Abstract
N-acetylserotonin (NAS) is synthesized from serotonin by arylalkylamine N-acetyltransferase (AANAT), which is predominantly expressed in the pineal gland and retina. NAS activates TrkB in a circadian manner and exhibits antidepressant effects in a TrkB-dependent manner. It also enhances neurogenesis in hippocampus in sleep-deprived mice. Here we report the identification of NAS derivatives that possess much more robust neurotrophic effects with improved pharmacokinetic profiles. The compound N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-2-oxopiperidine-3-carboxamide (HIOC) selectively activates TrkB receptor with greater potency than NAS. It potently protects retinas from light-induced retinal degeneration (LIRD), which is tightly coupled with pronounced TrkB activation in retinas. Pharmacokinetic studies demonstrate that this compound is stable in serum and liver microsomes. It can pass the blood–brain barrier and blood–retinal barrier. Hence, HIOC is a good lead compound for further drug development for treating retinal degenerative diseases.
Keywords: circadian rhythm, melatonin, small molecule, neurotrophins
Arylalkylamine N-acetyltransferase (AANAT) acetylates N-acetylserotonin (NAS) from the neurotransmitter serotonin in the pineal gland and retina. NAS is subsequently methylated and converted into melatonin by hydroxyindole-O-methyltransferase (HIOMT). The three components of the melatonin synthetic pathway, namely 5-HT, NAS, and melatonin, all display dramatic circadian rhythms (1). It has long been thought that NAS only acts as a precursor of melatonin in the process of melatonin biosynthesis. Recently, we demonstrated that NAS activates the TrkB receptor and exerts antidepressant effects in a TrkB-dependent manner (2). Moreover, we reported that NAS but not melatonin stimulates neurogenesis in the hippocampus of sleep-deprived mice via activating TrkB receptor in dentate gyrus (3). In addition to these findings, NAS has been shown to facilitate memory (4), regulate hypothermic body temperature (5), induce analgesia in central nervous system (6), and exert antioxidative actions (7).
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family that also includes nerve growth factor (NGF), Neurotrophin-3 (NT-3), and NT-4/5, exerts its biological functions through two transmembrane receptors: the p75 neurotrophin receptor (p75NTR) and TrkB receptor tyrosine kinase. BDNF binding to TrkB triggers its dimerization and autophosphorylation of tyrosine residues in its intracellular domain, leading to activation of the three major downstream signaling cascades, including mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and phospholipase C-γ1 (PLC-γ1) (8, 9). Through these pathways, BDNF mediates a variety of neuronal activities involved in neuronal survival, neurogenesis, and synaptic plasticity. Accumulating evidence demonstrates that neurotrophins play critical roles in the development of the retina and visual system (10–12). Limiting amounts of neurotrophins in the target area of the superior colliculus may control the apoptosis of retinal ganglion cells (RGCs) during early postnatal period. NT-4/5 or BDNF injected into superior colliculus promotes the survival of neonatal RGCs (13, 14). Intraocular injection of NGF, BDNF, and NT-4/5 rescues RGCs after axotomy (15–18). NT-4/5, in combination with other trophic factors, is involved in the postnatal survival of retinal neurons during both development and degeneration (19). Despite the fact that BDNF is not required for RGC survival during development (20), it promotes survival of RGCs in a variety of experimental lesion models both in vitro and in vivo (15, 21–24). This is consistent with the findings that RGCs express the BDNF receptor TrkB (20, 25, 26). Furthermore, BDNF is a potent stimulator of neurite growth in RGCs (27). TrkB receptors are also known to be expressed on a variety of other retinal cell types including Müller glia, horizontal cells, amacrine cells, and the retinal pigment epithelial cells (28), and BDNF has been shown to protect photoreceptors from light-induced retinal degeneration (LIRD) (29, 30).
In the present study, we have identified and characterized a NAS derivative that possesses much stronger stimulatory effect on TrkB receptor than NAS. It specifically activates TrkB but not TrkA receptor in mouse brain and retina upon i.p. administration. It exerts neuroprotective actions in a TrkB-dependent manner. Moreover, the compound strongly protects retinal photoreceptors in a LIRD animal model, and this action is associated with potent TrkB receptor activation in retinas. We also show that this compound is quite stable in serum and liver microsomes, and it can pass the blood–brain barrier (BBB) and blood–retinal barrier (BRB), and exhibits half-lives of ∼4 h and 1 h in brain and retinas, respectively.
Results
NAS Derivatives Trigger TrkB Signal Cascades in Primary Neurons.
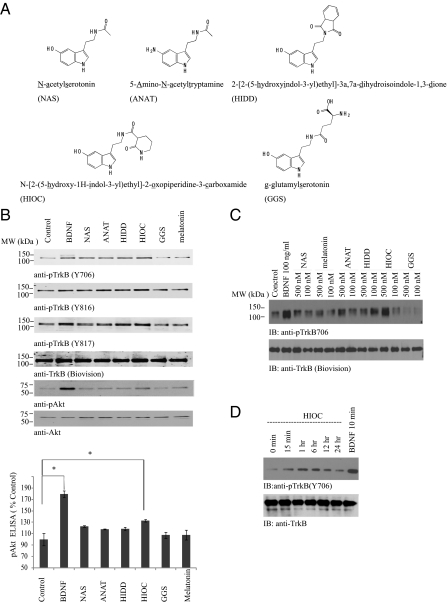
Recently, we showed that NAS but not melatonin or serotonin selectively activates TrkB but not other Trk receptors (2, 3). Nonetheless, NAS is labile, and its half-life in the circulatory system is relatively short (31). To identify more stable and robust compounds that provoke TrkB activation, we tested numerous NAS derivatives (Fig. 1A). Among these compounds, -[2-(5-hydroxy-1H-indol-3-yl)ethyl]-2-oxopiperidine-3-carboxamide (HIOC) elicited the strongest TrkB phosphorylation, which was followed by HIDD, NAS, and ANAT. By contrast, GGS or melatonin did not elevate TrkB phosphorylation compared with vehicle control (Fig. 1B, Top three). The downstream effector Akt was also robustly activated by HIOC as well (Fig. 1B, second from Bottom). The quantitative p-Akt ELISA with the neuronal cell lysates also confirmed that HIOC strongly increased Akt activation (Fig. 1B Bottom). Dosage assay revealed that HIOC elicited prominent TrkB activation at 500 nM, which was comparable to BDNF. By contrast, the other compounds including NAS exhibited much lower effect than HIOC. At 100 nM, the TrkB stimulatory activities by these compounds were marginal (Fig. 1C). We also examined HIOC's kinetic profiles in primary neuronal cultures. HIOC activated TrkB receptor and its downstream Erk1/2 in a time-dependent manner. HIOC increased p-TrkB at 15 min, and the signal increased at 1 h and peaked at 6 h; p-TrkB activity declined at 12 h and faded away at 24 h (Fig. 1D). Thus, HIOC selectively and potently activates TrkB receptor and its downstream effector Akt in neurons.
Fig. 1.
HIOC reveals the most potent TrkB activation activity in primarily cultured cortical neurons. (A) Chemical structures of NAS and its four derivatives (ANAT, HIDD, HIOC, and GGS). (B) TrkB activation and downstream signaling in primary cortical neurons treated with various compounds. Primary rat cortical neurons from E18 embryos (12 DIV) were treated with 500 nM of the NAS derivatives for 30 min. As positive controls, neurons were treated with 100 ng /mL BDNF for 10 min. The neuron cell lysates were collected and resolved by 10% SDS/PAGE. Immunoblotting was conducted with various antibodies (Upper). The neuronal lysates were also subjected to p-Akt ELISA analysis (Lower). (C) The dosage titration assay. The primary cultures were treated with 100 and 500 nM of various compounds for 30 min. The cell lysates from the neurons were analyzed by immunoblotting with p-TrkB antibody. HIOC activated TrkB much stronger at 500 nM than at 100 nM. (D) Time course assay of TrkB activation in primary cortical neurons treated with HIOC. Primary rat cortical neurons from E18 embryos (12 DIV) were treated with 500 nM compound for indicated time. As positive controls, neurons were treated with 100 ng/mL BDNF for 10 min. The neuron cell lysates were collected and resolved by 10% SDS/PAGE. Immunoblotting was conducted with various antibodies.
HIOC Activates TrkB More Potently Than NAS in Mouse Brain.
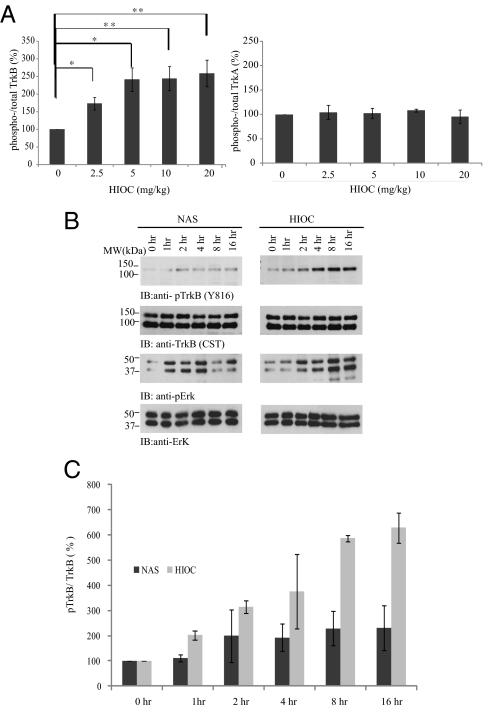
Among these NAS derivatives, HIOC exhibits the most robust TrkB stimulatory effect. As expected, HIOC displays robust neuroprotection against glutamate excitotoxicity in primary neuronal cell cultures (Fig. S1). Thus, we chose this compound to focus on our following studies. To assess whether HIOC can provoke TrkB activation in the brain, we injected mice (i.p.) with different doses of the compounds, killed the animals at 2 h, and monitored TrkB and TrkA activity in the brain lysates by immunoblotting. Quantitative analysis of TrkB and TrkA phosphorylation signals showed that TrkB but not TrkA was selectively phosphorylated in the brain in a dose-dependent manner. Maximal TrkB activation was observed with 5–20 mg/kg of HIOC (Fig. 2A). Hence, HIOC specifically activates TrkB receptor in mouse brain. Kinetic analysis demonstrated that both NAS and HIOC activated TrkB receptor at 1–2 h. NAS-induced TrkB phosphorylation reached a peak at about 2 h, and the p-TrkB 816 signals were still detectable at 16 h. Accordingly, Erk1/2 was also markedly activated by NAS at 1 h; activation was pronounced up to 4 h after injection and decreased thereafter (Fig. 2B Left Top and Upper Middle). HIOC induced longer lasting activation of TrkB and Erk1/2 than did NAS. Activation of TrkB was observed within 1 h of HIOC injection and peaked between 8 and 16 h. This temporal pattern tightly correlated with the downstream MAPK activation in the mouse brain tissues (Fig. 2B Right Top and Upper Middle). The quantitative analysis of the ratios of p-TrkB/total TrkB in mouse brain is shown (Fig. 2C). Hence, these data suggest that NAS and HIOC can penetrate the BBB and selectively activate TrkB.
Fig. 2.
HIOC activates TrkB receptor in animals. (A) HIOC activates TrkB in a dose-dependent manner in vivo. C57BL/6J mice were killed at 2 h after i.p. injection with indicated dose of HIOC and hippocampal lysates were analyzed by immunoblotting with anti-pTrkB (Y816), anti-pTrkA (Y785), anti-TrkB, and anti-TrkA antibodies. The ratios of p-TrkB/total TrkB and p-TrkA/total TrkA were calculated according to the specific bands using Image J software. HIOC activated TrkB in a dose-dependent manner, whereas it did not activate TrkA receptor (n = 4)(mean ± SEM, *P < 0.05, **P < 0.01, One-way ANOVA). (B and C) HIOC reveals long lasting TrkB activation activity than NAS in vivo. C57BL/6J mice were i.p. injected with 20 mg/kg of NAS or HIOC for indicated time, and hippocampal and cortical lysates were analyzed by immunoblotting with various antibodies. The ratios of p-TrkB/total TrkB were calculated according to the density of specific bands using Image J software. The data were from two sets of replicated experiments (n = 3; mean ± SEM).
HIOC Protects Neurons From Excitotoxicity in a TrkB-Dependent Manner.
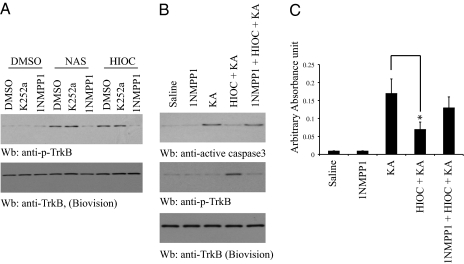
To explore whether HIOC like NAS could exert its neuroprotective actions via triggering TrkB activation in vivo, we used TrkB F616A knock-in mice, where it has been shown before that TrkB F616A can be selectively blocked by 1NMPP1, leading to a TrkB-null phenotype (32). To assess whether HIOC could mimic NAS, we prepared cortical neurons from TrkB F616A knock-in mice. As expected, HIOC-provoked TrkB phosphorylation was selectively reduced by 1NMPP1, but not by K252a. As a positive control, NAS exhibited the similar pattern (Fig. 3A). These findings suggest that HIOC strongly provokes both wild-type TrkB and TrkB F616A tyrosine phosphorylation. To address whether HIOC exerts a neuroprotective effect in vivo, we used the kainic acid (KA) excitotoxicity model in TrkB F616A knock-in mice. We hypothesized that blockade of TrkB F616A signaling by 1NMPP1 in mice would make the neurons vulnerable to KA-provoked neuronal cell death. Injection of KA caused marked caspase-3 activation in the brain (Fig. 3 B and C). HIOC administration suppressed KA-induced caspase-3 activation and 1NMPP1 pretreatment greatly diminished HIOC's protective effect in TrkB F616A mice. Caspase-3 activation was inversely correlated with TrkB activation by HIOC (Fig. 3B). Hence, these data indicate that HIOC selectively activates TrkB receptor and enhances neuronal survival in mice in a TrkB-dependent manner.
Fig. 3.
HIOC exerts its neuroprotective actions via TrkB receptor. (A) HIOC activates TrkB F616A mutant. Cortical neurons from TrkB F616A knockin mice were prepared and pretreated with 1NMPP1 (100 nM) for 2 h, followed by stimulation with NAS and its derivative HIOC. Cell lysates were analyzed by immunoblotting with anti-p-TrkB. (B) HIOC suppresses kainic acid (KA)-induced neuronal cell death in TrkB F616A mutant mice, which can be blocked by 1NMPP1. TrkB F616A mice were pretreated with 1NMPP1 (50 μM in drinking water) or water 1 d before the experiment. HIOC (20 mg/kg, i.p.) was injected into TrkB F616A mice 1 h before KA (20 mg/kg). Brain lysates were prepared 4 h after KA treatment and analyzed by immunoblotting with anti-active caspase 3 and anti-p-TrkB. (C) Caspase-3 ELISA. The above brain lysate samples were subjected to quantitative active caspase-3 ELISA analysis. The data were from three sets of replicated experiments (mean ± SEM). (*P < 0.05, Student t test).
HIOC Preserves Visual Function and Photoreceptor Morphology in a LIRD Animal Model.
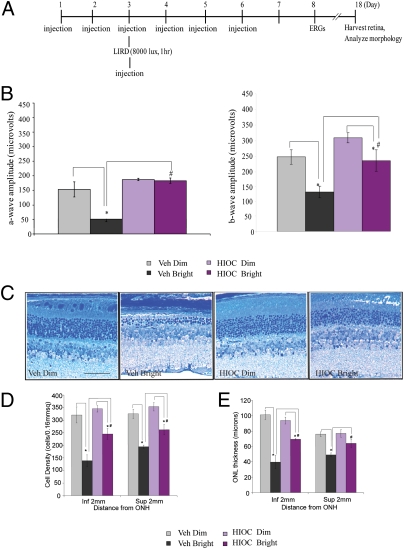
Since NAS is mainly generated in pineal gland and retinas, we extended our neuroprotective studies into retinas. To test the possible neuroprotective actions, we examined the effects of HIOC on LIRD in Balb/C mice. The experimental schematic plan is outlined in Fig. 4A. We observed significant reductions in scotopic a- and b-wave amplitudes (67% and 50%, respectively) in mice exposed to bright light compared with dim light controls (P < 0.001). In animals injected with HIOC, the reduction in a-wave and b-wave ERG amplitudes was 2 and 27%, respectively, compared with mice treated with HIOC under dim light (P < 0.001) (Fig. 4B). Therefore, treatment with 40 mg/kg of HIOC (i.p.) significantly attenuated loss of visual function as assessed by ERG a-wave and b-wave amplitudes. Bright light also triggered marked reduction of outer nuclear layer (ONL) thickness and loss of photoreceptor outer segments compared with the dim light controls (Fig. 4 C–E). Strikingly, HIOC inhibited the light-induced photoreceptor disruption (Fig. 4C). Quantitative analysis of cell density of ONL cells at a distance of 2 mm superior and inferior from the optical nerve head (ONH) showed that LIRD resulted in significant photoreceptor cell loss in inferior and superior retina, in both vehicle-treated animals and in HIOC-treated animals (*P < 0.05). However, vehicle-treated animals had much more photoreceptor cell loss than HIOC-treated animals subjected to LIRD (# P < 0.05) (Fig. 4D). We also measured the ONL thickness and found similar protective effects in HIOC-treated animals (Fig. 4E). Hence, these data indicate that HIOC protects the retinas from bright light-induced cell loss.
Fig. 4.
HIOC mitigates retinal damage induced by bright light. (A) Schematic experimental schedule. BALB/c mice (n = 4–5 per group) were administered i.p. injections of 40 mg/kg of HIOC once daily, for 6 consecutive days. On day 3, they were exposed to bright light (1 h at 8,000 lux) in a cylindrical light damage apparatus. HIOC was injected 30 min before and after light exposure. Electroretinogram (ERG) recordings were conducted on day 8 and retinas were harvested on day 18 to determine the extent of cellular and tissue damage. (B) ERG recordings. ERG a-wave amplitudes (Left) were measured at a flash intensity of 6.28 cd-s /m2. Light-induced retinal damage (LIRD) results in significant degradation of ERG a-wave amplitude in vehicle-treated animals (*P < 0.05), but not in HIOC treated animals. Furthermore, vehicle-treated animals have degraded ERG a-wave amplitudes compared with HIOC treated animals subjected to LIRD (#P < 0.05). ERG b-wave (Right) amplitudes were measured at a flash intensity of 6.28 cd-s/m2. LIRD results in significant degradation of ERG b-wave amplitude in both vehicle-treated animals and in HIOC-treated animals (*P < 0.05). Furthermore, vehicle-treated animals have degraded ERG b-wave amplitudes compared with HIOC treated animals subjected to LIRD (#P < 0.05). (C) Toluidine blue stain of retinas. Eyes were harvested, fixed in 2.5% glutaraldehyde/sodium cacodylate buffer, and processed for toluidine blue staining. Vertical sections (10 μm) of the retina were obtained through the optic nerve head (ONH) for eyes from all experimental groups of mice. (D) The cell density of outer nuclear layer (ONL) cells was measured at a distance of 2 mm superior and inferior from the ONH. LIRD results in significant photoreceptor cell loss in inferior and posterior retina, in both vehicle-treated animals and in HIOC treated animals (*P < 0.05). Further, vehicle-treated animals have more photoreceptor cell loss compared with HIOC treated animals subjected to LIRD (#P < 0.05). (E) The thickness of the ONL was measured at a distance of 2mm superior and inferior from the ONH. LIRD results in reduced ONL thickness in the inferior retina in both vehicle-treated animals and in HIOC treated animals (*P < 0.05). LIRD results in reduced ONL thickness in the posterior retina only in vehicle-treated animals (*P < 0.05), but not in HIOC-treated animals. Further, vehicle-treated animals have much thinner ONLs in inferior and superior retina compared with HIOC treated animals subjected to LIRD (# P < 0.05).
HIOC Strongly Activates TrkB Receptor in Retinas.
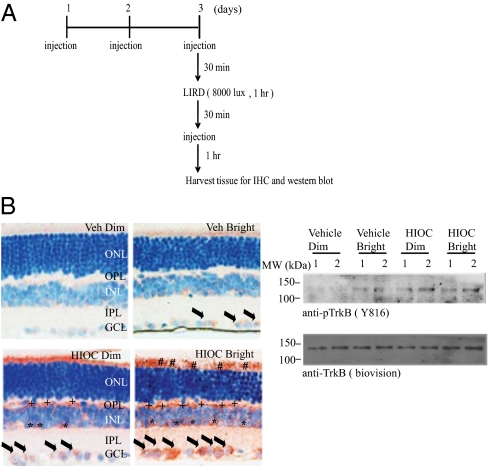
To assess whether the neuroprotective actions by HIOC is associated with TrkB receptor activation in the central nervous system (CNS), we monitored TrkB phosphorylation in the mice by immunohistochemistry and immunoblotting. The experimental schematic plan is summarized in Fig. 5A. Compared with dim light vehicle control, bright light slightly elevated TrkB phosphorylation signals mainly in ganglion cell layer (GCL) (Fig. 5B Left). Notably, HIOC treatment strongly increased p-TrkB signals in an area just above the outer nuclear layer, corresponding to the location of the photoreceptor inner segments, at the border of the inner nuclear (INL) and outer plexiform layer (OPL) and in the GCL. The TrkB receptor activation was further increased in HIOC-treated retina upon bright light stimulation. We also determined p-TrkB signals by immunoblotting. Similar p-TrkB activation patterns were observed in HIOC-treated retinas versus vehicle control (Fig. 5B Right).
Fig. 5.
HIOC activates TrkB receptor in the retinas. (A) Schematic experimental design. BALB/c mice were administered i.p. injections of 40 mg/kg of HIOC once daily, for 3 consecutive days. On day 3, they were exposed to bright light (1 h at 8,000 lux) in a cylindrical light damage apparatus. HIOC was injected 30 min before and after light exposure. One hour after the second administration of HIOC, mice were killed. Retinas from two mice per group were harvested for Western blotting. Two mice per group for immunohistochemistry staining were perfused with 4% PFA, and tissues were harvested for paraffin sections. (B) HIOC activates TrkB receptor in the retinas. Immunohistochemistry staining was conducted (Left). Phospho-TrkB was detected in ganglion cells (black arrows), possibly amacrine cells, and Müller glial cells (asterisks), in the outer plexiform layer (+ signs; possibly Müller glial and microgial processes), and in outer segments (# signs). Phospho-TrkB was demonstrable to some extent in HIOC-treated animals exposed to dim light (HIOC, Dim) and in some ganglion cells in vehicle-treated animals exposed to bright light (Vehicle, Bright). The nucleus was stained with hematoxylin. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. TrkB in retinas was potently activated by HIOC using Western blot assay. Fifty micrograms of retinal lysates were subjected to 10% SDS/PAGE and immunoblotted with anti-pTrkB (Y816) and anti-TrkB.
HIOC Is Stable in Vitro and Displays Longer Half-Life Than NAS in Mouse Brain.
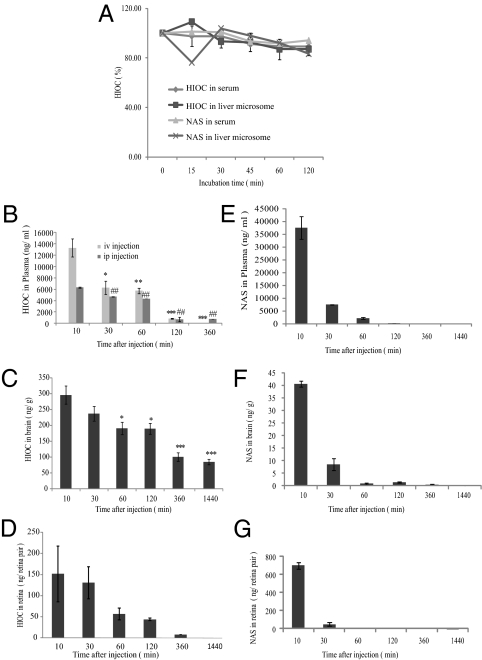
Both melatonin and NAS are labile and their half-lives in plasma are ∼30 min or less in rats (31, 33). To investigate whether HIOC is more stable and has longer half-life, we incubated HIOC with mouse serum and liver microsomes, respectively, extracted the compounds at several time points, and analyzed the concentrations of HIOC by HPLC. We found that, over the 120-min incubation period, the concentrations of HIOC remained relatively stable (Fig. 6A). We also extended our experiments into animals. We extracted the compounds from the plasma at different time points after i.p. injection of 40 mg/kg of HIOC. Quantitative analysis demonstrated that 10 min after i.p. injection, HIOC reached 6,200 ng/mL in the plasma, and its concentrations declined gradually from 30 to 60 min (Fig. 6B). The concentration sharply declined from 60 min to 120 min. The compound completely disappeared at 24 h. We made similar observations for the i.v. injected HIOC. However, 10 min after i.v. injection, the HIOC plasma concentration was approximately 13,000 ng/mL, and it decayed swiftly at 30–60 min (Fig. 6B). We also examined HIOC concentrations in mouse brain (Fig. 6C). At 10 min, about 300 ng/g of HIOC was detected in wet brain tissue after i.p. administration. The concentrations progressively decreased from 30 min to 120 min (from 250 to 195 ng/g). Remarkably, the compound was still detectable in brain 24 h after injection. The half-life for HIOC in brain tissues was estimated to be ∼4 h, but did not follow first order kinetics (Fig. 6C). We also assessed its pharmacokinetic profile in retinas. The half-life for HIOC in retina is estimated to be ∼45 min (Fig. 6D). We have also monitored NAS pharmacokinetic profiles in plasma, brain and retinas (Fig. 6 E–G). Clearly, HIOC is much stable than NAS. Moreover, it can penetrate the BBB and BRB, and distributes meaningful doses into the CNS to trigger TrkB receptor activation.
Fig. 6.
Pharmacokinetic profiles of HIOC. (A) HIOC and NAS stability assay in mouse serum and liver microsomes in vitro. HIOC and NAS were incubated in mouse serum or liver microsomes for 15, 30, 45, 60 and 120 min at 37 ºC. Data present mean of 2–3 samples per time point ± SEM of two duplicated samples. (B–D) Pharmacokinetics of HIOC in the plasma (B) (n = 3; Results were expressed as mean ± SEM, *, **, or ##, P <0.01; *** or ##, P < 0.001, compared the groups detected at 10 min; one-way ANOVA), the brain (C) (n = 3; Results were expressed as mean ± SEM, *, ** and ***P < 0.01, One-way ANOVA) and the retinas (D) after i.p. injection. Adult BALB/c mice (n = 3 per time point) were i.p. injected with 40 mg/kg of HIOC. Blood, retinas and brains were collected at 10, 30, 60, 120, 360, and 1,440 min after single injection. (E–G) Pharmacokinetics of NAS in the plasma (E), the brain (F), and the retinas (G) after i.p. injection. Adult BALB/c mice (n = 3 per time point) were i.p. injected with 90 mg/kg of NAS. Blood, retinas, and brains were collected at 10, 30, 60, 120, 360, and 1,440 min after a single injection. Data for B–G represent mean of three animals per time point ± SEM.
Discussion
We have identified and characterized a NAS derivative HIOC that exhibits a much stronger effect in promoting TrkB activation in mouse brain and retinas. Moreover, it selectively triggers TrkB but not TrkA activation and exerts its neuroprotective actions in a TrkB-dependent way. Furthermore, we found that HIOC prevents retinal degeneration induced by LIRD, and this effect is closely correlated with TrkB activation in retina.
HIOC is much more stable than NAS and it displays a half-life in mouse brain of ∼4 h. The discrepant half-lives for HIOC in plasma, brain, and retinas may reflect different biochemical machinery implicated in metabolizing this compound. It has been shown before that NAS can be modified through glucuronidation and sulfation on hydroxy group of the indole ring. Moreover, the acetylamide group can be oxidized into 5-HIAL or cyclic NAS with glucuronidation (34). Conceivably, the hydroxy group in HIOC can also be modified through glucuronidation and sulfation, which might account for its relatively short half-life in the plasma. Nonetheless, the acetylamide group of NAS is replaced with a spatial 2-oxopiperidine-3-carboxamide group in HIOC, which is much bigger in size. It could exert steric hindrance to block the hydrolysis of the amide bond to prevent 5-HIAL formation or cyclization, resulting in stabilization of HIOC. The escalated stability of HIOC might shed light on its sustained TrkB stimulatory effect in mouse brain (Fig. 2B).
A large number of studies support the findings that BDNF strongly protects the retina from damaging stimuli. For instance, BDNF protects retinal neurons against ischemia-induced and light-induced damage (29, 35, 36). It also promotes the survival and regeneration of axotomized retinal ganglion cells (RGCs) (17, 37). Moreover, pre-exposure of the retina to BDNF has a neuroprotective effect against the KCN-triggered retinal degeneration (38). Our findings support the hypothesis that NAS but not serotonin or melatonin mimics BDNF and activates TrkB receptors in the brain and retina. Moreover, it would be beneficial to use this small compound and its derivatives in recipients, rather than BDNF, which has poor BBB penetration capability and is rapidly degraded in the circulatory system (39). We report here that HIOC is a stable and potent NAS derivative. It can pass the BBB and promote TrkB activation in retina (Figs. 5 and 6). It does not trigger TrkB degradation in primary neurons even 24 h after incubation (Fig. 1D), whereas BDNF induces swift TrkB polyubiquitination and degradation (40, 41). Hence, it exerts pronounced neuroprotective effect against excitotoxicity and LIRD (Figs. 3 and 4). Our data demonstrate that HIOC can protect the retina from light-induced damage. Since BDNF/TrkB signalings are implicated in various neurological diseases, identification of HIOC as a stable and potent stimulator that activates TrkB receptors provides a promising lead compound for further medicinal chemistry and drug development for various neurodegenerative diseases in addition to retinal degeneration.
Materials and Methods
Reagents, Cells, and Mice.
BDNF was from PeproTech. Anti-TrkA, Phospho-Akt-473, anti-Akt, and anti-phospho-Erk1/2 antibodies were from Cell Signaling. Anti-p-TrkB Y817 antibody was from Epitomics. Anti-TrkB antibody was from Biovision and Cell Signaling. Phospho-TrkB Y816 antibody has been characterized and reported (3, 42). The anti-serum was affinity purified. This phospho-TrkB Y816 was used for immunostaining the brain and retinal sections. Phospho-Akt 473 Sandwich ELISA was from Cell Signaling. TrkB F616A mice and wild-type C57BL/6 mice were bred in a pathogen-free environment. Animal experiments were conducted according to the institutional ethical guidelines for animal experiments and approved by the Institutional Animal Care and Use Committee at Emory University.
Primary Rat Cortical Neuron Culture.
Primary rat cortical neurons were prepared as follows. E18 rat pups were decapitated and cortex was extirpated, cross chopped and suspended by pipetting for separation in 5% (vol/vol) FCS, 5% (vol/vol) horse serum (HS) DMEM. The cell suspension was then centrifuged at 250 × g for 5 min. This operation was repeated. Cells were seeded into polyethyleneimine-coated six-well plates at 37 ºC in 5% (vol/vol) CO2 and 95% (vol/vol) air. After 3 h, the culture medium was changed to Neurobasal containing B-27 supplement (Invitrogen) and incubated for 4 d. For maintenance, half of the medium is changed to fresh Neurobasal/ B27 every 4 d. After 12 d, the cultured neurons were used in various experiments.
Statistical Analysis.
Data are presented as mean ± SEM. Statistical evaluation was carried out by Student t test or two-way analysis of variance with Tukey multiple comparison test. Data were considered statistically significant when P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. David Ginty at Johns Hopkins University for TrkB knock-in mice. This work is supported by National Institutes of Health Grants R01 CA127119 (to K.Y.) and R01 EY004864 and P30 EY006360 (to P.M.I.), and an unrestricted departmental grant from Research to Prevent Blindness (RPB). P.M.I. is a recipient of a Senior Scientific Investigator Award from RPB.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119201109/-/DCSupplemental.
References
- 1.Chattoraj A, Liu T, Zhang LS, Huang Z, Borjigin J. Melatonin formation in mammals: In vivo perspectives. Rev Endocr Metab Disord. 2009;10:237–243. doi: 10.1007/s11154-009-9125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang SW, et al. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc Natl Acad Sci USA. 2010;107:3876–3881. doi: 10.1073/pnas.0912531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sompol P, et al. N-acetylserotonin promotes hippocampal neuroprogenitor cell proliferation in sleep-deprived mice. Proc Natl Acad Sci USA. 2011;108:8844–8849. doi: 10.1073/pnas.1105114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satake N, Morton BE. Scotophobin A causes dark avoidance in goldfish by elevating pineal N-acetylserotonin. Pharmacol Biochem Behav. 1979;10:449–456. doi: 10.1016/0091-3057(79)90216-8. [DOI] [PubMed] [Google Scholar]
- 5.Morton DJ. Both hydroxy- and methoxyindoles modify basal temperature in the rat. J Pineal Res. 1987;4:1–5. doi: 10.1111/j.1600-079x.1987.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 6.Psarakis S, Brown GM, Grota LJ. Analgesia induced by N-acetylserotonin in the central nervous system. Life Sci. 1988;42:1109–1116. doi: 10.1016/0024-3205(88)90567-x. [DOI] [PubMed] [Google Scholar]
- 7.Yan XY. [Freeze fracture study of the retinal pigment epithelium of the chick embryo] Zhonghua Yan Ke Za Zhi. 1988;24:168–170. [PubMed] [Google Scholar]
- 8.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 9.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parada LF, et al. The Trk family of tyrosine kinases: Receptors for NGF-related neurotrophins. Cold Spring Harb Symp Quant Biol. 1992;57:43–51. doi: 10.1101/sqb.1992.057.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 12.von Bartheld CS. Neurotrophins in the developing and regenerating visual system. Histol Histopathol. 1998;13:437–459. doi: 10.14670/HH-13.437. [DOI] [PubMed] [Google Scholar]
- 13.Cui Q, Harvey AR. At least two mechanisms are involved in the death of retinal ganglion cells following target ablation in neonatal rats. J Neurosci. 1995;15:8143–8155. doi: 10.1523/JNEUROSCI.15-12-08143.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma YT, Hsieh T, Forbes ME, Johnson JE, Frost DO. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J Neurosci. 1998;18:2097–2107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6:3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmignoto G, Maffei L, Candeo P, Canella R, Comelli C. Effect of NGF on the survival of rat retinal ganglion cells following optic nerve section. J Neurosci. 1989;9:1263–1272. doi: 10.1523/JNEUROSCI.09-04-01263.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602:304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- 18.Sawai H, Clarke DB, Kittlerova P, Bray GM, Aguayo AJ. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J Neurosci. 1996;16:3887–3894. doi: 10.1523/JNEUROSCI.16-12-03887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harada C, et al. Role of neurotrophin-4/5 in neural cell death during retinal development and ischemic retinal injury in vivo. Invest Ophthalmol Vis Sci. 2005;46:669–673. doi: 10.1167/iovs.04-0826. [DOI] [PubMed] [Google Scholar]
- 20.Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced size of retinal ganglion cell axons and hypomyelination in mice lacking brain-derived neurotrophic factor. Mol Cell Neurosci. 1997;9:397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- 21.Thanos S, Bähr M, Barde YA, Vanselow J. Survival and axonal elongation of adult rat retinal ganglion cells. Eur J Neurosci. 1989;1:19–26. doi: 10.1111/j.1460-9568.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 22.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci USA. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 24.Peinado-Ramón P, Salvador M, Villegas-Pérez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 25.Jelsma TN, Friedman HH, Berkelaar M, Bray GM, Aguayo AJ. Different forms of the neurotrophin receptor trkB mRNA predominate in rat retina and optic nerve. J Neurobiol. 1993;24:1207–1214. doi: 10.1002/neu.480240907. [DOI] [PubMed] [Google Scholar]
- 26.Rickman DW, Brecha NC. Expression of the proto-oncogene, trk, receptors in the developing rat retina. Vis Neurosci. 1995;12:215–222. doi: 10.1017/s0952523800007896. [DOI] [PubMed] [Google Scholar]
- 27.Bonnet D, et al. Brain-derived neurotrophic factor signalling in adult pig retinal ganglion cell neurite regeneration in vitro. Brain Res. 2004;1007:142–151. doi: 10.1016/j.brainres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci. 1999;19:8919–8930. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaVail MM, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 30.Gauthier R, Joly S, Pernet V, Lachapelle P, Di Polo A. Brain-derived neurotrophic factor gene delivery to muller glia preserves structure and function of light-damaged photoreceptors. Invest Ophthalmol Vis Sci. 2005;46:3383–3392. doi: 10.1167/iovs.05-0362. [DOI] [PubMed] [Google Scholar]
- 31.Chan MY, Pang SF, Tang PL, Brown GM. Studies on the kinetics of melatonin and N-acetylserotonin in the rat at mid-light and mid-dark. J Pineal Res. 1984;1:227–236. doi: 10.1111/j.1600-079x.1984.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki Y, Lynch HJ, Wurtman RJ. Melatonin in rat pineal, plasma, and urine: 24-hour rhythmicity and effect of chlorpromazine. Endocrinology. 1976;98:1418–1424. doi: 10.1210/endo-98-6-1418. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Chen C, Krausz KW, Idle JR, Gonzalez FJ. A metabolomic perspective of melatonin metabolism in the mouse. Endocrinology. 2008;149:1869–1879. doi: 10.1210/en.2007-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unoki K, LaVail MM. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994;35:907–915. [PubMed] [Google Scholar]
- 36.LaVail MM, et al. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang CW, et al. CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury. Invest Ophthalmol Vis Sci. 2005;46:1497–1503. doi: 10.1167/iovs.04-0664. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda K, et al. BDNF attenuates retinal cell death caused by chemically induced hypoxia in rats. Invest Ophthalmol Vis Sci. 1999;40:2130–2140. [PubMed] [Google Scholar]
- 39.Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- 40.Geetha T, Jiang J, Wooten MW. Lysine 63 polyubiquitination of the nerve growth factor receptor TrkA directs internalization and signaling. Mol Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Arévalo JC, et al. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 42.Jang SW, et al. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PLoS ONE. 2010;5:e11528. doi: 10.1371/journal.pone.0011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.