Abstract
Dysregulation in cellular redox systems results in accumulation of reactive oxygen species (ROS), which are causally associated with a number of disease conditions. Transforming growth factor β-activated kinase 1 (TAK1) is a signaling intermediate of innate immune signaling pathways and is critically involved in the redox regulation in vivo. Ablation of TAK1 causes accumulation of ROS, resulting in epithelial cell death and inflammation. Here we determine the mechanism by which TAK1 kinase is activated in epithelial tissues. TAB1 and TAB2 are structurally unrelated TAK1 binding protein partners. TAB2 is known to mediate polyubiquitin chain-dependent TAK1 activation in innate immune signaling pathways, whereas the role of TAB1 is not defined. We found that epithelial-specific TAB1 and TAB2 double- but not TAB1 or TAB2 single-knockout mice phenocopied epithelial-specific TAK1 knockout mice. We demonstrate that phosphorylation-dependent basal activity of TAK1 is dependent on TAB1. Ablation of both TAB1 and TAB2 diminished the activity of TAK1 in vivo and causes accumulation of ROS in the epithelial tissues. These results demonstrate that epithelial TAK1 activity is regulated through two unique, TAB1-dependent basal and TAB2-mediated stimuli-dependent mechanisms.
Oxidative stress is the major cause of chronic inflammatory diseases such as inflammatory bowel disease and is associated with a number of other diseases including cancers (1–3). Reactive oxygen species (ROS) are constantly generated through mitochondrial respiration in a cell-intrinsic manner and epithelial cells are additionally exposed to exogenous ROS from environmental factors such as commensal bacteria. ROS are normally reduced through antioxidant enzymes including superoxide dismutase and catalase as well as scavenger glutathione. Dysregulation in this cellular redox system causes oxidative stress and inflammation. Several proteins are known to be critically involved in the cellular redox regulation. Transcription factors NF-κB, AP-1, and Nrf2 transcriptionally regulate antioxidant enzymes and deletion of these genes increases oxidative stress in several tissues (4–9). Transforming growth factor β-activated kinase 1 (TAK1) is one of the major upstream activators of NF-κB and AP-1 in cytokine and Toll-like receptor pathways (10–13), and we recently reported that ablation of TAK1 down-regulates the level of Nrf2 (14). Intestinal epithelium- and epidermal-specific deletion of TAK1 causes accumulation of ROS, resulting in epithelial cell death and inflammation (14–16). However, it is not determined how TAK1 is appropriately activated in vivo to prevent ROS accumulation.
TAK1 kinase is a member of mitogen-activated protein kinase kinase kinases, which is an indispensable intermediate of several cytokine and Toll-like receptor pathways (12, 13, 17,19). TAK1 is recruited to and activated by the receptor proximal complex of TNF, IL-1, and Toll-like receptors through a polyubiquitin chain-mediated mechanism (17). TAK1 binding protein TAB2 confers ubiquitin binding domains and tethers TAK1 to the polyubiquitin chain (20–22). TAB3 is a closely related protein of TAB2 and can also recruit TAK1 to the polyubiquitin chain (22–26). Thus, TAB2 and TAB3 may redundantly function to activate TAK1. However, deficiency of TAB2 down-regulates NF-κB activation (21, 27), and TAB2 germ-line knockout causes embryonic lethality (28). Thus, it is likely that TAB2 plays a predominant role in TAK1 activation in some cell types. The specific roles of TAB2 in TAK1 activation in in vivo tissues still remain to be determined.
TAK1 has another binding partner, TAB1, which is structurally unrelated to TAB2 and binds to TAK1 at a site different from the TAB2 binding site (29, 30). TAB1 is found to be constantly associated with TAK1 and can highly activate TAK1 kinase activity when exogenously expressed together with TAK1 in cultured cells (29, 31). Co-overexpression of TAK1 and TAB1 causes oligomerization of TAK1 protein and induces autophosphorylation of TAK1, which is known to be required for TAK1 activation (30–32). However, TAB1 deficiency does not impair TNF-, IL-1–, and Toll-like receptor-induced NF-κB or AP-1 pathways (10, 33). Thus, TAB1 is dispensable for the TAB2/3-mediated polyubiquitin mechanism for TAK1 activation. We recently reported that ablation of TAB1 reduces the osmotic stress-induced TAK1-JNK pathway in mouse embryonic fibroblasts (MEFs) (33). Thus, TAB1 mediates TAK1 activation in pathways different from TAB2, but the specific role of TAB1 in TAK1 activation in vivo is still elusive.
Phosphorylation of TAK1 within the activation loop of the kinase is absolutely required for TAK1 activity (30, 31, 34), which is a conserved activation mechanism in many protein kinases (35). When TAK1 is activated, TAK1 autophosphorylates its activation loop through conformational changes that are induced by the association with a complex of TAB2-polyubiquitin chains (20) or by TAB1-dependent oligomerization (30, 33). Phosphorylation is known to be regulated not only by kinases but also by phosphatases. Indeed, phosphorylation of TAK1 is modulated by types 2A and 2C protein phosphatases including protein phosphatase 6 (PP6) (34), protein phosphatase 2A (PP2A) (36), and protein phosphatase 2C (37, 38). Inhibition of type 2A protein phosphatases up-regulates TAK1 phosphorylation and activity even under unstimulated conditions (34), indicating that TAK1 may be constantly phosphorylated and activated, but at the same time constantly deactivated by protein phosphatases. The role and regulation of protein phosphatase-dependent TAK1 deactivation are not yet known.
In this study, we investigated whether TAB2- or TAB1-mediated mechanisms are critically involved in the maintenance of TAK1 activity and the reduction of ROS in the epithelial tissues. Because epithelial tissues constantly express several cytokines including TNF and IL-1 and are exposed to Toll-like receptor ligands from commensal bacteria, we initially anticipated that Tab2 deletion would at least partially impair TAK1 activation and would cause similar abnormalities to those caused by TAK1 single deletion in the epithelial tissues. However, we did not observe any abnormalities in the Tab2-deficient epidermis or intestinal epithelium as shown in this study. We also found that the Tab1-deficient epidermis or intestinal epithelium did not exhibit any abnormalities as also shown later. We next hypothesized that both TAB1 and TAB2 participate in the activation of TAK1 and generated and characterized the epidermis and the intestinal epithelium having Tab1 and Tab2 double deletion.
Results and Discussion
Epithelial-Specific Double Deletion of Tab1 and Tab2 Phenocopies Epithelial-Specific Tak1 Knockout Mice.
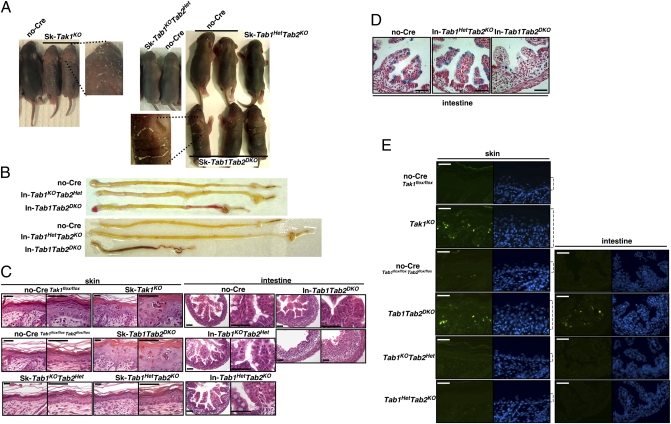
To determine the role of TAB1 and TAB2 in the epidermis and in the intestinal epithelium, we used the keratin 5-Cre epidermal-specific gene deletion system (K5-Cre) (39) and the villin-Cre intestinal epithelial-specific gene deletion system (Vil-Cre) (40), which are the same systems we used for characterization of TAK1 deficiency (16, 41). We generated and characterized K5-Cre and Vil-Cre transgenic mice having a Tab1 and Tab2 double-floxed allele (K5-Cre or Vil-Cre Tab1flox/flox Tab2flox/flox, skin- or intestinal epithelial-specific double knockouts, Sk- or In-Tab1Tab2DKO), and control littermate mice having Tab1 homo and Tab2 hetero-floxed (K5-Cre or Vil-Cre Tab1flox/flox Tab2flox/+, skin- or intestinal epithelial-specific single Tab1 knockouts, Sk- or In-Tab1KOTab2Het), and Tab1 hetero and Tab2 homo-floxed (K5-Cre or Vil-Cre Tab1flox/+ Tab2flox/flox, skin- or intestinal epithelial-specific single Tab2 knockouts, Sk- or In-Tab1HetTab2KO) and no-Cre (Tab1flox/flox Tab2flox/flox) mice. We analyzed three and five independent litters of each epidermal and intestinal epithelial version of single- and double-knockout neonatal mice and compared them with epidermis and intestinal epithelium-specific TAK1 single-knockout, K5-Cre and Vil-Cre Tak1flox/flox (Sk-Tak1KO and In-Tak1KO), mice. Either epidermal- or intestinal epithelium-specific single knockout, Tab1KOTab2Het or Tab1HetTab2KO, mice did not exhibit any abnormality. The reduction of proteins in the knockout epidermis was confirmed by immunoblots as shown later, and the reduction of Tab1 and Tab2 mRNA in the intestinal epithelium was confirmed in In-Tab1KOTab2Het and In-Tab1HetTab2KO (Fig. S1). We found that, although Sk- and In-Tab1Tab2DKO were born at a Mendelian ratio, they exhibited severe tissue damage at postnatal day 5 (P5) for Sk-Tab1Tab2DKO and at P0 for In-Tab1Tab2DKO, which resembles the damage found for Sk- and In-Tak1KO (Fig. 1). We observed inflexible skin with scaling in Sk-Tab1Tab2DKO (Fig. 1A). The intestine was shorter in In-Tab1Tab2DKO mice than those in single- or no-Cre littermate mice and hemorrhage was frequently observed in the double-deficient intestine (Fig. 1B), indicating severe structural damage in the intestine. Because the damage was very severe, we were not able to obtain tissue samples beyond P5 in Sk-Tab1Tab2DKO and P0 in In-Tab1Tab2DKO, and we assume that they are lethal at P6–8 in Sk-Tab1Tab2DKO and at P1–2 in In-Tab1Tab2DKO, which are very similar to Sk- and In-Tak1KO mice (16, 41). The Sk-Tab1Tab2DKO epidermis was hyperplastic and intraepidermal microabscesses and thickened hyperkeratotic stratum corneum were observed (Fig. 1C). The architecture of villi in the small intestine was found to be abnormal in all In-Tab1Tab2DKO intestines or completely destroyed in some In-Tab1Tab2DKO (Fig. 1C, Lower Right) intestines, and widespread infiltration of immune cells was observed at P0. The number of goblet cells was reduced in In-Tab1Tab2DKO but still detectable at P0 (Fig. 1D), which is also similar to that in In-Tak1KO (16). Apoptotic cells were highly increased in both Tab1 and Tab2 double-deficient epidermis and intestinal epithelium, which was also identical to Tak1 deficiency (Fig. 1E). Apoptotic cells were abundantly found in the crypts of Tab1 and Tab2 double-deficient intestine, and goblet cells as well as other epithelial cells underwent apoptosis (Fig. S2). These results demonstrate that double deletions of Tab1 and Tab2 cause the same abnormalities as single deletion of Tak1 in the epidermis and the intestinal epithelium.
Fig. 1.
Double deficiency of Tab1 and Tab2 phenocopies Tak1 deficiency. (A and B) no-Cre control, epidermal- or intestinal epithelial-specific single- and double-knockout mice, Sk-Tab1KOTab2Het, Sk-Tab1HetTab2KO, Sk-Tab1Tab2DKO, In-Tab1KOTab2Het, In-Tab1HetTab2KO, and In-Tab1Tab2DKO at postnatal day 5 (skin) or postnatal day 0 (intestine). Epidermal-specific Tak1-deficient mice (Sk-Tak1KO) at P5 are also shown. Eight double-knockout mice from three litters (skin) and 12 double-knockout mice from five litters (intestine) and their control littermate mice were analyzed and representative mice and the intestines are shown. Each separate photograph shows mice or the intestines from one litter. (C) The dorsal skin sections at P5 or cross-sections of intestine at P0 from control, single-deletion, and double-deletion mice were stained with hematoxylin/eosin. (D) The cross-sections of intestine at P0 were stained with Alcian Blue. (E) Apoptotic cells were detected with TUNEL staining (green). Nuclei were stained by DAPI (blue). Open brackets indicate epidermis. (Scale bars, 40 μm.)
Deficiency of TNF Signaling Rescues the Abnormalities in the Tab1 and Tab2 Double-Deficient Epithelium As It Does in the Tak1-Deficient Epithelium.
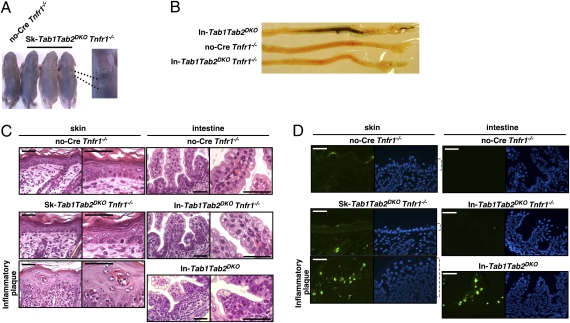
We further evaluated the phenotype of the double deficiency of Tab1 and Tab2. We previously reported that tissue damage in the Tak1-deficient epidermis and intestinal epithelium is largely rescued by TNF receptor 1 (Tnfr1) deficiency (16, 41). We reported that Tak1 deficiency highly increases sensitivity to TNF-induced cell death, and TNF-induced cell death induces inflammation in the Tak1-deficient epithelium . Thus, ablation of TNF signaling prevents cell death and subsequent inflammatory conditions in Sk- and In-Tak1KO mice (16, 41). If double deficiency of Tab1 and Tab2 causes the abnormalities due to reduced activation of TAK1, Sk-Tab1Tab2DKO and In-Tab1Tab2DKO mice should also be rescued by Tnfr1 deletion. We generated Sk-Tab1Tab2DKO and In-Tab1Tab2DKO mice on a Tnfr1−/− background. Sk-Tab1Tab2DKO Tnfr1−/− mice were alive at least until weaning age and did not exhibit pronounced tissue damage at P5 (Fig. 2 A and C). Although we observed spotty inflammation in the skin of Sk-Tab1Tab2DKO Tnfr1−/− mice, a large area in the skin was normal (Fig. 2 A and C). We note here that similar inflammatory plaques are seen in Sk-Tak1 Tnfr1−/− mice (41). The intestinal epithelia from In-Tab1Tab2DKO Tnfr1−/− mice were not distinguishable from those of single-knockout or no-Cre littermate controls at P0 (Fig. 2 B and C), and In-Tab1Tab2DKO Tnfr1−/− mice were alive at least until weaning age. The cell death was greatly reduced by Tnfr1 deficiency in both the epidermis and the intestinal epithelium (Fig. 2D). All observations are identical to those in Sk- and In-Tak1 Tnfr1−/− mice. These results further demonstrate that double deficiency of Tab1 and Tab2 causes the same defects as those caused by the single deficiency of Tak1. Thus, it is likely that TAK1 is activated through TAB1 and TAB2 in the epidermis and the intestinal epithelium.
Fig. 2.
Tnfr1 deficiency rescues abnormalities in Tab1Tab2DKO. (A and B) TNF receptor-deficient control (Tnfr1−/−) and epidermal- or intestinal epithelial-specific triple-knockout (Sk-Tab1Tab2DKO Tnfr1−/− and In-Tab1Tab2DKO Tnfr1−/−) mice at postnatal day 5 (skin) or postnatal day 0 (intestine). Nine (skin) and five (intestine) triple-knockout mice and their littermate mice were analyzed. Representative mice and the intestines are shown. (C) The dorsal skin sections at P5 or cross-sections of intestine at P0 from control and triple-knockout mice were stained with hematoxylin/eosin. (D) Apoptotic cells were detected with TUNEL staining (green). Nuclei were stained by DAPI (blue). Open brackets indicate epidermis. (Scale bars, 40 μm.)
The epidermis and the intestinal epithelium are constantly exposed to commensal bacteria. Thus, we assumed that commensal bacteria-derived innate immune stimuli directly or indirectly activate TAK1 through the Toll-like, TNF, and IL-1 receptors polyubiquitin-TAB2–dependent mechanism in the epithelial cells. However, our results indicate that the TAB2-dependent pathway is only a part of the TAK1 activation mechanisms in the epithelial tissues and that TAB1-dependent activation of TAK1 occurs during normal development. We next attempted to determine what activates TAK1 through TAB1 in the epithelial tissues.
TAB1 but Not TAB2 Is Essential for Phosphorylation-Dependent TAK1 Activation in Epithelial Cells.
To investigate the TAB1-dependent activation of TAK1 in the epithelial tissue, we isolated and cultured skin epithelial cells, keratinocytes, from Sk-Tab1KOTab2Het, Tab1HetTab2KO, and Tab1Tab2DKO and used them to identify the mechanism of TAB1-dependent TAK1 activation. Because we previously reported that TAB1 is important for osmotic stress-induced activation of TAK1 in MEFs (33), we examined whether osmotic stress is the TAB1-dependent activator of TAK1 in epithelial cells. Tab1KOTab2Het, Tab1HetTab2KO, and Tab1Tab2DKO keratinocytes were treated with osmotic stress, 0.5 M NaCl, to examine the activation of TAK1. The activation of TAK1 was monitored by phosphorylation of Thr-187, which is correlated with TAK1 activity (32, 34). The activation of TAK1 was only moderately reduced by Tab1 single or Tab1 and Tab2 double deficiency (Fig. S3). Thus, osmotic stress is not the major TAB1-dependent activator in the epithelial cells.
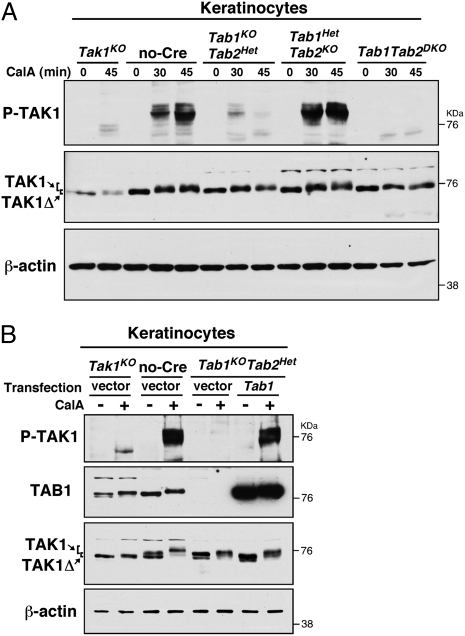
TAK1 is also known to be regulated by type 2A protein phosphatases (34, 36). Inhibitors of protein phosphatase such as calyculin A, which is a potent inhibitor of the type 2A family of protein phosphatases, can greatly up-regulate TAK1 activity even in the absence of stimuli (34). Thus, we assume that TAK1 is constantly deactivated by protein phosphatases and that differences in the levels of protein phosphatases may alter TAK1 activity under basal conditions. We hypothesize that TAK1 activity is basally regulated by protein phosphatases in a TAB1-dependent manner in epithelial tissues. Tak1KO, no-Cre control, Tab1KOTab2Het, Tab1HetTab2KO, and Tab1Tab2DKO keratinocytes were treated with calyculin A (Fig. 3A). We found that Tab1 deficiency greatly reduced calyculin A-induced TAK1 activation, whereas Tab2 deficiency did not affect the level of TAK1 activity. Double deficiency of Tab1 and Tab2 abolished TAK1 activation. Thus, protein phosphatase-dependent TAK1 regulation is largely dependent on TAB1. To confirm the role of TAB1 in the protein phosphatase regulation of TAK1 kinase activity, TAB1 was transiently reexpressed in the Tab1KOTab2Het keratinocytes (Fig. 3B). The protein phosphatase inhibitor-induced TAK1 activation was restored in a TAB1-dependent manner. These results demonstrate that TAK1 kinase activity is regulated by protein phosphatases in a TAB1- but not TAB2-dependent manner in epithelial cells. Thus, we hypothesize that two independent mechanisms, namely TAB1-dependent basal and stimuli-TAB2–dependent mechanisms, activate TAK1 in in vivo epithelial tissues.
Fig. 3.
Inhibition of type 2A protein phosphatases activates TAK1 in a TAB1-dependent manner. (A) Tak1KO, no-Cre, Tab1KOTab2Het, Tab1HetTab2KO, and Tab1Tab2DKO keratinocytes were stimulated with 20 nM calyculin A for 30 or 45 min. Immunoblotting was performed using the indicated antibodies. (B) pCMV-Flag vector or pCMV-Flag-TAB1 was transfected into Tak1KO, no-Cre, or Tab1KOTab2Het keratinocytes and cells were stimulated with 20 nM calyculin A for 30 min. Immunoblotting was performed using the indicated antibodies.
Ablation of Tab1 Largely Down-Regulates and Double Ablation of Tab1 and Tab2 Abolishes TAK1 Activity in the in Vivo Epithelium.
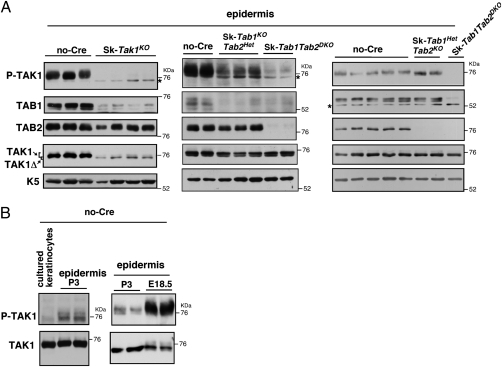
If TAB1-dependent basal regulation of TAK1 is attributed to the in vivo TAK1 activity, TAK1 should be constitutively active in the in vivo epithelium. The protein extracts from SK-Tak1KO, Sk-Tab1KOTab2Het, Sk-Tab1HetTab2KO, and Sk-Tab1Tab2DKO and control no-Cre skin at P3 were prepared and examined for TAK1 activity. We analyzed seven litters; the results from six litters are shown and each lane represents proteins from one mouse in Fig. 4A and Fig. S4. TAK1 activity was found to be high in the control no-Cre skin protein extracts, and epidermal-specific Tak1 deficiency almost completely diminished TAK1 activity (Fig. 4A, Left). The TAK1 activity in the epidermis was much higher than that in cultured keratinocytes (Fig. 4B), indicating that TAK1 is constitutively activated in the in vivo epidermis. Epidermal TAK1 was found to be active in both neonatal and embryonic epidermis (Fig. 4B), indicating that not only environmental stimuli such as commensal bacteria but also other mechanisms activate TAK1 in epidermis. Tab2 deficiency did not reduce the level of TAK1 activity, whereas Tab1 deficiency greatly reduced TAK1 activity (Fig. 4A, Center and Right, and Fig. S4). Double deficiency of Tab1 and Tab2 diminished TAK1 activity (Fig. 4A, Center and Right, and Fig. S4). Importantly, the levels of TAK1 protein were not altered by Tab1 and/or Tab2 deficiency [Fig. 4A, Center and Right (fourth row) and Fig. S4], indicating that TAB1 and/or TAB2 regulate TAK1 activity but not protein stability. The remaining very low TAK1 activity in Tab1Tab2DKO skin may be derived from nonepithelial cells in the skin or from TAB3-dependent activity. These results are consistent with our hypothesis that TAB1-dependent basal activation of TAK1 is important for the maintenance of TAK1 activity in the in vivo epithelium. TAB2 seems to play a compensatory role to maintain the level of TAK1 activity. We note here that the levels of TAB1 protein were reduced in Tak1-deficient epidermis (Fig. 4A, Left, second row), suggesting that TAB1 is stabilized by binding to TAK1.
Fig. 4.
Basal activity of TAK1 in in vivo epidermis is largely dependent on TAB1. (A) Immunoblotting of phosphorylated TAK1, total TAK1, TAB1, and TAB2 in epidermal extracts from no-Cre control, Sk-Tak1KO, Sk-Tab1KOTab2Het, Sk-Tab1HetTab2KO, and Sk-Tab1Tab2DKO mice. Each lane shows proteins from one mouse and each set of panels is a mouse group from the same litter. Keratin 5 (K5) was used as a loading control. Asterisks indicate nonspecific bands. (B) Protein extracts from no-Cre cultured keratinocytes (Left), no-Cre epidermis at P3 (Left and Right), and no Cre epidermis at embryonic day 18.5 (Right) were subjected to immunoblot analysis. Each lane represents a sample from one mouse.
Double Deletion of Tab1 and Tab2 Causes Accumulation of ROS in the Intestinal Epithelium.
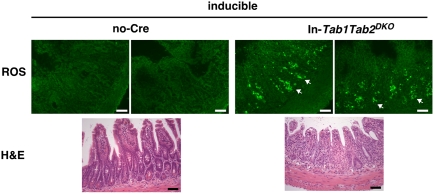
Finally, we asked whether double deficiency of Tab1 and Tab2 causes ROS accumulation as does Tak1 single deletion. To this end, we generated and analyzed an inducible version of In-Tab1Tab2DKO mice, because ROS accumulation is most clearly observed in the Tak1-deficient intestinal epithelium in adult mice (14). We observed highly increased ROS in the Tab1Tab2DKO intestine (Fig. 5 and Fig. S5A). Thus, TAB1- and TAB2-dependent activation of TAK1 is essential for preventing accumulation of ROS. We also examined whether Tnfr1 deficiency rescues ROS accumulation in the adult Tab1Tab2DKO intestine (Fig. S5B). The ROS levels might be less but were not completely reduced by Tnfr1 deficiency, suggesting that ROS are induced not only by TNF but also by other inducers in the adult intestine. This observation is consistent with that in the Tak1-deficient Tnfr1−/− intestinal epithelium (14). Tissue damage was observed in both inducible Sk-Tab1Tab2DKO and inducible In-Tab1Tab2DKO adult mice upon gene deletion (Fig. 5 and Fig. S6 A and B), which is similar to damage in inducible Sk-Tak1KO (15) and In-Tak1KO (16) mice. In the Tab1 and Tab2 double-deficient adult intestinal epithelium, intestinal epithelial cells including paneth cells were damaged and morphologically apoptotic (Fig. S6C).
Fig. 5.
Double deficiency of Tab1 and Tab2 causes ROS accumulation. Shown are ROS detection (Upper) and H&E staining (Lower) in no-Cre control and inducible In-Tab1Tab2DKO adult mice. Mice were treated with tamoxifen for 2 consecutive days and the intestine sections were prepared 2 d after the second tamoxifen treatment. ROS were detected in unfixed fresh frozen intestine sections by CM-H2DCFDA. Photomicrographs are representative images of six mice. Two images from different portions of one mouse are shown. Other images are shown in Fig. S5. Arrows indicate examples of ROS-positive cells. (Scale bars, 50 μm.)
In this study, we demonstrated that TAB1 regulates basal activity of TAK1 and plays an essential role in the maintenance of TAK1 activity in epithelial tissues. TAK1 is constitutively associated with TAB1 in cells (31). Overexpression of TAK1 together with TAB1 highly activates TAK1 (31). As shown in this study, inhibition of protein phosphatases activates TAK1 in a TAB1-dependent manner (Fig. 3). Taken together, we propose that the TAK1-TAB1 complex is an active form of TAK1 and stably presents even under basal conditions, but it is constantly deactivated by type 2A protein phosphatases. Indeed, we have previously demonstrated that one of the type 2A protein phosphatases, PP6, is constitutively associated with TAK1 (34).
Type 2A protein phosphatases are abundantly and ubiquitously expressed in many cell types (42). Alterations in expression levels and/or activities of type 2A protein phosphatases perhaps modulate the activity of the TAK1-TAB1 complex in tissues. It is well established that cytokines and bacteria activate TAK1 through the TAB2-polyubiquitin mechanism, resulting in activation of proinflammatory responses in cells. The TAB2-dependent mechanism is primarily important for acute induction of inflammation in response to insulting challenges such as bacterial infection. However, because TAK1 activity is essential for reduction of ROS and tissue integrity, we assume that this inflammation-associated mechanism is able to play a compensatory role to maintain TAK1 activity. Two independent mechanisms for TAK1 activation are used to securely maintain the TAK1 activity.
Methods
Mice.
Tak1-floxed (Tak1flox/flox) (11), Tab1-floxed (Tab1flox/flox) (43), and Tab2-floxed (Tab2flox/flox) (28) mice were previously described. Tnfr1-deficient (Tnfr1−/−), K5-Cre, Villin-Cre, K14-CreERT, and Villin-CreERT2 mice were obtained from the Jackson Laboratories or as described previously (39, 40, 44, 45). Induction of the Tak1, Tab1, and Tab2 deletion in the inducible versions of hetero- and double-knockout mice was achieved by i.p. injection of tamoxifen (50 mg/kg mouse weight) for 2–3 consecutive days in the intestinal epithelial-specific inducible gene deletion mice and by topical treatment of 4-hydroxy-tamoxifen (1 mg per mouse per day) for 5 consecutive days in the epidermal-specific inducible gene deletion mice. Deletion of the genes was confirmed by PCR. All animal experiments were conducted with the approval of the North Carolina State University Institutional Animal Care and Use Committee.
Cell Culture.
Tab1Tab2DKO, Tab1KOTab2Het, Tab1HetTab2KO, and no-Cre control keratinocytes were isolated from K5-Cre Tab1flox/floxTab2flox/flox, K5-Cre Tab1flox/floxTab2flox/+, K5-Cre Tab1flox/+Tab2flox/flox, and Tab1flox/floxTab2flox/flox mice, respectively, as described previously (41). Spontaneously immortalized keratinocytes derived from the skin of postnatal day 0–2 mice were cultured in Ca2+-free MEM (Lonza) supplemented with 4% Chelex-treated bovine growth serum (HyClone), 10 ng/mL of human or murine epidermal growth factor (Peprotech), and 0.05 mM calcium chloride and penicillin–streptomycin at 33 °C in 8% CO2.
Reagents.
Reagents used were calyculin A (EM Science) and tamoxifen (MP Biomedicals). Polyclonal antibodies were TAK1, TAB1, and TAB2 described previously (13, 46). Phosphorylated TAK1 (Cell Signaling), keratin 5 (Convance), and β-actin (Sigma) were used.
Immunoblotting.
Cell extracts were prepared using a lysis buffer [20 mM Hepes (pH 7.4), 150 mM NaCl, 12.5 mM β-glycerophosphate, 100 nM calyculin A, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 μM aprotinin, and 0.5% Triton X-100] and then incubated for 30 min at 4 °C, resolved on SDS/PAGE, and transferred to Hybond-ECL or Hybond-P membranes (GE Healthcare). The membranes were immunoblotted with various antibodies and the bound antibodies were visualized with horseradish peroxidase-conjugated antibodies against rabbit or mouse IgG, using the ECL or ECL advance Western blotting detection kit (GE Healthcare).
Immunohistochemistry.
dUTP nick-end labeling (TUNEL) assay was performed on formalin-fixed paraffin sections, using an apoptotic cell death detection kit (Promega) according to the manufacturer's instructions. For the detection of intestinal goblet cells, paraffin-embedded sections were stained with Alcian Blue and with Nuclear Fast Red as a counterstain.
Real-Time PCR.
Total RNAs were isolated using an RNeasy Mini kit (Qiagen). cDNA was synthesized using TaqMan reverse transcription reagents (Applied Biosystems). mRNA levels of Tab1, Tab2, and Gapdh were analyzed by real-time PCR with SYBR Green (Applied Biosystems). Tab1 primer, 5′-ACCCTGCTGGTGAGGAACT-3′ and 5′-AGGGACAGAGTCACACTAGTCTT-3′; Tab2 primer, 5′-GGATAGAATAAGCGAAGCCCGGAA-3′ and 5′-CTCTTTGAAGCCGTTCCATCCT-3′; and Gapdh primers, GAAGGTCGCTGTGAACGGA and GTTAGTGGGGTCTCGCTCCT were used. Results were analyzed using the comparative Ct Method. Values were normalized to the level of Gapdh mRNA.
ROS.
Freshly harvested small intestines were embedded and frozen in OCT compound (Sakura Finetechnical) without any fixation. Sections were stained with 5 μM CM-H2DCFDA (Invitrogen) for 40 min at 37 °C. Images were taken using a fluorescent microscope (XM10; Olympus) controlled by CellSens standard (Olympus). At least 10 areas were observed in each section and photographed with the same exposure time.
Supplementary Material
Acknowledgments
We thank S. Akira for Tak1-floxed and Tab2-floxed mice and S. Elliott, L. Hester, and M. Mattmuler for support. This work was supported by National Institutes of Health Grant GM068812 and GM084406 (to J.N.-T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116188109/-/DCSupplemental.
References
- 1.Benz CC, Yau C. Ageing, oxidative stress and cancer: Paradigms in parallax. Nat Rev Cancer. 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 3.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji Y. JunD activates transcription of the human ferritin H gene through an antioxidant response element during oxidative stress. Oncogene. 2005;24:7567–7578. doi: 10.1038/sj.onc.1208901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 8.Pham CG, et al. Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, et al. Role of AP-1 in the coordinate induction of rat glutamate-cysteine ligase and glutathione synthetase by tert-butylhydroquinone. J Biol Chem. 2002;277:35232–35239. doi: 10.1074/jbc.M203812200. [DOI] [PubMed] [Google Scholar]
- 10.Shim JH, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato S, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 12.Takaesu G, et al. TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway. J Mol Biol. 2003;326:105–115. doi: 10.1016/s0022-2836(02)01404-3. [DOI] [PubMed] [Google Scholar]
- 13.Ninomiya-Tsuji J, et al. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 14.Kajino-Sakamoto R, et al. TGF-β-activated kinase 1 signaling maintains intestinal integrity by preventing accumulation of reactive oxygen species in the intestinal epithelium. J Immunol. 2010;185:4729–4737. doi: 10.4049/jimmunol.0903587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omori E, Morioka S, Matsumoto K, Ninomiya-Tsuji J. TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J Biol Chem. 2008;283:26161–26168. doi: 10.1074/jbc.M804513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kajino-Sakamoto R, et al. Enterocyte-derived TAK1 signaling prevents epithelium apoptosis and the development of ileitis and colitis. J Immunol. 2008;181:1143–1152. doi: 10.4049/jimmunol.181.2.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen ZJ, Bhoj V, Seth RB. Ubiquitin, TAK1 and IKK: Is there a connection? Cell Death Differ. 2006;13:687–692. doi: 10.1038/sj.cdd.4401869. [DOI] [PubMed] [Google Scholar]
- 18.Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Xia ZP, et al. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 2009;461:114–119. doi: 10.1038/nature08247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishida S, Sanjo H, Akira S, Matsumoto K, Ninomiya-Tsuji J. TAK1-binding protein 2 facilitates ubiquitination of TRAF6 and assembly of TRAF6 with IKK in the IL-1 signaling pathway. Genes Cells. 2005;10:447–454. doi: 10.1111/j.1365-2443.2005.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanayama A, et al. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Jin G, et al. Identification of a human NF-κB-activating protein, TAB3. Proc Natl Acad Sci USA. 2004;101:2028–2033. doi: 10.1073/pnas.0307314101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung PC, Nebreda AR, Cohen P. TAB3, a new binding partner of the protein kinase TAK1. Biochem J. 2004;378:27–34. doi: 10.1042/BJ20031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishitani T, et al. Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 2003;22:6277–6288. doi: 10.1093/emboj/cdg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz-Sanjuán I, Bell E, Altmann CR, Vonica A, Brivanlou AH. Gene profiling during neural induction in Xenopus laevis: Regulation of BMP signaling by post-transcriptional mechanisms and TAB3, a novel TAK1-binding protein. Development. 2002;129:5529–5540. doi: 10.1242/dev.00097. [DOI] [PubMed] [Google Scholar]
- 27.Broglie P, Matsumoto K, Akira S, Brautigan DL, Ninomiya-Tsuji J. Transforming growth factor β-activated kinase 1 (TAK1) kinase adaptor, TAK1-binding protein 2, plays dual roles in TAK1 signaling by recruiting both an activator and an inhibitor of TAK1 kinase in tumor necrosis factor signaling pathway. J Biol Chem. 2010;285:2333–2339. doi: 10.1074/jbc.M109.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanjo H, et al. TAB2 is essential for prevention of apoptosis in fetal liver but not for interleukin-1 signaling. Mol Cell Biol. 2003;23:1231–1238. doi: 10.1128/MCB.23.4.1231-1238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono K, et al. An evolutionarily conserved motif in the TAB1 C-terminal region is necessary for interaction with and activation of TAK1 MAPKKK. J Biol Chem. 2001;276:24396–24400. doi: 10.1074/jbc.M102631200. [DOI] [PubMed] [Google Scholar]
- 30.Scholz R, et al. Autoactivation of transforming growth factor β-activated kinase 1 is a sequential bimolecular process. J Biol Chem. 2010;285:25753–25766. doi: 10.1074/jbc.M109.093468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem. 2000;275:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- 32.Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-β-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem. 2005;280:7359–7368. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki M, et al. TAK1-binding protein 1, TAB1, mediates osmotic stress-induced TAK1 activation but is dispensable for TAK1-mediated cytokine signaling. J Biol Chem. 2008;283:33080–33086. doi: 10.1074/jbc.M807574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kajino T, et al. Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J Biol Chem. 2006;281:39891–39896. doi: 10.1074/jbc.M608155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson G, et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 36.Kim SI, Kwak JH, Wang L, Choi ME. Protein phosphatase 2A is a negative regulator of transforming growth factor-β1-induced TAK1 activation in mesangial cells. J Biol Chem. 2008;283:10753–10763. doi: 10.1074/jbc.M801263200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li MG, et al. Regulation of the interleukin-1-induced signaling pathways by a novel member of the protein phosphatase 2C family (PP2Cepsilon) J Biol Chem. 2003;278:12013–12021. doi: 10.1074/jbc.M211474200. [DOI] [PubMed] [Google Scholar]
- 38.Hanada M, et al. Regulation of the TAK1 signaling pathway by protein phosphatase 2C. J Biol Chem. 2001;276:5753–5759. doi: 10.1074/jbc.M007773200. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez A, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 40.Madison BB, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 41.Omori E, et al. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssens V, Goris J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inagaki M, et al. Generation of a conditional mutant allele for Tab1 in mouse. Genesis. 2008;46:431–439. doi: 10.1002/dvg.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeffer K, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 45.el Marjou F, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 46.Takaesu G, et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.