h urine specimens from each subject were analyzed for calcium on the same day, yielding an intra-assay coefficient of variation of 3.6%. Meriter Laboratories (Madison, WI) measured all spot calcium-to-creatinine ratios using COBAS INTEGRA 400/800 systems (coefficients of variation of 2.2% (within) and 3.8% (between)). Assay characteristics of other laboratory tests are detailed elsewhere [18].
Study objectives and statistical analysis
The objectives of this study were to determine whether the postprandial SUCCR would be used interchangeably with 24HUC values and to validate two formulas derived in a separate population to improve diagnostic performance of the fasting SUCCR [17]. We compared 24HUC with fasting, postprandial, and average SUCCR measurements collected during the same study visit using paired t tests, Pearson’s correlation coefficients, and Bland–Altman analyses. We assessed sensitivity and specificity of the fasting, postprandial, and average SUCCR values to diagnose hypercalciuria using the gold standard 24HUC result as the indicator of hypercalciuria. We assessed sensitivity and specificity of the calculated 24HUC values using an upper limit of normal of 250 mg/24-h [24], an upper limit of 4 mg/kg/24-h [25], and upper limits based on 95th percentile ranges established from a reference population of postmenopausal women with low, moderate, and high calcium intake [26]. We employed linear mixed models to determine variability in SUCCR measures due to individual differences between women and to assess relationships between 24HUC and demographic, laboratory, and dietary variables including calcium intake. All analyses were conducted using Analyze-IT (version 2.12) and the R statistical analysis system (version 2.11.1).
Results
Basic data values
Twenty-one women with a mean ± one SD age of 58± 7 years completed all three 24-h visits. Seventeen women were Caucasian and two each were Black and Hispanic. Subjects’ mean height, weight, and body mass index were 163±6 cm, 78±13 kg, and 29±5 kg/m2, respectively. As stated in the “Methods” Section, women consumed meals during their inpatient stays that replicated their outpatient intake of macronutrients and micronutrients [18]; each woman consumed the same meals at each of her three inpatient visits. Through the combination of diet and supplements, women ingested 1,400±650 mg of calcium per day, approximately half (46%) with breakfast. Although the calcium content of the breakfast varied between subjects (700±600 mg), each woman’s calcium intake from diet and supplements was identical to her outpatient consumption. Twelve subjects (57%) took supplements containing calcium prior to the research study; these were continued during inpatient stays.
Subjects provided postprandial SUCCR specimens an average of 18±12 min after eating lunch. If a woman provided a 24HUC and fasting SUCCR but no postprandial SUCCR at a study visit, we included her paired 24HUC and fasting SUCCR in the analysis of all data. Thus, a total of 62 paired 24HUC and fasting SUCCR, 62 paired 24HUC and postprandial SUCCR, and 61 paired 24HUC and average SUCCR were analyzed for this study.
Table 2 summarizes subjects’ mean 24HUC, fasting SUCCR, postprandial SUCCR, and average SUCCR measurements. The mean value of all 24HUC measures (n=63) was 190±75 mg/24-h (2.5±1.1 mg/kg/24-h). The mean value of all converted [5] fasting SUCCR measures (n=62) was 120±65 mg/24-h, while the mean value of all converted postprandial SUCCR measures (n=62) was 250±120 mg/24-h. Lastly, the mean value of all converted, averaged SUCCR measures (n=61) was 180±80 mg/24-h.
Table 2.
Summary of urine calcium measurements across study visits
| Visit 1 | Visit 2 | Visit 3 | p value | ||
|---|---|---|---|---|---|
| Spot urine tests | Fasting calcium-to-creatinine ratio | 0.11±0.06 | 0.12±0.06 | 0.14±0.07 | 0.25 |
| Postprandial calcium-to-creatinine ratio | 0.24±0.12 | 0.26±0.13 | 0.25±0.1 | 0.69 | |
| Average calcium-to-creatinine ratio | 0.17±0.08 | 0.19±0.09 | 0.2±0.08 | 0.45 | |
| 24-h urine | Calcium, mg/24-h | 190±85 | 200±64 | 190±76 | 0.58 |
Data are presented as the mean ± standard deviation. P values are reported using within-subject analysis of variance. The SUCCR is defined as the ratio of calcium in milligrams per deciliter to creatinine in milligrams per deciliter. Authors [4–7] recommend multiplying the SUCCR by 1,000 to determine 24HUC levels, and report that the SUCCR is interchangeable with the 24HUC
Using ANOVA, within-subject 24HUC values were not significantly different across the three study visits (p=0.58), with good agreement by Bland–Altman analysis. 24HUC values from visit 1 agreed with visit 2 (Bland–Altman bias=+15 mg), visit 1 agreed with visit 3 (Bland–Altman bias=+8 mg), and visit 2 agreed with visit 3 (Bland–Altman bias=−7 mg). By ANOVA, within-subject fasting, postprandial, and average SUCCR values were not significantly different across the three study visits (p=0.25, p=0.69, and p=0.45, respectively).
Using the gold standard 24HUC measurement, hypercalciuria was present in 13 samples (21%) from seven women using an upper limit of 250 mg/24-h [24], seven samples (11%) from four women using an upper limit of 4 mg/kg/24-h [25], and one sample (2%) using upper limits established from a reference population [26].
Performance of the fasting, postprandial, and average SUCCR
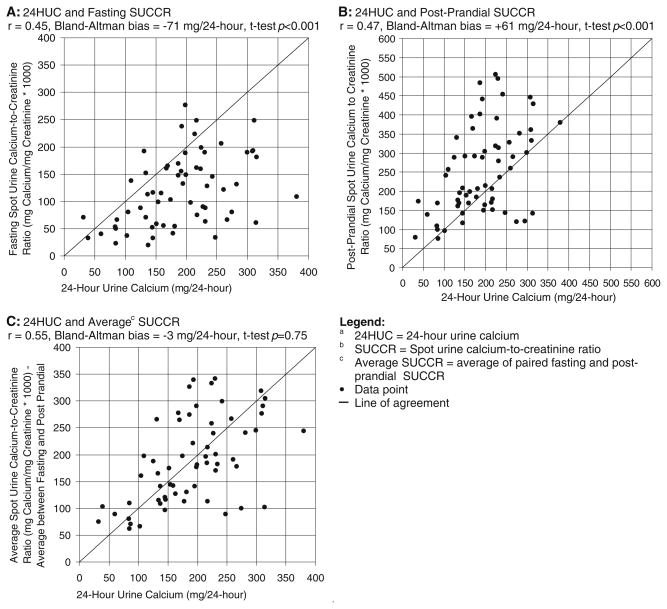
The fasting SUCCR and measured 24HUC values were significantly different (paired t test p<0.001) and demonstrated poor correlation and poor agreement (Pearson’s correlation r=0.45, Bland–Altman bias=−71 mg/24-h; Fig. 1a). The fasting SUCCR demonstrated a diagnostic sensitivity of 0% using all hypercalciuria definitions (Table 3). The postprandial SUCCR was also significantly different from measured 24HUC values (paired t test p<0.001), demonstrating poor correlation and poor agreement with measured 24HUC values (Pearson’s correlation r =0.47, Bland–Altman bias=+61 mg/24-h; Fig. 1b) and diagnostic sensitivities of 77% (upper limit 250 mg/24-h), 77% (upper limit 4 mg/kg/24-h), and 100% (upper limit from reference population; Table 3). Values obtained by averaging paired fasting and postprandial SUCCR measurements demonstrated poor correlation but an improved agreement with 24HUC measurements (paired t test p=0.75, Pearson’s correlation r=0.55, Bland–Altman bias=−3 mg/24-h; Fig. 1c). Despite better agreement, average SUCCR values demonstrated sensitivities of 42% (upper limit 250 mg/24-h), 67% (upper limit 4 mg/kg/24-h), and 0% (upper limit from reference population).
Fig. 1.
Correlation between the 24HUC and the SUCCR
Table 3.
The sensitivity and specificity of urine tests to diagnose hypercalciuria
| Upper limit of normal of 24HUC | Prevalence of hypercalciuria using 24HUCa | Variable tested against 24HUC | Prevalence of hypercalciuria using SUCCR | Sensitivityb | Specificityb | Degree of misclassificationc |
|---|---|---|---|---|---|---|
| 250 mg/24-h [24] | 21% (n=12) | Fasting SUCCR | 2% (n=1) | 0% | 98% | 21% |
| postprandial SUCCR | 47% (n=29) | 77% | 61% | 35% | ||
| Average SUCCR | 26% (n=16) | 42% | 78% | 30% | ||
| 4 mg/kg/24-h [25] | 11% (n=7) | Fasting SUCCR | 0% (n=0) | 0% | 100% | 10% |
| postprandial SUCCR | 32% (n=21) | 71% | 71% | 29% | ||
| Average SUCCR | 11% (n=7) | 67% | 95% | 8% | ||
| Reference population, mg/24-h [26] | 2% (n=1) | Fasting SUCCR | 0% (n=0) | 0% | 100% | 6% |
| postprandial SUCCR | 26% (n=16) | 100% | 75% | 32% | ||
| Average SUCCR | 8% (n=5) | 0% | 92% | 16% | ||
| Reference population mg/kg/24-h [26] | 0% (n=0) | Fasting SUCCR | 0% (n=0) | NA | 100% | 0% |
| postprandial SUCCR | 13% (n=8) | NA | 87% | 13% | ||
| Average SUCCR | 3% (n=2) | NA | 97% | 3% |
24HUC 24-h urine calcium, SUCCR spot urine calcium-to-creatinine ratio (milligrams calcium/milligrams creatinine
Prevalence of hypercalciuria was based on analysis of 63 24-h urine collections
Sensitivity and specificity refer to the various SUCCR tests’ ability to diagnose hypercalciuria compared to the gold standard 24HUC
Degree of misclassification calculated as (false positives+false negatives)/total tested
We multiplied the SUCCR by individual estimates of urine creatinine from the Cockcroft–Gault equation [27]. This adjustment did little to improve the correlation between the SUCCR and the 24HUC values (Pearson’s correlation r=0.43). Additionally, spot urine calcium alone correlated poorly with 24HUC values (Pearson’s correlation r=0.23). Thus, neither the SUCCR alone, SUCCR multiplied by estimated urine creatinine, nor the spot urine calcium alone could adequately predict 24HUC.
Test of formulas to correct the SUCCR
We corrected the fasting SUCCR using previously published formulas [17]:
and
The calculated mean calcium values based on fasting SUCCR measurements and the formulas above were 150± 80 mg/24-h and 2.6±1.1 mg/kg/24-h. We compared these calculated calcium values to our measured 24HUC values, and results were significantly different, demonstrating poor correlation and poor agreement (t test p=0.004, Pearson’s correlation r=0.37, Bland–Altman bias=−44 mg/24-h). Likewise, calculated 24HUC values in milligrams per kilogram in 24-h were significantly different than measured 24HUC values in milligrams per kilogram in 24-h (t test p=0.02, Pearson’s correlation r=0.30, Bland–Altman bias=+0.10 mg/kg/24-h). Formula-derived 24HUC in milligrams in 24-h and in milligrams per kilogram in 24-h demonstrated unacceptable sensitivities ranging from 0–17% (summarized in Table 3). Substituting the postprandial and average SUCCR values in these two formulas did not improve correlation, agreement, diagnostic sensitivity, or specificity (data not reported).
Linear mixed modeling
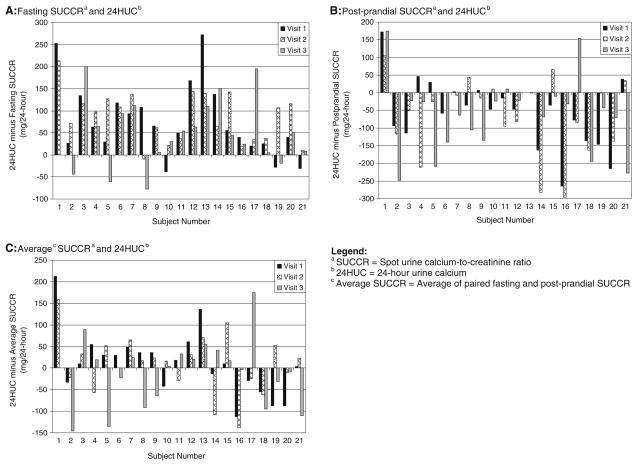
Because we collected samples from each woman at three inpatient stays, we used linear mixed models to measure variance in the ability of the SUCCR to predict 24HUC between women. When using the linear mixed model to compare the 24HUC with the fasting SUCCR, the estimated standard deviation between women was 53 mg and within women was 44 mg. When comparing the 24HUC to the postprandial SUCCR, the estimated standard deviation between women was 51 mg and within women was 46 mg. Such large variation between SUCCR and 24HUC values is illustrated in Fig. 2.
Fig. 2.
Inter-individual variability in the ability of the SUCCR to predict 24HUC
We used linear mixed models to investigate whether other variables affected the relationship between the 24HUC and the SUCCR. The variable that most greatly reduced the estimated standard deviation between SUCCR and 24HUC values was dietary calcium. Incorporation of dietary calcium into models using the fasting and postprandial SUCCR lowered the estimated standard deviation between women to 27 mg and 32 mg, respectively. We tested a formula incorporating dietary calcium and the fasting SUCCR to predict 24HUC. This formula was:
When we compared the calculated 24HUC from this formula to measured 24HUC values, we found a diagnostic sensitivity and specificity of 50% and 94%, respectively (using an upper normal limit of 250 mg/24-h).
Although dietary sodium increases calcium excretion [28], a model incorporating dietary sodium did not improve the ability of spot specimens to predict 24HUC values. After incorporating dietary sodium into the model, the estimated standard deviation between women was 54 mg.
Discussion
Some experts report that the SUCCR, multiplied by 1,000, is interchangeable with 24HUC values [5, 8, 13]. In this study, we compared fasting and postprandial SUCCR measurements to carefully collected inpatient 24HUC measurements. The SUCCR was not interchangeable with measured 24HUC levels, regardless of collection time, unit conversion (milligrams in 24-h or milligrams per kilogram in 24-h), or change in hypercalciuria definition. The fasting SUCCR systematically underestimated 24HUC, while the postprandial SUCCR systematically overestimated the 24HUC. The average of the fasting and postprandial SUCCR measurements demonstrated a lower Bland–Altman bias when compared to 24HUC measurements, but did not demonstrate improved sensitivity and specificity in diagnosing hypercalciuria. Figure 2 illustrates the substantial between-subject variability between average SUCCR and measured 24HUC values. Formulas [17] did not improve the diagnostic performance or agreement of spot urine calcium-to-creatinine values with measured 24HUC values. Moreover, we detected large between-subject variations in the ability of the SUCCR to predict 24HUC, and this variation was impossible to correct using various models.
Strengths of our study include the collection of complete 24-h urine specimens from subjects during inpatient research visits, the use of both fasting and postprandial specimens to predict 24HUC values, and replication of typical diet based on 7-day food records. Study limitations include the evaluation of a small, homogenous group of postmenopausal women, most of whom did not have hypercalciuria. We also excluded subjects with chronic kidney disease and those taking medications known to interfere with calcium metabolism. We therefore cannot apply our results to women with kidney disease or nephrolithiasis, premenopausal women, men, or children. We collected only two spot urine specimens, and it is possible that samples collected at other times within a 24-h period would exhibit improved test performance. Finally, because we collected 24HUC specimens in a research setting, 24HUC specimens collected in clinical practice may demonstrate different results when compared to spot urine specimens.
In summary, the fasting, postprandial, and average SUCCR were not interchangeable with 24HUC values and demonstrated an unacceptably low diagnostic sensitivity for detecting hypercalciuria. Likewise, formulas incorporating SUCCR and other variables did not improve the SUCCR test performance to a degree permitting its use in clinical practice. Because the SUCCR performs poorly in diagnosing hypercalciuria, clinicians should stop using the SUCCR to estimate urine calcium loss and diagnose hypercalciuria. Future studies should focus on methods to ensure complete 24-h urine specimens in outpatients.
Acknowledgments
Grant support KEH received salary support from the NIH (K23 AR050995 and R01 AG028739) during the conduct of this study. The project was supported by grants from the GCRC (NCRR M01 RR03186), the American College of Rheumatology/Research Education Foundation, and American Society for Specialty Physicians through the Hartford Foundation and Atlantic Philanthropies (Junior Career Development Award in Geriatric Medicine), and the University of Wisconsin Institute for Clinical and Translational Research, funded through an NIH Clinical and Translational Science Award (1UL 1RR025011). Sponsors had no role in any portion of the study, including its design, conduct, data analysis, and manuscript preparation.
Footnotes
Conflicts of interest None.
Contributor Information
A. N. Jones, Department of Medicine, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA
M. M. Shafer, Wisconsin State Lab of Hygiene, 2601 Agriculture Drive, Madison, WI 53718, USA
N. S. Keuler, Department of Statistics, University of Wisconsin College of Letters and Science, 1300 University Avenue, Madison, WI 53706, USA
E. M. Crone, Department of Medicine, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA
K. E. Hansen, Email: keh@medicine.wisc.edu, Department of Medicine, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA. UW School of Medicine and Public Health, Room 4124, 1685 Highland Avenue, Madison, WI 53705-2281, USA
References
- 1.Park S, Pearle MS. Pathophysiology and management of calcium stones. Urol Clin North Am. 2007;34(3):323–334. doi: 10.1016/j.ucl.2007.04.009. 10.1016/j.ucl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Adams JS, Song CF, Kantorovich V. Rapid recovery of bone mass in hypercalciuric, osteoporotic men treated with hydrochlorothiazide. Ann Intern Med. 1999;130(8):658–660. doi: 10.7326/0003-4819-130-8-199904200-00012. 199904200-00006. [DOI] [PubMed] [Google Scholar]
- 3.Strohmaier WL, Hoelz KJ, Bichler KH. Spot urine samples for the metabolic evaluation of urolithiasis patients. Eur Urol. 1997;32 (3):294–300. [PubMed] [Google Scholar]
- 4.Topal C, Algun E, Sayarlioglu H, Erkoc R, Soyoral Y, Dogan E, Sekeroglu R, Cekici S. Diurnal rhythm of urinary calcium excretion in adults. Ren Fail. 2008;30(5):499–501. doi: 10.1080/08860220802060471. 10.1080/08860220802060471. [DOI] [PubMed] [Google Scholar]
- 5.Gokce C, Gokce O, Baydinc C, Ilhan N, Alasehirli E, Ozkucuk F, Tasci M, Atilkeler MK, Celebi H, Arslan N. Use of random urine samples to estimate total urinary calcium and phosphate excretion. Arch Intern Med. 1991;151(8):1587–1588. doi: 10.1001/archinte.1991.00400080083015. [DOI] [PubMed] [Google Scholar]
- 6.Nordin BE. Assessment of calcium excretion from the urinary calcium/creatinine ratio. Lancet. 1959;2(7099):368–371. doi: 10.1016/s0140-6736(59)91635-6. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Yonou H, Hokama S, Oda M, Morozumi M, Sugaya K. Urinary saturation and risk factors for calcium oxalate stone disease based on spot and 24-hour urine specimens. Front Biosci. 2003;8:a167–a176. doi: 10.2741/1139. [DOI] [PubMed] [Google Scholar]
- 8.Ghazali S, Barratt TM. Urinary excretion of calcium and magnesium in children. Arch Dis Child. 1974;49(2):97–101. doi: 10.1136/adc.49.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaacson LC, Jackson WP. The urinary excretion of calcium and magnesium, with special reference to the urinary calcium/creatinine ratio and calcium/osmolar ratio. Clin Sci. 1963;24:223–227. [PubMed] [Google Scholar]
- 10.Wills MR. The urinary calcium–creatinine ratio as a measure of urinary calcium excretion. J Clin Pathol. 1969;22(3):287–290. doi: 10.1136/jcp.22.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyun M, Guven AG, Filiz S, Akman S, Akbas H, Baysal YE, Dedeoglu N. Screening for hypercalciuria in schoolchildren: what should be the criteria for diagnosis? Pediatr Nephrol. 2007;22 (9):1297–1301. doi: 10.1007/s00467-007-0528-9. 10.1007/s00467-007-0528-9. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 13.Colon-Emeric CS, Saag KG. Osteoporotic fractures in older adults. Best Pract Res Clin Rheumatol. 2006;20(4):695–706. doi: 10.1016/j.berh.2006.04.004. 10.1016/j.berh.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brazier M, Kamel S, Maamer M, Agbomson F, Elesper I, Garabedian M, Desmet G, Sebert JL. Markers of bone remodeling in the elderly subject: effects of vitamin D insufficiency and its correction. J Bone Miner Res. 1995;10(11):1753–1761. doi: 10.1002/jbmr.5650101119. 10.1002/jbmr.5650101119. [DOI] [PubMed] [Google Scholar]
- 15.Suitor C, Meyers L. Dietary reference intakes research synthesis: Workshop summary (2006) Food and Nutrition Board, Institute of Medicine of the National Academies. The National Academies Press; Washington DC: 2006. [Google Scholar]
- 16.Hong YH, Dublin N, Razack AH, Mohd MA, Husain R. Twenty-four hour and spot urine metabolic evaluations: correlations versus agreements. Urology. 2010;75(6):1294–1298. doi: 10.1016/j.urology.2009.08.061. 10.1016/j.urology.2009.08.061. [DOI] [PubMed] [Google Scholar]
- 17.Jones AN, Blank RD, Lindstrom MJ, Penniston KL, Hansen KE. Adjustment for body mass index and calcitrophic hormone levels improves the diagnostic accuracy of the spot urine calcium-to-creatinine ratio. Osteoporos Int. 2010;21(8):1417–1425. doi: 10.1007/s00198-009-1058-z. 10.1007/s00198-009-1058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Ziegler TE, Penniston KL, Alvig AL, Shafer MM. Do proton pump inhibitors decrease calcium absorption? J Bone Miner Res. 2010;25 (12):2510–2519. doi: 10.1002/jbmr.166. 10.1002/jbmr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yilmaz G, Yilmaz FM, Hakligor A, Yucel D. Are preservatives necessary in 24-hour urine measurements? Clin Biochem. 2008;41 (10–11):899–901. doi: 10.1016/j.clinbiochem.2008.03.002. 10.1016/j.clinbiochem.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Scott PJ, Hurley PJ. Demonstration of individual variation in constancy of 24-hour urinary creatinine excretion. Clin Chim Acta. 1968;21(3):411–414. doi: 10.1016/0009-8981(68)90069-7. 0009-8981(68)90069-7. [DOI] [PubMed] [Google Scholar]
- 21.Paterson N. Relative constancy of 24-hour urine volume and 24-hour creatinine output. Clin Chim Acta. 1967;18(1):57–58. doi: 10.1016/0009-8981(67)90245-8. [DOI] [PubMed] [Google Scholar]
- 22.Cote AM, Firoz T, Mattman A, Lam EM, von Dadelszen P, Magee LA. The 24-hour urine collection: gold standard or historical practice? Am J Obstet Gynecol. 2008;199(6):625, e621–626. doi: 10.1016/j.ajog.2008.06.009. 10.1016/j.ajog.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Bingham SA, Cummings JH. The use of creatinine output as a check on the completeness of 24-hour urine collections. Hum Nutr Clin Nutr. 1985;39(5):343–353. [PubMed] [Google Scholar]
- 24.Chandhoke PS. Evaluation of the recurrent stone former. Urol Clin North Am. 2007;34(3):315–322. doi: 10.1016/j.ucl.2007.04.007. 10.1016/j.ucl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59(6):2290–2298. doi: 10.1046/j.1523-1755.2001.00746.x. 10.1046/j.1523-1755.2001.00746.x. [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP, Recker RR, Ryan RA. Urinary calcium in perimenopausal women: normative values. Osteoporos Int. 1999;9 (1):13–18. doi: 10.1007/s001980050110. [DOI] [PubMed] [Google Scholar]
- 27.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 28.Nouvenne A, Meschi T, Prati B, Guerra A, Allegri F, Vezzoli G, Soldati L, Gambaro G, Maggiore U, Borghi L. Effects of a low-salt diet on idiopathic hypercalciuria in calcium-oxalate stone formers: a 3-mo randomized controlled trial. Am J Clin Nutr. 2010;91 (3):565–570. doi: 10.3945/ajcn.2009.28614. 10.3945/ajcn.2009.28614. [DOI] [PubMed] [Google Scholar]